Abstract
Shiga-toxigenic Escherichia coli (STEC) O113:H21 strains that lack the locus of enterocyte effacement (LEE) efficiently invade eukaryotic cells in vitro, unlike LEE-positive O157:H7 strains. We used a fliC deletion mutant of the O113:H21 STEC strain 98NK2 (98NK2ΔfliC) to show that invasion of colonic epithelial (HCT-8) cells is heavily dependent on production of flagellin, even though adherence to the cells was actually enhanced in the mutant. Flagellin binds and signals through Toll-like receptor 5 (TLR5), but there was no evidence that either TLR5, the adaptor protein myeloid differentiation primary response gene 88 (MyD88), or the serine kinase interleukin-1 receptor-associated kinase (IRAK) were required for invasion of HCT-8 cells by strain 98NK2, as judged by transfection, RNA knockdown, or inhibitor studies. However, pretreatment of cells with anti-asialo-GM1 significantly decreased 98NK2 invasion (by 40.8%), while neuraminidase treatment (which cleaves terminal sialic acid residues, thus converting GM1 into asialo-GM1) significantly increased invasion (by 70.7%). Pretreatment of HCT-8 cells with either the cholesterol-depleting agent methyl-β-cyclodextrin (MβCD) or the tyrosine kinase inhibitor genistein significantly decreased invasion by 98NK2, indicating a potential role for lipid rafts in the invasion mechanism. Confocal microscopy also showed invading 98NK2 colocalized with lipid raft markers caveolin-1 and GM1. Interestingly, anti-asialo-GM1, neuraminidase, MβCD, and genistein have similar effects on the vestigial level of STEC invasion seen for STEC strain 98NK2ΔfliC, indicating that lipid rafts mediate a common step in flagellin-dependent and flagellin-independent cellular invasion.
INTRODUCTION
Shiga-toxigenic Escherichia coli (STEC) strains are a major cause of severe gastrointestinal disease in humans, as well as of hemolytic-uremic syndrome (HUS), a life-threatening systemic sequela, characterized by a triad of acute renal failure, microangiopathic hemolytic anemia, and thrombocytopenia (14, 34). STEC strains are generally considered to be noninvasive pathogens, and after ingestion and establishment of intestinal colonization, they release Shiga toxin (Stx) into the gut lumen. The Stx is then absorbed into the circulation system, where it targets host cells expressing its specific glycolipid receptor globotriaosylceramide (Gb3) (18). In humans, Gb3 receptors are present at high levels in renal tubular epithelium; significant levels are also expressed in microvascular endothelial cells of the kidney, intestine, pancreas, and brain, particularly after exposure to inflammatory cytokines, and Stx-mediated damage at these sites accounts for the pathological features of STEC disease and HUS (34). Many STEC strains associated with serious pathology in humans, including those belonging to serotype O157:H7, form attaching and effacing (A/E) lesions on enterocytes, a property mediated by the locus of enterocyte effacement (LEE) pathogenicity island (23). It is thought that these lesions contribute significantly to host colonization and disease pathogenesis (22, 24, 40). However, some LEE-negative strains are also responsible for cases of severe disease, including outbreaks of HUS (33, 34). Aside from the production of Shiga toxin (34, 48) and the subtilase cytotoxin (31, 32), the virulence factors important in pathogenicity of LEE-negative strains are poorly defined.
LEE-positive STEC strains such as O157:H7 are generally considered to be noninvasive (14, 25, 43). However, certain clinically isolated LEE-negative STEC strains have been shown to invade Chinese hamster ovary K1 (CHO-K1) cells at levels comparable to that of enteroinvasive E. coli (EIEC) (20). Transmission electron microscopy observed LEE-negative O113:H21 STEC located within a membrane-bound vacuole in CHO-K1 cells, whereas O157:H7 STEC remained extracellular (20). Invasion in CHO-K1 cells was found to be dependent on the host cytoskeleton and required intact actin microfilaments, microtubules, and pan-Rho GTPases, but not tyrosine kinases (20). Invasion assays also showed O113:H21 to be invasive in more relevant cell lines such as Caco-2 and HCT-8 cells (both derived from human colonic epithelium) (20). A large number of the highly invasive clinical isolates in this study were O113:H21 strains, and further studies showed that H21 flagellin was essential for invasion of HCT-8 cells by these strains (19).
Recognition of flagellin is mediated by Toll-like receptor 5 (TLR5) (10). TLR5 signaling, through the adaptor protein myeloid differentiation primary response gene 88 (MyD88) and the interleukin-1 receptor (IL-1R)-associated kinase (IRAK), results in the activation of the mitogen-activated protein kinases (MAPKs) and nuclear factor κB (NF-κB) and the upregulation of several cytokines, including interleukin-8 (IL-8) (2–4). This may also result in the upregulation of receptors on endothelial cells that are involved in mediating inflammatory responses such as the recruitment of neutrophils (21, 28), and the superinduction of IL-8 (12).
We have previously shown that H21 flagellin from the LEE-negative O113:H21 STEC strain 98NK2 was responsible for the majority of IL-8 mRNA and IL-8 protein upregulation in HCT-8 cells by this strain (35). More recently, we have shown that both Shiga toxin 2 (Stx2) and H21 flagellin can synergistically upregulate MAPKs, particularly the stress-activated protein kinases c-Jun N-terminal protein kinases (JNKs) and p38 (12). Interestingly, a 98NK2 mutant with a deletion in the flagellin structural gene fliC (98NK2ΔfliC) was also significantly less virulent than its parent in the streptomycin-treated mouse model of fatal STEC disease (36). There were no differences in the level of intestinal colonization by these strains or the level of recruitment of neutrophils to the site of infection. However, STEC strain 98NK2ΔfliC appeared to be less capable of forming an intimate association with the colonic epithelium (36), consistent with the defect in in vitro invasion. Since flagellin-mediated invasion may be highly important in virulence of LEE-negative STEC, the present study was aimed at determining the mechanism by which 98NK2 utilizes H21 flagellin to invade human colonic epithelial cells in vitro.
MATERIALS AND METHODS
Bacterial strains.
STEC strain 98NK2 (O113:H21) was isolated from a patient with HUS at the Women's and Children's Hospital, Adelaide, South Australia, Australia, as previously described (33). A flagellin deletion mutant of strain 98NK2 (98NK2ΔfliC) has been previously described (35). E. coli strains were routinely cultured in Luria-Bertani broth with appropriate antibiotics as needed.
Reagents and antibodies.
4′,6-Diamidino-2-phenylindole (DAPI), dimethyl sulfoxide (DMSO), filipin, genistein, neuraminidase from Clostridium perfringens, methyl-β-cyclodextrin (MβCD), and rabbit serum were from Sigma Chemical Co. (St. Louis, MO). Blasticidin S HCl, Lipofectamine 2000, and gentamicin were from Invitrogen Life Technologies. Monoclonal anti-hTLR5 (hTLR5 stands for human TLR5) IgA and control IgA2 were from InvivoGen (San Diego, CA). Anti-asialo-GM1 antibody was from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). O113 monospecific O rabbit antiserum was from Statens Serum Institute (Copenhagen, Denmark). Polyclonal mouse anti-caveolin-1 antibody was from BD Transduction Laboratories (Franklin Lakes, NJ). Cholera toxin subunit B (recombinant) Alexa Fluor 594 conjugate, Alexa Fluor 488 donkey anti-rabbit IgG, Alexa Fluor 594 goat anti-mouse IgG, and Alexa Fluor 647 goat anti-rabbit IgG were from Molecular Probes (Invitrogen Life Technologies). Vectashield mounting medium was from Vector Laboratories (Burlingame, CA). Mission small interfering RNAs (siRNAs) directed against MyD88 and TLR5 or universal negative-control siRNAs were obtained from Sigma Chemical Co. All oligonucleotides were from Sigma Chemical Co.
Cell culture media and conditions for HCT-8 cells.
All tissue culture media and reagents were obtained from Gibco BRL-Life Technologies (Grand Island, NY). Unless otherwise indicated, HCT-8 cells were maintained at 37°C in an atmosphere of 5% CO2 in RPMI 1640 medium supplemented with 10 mM HEPES, 1 mM sodium pyruvate, 10% heat-inactivated fetal calf serum (FCS), 50 IU of penicillin per ml, and 50 μg of streptomycin per ml and used at 90 to 100% confluence.
Transfection of HCT-8 cells.
The DNA constructs used in the transfection of HCT-8 cells (pEF6/V5-IRAK Wt [Wt stands for wild type] and DN [DN stands for dominant negative], pEF6/V5-MyD88 DN and pEF6/V5-TLR5 Wt and DN, and the empty vector pEF6/V5-His) were a kind gift from A. T. Gewirtz (Emory University, Atlanta, GA). For transient transfection, HCT-8 cells were seeded in 24-well trays at 2 × 105 cells/well overnight (to 50% confluence). Plasmids were transfected with DNA and Lipofectamine 2000 at a ratio of 1:5 (DNA in micrograms and Lipofectamine 2000 in microliters) for 6 h per the manufacturer's instructions (Invitrogen Life Technologies). After 6 h of incubation, the cells were rescued by adding RPMI 1640 medium containing 20% FCS (no antibiotics) and incubated overnight. The following day, the cells were used in invasion assays. Stable transfection of HCT-8 cells was performed as for transient transfection but with the following modifications. The cells were seeded in 6-well plates at a density of 1 × 106 cells/well and incubated overnight (to 50% confluence). After transfection and overnight incubation as described above, the cells were passaged and selective medium containing 6 μg/ml blasticidin S HCl was added 16 to 48 h after transfection. The cells were left to develop foci, and the medium was changed every 48 to 72 h to maintain selection.
Small interfering RNA knockdown.
RNA-mediated interference for downregulating MyD88 or TLR5 was done by transfection of duplexes of MyD88 siRNA (PDSIRNA 1228812 and 1228813) or TLR5 (PDSIRNA 1262181 and 1262182) (Sigma). The nontargeting Universal Negative Control 1 (SIC001) was used as a control (Sigma). HCT-8 cells were seeded in 6-well trays at 4 × 105 cells/well and incubated overnight (to 20% confluence). The cells were then transfected with 130 nM siRNA per well using Transit TKO reagent per the manufacturer's instructions (Mirus Bio Corp, Madison, WI) in serum- and antibiotic-free RPMI 1640 medium. One milliliter of fresh RPMI 1640 medium (without antibiotics) was added the next morning, and after knockdown, the cells were plated for invasion assays and used at 48 h. Approximately 24 h after knockdown, RNA was extracted from cells, and knockdown was confirmed by real-time PCR with TLR5- or MyD88-specific primers as described below.
RNA extraction and real-time RT-PCR.
RNA was extracted using RNeasy minicolumns and QIAshredder columns per the manufacturer's instructions (Qiagen, Valencia, CA). RNasin RNase inhibitor (Promega, Madison, WI) was added to samples. Contaminating DNA was digested with RNase-free DNase I (Roche Applied Science, Sydney, Australia) per the manufacturer's instructions. The absence of DNA contamination in all RNA preparations was confirmed by reverse transcription-PCR (RT-PCR) analysis using primers specific for the gene encoding the housekeeping enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The levels of mRNA produced by HCT-8 cells were determined by quantitative real-time RT-PCR using the SuperScript III Platinum SYBR green one-step quantitative RT-PCR (qRT-PCR) kit (Invitrogen). The quantitative RT-PCR was performed on a Rotorgene RG-2000 cycler (Corbett Research, Mortlake, New South Wales, Australia). Each RNA sample was assayed in triplicate using primers specific for the internal control GAPDH mRNA and IL-8 mRNA (both sets of primers previously described [35]), TLR5 mRNA (sense [5′-GAGCCCCTACAAGGGAAAAC-3′] and antisense [5′-TGCTGATGGCATTGCTAAAG-3′]), or MyD88 mRNA (42). Results were calculated using the comparative cycle threshold (2ΔΔCT) method, as previously described (35).
Invasion assays.
Invasion assays were performed as described previously, with some modifications (20, 41). HCT-8 cells were seeded in 24-well trays at 1.3 × 105 cells/well and left to attach overnight. STEC strains were grown overnight at 25°C. The following day, overnight cultures of STEC were diluted in RPMI 1640 medium containing 0.5% mannose (no FCS and no antibiotics) and added at approximately 107 CFU/well to washed HCT-8 cells that were >90% confluent and then incubated for 3 h. To determine the total number of adherent bacteria, infected-cell monolayers were gently washed 3 times with phosphate-buffered saline (PBS) followed by detachment by the addition of 100 μl of 0.25% trypsin–0.02% EDTA followed by lysis with 400 μl of 0.025% Triton X-100 per well. Dilutions were plated onto LB agar for quantitation of adherent bacteria. To determine the number of intracellular bacteria, replicate infected HCT-8 cell cultures were washed 3 times with PBS, incubated with 1 ml of 100 μg/ml gentamicin in RPMI 1640 medium for 1 h, washed 3 times with PBS, detached, and lysed as described above before dilutions were plated onto LB agar for quantitation of intracellular bacteria. Where required, HCT-8 cells were pretreated with the appropriate concentration of chemical or antibody (or an appropriate vehicle control) for 60 min prior to infection and maintained in the supplemented medium throughout the invasion period. Adherent bacteria were defined as the percentage of cell-associated bacteria from the original inoculum, and invasive bacteria were defined as the percentage of total adherent bacteria that resist gentamicin treatment. Results are expressed as the percent invasion (mean ± standard deviation [SD]). All experiments shown were performed at least three times with a minimum of duplicate wells in each experiment.
Fluorescence microscopy.
HCT-8 cells were seeded onto 8-well chamber slides (Lab-Tek; Thermo Fisher Scientific, San Jose, CA) at 1.3 × 105 cells/well and allowed to attach overnight (up to 70% confluence). The following day, the cells were washed with PBS and infected with bacteria for up to 3 h. The cells were washed four times with PBS to remove nonadherent bacteria. The cells were fixed in 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 where required. Inside-out staining of bacteria was performed essentially as previously described (16), with some modifications. Bacteria were labeled with O113 monospecific rabbit antiserum (1:250) followed by Alexa Fluor 647 goat anti-rabbit IgG (1:400). The cells were then permeabilized and incubated with O113 antiserum followed by Alexa Fluor 488 donkey anti-rabbit IgG (1:400). In nonpermeabilized cells, after the addition of secondary antibodies conjugated to Alexa Fluor 647, bacteria appear purple, and in permeabilized cells after the addition of secondary antibody conjugated with Alexa Fluor 488, bacteria appear green. Thus, external STEC will have bound both secondary antibodies and be visible as both green and purple (with colocalization shown as white), whereas invaded STEC bacteria were stained green only (and appear green). The nuclei of cells have been counterstained with DAPI. For GM1 colocalization, cells were incubated for 1 h with 5 μg/ml CtxB-Alexa Fluor 594 before proceeding with inside-out staining. For caveolin-1 labeling, fixed and permeabilized cells were incubated with mouse anti-caveolin-1 (1:50) followed by incubation with Alexa Fluor 594 goat anti-mouse (1:400). Images were obtained using a Leica Spectral SP5 confocal microscope (Leica Microsystems, Wetzlar, Germany) at Adelaide Microscopy using a 100× 1.4 numerical aperture oil objective. The pixel intensity was adjusted so that minimal and maximal values were ∼0 and 256, respectively, in each channel. Images were captured and analyzed using LAS AF software (Leica).
Statistical analysis.
Statistical analyses were performed using Prism 5.01 software (GraphPad Software, San Diego, CA). For Fig. 4A and 6B, significance was compared using a one-way analysis of variance (ANOVA) with Tukey's multiple comparison test. For Fig. 1, 3, 4, 5, and 6, comparisons between two values were analyzed using Student's t test. Differences were considered significant at P values of <0.05.
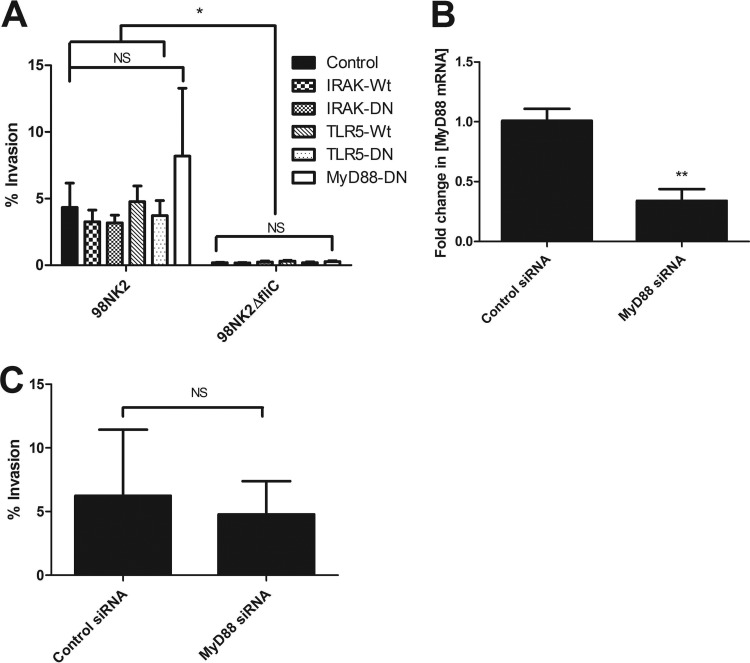
Fig 4.
MyD88 is not involved in invasion of cells by STEC strain 98NK2 or 98NK2ΔfliC. (A) Invasion assays with STEC strains 98NK2 and 98NK2ΔfliC in HCT-8 cells transiently transfected with MyD88-DN, IRAK-Wt, IRAK-Wt, TLR5-DN, or TLR5-Wt approximately 24 to 36 h prior to invasion assays (as described in Materials and Methods). Results shown are the means plus SDs from three independent experiments in duplicate wells. Significant differences are indicated as follows: *, P values ranged from 0.0002 to 0.0494; NS, not significant. (B) HCT-8 cells were transfected with siRNA directed against MyD88 or with negative-control siRNA, and RNA was extracted 24 h later (as described in Materials and Methods). MyD88 mRNA was quantitated by real-time RT-PCR. Results are expressed as the fold increase in the concentration of MyD88 mRNA relative to control cells. Data shown are the means plus SDs from three independent experiments performed in triplicate. Significant differences are indicated as follows: **, P = 0.0028. (C) HCT-8 cells were transfected with siRNA directed against MyD88 or with negative-control siRNA and used in invasion assays performed with strain 98NK2 approximately 48 h later (as described in Materials and Methods). Results shown are the means plus SDs from three independent experiments, each performed in quadruplicate. NS, not significant.
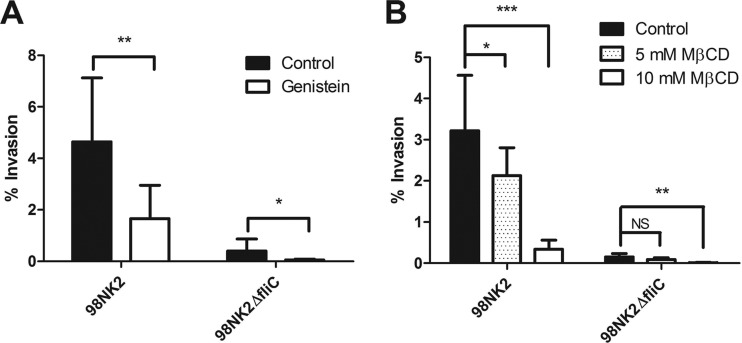
Fig 6.
Role of genistein and MβCD in invasion of HCT-8 cells by STEC strains 98NK2 and 98NK2ΔfliC. (A) HCT-8 cells were pretreated with 100 μg/ml genistein or vehicle control (water) for 1 h prior to infection. Invasion assays were then performed with STEC strain 98NK2 or 98NK2ΔfliC. Results shown are the means plus SDs from three independent experiments in triplicate. **, P = 0.0056; *, P = 0.0459. (B) HCT-8 cells were pretreated with either 5 or 10 mM MβCD or with vehicle control (DMSO) for 1 h prior to infection. Invasion assays were then performed with strain 98NK2 or 98NK2ΔfliC. Results shown are the means plus SDs from three independent experiments in triplicate. ***, P < 0.0001; **, P = 0.0002; *, P = 0.0456; NS, not significant.
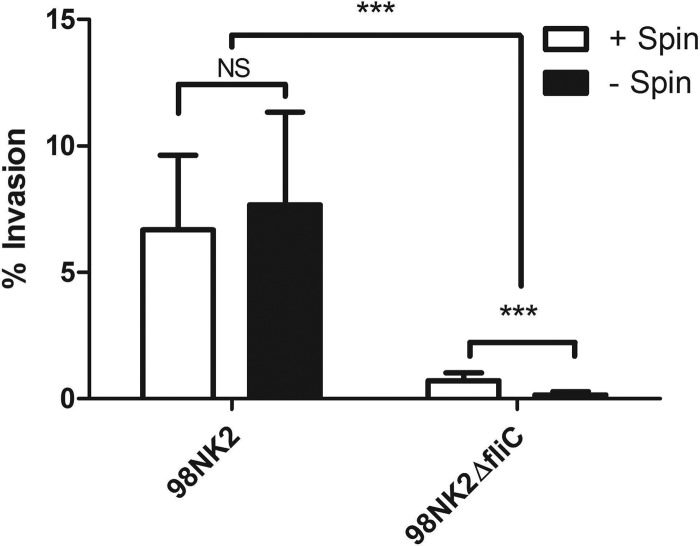
Fig 1.
Invasion of HCT-8 cells by STEC strains 98NK2 and 98NK2ΔfliC. Invasion assays with either 98NK2 or 98NK2ΔfliC were carried out with (+) or without (−) centrifugation (Spin) for 5 min at 150 × g onto HCT-8 cells. Invasion is expressed as the percentage of total adherent bacteria that were resistant to gentamicin. Data are the means plus SDs (error bars) from three independent experiments, each performed in quadruplicate. Values that are significantly different (P < 0.0001) are indicated by brackets and three asterisks. NS, not significant.
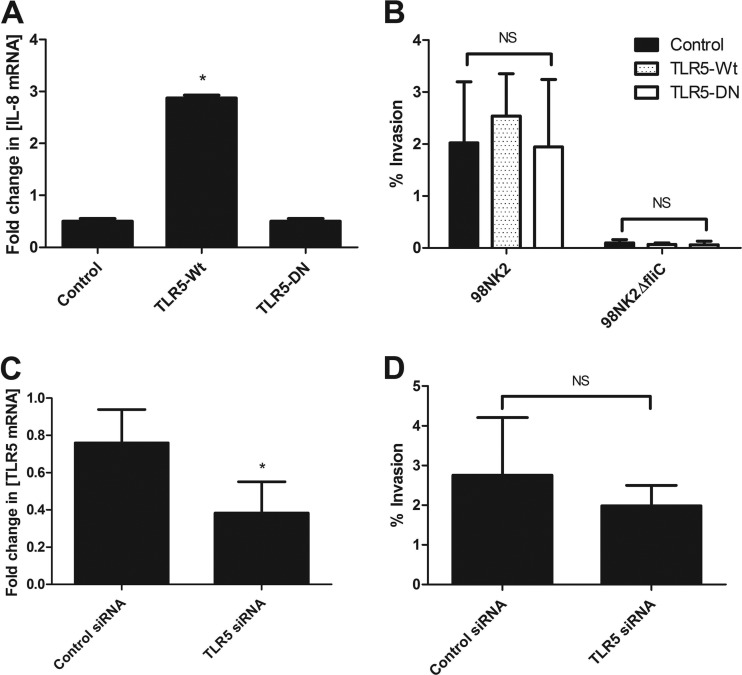
Fig 3.
TLR5 is not involved in invasion of cells by STEC strain 98NK2 or 98NK2ΔfliC. (A) Untransfected HCT-8 cells or cells stably transfected with TLR5-Wt, TLR5-DN, or empty vector (control) were stimulated with strain 98NK2, and RNA was extracted after 4 h. IL-8 mRNA was quantitated by real-time RT-PCR. Results are expressed as the fold increase in the concentration of IL-8 mRNA relative to that in untransfected HCT-8 cells, and data are shown as the means plus SDs for triplicate assays. *, P = 0.0257 compared to control (empty vector) cells or P = 0.0265 compared to cells transfected with TLR5-DN. (B) Invasion assays with STEC strains 98NK2 and 98NK2ΔfliC in HCT-8 cells stably transfected with TLR5-Wt, TLR5-DN, or empty vector. Data shown are the means plus SDs from three independent experiments, each performed in at least duplicate assays (n = 8). NS, not significant. (C) HCT-8 cells were transfected with siRNA directed against TLR5 or with negative-control siRNA, and RNA was extracted 24 h later (as described in Materials and Methods). TLR5 mRNA was quantitated by real-time RT-PCR. Results are expressed as the fold increase in the concentration of TLR5 mRNA relative to control cells. Data shown are the means plus SDs from three independent experiments performed in triplicate. *, P = 0.0106. (D) HCT-8 cells were transfected with siRNA directed against TLR5 or with negative-control siRNA and used in invasion assays performed with strain 98NK2 approximately 48 h later (as described in Materials and Methods). Results shown are the means plus SDs from three independent experiments, each performed in quadruplicate. NS, not significant.
Fig 5.
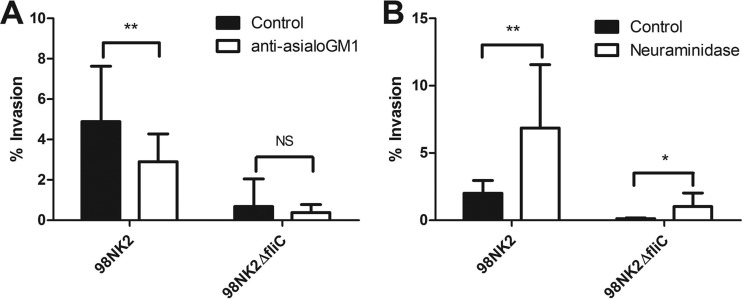
Role of asialo-GM1 and neuraminidase in invasion of HCT-8 cells by STEC strains 98NK2 and 98NK2ΔfliC. (A) HCT-8 cells were pretreated with anti-asialo-GM1 antibody (1:100) or normal rabbit serum (control) for 1 h prior to infection. Invasion assays were then performed with STEC strain 98NK2 or 98NK2ΔfliC. Results shown are the means plus SDs from five independent experiments, each performed in triplicate. **, P = 0.0093; NS, not significant. (B) HCT-8 cells were pretreated with 2.5 U/ml neuraminidase or with vehicle control (PBS containing 25 mM KCl [pH 6.0]) for 1 h prior to infection. Invasion assays were then performed with 98NK2 or 98NK2ΔfliC. Results shown are the means plus SDs from three independent experiments, each performed at least in triplicate. **, P = 0.0051; *, P = 0.0115.
RESULTS
HCT-8 cell invasion by STEC strains 98NK2 and 98NK2ΔfliC.
In an initial experiment, HCT-8 cell monolayers were infected with 107 CFU/ml 98NK2 or 98NK2ΔfliC, and total bacterial adherence and invasion were assayed as described in Materials and Methods. Interestingly, strain 98NK2ΔfliC showed significantly higher adherence than wild-type strain 98NK2 (81.4% ± 5.0% and 48.7% ± 9.0% of the original inocula, respectively [P = 0.004]2). However, 7.67% of the adhered wild-type 98NK2 were able to invade HCT-8 cells compared with only 0.16% for 98NK2ΔfliC (P < 0.0001) (Fig. 1). To ensure that this 48-fold difference in invasion was not due to a defect in the ability of (nonmotile) 98NK2ΔfliC to make close contact with HCT-8 cells, assays were also conducted in which bacteria were centrifuged onto the HCT-8 monolayers at the commencement of the 3-h incubation period. Centrifugation of 98NK2ΔfliC onto HCT-8 monolayers resulted in a significant increase in both adherence (3.6-fold) (P = 0.0003) (data not shown) and invasion (4.4-fold) (P < 0.0001) (Fig. 1) compared to noncentrifuged monolayers. However, 98NK2ΔfliC was still 9.4-fold less invasive than 98NK2 (0.71% versus 6.68%, respectively [P < 0.0001]; Fig. 1). In contrast, centrifugation of 98NK2 onto HCT-8 monolayers had no significant effect on invasion, although adherence did increase 1.8-fold (P = 0.0131) (data not shown). These data suggest that although H21 flagellin is not required for adherence, it plays an important role in the invasion of HCT-8 cells by 98NK2.
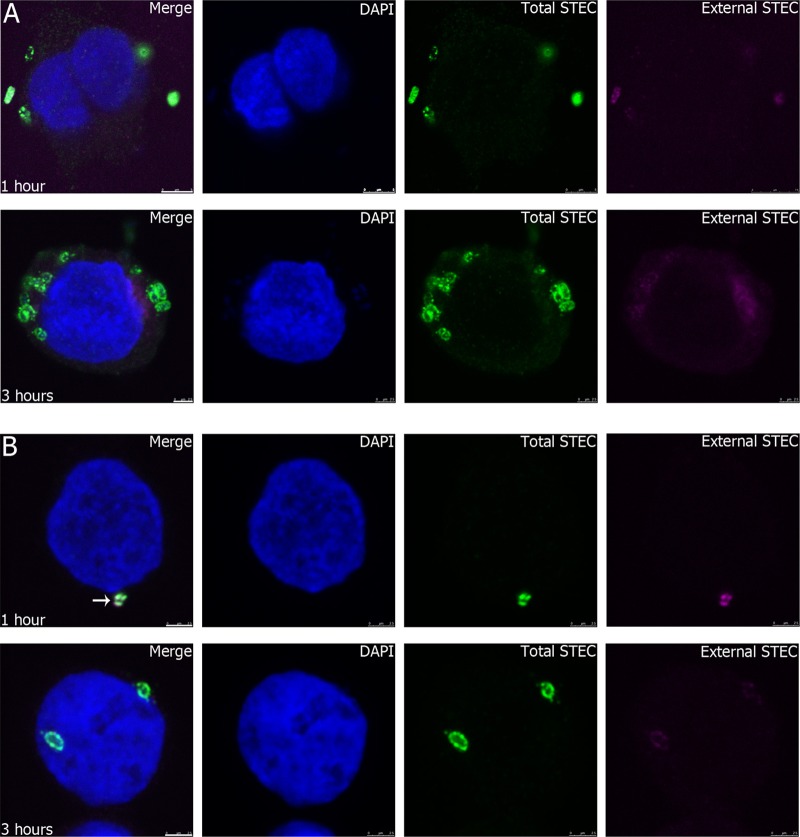
Invasion of HCT-8 cells infected with either 98NK2 or 98NK2ΔfliC was also investigated microscopically using “inside-out” staining with polyclonal anti-O113 rabbit serum to label bacteria followed by secondary antibodies tagged with either Alexa Fluor 647 or Alexa Fluor 488 used before and after permeabilization of the HCT-8 cells, respectively. This distinguishes between internal bacteria (stained with Alexa Fluor 488 only) and external bacteria (stained largely with Alexa Fluor 647) (see Materials and Methods). At 1 h after infection with STEC strain 98NK2, there was a mean of 1.7 ± 1.2 bacteria associated with each cell (the maximum observed for any single cell was 11), and many of these bacteria were already intracellular. By 3 h postinfection, the majority of 98NK2 bacteria associated with HCT-8 cells were intracellular (mean, 2.6 ± 1.6 bacteria/cell) (Fig. 2A). However, for 98NK2ΔfliC-infected cells, there were significantly fewer bacteria associated with HCT-8 cells at 1 h (mean, 1.1 ± 0.3 bacteria/cell) (P < 0.0001) than for 98NK2-infected cells, and there was a higher proportion of extracellular bacteria than for 98NK2-infected cells (Fig. 2B). At 3 h, there were still significantly fewer bacteria/cell than for 98NK2-infected cells (mean, 1.4 ± 0.7) (P < 0.0001), and the majority of the 98NK2ΔfliC bacteria still showed some binding to both the secondary antibodies, implying that they remain at least partially extracellular, even 3 h postinfection (Fig. 2B). This finding appears to differ from the adherence data determined by viable counts referred to previously, where STEC strain 98NK2ΔfliC showed increased adherence (but poorer invasion) relative to the wild-type strain. This may be attributable to the greater number of washing steps in the immunofluorescence experiments or perhaps to differences in the adherence of strain 98NK2ΔfliC to >90% confluent monolayers versus isolated cells. The fields shown for 98NK2ΔfliC are not representative, as the majority of HCT-8 cells did not have any bound bacteria. Indeed, only 10.4% of cells had adherent 98NK2ΔfliC at 1 h compared to 38.1% for 98NK2 (P < 0.0001). At 3 h postinfection, 71.8% of HCT-8 cells had bound 98NK2, whereas only 41.1% of cells had 98NK2ΔfliC (P < 0.0001). These data support the previous data showing that there are marked differences in the ability of 98NK2 to invade HCT-8 cells, with 98NK2 invading more frequently and earlier than 98NK2ΔfliC.
Fig 2.
Association of STEC strains 98NK2 and 98NK2ΔfliC with HCT-8 cells. HCT-8 cells were infected with STEC strain 98NK2 (A) or 98NK2ΔfliC (B) for 1 or 3 h in 8-well chamber slides. Slides were fixed and processed for inside-out immunofluorescence, as described in Materials and Methods. Nuclei are blue (DAPI), extracellular STEC is purple (Alexa Fluor 647), and total STEC is green (Alexa Fluor 488). Bars, 5 μm (top) and 2.5 μm (bottom) (A) and 2.5 μm (B). The white arrow in the merged image in panel B shows colocalized fluorescence indicative of external STEC.
TLR5 is not involved in invasion of HCT-8 cells by STEC strain 98NK2.
To investigate whether flagellin-mediated invasion of cells by STEC strain 98NK2 is either directly or indirectly dependent on TLR5, the only known Toll-like receptor capable of binding flagellin, HCT-8 cells were stably transfected with plasmids expressing wild-type TLR5 (TLR5-Wt) or dominant-negative TLR5 (TLR5-DN) or control plasmid (empty vector) (see Materials and Methods). After selection and expansion in the presence of blasticidin, stable cells were screened for their ability to induce IL-8 mRNA expression in the presence of H21 flagellin. The cells were infected with 98NK2, and RNA was extracted and analyzed by real-time RT-PCR. Infected HCT-8 cells expressing TLR5-Wt showed a significant (5.7-fold) increase in IL-8 mRNA compared to empty vector control cells (P = 0.0257) (Fig. 3A). This increase in TLR5 -signaling pathway activity is most likely attributable to an increase in TLR5 expression by these cells. Infection of HCT-8 cells expressing TLR5-DN showed a 0.49-fold change in IL-8 mRNA compared to Wt HCT-8 cells, which was almost identical to that seen for the control (empty-vector-transfected) cells (0.50-fold change) (Fig. 3A). This suggests that the TLR5-DN construct may be ineffective.
Invasion assays performed with these cells showed that there were no significant differences in the level of invasion of cells stably expressing TLR5-Wt or TLR5-DN or control cells, by either 98NK2 or 98NK2ΔfliC. However, STEC strain 98NK2 was significantly more invasive than STEC strain 98NK2ΔfliC in all cell lines (P < 0.001) (Fig. 3B). These results suggest that increasing TLR5 expression does not impact on HCT-8 invasion by 98NK2. However, since IL-8 mRNA expression in response to 98NK2 infection was not reduced in cells transfected with plasmid expressing TLR5-DN, a role for TLR5 in invasion could not be eliminated.
Accordingly, we adopted an independent RNA interference (RNAi) knockdown approach (see Materials and Methods). Knockdown of TLR5 by siRNA transfection significantly decreased the expression of TLR5 mRNA by 51.3% ± 13.5% compared with negative-control siRNA-treated cells (P = 0.0106) (Fig. 3C). Invasion assays showed that knockdown of TLR5 resulted in no significant differences in either the adherence or invasion of cells by STEC strain 98NK2 compared with control cells (Fig. 3D). Furthermore, preincubation of HCT-8 cells with 10 μg/ml of an anti-human TLR5 antibody known to neutralize TLR5 signaling, or isotype control, did not significantly inhibit invasion either by 98NK2 (2.29% ± 0.65% versus 1.75 ± 0.35%, respectively) or 98NK2ΔfliC (0.12% ± 0.01% versus 0.13% ± 0.07%, respectively). Collectively, these data strongly suggest that TLR5 is not essential for flagellin-mediated invasion of HCT-8 cells by 98NK2.
MyD88 is not required for flagellin-mediated invasion of HCT-8 cells.
All TLRs can utilize the adaptor protein MyD88 and signal through the serine kinase IRAK. We therefore examined the effect of MyD88/IRAK on invasion by transiently transfecting HCT-8 cells with plasmids expressing IRAK-Wt and IRAK-DN, MyD88-DN, and TLR5-Wt and TLR5-DN or with control (empty vector) plasmids and then using them for invasion assays 24 h later. There were no significant differences in the ability of either 98NK2 or 98NK2ΔfliC to invade the transfected cells compared to control cells (Fig. 4A). However, STEC strain 98NK2 was significantly more invasive than STEC strain 98NK2ΔfliC in all cell types, except MyD88-DN (P values ranged from 0.0007 to 0.0494). Transient transfection of HCT-8 cells with TLR5-Wt and -DN constructs also confirmed the results for TLR5 obtained above using stable transfectants. Cotransfection with pEGFP (EGFP stands for enhanced green fluorescent protein) showed that the transfection efficiency in HCT-8 cells was relatively low (15 to 20%), which may have masked small differences in invasion. Nevertheless, the absence of any difference between cells transfected with wild-type versus dominant-negative constructs mitigates against any significant involvement.
RNAi knockdown was also used to examine the involvement or noninvolvement of MyD88 in invasion of HCT-8 cells by STEC strain 98NK2. Transfection with MyD88 siRNA significantly decreased the expression of MyD88 mRNA by 66.4% ± 7.5% compared with negative-control cells (P = 0.0028) (Fig. 4B). Invasion assays performed with these cells showed that knockdown of MyD88 had no significant effect on invasion by 98NK2 compared to invasion of control cells (Fig. 4C). Collectively, the above data show that flagellin-mediated invasion of HCT-8 cells by 98NK2 is independent of both MyD88 and IRAK.
Asialo-GM1 is involved in invasion of cells by STEC strain 98NK2.
In addition to TLR5, the glycolipids/gangliosides asialo-GM1, GM1, and GD1a have been shown to act as coreceptors for Pseudomonas aeruginosa flagellin (26). Accordingly, invasion assays were conducted using HCT-8 cells pretreated with polyclonal rabbit anti-asialo-GM1 or control rabbit serum. Pretreatment with anti-asialo-GM1 significantly decreased the invasion of STEC strain 98NK2 into HCT-8 cells by 40.8% compared to control antibody-treated cells (P = 0.0093) (Fig. 5A) without affecting levels of adherence (not shown). Pretreatment of cells with anti-asialo-GM1 did not significantly reduce invasion by STEC strain 98NK2ΔfliC, although the percentage was lower (Fig. 5A). Invasion was also investigated in HCT-8 cells pretreated with neuraminidase to cleave terminal sialic acid residues from gangliosides, including GM1, thereby generating additional asialo-GM1 on the cell surface. Pretreatment with neuraminidase significantly increased invasion by strain 98NK2 by 70.7% compared to untreated control cells (P = 0.0051; Fig. 5B). Interestingly, invasion of neuraminidase-treated cells by strain 98NK2ΔfliC was also increased by 88.6% (P = 0.0115). No significant differences were observed in adherence of either 98NK2 or 98NK2ΔfliC to neuraminidase-treated or control cells (not shown). Together these data suggest that asialo-GM1 and/or other desialyated gangliosides play an important role in invasion of HCT-8 cells by 98NK2.
Lipid rafts and invasion of cells by STEC strain 98NK2.
The above findings raise the possibility that invasion of HCT-8 cells by STEC strain 98NK2 is dependent on lipid raft-mediated signaling pathways, as reported for other pathogens (17). To examine this, HCT-8 cells were preincubated with the lipid raft inhibitors MβCD (which depletes cholesterol) and genistein (which inhibits tyrosine kinases) before the addition of bacteria for invasion assays. Preincubation with genistein significantly decreased the invasion of cells by strain 98NK2 by 64.4% compared to control (vehicle-treated) cells (P = 0.0056) (Fig. 6A). Preincubation with MβCD also reduced invasion of cells by strain 98NK2 in a dose-dependent manner (Fig. 6B). Invasion of cells by 98NK2 was decreased by 89.5% (P < 0.0001) with 10 mM MβCD and by 33.9% with 5 mM MβCD (P = 0.0456). There were no significant differences in the adherence of 98NK2 with any of the treatments compared to control cells (data not shown). Interestingly, the vestigial level of invasion of HCT-8 cells by STEC strain 98NK2ΔfliC was also significantly inhibited by pretreatment with genistein (P = 0.0459) (Fig. 6A) or 10 mM MβCD (P = 0.0002) (Fig. 6B). Interestingly, the decreased invasion in the presence of 10 mM MβCD occurred in spite of a significant increase in adherence of 98NK2ΔfliC to HCT-8 cells under these conditions compared to control (vehicle-treated) cells (P = 0.0031) (data not shown). These data suggest that invasion of cells by strain 98NK2 is dependent on both cholesterol and tyrosine kinase and, therefore, most likely lipid raft mediated, and that lipid rafts may also be involved in flagellin-independent invasion in both 98NK2 and 98NK2ΔfliC.
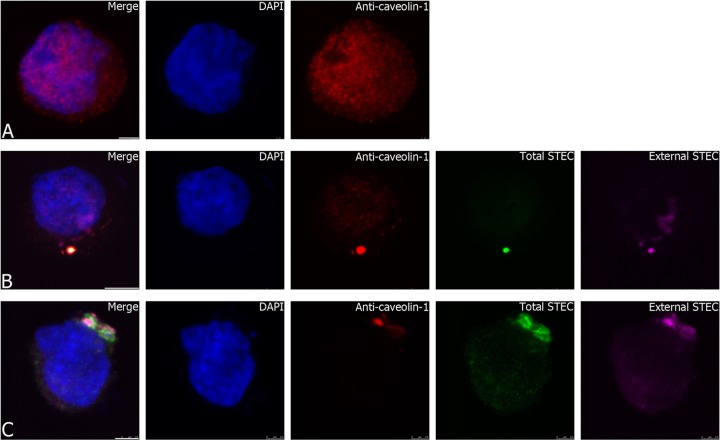
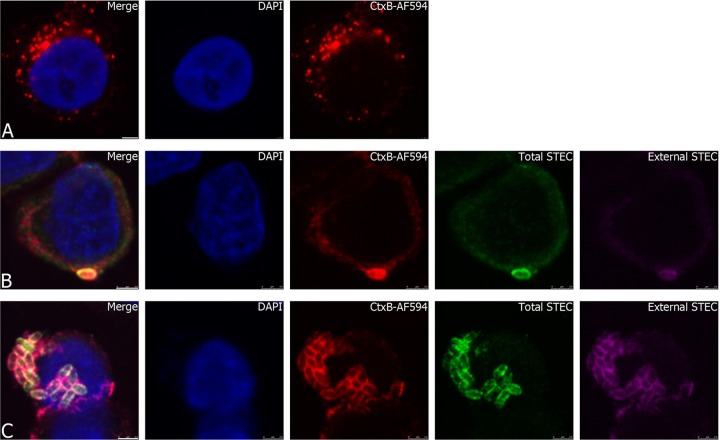
To further examine the association between invading STEC strain 98NK2 and lipid rafts, colocalization of bacteria with lipid raft markers caveolin-1 and GM1 was examined at 1 to 3 h postinfection. Uninfected HCT-8 cells showed a ubiquitous distribution of caveolin-1 (Fig. 7A). In contrast, HCT-8 cells infected with strain 98NK2 showed accumulation and colocalization of caveolin-1 around bacteria in the process of invading cells (Fig. 7B and C). Quantitative analysis of confocal images indicated 100% and 94.7% colocalization of total STEC and anti-caveolin-1 signals at 1 and 3 h, respectively. HCT-8 cells were also treated with Alexa Fluor 594-labeled cholera toxin B subunit (594-CtxB), which specifically binds GM1 in lipid rafts. In uninfected HCT-8 cells, 594-CtxB staining was not homogeneous, but it was nevertheless widely distributed (Fig. 8A). However, in HCT-8 cells infected with 98NK2, 594-CtxB staining colocalized strongly with invading bacteria from 2 h after infection (Fig. 8B and C). Quantitative analysis indicated 99.9% and 97.2% colocalization of total STEC and CtxB signals at 2 and 3 h, respectively. Neither anti-caveolin-1 nor 594-CtxB stained 98NK2 in the absence of HCT-8 cells (results not shown). These data strongly implicate lipid rafts in invasion of HCT-8 cells by 98NK2.
Fig 7.
Association of STEC strain 98NK2 with the lipid raft marker caveolin-1. (A) Uninfected HCT-8 cells in chamber slides were fixed and processed for caveolin-1 labeling as described in Materials and Methods. (B and C) HCT-8 cells were infected with strain 98NK2 for 1 h (B) or 3 h (C) in chamber slides. Slides were fixed and processed for inside-out immunofluorescence and caveolin-1 labeling as described in Materials and Methods. Nuclei are blue (DAPI), extracellular STEC is purple (Alexa Fluor 647), total STEC is green (Alexa Fluor 488), and caveolin-1 is red (Alexa Fluor 594). Bars, 2.5 μm (A and C) and 5 μm (B).
Fig 8.
Association of STEC strain 98NK2 with the lipid raft marker GM1. (A) Uninfected HCT-8 cells in chamber slides were incubated for 1 h at 37°C in the presence of CtxB-AF594 and then fixed and processed as described in Materials and Methods. Bar, 2.5 μm. (B and C) HCT-8 cells were infected with 98NK2 for 2 h (B) or 3 h (C) in chamber slides and also incubated with CtxB-AF594 (red) for 1 h at 37°C. Slides were then fixed and processed for inside-out immunofluorescence as described in Materials and Methods. Nuclei are blue (DAPI), extracellular STEC is purple (Alexa Fluor 647), and total STEC is green (Alexa Fluor 488). Bars, 2.5 μm.
DISCUSSION
Most STEC strains associated with severe human disease (e.g., strains belonging to serotype O157:H7) are LEE positive and are generally thought to be noninvasive pathogens. These strains colonize the human gut, adhere intimately to intestinal epithelial cells, and also inject effector proteins into host cells via the LEE-encoded type III secretion apparatus. However, a distinct group of STEC strains that lack the LEE locus (e.g., serotype O113:H21) are also capable of causing severe human disease, and these strains utilize distinct mechanisms for colonization of the gut. Unlike O157:H7 strains, O113:H21 STEC strains are capable of invading intestinal epithelial cells in vitro in a process that is largely dependent on expression of flagellin, and this may be an important mechanism of colonization. In previous studies, we have shown that expression of flagellin facilitates intimate contact of the O113:H21 strain 98NK2 with colonic epithelium and confers increased lethality in a streptomycin-treated mouse model (37). Many other intestinal pathogens, including atypical enteropathogenic E. coli (EPEC) (37), Listeria monocytogenes (7, 30), and Salmonella enterica serovar Typhimurium (15, 45), utilize flagellin (to some degree) to invade host cells. Likewise, uropathogenic E. coli (UPEC) utilizes flagellin for invasion of renal collecting duct cells (35). However, the precise mechanism whereby flagellin facilitates invasion by these diverse pathogens is not well understood.
We previously showed that H21 flagellin from STEC strain 98NK2 is an important stimulator of inflammatory cytokines and chemokines, in particular IL-8 (35), and that NF-κB, extracellular signal-regulated kinases (ERKs), and JNKs are required for flagellin-induced transcription of IL-8 message, while the role of p38 was posttranscriptional (12). Since flagellin signaling is mediated through TLR5, we hypothesized that interaction with this Toll-like receptor may be essential for triggering invasion of host cells by STEC strains such as 98NK2. However, experiments with TLR5 transfections, RNA knockdowns, and blocking antibodies failed to provide any evidence in support of this proposition. We also examined the role of the adaptor protein MyD88, through which all TLRs can signal. MyD88 knockdown, which was more efficient than TLR5 knockdown, again showed that MyD88 was not involved in invasion of 98NK2 in HCT-8 cells. Similarly, IRAK, which functions downstream of MyD88 in TLR signaling pathways, appeared to have no significant role in invasion. Collectively, these data strongly mitigate against involvement of TLR5 or any other TLRs in flagellin-mediated invasion of HCT-8 cells by 98NK2.
The glycolipids/gangliosides asialo-GM1, GM1, and GD1a are also potential receptors for flagellin (9). It has previously been shown that the flagellin of Pseudomonas aeruginosa can induce IL-8 signaling in cells in an asialo-GM1-dependent manner (1). It was initially thought that asialo-GM1 may be a coreceptor for TLR5 (1, 29), but this was subsequently found not to be the case (26, 46). Our data clearly show that blocking asialo-GM1 in HCT-8 cells with exogenous antibody significantly decreases invasion by STEC strain 98NK2. Conversely, desialating gangliosides by pretreating HCT-8 cells with neuraminidase, thereby presumably increasing the amount of asialo-GM1 on the surface, significantly increased invasion. Interestingly, parallel effects were also seen with these two treatments when invasion by STEC strain 98NK2ΔfliC was assayed. Anti-asialo-GM1 reduced invasion by strain 98NK2ΔfliC to a degree similar to that seen for strain 98NK2, although the low total level of invasion in the absence of flagellin meant that this did not reach statistical significance. However, the stimulation of invasion by 98NK2ΔfliC by neuraminidase treatment was statistically significant. Thus, asialo-GM1 appears to play an important role in invasion of HCT-8 cells by 98NK2, and it appears that this effect is not absolutely dependent on flagellin.
Glycosphingolipids such as GM1 and asialo-GM1 are important components of lipid rafts (17, 47), which are specialized cholesterol-rich membrane microdomains that serve as organizing centers for the assembly of signaling molecules. This raised the question of whether invasion of HCT-8 cells by STEC strain 98NK2 might be triggered by interactions with lipid raft-associated cell surface receptors. Pretreating HCT-8 cells either with MβCD, which disrupts lipid rafts by depleting cholesterol, or with genistein, a tyrosine kinase inhibitor that blocks raft-mediated signaling, significantly decreased invasion by strain 98NK2. Further evidence for lipid raft-mediated invasion was also obtained by fluorescence colocalization of invading 98NK2 with the lipid raft markers caveolin-1 and GM1 on the HCT-8 cell surface.
Lipid rafts have been shown to be involved in cellular invasion by several enteric pathogens, including EPEC (6), Shigella flexneri (5, 16), Salmonella enterica serovar Typhimurium (5), Listeria monocytogenes (38), and Campylobacter jejuni (13). Lipid rafts are often used by bacteria as signaling platforms, since they contain a concentration of transmembrane and lipid-anchored proteins that are important in several different signaling pathways (8, 17, 47). Flagellin from P. aeruginosa is also able to elicit host cell responses, including ERK phosphorylation, through binding to asialo-GM1 (27). However, asialo-GM1 lacks both transmembrane and intracellular domains and therefore cannot directly contact cytoplasmic signaling platforms, implying the involvement of other macromolecules in the process (27). Interestingly, some TLRs have been shown to be recruited to lipid rafts, including TLR4 (44) and TLR2 (39). Thus, it is tempting to speculate that binding of STEC strain 98NK2 to asialo-GM1 leads to the recruitment of TLR5 to lipid rafts and facilitation of intracellular signaling. Indeed, secretion of IL-8 by A549 cells in response to S. Typhimurium flagellin is dependent on lipid raft formation (11). Asialo-GM1 and flagellin have also been shown to cooperatively induce nucleotide signaling to activate Erk1/2 (26).
In the present study, pretreatment of HCT-8 cells with MβCD, genistein, or anti-asialo-GM1 also significantly decreased invasion by STEC strain 98NK2ΔfliC, while treatment with neuraminidase significantly increased 98NK2ΔfliC invasion, although overall invasion was still massively reduced compared to that for strain 98NK2. Thus, both flagellin-dependent and flagellin-independent invasion utilize a common lipid raft-mediated cellular signaling pathway. At present, the identity of the nonflagellar 98NK2 ligand required for the vestigial invasion activity is unknown, although type I pili can be eliminated from the list of candidates, since culture media used in these studies contained mannose to prevent type I pilus-mediated binding to cells. Thus, although we have clearly shown a role for asialo-GM1 and lipid rafts, but not TLRs, in invasion of HCT-8 cells by STEC strain 98NK2, further studies are needed to fully elucidate the mechanism of cellular invasion by these highly virulent LEE-negative STEC strains.
ACKNOWLEDGMENTS
This work was supported by Program Grant 565526 from the National Health and Medical Research Council of Australia (NHMRC). A.W.P. is an Australian Research Council DORA Fellow; J.C.P. is a NHMRC Australia Fellow.
Footnotes
Published ahead of print 11 June 2012
REFERENCES
- 1. Adamo R, Sokol S, Soong G, Gomez MI, Prince A. 2004. Pseudomonas aeruginosa flagella activate airway epithelial cells through asialoGM1 and Toll-like receptor 2 as well as Toll-like receptor 5. Am. J. Respir. Cell Mol. Biol. 30:627–634 [DOI] [PubMed] [Google Scholar]
- 2. Aderem A, Ulevitch RJ. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782–787 [DOI] [PubMed] [Google Scholar]
- 3. Akira S, Takeda K, Kaisho T. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675–680 [DOI] [PubMed] [Google Scholar]
- 4. Armant MA, Fenton MJ. 2002. Toll-like receptors: a family of pattern-recognition receptors in mammals. Genome Biol. 3:3011–3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brumell JH, Grinstein S. 2003. Role of lipid-mediated signal transduction in bacterial internalization. Cell. Microbiol. 5:287–297 [DOI] [PubMed] [Google Scholar]
- 6. Bulgin R, et al. 2009. The T3SS effector EspT defines a new category of invasive enteropathogenic E. coli (EPEC) which form intracellular actin pedestals. PLoS Pathog. 5:e1000683 doi:10.1371/journal.ppat.1000683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dons L, et al. 2004. Role of flagellin and the two-component CheA/CheY system of Listeria monocytogenes in host cell invasion and virulence. Infect. Immun. 72:3237–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dykstra M, Cherukuri A, Sohn HW, Tzeng SJ, Pierce SK. 2003. Location is everything: lipid rafts and immune cell signaling. Annu. Rev. Immunol. 21:457–481 [DOI] [PubMed] [Google Scholar]
- 9. Feldman M, et al. 1998. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect. Immun. 66:43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hayashi F, et al. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099–1103 [DOI] [PubMed] [Google Scholar]
- 11. Im J, et al. 2009. Induction of IL-8 expression by bacterial flagellin is mediated through lipid raft formation and intracellular TLR5 activation in A549 cells. Mol. Immunol. 47:614–622 [DOI] [PubMed] [Google Scholar]
- 12. Jandhyala DM, et al. 2010. Shiga toxin 2 and flagellin from Shiga-toxigenic Escherichia coli superinduce interleukin-8 through synergistic effects on host stress-activated protein kinase activation. Infect. Immun. 78:2984–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kalischuk LD, Inglis GD, Buret AG. 2009. Campylobacter jejuni induces transcellular translocation of commensal bacteria via lipid rafts. Gut Pathog. 1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaper JB, Nataro JP, Mobley HL. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123–140 [DOI] [PubMed] [Google Scholar]
- 15. Kim M, Lim S, Kim D, Choy HE, Ryu S. 2009. A tdcA mutation reduces the invasive ability of Salmonella enterica serovar Typhimurium. Mol. Cells 28:389–395 [DOI] [PubMed] [Google Scholar]
- 16. Lafont F, Tran Van Nhieu G, Hanada K, Sansonetti P, van der Goot FG. 2002. Initial steps of Shigella infection depend on the cholesterol/sphingolipid raft-mediated CD44-IpaB interaction. EMBO J. 21:4449–4457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lafont F, van der Goot FG. 2005. Bacterial invasion via lipid rafts. Cell. Microbiol. 7:613–620 [DOI] [PubMed] [Google Scholar]
- 18. Lindberg AA, et al. 1987. Identification of the carbohydrate receptor for Shiga toxin produced by Shigella dysenteriae type 1. J. Biol. Chem. 262:1779–1785 [PubMed] [Google Scholar]
- 19. Luck SN, Badea L, Bennett-Wood V, Robins-Browne R, Hartland EL. 2006. Contribution of FliC to epithelial cell invasion by enterohemorrhagic Escherichia coli O113:H21. Infect. Immun. 74:6999–7004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luck SN, Bennett-Wood V, Poon R, Robins-Browne RM, Hartland EL. 2005. Invasion of epithelial cells by locus of enterocyte effacement-negative enterohemorrhagic Escherichia coli. Infect. Immun. 73:3063–3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maaser C, et al. 2001. Colonic epithelial cells induce endothelial cell expression of ICAM-1 and VCAM-1 by a NF-κB-dependent mechanism. Clin. Exp. Immunol. 124:208–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marchès O, et al. 2000. Role of Tir and intimin in the virulence of rabbit enteropathogenic Escherichia coli serotype O103:H2. Infect. Immun. 68:2171–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McDaniel TK, Jarvis KG, Donnenberg MS, Kaper JB. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. U. S. A. 92:1664–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McKee L, Melton-Celsa AR, Moxley RA, Francis DH, O'Brien AD. 1995. Enterohemorrhagic Escherichia coli O157:H7 requires intimin to colonize the gnotobiotic pig intestine and to adhere to HEp-2 cells. Infect. Immun. 63:3739–3744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McKee ML, O'Brien AD. 1995. Investigation of enterohemorrhagic Escherichia coli O157:H7 adherence characteristics and invasion potential reveals a new attachment pattern shared by intestinal E. coli. Infect. Immun. 63:2070–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McNamara N, et al. 2006. AsialoGM1 and TLR5 cooperate in flagellin-induced nucleotide signaling to activate Erk1/2. Am. J. Respir. Cell Mol. Biol. 34:653–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McNamara N, et al. 2001. ATP transduces signals from ASGM1, a glycolipid that functions as a bacterial receptor. Proc. Natl. Acad. Sci. U. S. A. 98:9086–9091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nasreen N, et al. 2001. Polar production of interleukin-8 by mesothelial cells promotes the transmesothelial migration of neutrophils: role of intercellular adhesion molecule-1. J. Infect. Dis. 183:1638–1645 [DOI] [PubMed] [Google Scholar]
- 29. Ogushi K, et al. 2004. Gangliosides act as co-receptors for Salmonella enteritidis FliC and promote FliC induction of human β-defensin-2 expression in Caco-2 cells. J. Biol. Chem. 279:12213–12219 [DOI] [PubMed] [Google Scholar]
- 30. O'Neil HS, Marquis H. 2006. Listeria monocytogenes flagella are used for motility, not as adhesins, to increase host cell invasion. Infect. Immun. 74:6675–6681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paton AW, et al. 2006. AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature 443:548–552 [DOI] [PubMed] [Google Scholar]
- 32. Paton AW, Srimanote P, Talbot UM, Wang H, Paton JC. 2004. A new family of potent AB5 cytotoxins produced by Shiga toxigenic Escherichia coli. J. Exp. Med. 200:35–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Paton AW, Woodrow MC, Doyle RM, Lanser JA, Paton JC. 1999. Molecular characterization of a Shiga toxigenic Escherichia coli O113:H21 strain lacking eae responsible for a cluster of cases of hemolytic-uremic syndrome. J. Clin. Microbiol. 37:3357–3361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paton JC, Paton AW. 1998. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 11:450–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rogers TJ, Paton AW, McColl SR, Paton JC. 2003. Enhanced CXC chemokine responses of human colonic epithelial cells to locus of enterocyte effacement-negative Shiga-toxigenic Escherichia coli. Infect. Immun. 71:5623–5632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rogers TJ, Paton JC, Wang H, Talbot UM, Paton AW. 2006. Reduced virulence of an fliC mutant of Shiga-toxigenic Escherichia coli O113:H21. Infect. Immun. 74:1962–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sampaio SC, et al. 2009. The flagella of an atypical enteropathogenic Escherichia coli strain are required for efficient interaction with and stimulation of interleukin-8 production by enterocytes in vitro. Infect. Immun. 77:4406–4413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seveau S, Bierne H, Giroux S, Prévost MC, Cossart P. 2004. Role of lipid rafts in E-cadherin- and HGF-R/Met-mediated entry of Listeria monocytogenes into host cells. J. Cell Biol. 166:743–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Soong G, Reddy B, Sokol S, Adamo R, Prince A. 2004. TLR2 is mobilized into an apical lipid raft receptor complex to signal infection in airway epithelial cells. J. Clin. Invest. 133:1482–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tacket CO, et al. 2000. Role of EspB in experimental human enteropathogenic Escherichia coli infection. Infect. Immun. 68:3689–3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Talbot UM, Paton AW, Paton JC. 1996. Uptake of Streptococcus pneumoniae by respiratory epithelial cells. Infect. Immun. 64:3772–3777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Taxman DJ, et al. 2006. Criteria for effective design, construction, and gene knockdown by shRNA vectors. BMC Biotechnol. 6:7 doi:10.1186/1472-6750-6-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Taylor MC. 2008. Enterohaemorrhagic Escherichia coli and Shigella dysenteriae type 1-induced haemolytic uraemic syndrome. Pediatr. Nephrol. 23:1425–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Triantafilou M, Miyake K, Golenbock DT, Triantafilou K. 2002. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J. Cell Sci. 115:2603–2611 [DOI] [PubMed] [Google Scholar]
- 45. van Asten FJAM, Hendriks HGCJM, Koninkx JFJG, van der Zeijst BAM, Gaastra W. 2004. Flagella-mediated bacterial motility accelerates but is not required for Salmonella serotype Enteritidis invasion of differentiated Caco-2 cells. Int. J. Med. Microbiol. 294:395–399 [DOI] [PubMed] [Google Scholar]
- 46. West AP, Dancho BA, Mizel SB. 2005. Gangliosides inhibit flagellin signaling in the absence of an effect on flagellin binding to Toll-like receptor 5. J. Biol. Chem. 280:9482–9488 [DOI] [PubMed] [Google Scholar]
- 47. Zaas DW, Duncan M, Rae Wright J, Abraham SN. 2005. The role of lipid rafts in the pathogenesis of bacterial infections. Biochim. Biophys. Acta 1746:305–313 [DOI] [PubMed] [Google Scholar]
- 48. Zoja C, Buelli S, Morigi M. 2010. Shiga toxin-associated hemolytic uremic syndrome: pathophysiology of endothelial dysfunction. Pediatr. Nephrol. 25:2231–2240 [DOI] [PubMed] [Google Scholar]