Abstract
Neuroinflammation has the capacity to alter normal central nervous system (CNS) homeostasis and function. The objective of the present study was to examine the effects of an inflammatory milieu on the electrophysiological properties of striatal astrocyte subpopulations with a mouse bacterial brain abscess model. Whole cell patch-clamp recordings were performed in striatal glial fibrillary acidic protein (GFAP)-green fluorescent protein (GFP)+ astrocytes neighboring abscesses at postinfection days 3 or 7 in adult mice. Cell input conductance (Gi) measurements spanning a membrane potential (Vm) surrounding resting membrane potential (RMP) revealed two prevalent astrocyte subsets. A1 and A2 astrocytes were identified by negative and positive Gi increments vs. Vm, respectively. A1 and A2 astrocytes displayed significantly different RMP, Gi, and cell membrane capacitance that were influenced by both time after bacterial exposure and astrocyte proximity to the inflammatory site. Specifically, the percentage of A1 astrocytes was decreased immediately surrounding the inflammatory lesion, whereas A2 cells were increased. These changes were particularly evident at postinfection day 7, revealing increased cell numbers with an outward current component. Furthermore, RMP was inversely modified in A1 and A2 astrocytes during neuroinflammation, and resting Gi was increased from 21 to 30 nS in the latter. In contrast, gap junction communication was significantly decreased in all astrocyte populations associated with inflamed tissues. Collectively, these findings demonstrate the heterogeneity of striatal astrocyte populations, which experience distinct electrophysiological modifications in response to CNS inflammation.
Keywords: hemichannels, whole cell patch clamp, gap junction communication
neuroinflammation leads to astrocyte activation and the initiation of complex cellular alterations referred to as reactive astrogliosis. The hallmarks of astrogliosis are astrocyte hypertrophy and a robust increase in glial fibrillary acidic protein (GFAP) expression (Sofroniew and Vinters 2010). Since astrocytes are critical for maintaining central nervous system (CNS) homeostasis, including K+ and glutamate buffering and redistribution (Takahashi et al. 2010; Wallraff et al. 2006), nutritional support for neurons via glucose delivery (Rouach et al. 2008), gap junction-mediated communication by Ca2+ waves and ATP release (MacVicar and Thompson 2010; Scemes and Giaume 2006), and information processing through tripartite synapses (Halassa and Haydon 2010; Hamilton and Attwell 2010), it is important to understand how reactive astrocytes adapt their activity to maintain tissue homeostasis. Electrophysiological techniques have been widely used to study ion exchange through cell membranes as well as gap junctions. Several models have been utilized to study the electrophysiological properties of activated astrocytes in situ (Anderova et al. 2004; Bordey et al. 2001; Schroder et al. 1999; Takahashi et al. 2010; Wang et al. 2008); however, the consequences of a rapid, fulminate neuroinflammatory stimulus on astrocyte electrophysiological properties remain incompletely understood. Previous studies from our laboratory have utilized a well-established model of bacterial brain abscess (Kielian et al. 2001, 2007a, 2007b) to investigate the effects of local inflammation on astrocyte gap junction communication (GJC) and hemichannel (HC) activity (Karpuk et al. 2011). Specifically, we reported distance-dependent alterations in GJC, HC opening, modulations in connexin and pannexin expression, as well as upregulation of glutamate transporters. We also noted correlations between general electrophysiological parameters of astrocytes in relation to their distance from the brain abscess margins. However, our prior study did not identify the nature of astrocytic electrophysiological modifications in the striatum or explain how a potent inflammatory milieu, modeled by acute bacterial infection, can alter inward and outward currents in activated astrocytes.
Previous studies in the striatum of young rats demonstrated that >95% of astrocytes displayed passive properties, which do not exhibit time- or voltage-dependent currents and therefore possess typical linear voltage-current (I-V) relationships (Adermark and Lovinger 2008). Passive astrocyte properties are thought to be mediated by extensive GJC that can distort whole cell recordings by currents escaping to neighboring cells. In contrast, earlier studies in hippocampal slices indicated that the passive profile of astrocytes is intrinsic and not influenced by GJC (Schools et al. 2006; Wallraff et al. 2006). However, voltage-dependent currents that are characteristic of complex astrocytes (Matthias et al. 2003) can also be revealed in reactive astrocytes after the application of various channel inhibitors and/or leak subtraction (Anderova et al. 2004; Perillan et al. 1999). Among them, K+ voltage-dependent currents are considered essential contributors to resting conductance (Bordey and Sontheimer 1997; Seifert et al. 2009).
The percentage of passive astrocytes can be altered during astrogliosis, which agrees with the idea of alterations in ionic membrane currents following astrocyte activation (Anderova et al. 2004). Indeed, inward-rectifying K+ (Kir) currents were reduced in activated astrocytes after an entorhinal cortex lesion (ECL) (Schroder et al. 1999) and in layer I astrocytes during cortical freeze lesion (CFL) (Bordey et al. 2001), whereas an increase in Kir current occurred in astrocytes after a cortical stab wound (CSW) (Anderova et al. 2004). However, astrocyte delayed-rectifying outward K+ current was enhanced in CFL and CSW models but not in ECL. These discrepancies may result from the diverse nature of the injury models, which elicit different degrees of astrogliosis (Bordey et al. 2001; Liberto et al. 2004; Sofroniew and Vinters 2010) and highlight the fact that currently we do not fully understand the intricate changes occurring in activated astrocytes.
Most electrophysiological studies have investigated astrocytic currents over a wide range of membrane potentials. However, in vivo experiments revealed minor 1- to 2-mV deviations of astrocytic resting membrane potential (RMP) associated with neuronal electrical activity (Amzica et al. 2002; Mishima and Hirase 2010). An interpretation of these findings is that astrocytic functioning in situ occurs over a very narrow membrane potential range but is associated with large currents passed through the astrocytic membrane. Regarding this issue, in the present study we investigated electrical events of astrocytes with a narrow range of membrane potentials surrounding RMP. This demonstrates the feasibility of our approach to query changes in intrinsic astrocyte properties in the face of neuroinflammation.
The objective of the present study was to identify alterations in astrocytic inward and outward membrane currents surrounding RMP in the inflammatory milieu generated during bacterial brain abscess formation. Whole cell voltage- and current-clamp recordings were used to measure properties of striatal astrocytes at varying distances from the abscess in acute brain slices from GFAP-green fluorescent protein (GFP) mice. We report that the number of astrocytes expressing preferentially inward or outward current components (referred to as A1 and A2 astrocytes, respectively) was altered during the course of neuroinflammation. The increased voltage-dependent conductance and capacitance in all astrocytes surrounding the inflammatory milieu coincided with increased HC activity at postinfection day 3. The possible functional role of A1 and A2 populations is discussed. Collectively, these studies identify novel adaptations of astrocytes to an inflammatory milieu that may impact CNS homeostasis and neuronal integrity in neighboring tissues.
MATERIALS AND METHODS
Generation of experimental brain abscesses.
Brain abscesses were established in GFAP-GFP mice (8–12 wk of age; The Jackson Laboratory, Bar Harbor, ME), by the intracerebral inoculation of live Staphylococcus aureus (strain USA300 CAV1002) (Sifri et al. 2007) encapsulated in agarose beads as previously described (Kielian et al. 2001). A rodent stereotaxic apparatus equipped with a Cunningham mouse adaptor (Stoelting, Kiel, WI) was used to implant S. aureus-encapsulated agarose beads into the dorsal striatum, with the following coordinates relative to bregma: +1.0 mm rostral, +2.0 mm lateral, and −3.0 mm deep from the surface of the brain. A burr hole was made, and a 5-μl Hamilton syringe fitted with a 26-gauge needle was used to slowly deliver 2-μl beads (103 colony-forming units) into the brain parenchyma over a 30-s period. The needle remained in place for 2–3 min after injection to prevent the efflux of injected material. Earlier studies from our laboratory have established that the introduction of sterile agarose beads does not induce detectable inflammation or peripheral immune cell infiltrates (Baldwin and Kielian 2004; Kielian et al. 2001). In addition, at the time points evaluated in the present study (i.e., postinfection days 3 and 7) the tissue within 1–2 mm from the initial stab wound is completely obliterated by necrosis, and, as such, comparisons with sham-injected animals (i.e., sterile agarose beads) are less relevant. Since astrocyte electrophysiological measurements were conducted adjacent to the brain abscess margins, it can be concluded that responses are elicited by inflammation originating from bacterial infection and not from the original stab wound used to inoculate bacteria into the brain parenchyma, since this region no longer exists. Therefore, comparisons between the intact parenchyma surrounding brain abscesses and identical regions from uninfected brains were considered the most accurate comparisons to reveal changes elicited by the inflammatory milieu after bacterial exposure. We utilized a similar strategy for our recent report, in which comparisons were made between infected and noninfected tissues (Karpuk et al. 2011). The animal use protocol, approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee, is in accord with National Institutes of Health guidelines for the use of rodents.
Acute brain slice preparation.
At the appropriate time points after infection, mice were killed by cervical dislocation and immediately decapitated. The brain was quickly removed and bathed in ice-cold artificial cerebrospinal fluid (ACSF, in mM: 124 NaCl, 26 NaHCO3, 3 KCl, 2 MgCl2, 2 CaCl2, 0.4 ascorbic acid, 10 glucose) for slice preparation. Next, horizontal slices (300–400 μm thick) were prepared with a Leica VT1000S vibrating-blade microtome (Leica Microsystems) and immediately placed in ACSF at 32°C. After a 20- to 30-min incubation period at 32°C, slices were held in ACSF at room temperature for at least 1 h before further use. All incubation solutions were equilibrated and continuously bubbled with carbogen (95% O2-5% CO2). In some experiments, slices were incubated with sulforhodamine 101 (SR101 acid chloride, 0.2–0.4 μM; Sigma, St. Louis, MO) as an additional marker for astrocyte identification (Nimmerjahn et al. 2004). In other experiments, brain slices were incubated with ethidium bromide (EtBr) for 10 min to identify cells with increased HC activity (Karpuk et al. 2011) that could be used for patch-clamp recordings.
Electrophysiology.
Electrophysiological recordings were performed in a submerged chamber (RC-27, Warner Instruments, Hamden, CT) continuously perfused with ACSF at a rate of 1–2 ml/min at 30°C. Patch pipettes for recording electrodes were pulled from borosilicate glass capillaries (1.5/0.84 mm OD/ID) with a micropipette puller (P-97, Sutter Instruments, Novato, CA). Recording electrodes were filled with a solution containing (in mM) 130 KCl, 0.2 CaCl2, 1 MgCl2, 5 EGTA, 10 HEPES, pH 7.2–7.3 with an electrical resistance of 6–10 MΩ. In other experiments, K-gluconate or CsCl (Sigma) was substituted in equimolar concentrations for K+ in the intracellular recording solution. The gap junction permeant dye Alexa Fluor 350 (0.5 mM, Invitrogen, San Diego, CA) was added to the intracellular recording solution to calculate the degree of astrocyte GJC. The following drugs were used (in mM): 1 CsCl, 6 KCl, and 0.1 gap junction/HC inhibitor carbenoxolone disodium salt (CBX) (all from Sigma).
Whole cell patch-clamp recordings were performed on GFAP-GFP+ striatal astrocytes with a computer-controlled amplifier (Multiclamp 700B, Molecular Devices, Sunnyvale, CA) and a video setup equipped with a motorized Axio Examiner Z1 fluorescent microscope, a high-sensitivity and -resolution digital camera (AxioCam MRm), and AxioVision software (all from Zeiss). Analog signals from the amplifier were digitized at a 5-to 10-kHz sampling rate with a 16-bit resolution Digidata-1440A acquisition system and pCLAMP-10 software (both from Molecular Devices), which allowed detection of picoampere changes in macroscopic currents passed through low-resistance astrocytes in living brain tissue. It was shown earlier (Zhou et al. 2009) and confirmed in this study that access resistance (Ra) exceeds the membrane resistance (Rm) of astrocytes up to 5 times, which can cause voltage-clamping errors of ∼80% depending on Rt/Rm, where Rt is total membrane resistance (Ra + Rm). Using the membrane test function integrated in pCLAMP-10, we estimated the astrocyte Rt/Rm as 5.2 ± 0.74 (n = 18) and 4.9 ± 0.42 (n = 27) in uninfected and infected brain slices, respectively. However, the remaining voltage [∼20% of command voltage (Vc)] applied to the cellular membrane can generate still large passive currents, which obscures voltage-dependent currents. The linear I-V relationship is a prominent characteristic of passive currents. Therefore, I-V differentiation negates passive conductance and may reveal voltage-dependent conductances, in particular, if the actual membrane potential (Vm) is taken into account [i.e., Vm = Vc × (Rm/Rt)].
Electrophysiological parameters of astrocytes were calculated off-line with Clampfit-10 and custom-designed software (Karpuk and Hayar 2008). The total resting membrane conductance (Gt) [Gm is estimated membrane conductance, i.e., Gm = Gt × (Rt/Rm)] and resistance (Rt) were calculated as the linear I-V slope by using 3–5 data points near the value of RMP (Fig. 1B). Utilizing this method, cell input conductance (Gi) was calculated at every Vc used in the recordings (on average, from −140 to +60 mV). Thus Gt is only one point within the numerous Gi range calculated at different Vc. Since Gi values (nS) were dependent on Vc, as shown in this study, the relationship between Gi and Vc revealed both positive and negative Gi increments or Gi slopes measured in picosiemens per millivolt or picoamperes per millivolt square (Fig. 1C). The latter indicates that real current changes at the cell membrane should be adjusted by the factor [Rt/Rm]2 to compensate for the voltage drop on Ra. Since I-V tails usually fluctuate because of inward and outward current activation at negative (i.e., from −90 to −140 mV) or positive (i.e., 0 to +60 mV) membrane potentials, respectively, we defined the basic Gi slope, which was calculated within the linear segment of the Gi plot within −90 to 0 mV of Vc and always included the RMP point (i.e., zero holding current) (Fig. 1C). The negative and positive Gi slopes (i.e., −Gi and + Gi, respectively) averaged from three or four consequential voltage-clamp recordings were the main criteria for grouping astrocytes into different subsets. This method enabled the identification of A1 astrocytes typified by −Gi as well as A2 astrocytes that were classified by +Gi. Cells that exhibited complex Gi patterns were included in appropriate subsets as dictated by their basic Gi slope (Fig. 1C; Fig. 2).
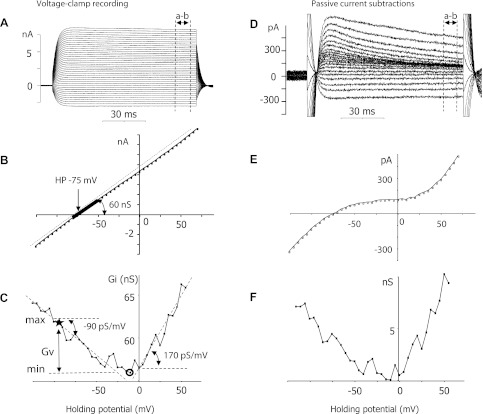
Fig. 1.
Methods for calculating astrocyte input conductance parameters. A: whole cell voltage-clamp recording in a striatal glial fibrillary acidic protein (GFAP)-green fluorescent protein (GFP)+ astrocyte located near an abscess at postinfection day 7. Holding potential (HP) and applied voltage steps (not shown) were −75 and 5 mV, respectively. B: voltage-current (I-V) relationship constructed from the current and voltage points that were obtained by averaging the corresponding segments (a–b) (70–90 ms) delineated in A. The total resting conductance (Gt) was taken as the linear I-V slope including 3–5 points near zero current (bold line, curved arrows, 60 nS). The dashed line was drawn to emphasize linear I-V. C: plot of cell input conductance (Gi) calculated from B vs. holding voltages. Dashed lines indicate negative and positive linear slopes (−90 ± 4 pS/mV and 170 ± 13 pS/mV, respectively). Left dashed line indicates the basic negative Gi slope [calculated at the linear segment of Gi plot within the voltage range −90 to 0 mV including the resting membrane potential (RMP) value (zero holding current)] that was used as a criterion to identify astrocyte subsets. Right dashed line indicates the positive Gi slope. The absolute differences between maximal (max, star) and minimal (min, circle) Gi found at the same voltage range (−90 to 0 mV) were defined as voltage-dependent conductance (Gv, straight arrows). The minimal Gi was used for passive current subtraction (see D–F). D: voltage-dependent currents were revealed in the recording depicted in A after current subtractions. E: I-V was calculated from current subtractions in D by the same method used in B. F: Gi plot calculated from I-V in E reveals identical Gi patterns compared with the original Gi pattern in C, which supports the conclusion that calculation of Gi slopes represents an efficient tool to explore Gv.
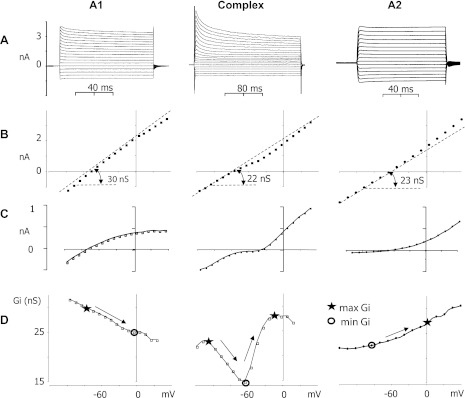
Fig. 2.
Electrophysiological heterogeneity of striatal astrocytes during neuroinflammation. Representative recordings and their parameters (from top to bottom) for different GFAP-GFP+ striatal astrocyte subsets located in infected tissues. A: whole cell voltage-clamp recordings of A1, complex, and A2 astrocytes [i.e., −75, −90, and −66 mV holding potentials (RMPs), respectively]. B: I-V relationships corresponding to recordings shown in A, with values indicating the total resting conductance calculated by I-V slopes. Dashed lines are used to depict I-V declinations. C: I-V plots constructed from recordings in A after the passive current subtraction display active components of inward and outward currents. D: Gi calculated from the original I-V plots depicted in B and plotted vs. holding voltage. Gi slopes (depicted by arrows) indicate the voltage dependence of inward and outward current vectors. The absolute difference between maximal (star) and minimal (circle) Gi found on linear plot segments within the voltage range of −90 to 0 mV was considered as Gv. However, the maximal Gi for complex astrocytes can be found at more negative holding potentials, where the sign of Gi derivatives is changed.
The minimal Gi detected at the Vc range from −90 to 0 mV was considered passive conductance and was used for current subtractions to visualize active current components (Fig. 1, D–F). The absolute difference between maximal and minimal Gi found in the Vc range from −90 to 0 mV was considered the voltage-dependent conductance (Gv) (Fig. 1C). It should be noted that maximal Gi for A1 cells was located at more negative potentials than minimal Gi and opposing maximal/minimal Gi locations were characteristic for A2 cells (Fig. 2D).
Cell membrane capacitance (Cm) values were taken from the amplifier readout in voltage-clamp mode as compensated capacitance. In current-clamp mode, Cm was calculated from I-V recordings with the time constant definition tm = RmCm, where tm is the time constant calculated by the declining membrane potential at 5–20 mV from RMP (i.e., time point at 63% of maximal declination) when negative rectangular current steps were injected into cells by patch pipette.
Quantitation of astrocyte coupling.
The gap junction permeant dye Alexa Fluor 350 was included in the patch pipette to visualize the degree of GJC in GFAP-GFP+ striatal astrocytes. Calculations of astrocyte coupling were performed by enumerating the number of superimposed cell images under appropriate filters for GFAP and Alexa Fluor 350 in microscopic fields of view (220 × 165 μm). Cell coupling was confirmed by the measurement of optical intensities with AxioVision software. Cells were considered coupled if the peak optical intensities for Alexa Fluor 350 overlapped with GFAP-GFP intensities and exceeded 10% of background levels. Astrocyte soma area was measured by manually outlining cell body images (distinguishable by GFAP-GFP and SR101 fluorescence) with appropriate tools in AxioVision.
Statistical analyses.
A Student's two-tailed t-test was used for data analyses (MS Excel 2007), with values reported as means ± SE compiled from independent experiments.
RESULTS
Characterization of striatal astrocyte hypertrophy during brain abscess formation.
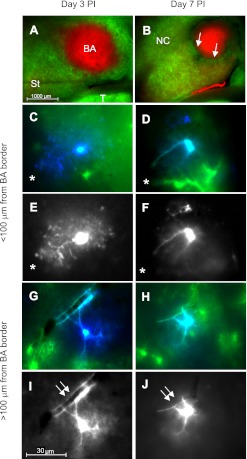
Representative fluorescent images of brain slices from GFAP-GFP mice harboring brain abscesses at postinfection day 3 and day 7 (Fig. 3, A and B, respectively) depict a central necrotic core delineated by nonspecific SR101 uptake, surrounded by intense astrocyte activation as demonstrated by robust GFP expression. In general, brain abscesses were larger at postinfection day 3 with irregular margins, whereas at day 7 lesions were more compact and exhibited a structured organization. By day 7 after infection, a narrow zone devoid of GFP signal was evident surrounding the central abscess core, which represents the region where fibrotic encapsulation occurs (Fig. 3B). Astrocytes stained by Alexa Fluor 350 during whole cell recordings revealed different morphologies based on their distance from the brain abscess margins (Fig. 3, C–J). To measure the degree of astrocyte hypertrophy, we next quantitated soma area. In agreement with our observations of distinct morphological changes nearest the inflammatory site, astrocytic soma were significantly larger closest to the brain abscess margins at both postinfection days 3 and 7 (Fig. 4A). Collectively, these morphological findings support the possibility of astrocyte heterogeneity emanating from a site of active inflammation, which supports recent work in our laboratory using the experimental brain abscess model (Karpuk et al. 2011).
Fig. 3.
Neuroinflammation induces region-dependent changes in astrocyte morphology. Acute brain slices were prepared from GFAP-GFP mice at day 3 (A, C, E, G, I) or 7 (B, D, F, H, J) after an intracerebral inoculation of live S. aureus and stained with SR101 to facilitate astrocyte identification in conjunction with GFAP-GFP expression. A and B: low-power magnification of brain slices at postinfection (PI) day 3 (A) or 7 (B), with the latter depicting fibrous capsule formation (arrows, B). C–F: images of GFAP-GFP+ astrocytes (C and D; green) stained with the fluorescent dye Alexa Fluor 350 (blue or white) during whole cell patch-clamp recording at days 3 (C and E) and 7 (D and F) after infection in close proximity (i.e., <100 μm) from the brain abscess margins (denoted with asterisks). G–J: GFAP-GFP+ astrocytes located at farther distances (i.e., >100 μm) from the brain abscess border at postinfection days 3 (G and I) and 7 (H and J) reveal similar morphological features (double arrows indicate a blood vessel). Anatomical regions are depicted in A and B: BA, brain abscess; St, striatum; NC, neocortex; T, thalamus.
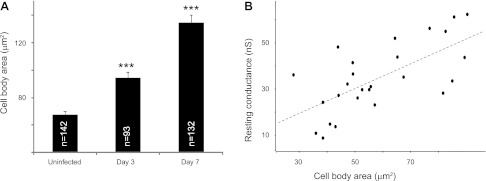
Fig. 4.
Astrocytes undergo hypertrophy over the course of brain abscess evolution. A: area of GFAP-GFP+ astrocyte soma was determined in uninfected slices (67.7 ± 2.2 μm2) as well as at postinfection days 3 (94.5 ± 4.2 μm2) and 7 (134.5 ± 5.6 μm2) (***P < 0.001 vs. uninfected). B: a positive correlation between astrocyte soma area and resting conductance was only observed in astrocytes from uninfected slices (slope = 540 ± 120 pS/μm2, correlation coefficient = 0.66, n = 26, P < 0.05).
Effects of neuroinflammation on basic astrocyte electrophysiological parameters.
Next, we evaluated basic electrophysiological parameters of GFAP-GFP+ astrocytes in acute brain slices from both uninfected GFAP-GFP mice and animals harboring S. aureus-induced brain abscesses at days 3 and 7 after infection. In our previous study, we found that some electrophysiological parameters of GFAP-GFP+ striatal astrocytes, as well as GJC and HC activity, were significantly modified immediately surrounding inflamed tissues (Karpuk et al. 2011). The depolarized RMP was due to an increased number of astrocytes with depolarizing potentials immediately surrounding abscesses, mainly in the range of −50 to −30 mV (i.e., 16% in uninfected slices vs. 33% at day 3 after infection). However, in the present study we restricted our analysis to astrocytes that displayed RMP values from −90 to −50 mV, since these ranges fall within the values typical for astrocytes under resting conditions with minimal activation of inward/outward currents (Perillan et al. 1999). Additionally, the Vm range was narrowed because of the effect of Ra in whole cell voltage-clamp configuration. This approach revealed both negative and positive Gi increments in response to depolarizing voltage steps that allowed us to divide GFAP-GFP+ astrocytes into two subsets, which we refer to as A1 and A2 astrocytes, respectively (Fig. 2; also refer to materials and methods for how these two astrocyte subtypes were classified). Since the frequency of complex astrocytes in the striatum was rather low (∼15% of the total cells analyzed at postinfection day 3), they were not defined as a separate category because statistical analysis could not be applied.
Impact of neuroinflammation on intrinsic properties of A1 and A2 astrocytes.
A1 and A2 astrocytes demonstrated different electrophysiological parameters in uninfected brain slices (Table 1). Specifically, A2 astrocytes had more hyperpolarized RMP compared with A1 astrocytes (−76.6 ± 1.12 and −69.2 ± 1.30 mV, respectively, P < 0.001), whereas A1 astrocytes had higher Gt compared with A2 cells (33.6 ± 1.64 and 21.1 ± 1.69 nS, respectively, P < 0.001). Accordingly, Cm was significantly higher in A1 compared with A2 astrocytes (37.9 ± 2.16 and 29.3 ± 2.43 pF, P < 0.05), since tm did not differ (Table 1). In addition, a positive relationship was observed between astrocyte soma area and Gt for both A1 and A2 subsets (slope = 540 ± 120 pS/μm2, P < 0.05; Fig. 4B). Gt values were decreased within 0–200 μm from the abscess margins and increased between a distance of 200 and 800 μm for both astrocyte subsets at postinfection day 3 [25.8 ± 1.8 (n = 15) and 36.1 ± 5.4 nS (n = 9), respectively; P < 0.05]. In contrast, astrocyte soma area was increased in close proximity to the abscess (Fig. 4A) and approached values characteristic of astrocytes from uninfected slices with increasing distance from the lesion (data not shown). Therefore, the opposing changes in Gt and soma area distorted their relationship as depicted in Fig. 4B.
Table 1.
Electrophysiological parameters of different GFAP-GFP+ astrocyte subsets
| Astrocyte Group | RMP, mV | Gt, nS | Gm, nS | Cm, pF | tm, ms | n |
|---|---|---|---|---|---|---|
| A1 | ||||||
| Uninfected | −69.2 ± 1.30 | 33.6 ± 1.64 | 168 ± 8 | 37.9 ± 2.16 | 1.05 ± 0.04 | 28 |
| Day 3 PI | −73.3 ± 1.15a | 30.9 ± 1.26 | 154 ± 6 | 68.9 ± 6.95c | 2.22 ± 0.24c | 18 |
| Day 7 PI | −67.2 ± 4.63 | 42.1 ± 5.44 | 210 ± 27 | 28.4 ± 3.08 | 0.91 ± 0.05b | 8 |
| A2 | ||||||
| Uninfected | −76.6 ± 1.12e | 21.1 ± 1.69e | 105 ± 8e | 29.3 ± 2.43d | 0.97 ± 0.05 | 21 |
| Day 3 PI | −64.2 ± 1.61c,e | 29.9 ± 2.25b | 149 ± 11b | 66.7 ± 4.84c | 2.45 ± 0.28c | 15 |
| Day 7 PI | −69.9 ± 1.71b | 21.3 ± 2.09e | 106 ± 10e | 34.6 ± 7.6 | 1.24 ± 0.19 | 14 |
Values are means ± SE. GFAP, glial fibrillary acidic protein; GFP, green fluorescent protein; PI, postinfection; RMP, resting membrane potential; Gt, total resting membrane conductance; Gm, estimated membrane conductance; Cm, cell membrane capacitance; tm, time constant.
P < 0.05;
P < 0.01,
P < 0.001 vs. uninfected slices;
P < 0.01,
P < 0.001 vs. A1 cells at same time point.
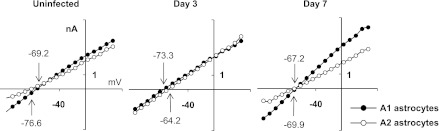
Compared with uninfected slices, A1 and A2 astrocytes surrounding the inflammatory brain abscess margins exhibited differences in several electrophysiological parameters (Table 1 and Fig. 5). For example, RMP was inversely modified in A1 and A2 astrocytes at postinfection day 3. Specifically, membrane hyperpolarization was observed in A1 cells (−73.3 ± 1.15 vs. −69.2 ± 1.30 mV in day 3 and uninfected slices, respectively; P < 0.001), whereas depolarization occurred in A2 astrocytes (−64.2 ± 1.61 vs. −76.6 ± 1.12 mV in day 3 and uninfected slices, respectively; P < 0.001). In parallel with RMP depolarization, Gt was increased only in A2 astrocytes (21.1 ± 1.69 to 29.9 ± 2.25 nS; P < 0.01), which was expected since these cells possess positive Gi increments under depolarizing conditions, which was observed in A2 cells during inflammation. However, Gt did not increase in A1 cells with negative Gi increments despite RMP hyperpolarization. As a result, averaged I-V were almost indistinguishable between A1 and A2 astrocytes at postinfection day 3 but did differ at day 7, reflecting major changes in astrocyte intrinsic properties at this time point (Fig. 5 and Table 1).
Fig. 5.
I-V relationships in A1 and A2 astrocytes. The differences between the I-V curves of A1 and A2 astrocytes were minimal at postinfection day 3 and maximal in uninfected brain slices as well as at day 7 after infection. I-V were averaged (using a bin value of 10 mV) for all appropriate astrocyte subsets presented in Table 1. Numbers with arrows indicate the extrapolated values of RMP for A1 (downward arrows) and A2 (upward arrows).
Despite the finding that Gt (Gm) was increased only in A2 astrocytes after infection, Cm and time constants were altered in both A1 and A2 subsets (Table 1). These measurements were significantly increased at postinfection day 3 and returned to values typical of uninfected slices by day 7 after bacterial exposure. Collectively, these data suggest that astrocyte membrane surface area was increased at postinfection day 3, since Cm is proportional to membrane area, and are in agreement with the astrocyte hypertrophy observed near the abscess margins (Fig. 3A). However, this relationship was not always observed, since astrocyte soma area was also increased at postinfection day 7, whereas Cm values returned to levels in uninfected slices at this time point (Table 1). This disconnect between Cm values and astrocyte hypertrophy may be explained by the lack of astrocytic fine processes (based on visual observations) in close proximity to the brain abscess margins at postinfection day 7.
Neuroinflammation alters percentages and distribution patterns of A1 and A2 astrocytes in time-dependent manner.
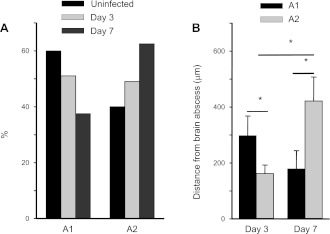
In uninfected slices, ∼60% of GFAP-GFP+ astrocytes displayed A1 characteristics, whereas 40% exhibited features of the A2 subset (Fig. 6A). The percentages of A1 astrocytes were decreased at both days 3 and 7 after infection (i.e., 51% and 37.5%, respectively), whereas A2 cells were increased at both time points (i.e., 49% and 62.5%, respectively; Fig. 6A). We also observed that A1 and A2 astrocytes exhibited different distribution patterns in the context of inflammation. In general, A1 astrocytes that displayed only negative Gi increments were located farther from the abscess margins compared with A2 cells at postinfection day 3 (280 ± 67 vs. 161 ± 30 μm; P < 0.05; Fig. 6B). Conversely, A1 astrocytes were more prominent closer to the inflammatory site than A2 cells at day 7 after bacterial exposure (243 ± 86 vs. 421 ± 86 μm; P = 0.05; Fig. 6B). Therefore, astrocyte activation was associated with a redistribution of A1 and A2 populations, whereas increased percentages of A2 astrocytes were observed during brain abscess development.
Fig. 6.
Percentage and distribution of A1 and A2 astrocytes are affected by the inflammatory milieu. A: % of A1 and A2 astrocytes in uninfected slices vs. abscess tissues reveal that the former are more abundant during early infection (i.e., day 3), whereas A2 astrocytes are more prevalent during later stages of inflammation (i.e., day 7). B: inverse relationship between the distribution patterns of A1 and A2 astrocytes relative to distance from the abscess margins. *P < 0.05.
Neuroinflammation modulates astrocyte electrophysiological reactivity.
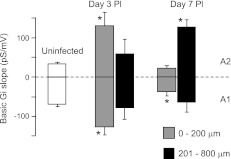
The differential distribution of A1 and A2 astrocytes from the brain abscess margins at postinfection days 3 and 7 (Fig. 6) was in accordance with the relationships found between astrocyte distances from the abscess and +/−Gi (Fig. 7 and Table 2). +/−Gi calculations allowed us to evaluate the reactivity of astrocytic inward and outward currents in response to depolarizing voltage steps, criteria that were initially used to separate astrocytes into A1 and A2 subsets. Gi increments, typically of a single pattern, were observed in serial I-V recordings, and thus they were not noise Gi deviations (Fig. 2). The rate of Gi changes was dependent upon Gv amplitude (data not shown) and the distance from the inflammatory site, with maximal expression within 200 μm of the abscess at postinfection day 3 (−124 ± 21 vs. −66 ± 6 for A1 and 129 ± 36 vs. 32 ± 3 pS/mV for A2; P < 0.05 vs. uninfected). The amplitude of both Gi slopes reached control values at a distance of 300–400 μm from the abscess margins at postinfection day 3 but revealed even smaller values at day 7 (35 ± 11 and 21 ± 9 pS/mV, respectively; Fig. 7). Overall, the negative Gi slope was more exaggerated at postinfection day 3 near the abscess but returned to levels observed in uninfected slices by day 7. Concomitantly, the positive Gi slope was heightened at postinfection day 3 and remained unchanged out to day 7 farther from the abscess.
Fig. 7.
Rate of cell conductance changes is heightened in both A1 and A2 astrocytes during brain abscess development. Both negative and positive Gi slopes were increased at day 3 postinfection nearest the brain abscess margins (i.e., 0 to 200 μm), while only positive slopes were increased at day 7 farther from the abscess (201 to 800 μm; *P < 0.05 vs. values from uninfected slices) with calculations presented in Table 2.
Table 2.
Parameters of Gi increments in GFAP-GFP+ striatal astrocytes
| Distance from Brain Abscess Margin |
|||||||
|---|---|---|---|---|---|---|---|
| 0–200 μm |
200–1,000 μm |
||||||
| Astrocyte Subset | Treatment Group | Vc, pS/mV | Vm, nS/mV | n | Vc, pS/mV | Vm, nS/mV | n |
| A1 | Uninfected | −67 ± 7 | −1.67 ± 0.17 | 34 | |||
| Day 3 PI | −124 ± 22* | −3.10 ± 0.55* | 14 | −76 ± 30 | −1.90 ± 0.75 | 7 | |
| Day 7 PI | −35 ± 12 | −0.87 ± 0.30 | 6 | −62 ± 25* | −1.55 ± 0.62 | 6 | |
| A2 | Uninfected | 32 ± 6 | 0.80 ± 0.15 | 20 | |||
| Day 3 PI | 129 ± 36* | 3.22 ± 0.90* | 9 | 58 ± 40 | 1.45 ± 1.0 | 6 | |
| Day 7 PI | 21 ± 9 | 0.52 ± 0.22 | 5 | 126 ± 20* | 3.15 ± 0.50 | 8 | |
Values are means ± SE. Results were calculated at the command voltages (Vc) and adjusted for membrane potentials (Vm) by the factor [Rt/Rm]2, where Rt is total membrane resistance and Rm is membrane resistance.
P < 0.05 vs. uninfected slices.
Of note, ∼15% of the total GFAP-GFP+ astrocytes examined possessed both + and −Gi and were only found within 100 μm of abscesses at postinfection day 3. These cells were classified as complex astrocytes based solely on electrophysiological properties, since they were not tested for the existence of sodium channels or glutamate receptors that have been described for complex cells by others (Matthias et al. 2003). Since such astrocytes possessed both positive and negative Gi slopes, it is possible that they represent a transition state and/or possess both A1 and A2 electrophysiological attributes in inflamed tissues; however, this remains speculative.
Neuroinflammation modulates astrocyte voltage-dependent conductance.
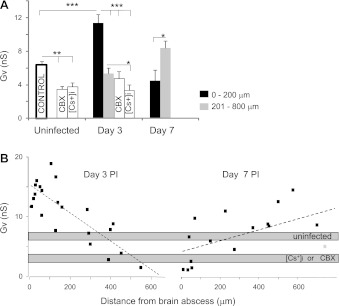
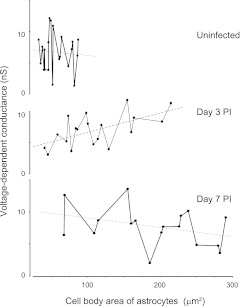
Because Gv amplitudes were not significantly different between A1 and A2 astrocytes, we combined Gv parameters from both groups to simplify additional calculations. With this approach, the Gv of astrocytes revealed distribution patterns similar to those observed for +/−Gi measurements. Specifically, maximal Gv amplitude was found within 200 μm of the abscess margins at postinfection day 3 (11.4 ± 1.02 vs. 6.3 ± 0.5 nS in uninfected; P < 0.001), whereas Gv approached values representative of uninfected tissues at farther distances from the abscess margins (Fig. 8A). In addition, a positive relationship between Gv and astrocyte soma area was observed at postinfection day 3 (slope = 30 ± 10 pS/μm2; P < 0.05) (Fig. 9), which suggests that voltage-dependent currents are activated in hypertrophied astrocytes. In contrast, Gv was reduced over twofold at postinfection day 7 (4.47 ± 1.31 nS; P < 0.05 vs. day 3; Fig. 8) but was still significantly higher farther from the abscess margins (8.37 ± 0.86 nS; P < 0.05 compared with uninfected), mainly because of outward currents (Fig. 8). These Gv modifications indicate that astrocyte activation was reduced in regions immediately surrounding the abscess at postinfection day 7, whereas astrocytes located at farther distances from the inflammatory milieu remained affected. Here Gv was calculated from original I-V with Vc values. The estimated Gv values on Vm had severalfold higher amplitude depending on Rt/Rm. Overall, the Gv percentage represented a significant portion of Gm in uninfected tissues (i.e., 23%), whereas the maximal Gv percentage was observed at postinfection day 3 near the abscess (i.e., 37%; Table 3).
Fig. 8.
Gv of astrocytes is differentially affected by the time after infection. A: Gv in uninfected and infected tissues were reduced after bath application of (CBX, 50–100 μM) and Cs+ ([Cs+]i, 130 mM). *P < 0.05; **P < 0.01, ***P < 0.001. B: dashed lines represent linear regressions for postinfection days 3 (−24 ± 4 pS/μm, correlation coefficient = 0.8, n = 23; P < 0.05) and 7 (12 ± 3 pS/μm, correlation coefficient = 0.7, n = 15; P < 0.05).
Fig. 9.
Astrocytic Gv and soma area exhibit a positive correlation only during early neuroinflammation. Dashed lines indicate linear regressions for astrocytes in uninfected tissue (slope = −3 ± 3.7 pS/μm2, correlation coefficient = 0.02; P > 0.05, n = 21) and at postinfection days 3 (slope = 30 ± 10 pS/μm2, correlation coefficient = 0.6; P < 0.05, n = 19) and 7 (slope = −14 ± 10 pS/μm2, correlation coefficient = 0.34; P > 0.05, n = 17).
Table 3.
Estimated Gv of GFAP-GFP+ striatal astrocytes at different distances from abscess
| 0–200 μm |
200–1,000 μm |
|||||
|---|---|---|---|---|---|---|
| Treatment Group | Gv, nS | n | % of Gm | Gv, nS | n | % of Gm |
| Uninfected | 32.0 ± 2.50 | 41 | 23 | |||
| Day 3 PI | 57.0 ± 5.10* | 18 | 37 | 26.5 ± 3.55 | 8 | 17 |
| Day 7 PI | 22.5 ± 6.55 | 6 | 14 | 42.0 ± 4.30 | 10 | 26 |
Values are means ± SE. Gv, voltage-dependent conductance.
P < 0.001 vs. uninfected slice.
We predicted that astrocytes with increased Gv neighboring an abscess would also display increased HC activity for two reasons. First, we have recently reported that astrocyte HC activity was increased in close proximity to abscesses at postinfection day 3 and was reduced at day 7 (Karpuk et al. 2011), in agreement with the redistribution of Gv shown in this study (Fig. 8). Second, astrocytes that displayed activated HCs also revealed increased Gv [8.97 ± 1.25 nS (n = 5); P < 0.05 vs. uninfected]. Gv steadily decreased at a distance up to 600 μm from the abscess at postinfection day 3 with a negative slope (−24 ± 4 pS/μm; P < 0.05; Fig. 8B), in accordance with the progressive reduction in inflammatory stimulus intensity farther from the lesion site. Conversely, resting conductance was increased at the same distance from the abscess at postinfection day 3, reflecting the passive current amplification. However, the opposite relationship between Gv and distance from the abscess was observed at postinfection day 7, an interval when inflammation is beginning to resolve (12 ± 3 pS/μm; P < 0.05; Fig. 8B). Collectively, these electrophysiological data suggest that astrocytic Gv modifications surrounding an abscess coincide with the extent of inflammation.
Gap junction activity and K+ modify astrocyte Gv.
To clarify the mechanisms involved in regulating astrocytic Gv expression, whole cell patch-clamp recordings were performed using a Cs+-based microelectrode to block the majority of K+ currents. This approach resulted in a significant decrease in Gv, similar to values observed in both uninfected slices (3.78 ± 0.47 nS; P < 0.01) and at postinfection day 3 (3.35 ± 0.64 nS; P < 0.01) (Figs. 6 and 7). Meanwhile, there was no significant change in resting conductance (19.7 ± 3.1 and 27.4 ± 4.8 nS, P > 0.05 for uninfected and postinfection day 3, respectively) compared with recordings made using a K+-based microelectrode (Table 1). Application of the gap junction/HC inhibitor CBX also resulted in a significant decrease in Gv (3.44 ± 0.4 nS and 4.7 ± 0.9 nS, P < 0.05 for uninfected and postinfection day 3, respectively) but did not significantly affect the residual difference in cell resting conductance at day 3 after infection (30.7 ± 4.25 and 23.5 ± 2.5 nS, P > 0.05 for control and CBX, respectively). Collectively, these results suggest that voltage-dependent currents, not passive currents, are the first targets for intracellular Cs+. The decrease in Gv following CBX application suggests the existence of functional links between connexin/pannexin channels and voltage-dependent K+ channels.
DISCUSSION
This study demonstrated that the robust inflammatory milieu generated during CNS parenchymal infection leads to significant electrophysiological and morphological changes in GFAP-GFP+ striatal astrocytes in the parenchyma surrounding brain abscesses. Specifically, astrocyte voltage-dependent currents were increased immediately surrounding the inflammatory site at postinfection day 3, which extended to distances further removed from the lesion at day 7. Electrophysiological recordings identified two astrocyte subsets classified by their characteristic negative and positive Gi increments. We referred to these as A1 and A2 populations, respectively, which is a relatively new approach for determining astrocyte electrophysiological profiles. Specifically, A1 and A2 astrocytes were characterized by dominating inward and outward voltage-dependent currents, respectively, at a Vc range of −90 to 0 mV and the percentages of each population changed dynamically during the course of infection. More A2 astrocytes with outward currents were observed at postinfection day 7, which was the final time point investigated in this study. All activated astrocytes demonstrated increased membrane capacitance and hypertrophy during the course of infection, whereas increased resting Gi was only evident in A2 astrocytes.
In this study we applied a novel method for the analysis of I-V relationships in astrocytes. This method takes into account the derivatives of currents recorded at all applied Vc, which allowed us to identify and quantify subtle I-V declinations evoked by voltage-gated channels. The derivatives of currents were calculated as I-V slopes, which represented the values of cell input conductance (i.e., Gi). The negative or positive Gi changes versus Vc determined by the sign of basic Gi slope indicated the direction of the summary current vector through the cell membrane, which was used to distinguish A1 from A2 astrocytes (Fig. 2D). It is highly probable that A1 and A2 current components include K+ channels underlining the passive conductance shown for hippocampal astrocytes (Seifert et al. 2009; Zhou et al. 2009). If this is taken together with the intrinsic properties presented in Table 1, it can be suggested that the different electrophysiological attributes of A1 and A2 astrocytes may be indicative of their functional specialization in the glial syncytium.
To our knowledge, this is the second study describing detailed electrophysiological parameters of striatal astrocytes in brain slices of adult mice during normal and pathological conditions (Wang et al. 2008). In prior reports, A1 and A2 astrocytes were identified in brain slices of young rats by visual analysis of I-V tails in the gliotic cortex (Anderova et al. 2004) and striatum (Adermark and Lovinger 2008). The method used in this study should facilitate A1 and A2 astrocyte as well as complex astrocyte identification using a standard mathematical algorithm.
Since Gi of A1 and A2 astrocytes was altered in opposite directions in response to incremental holding potential, it is probable that similar events may occur during natural fluctuations of astrocytic Vm in situ. In support of this possibility, spontaneous astrocytic Vm oscillations evoked by K+ concentration ([K+]) modifications in the extracellular space due to neuronal activity were observed in deeply anesthetized animals (Amzica et al. 2002; Mishima and Hirase 2010). Under such conditions, A1 and A2 astrocytes may reveal temporally distinct K+ permeabilities with identical Vm, which would be expected to increase transjunctional voltage and contribute to Vm oscillations. Thus we propose that, if directly connected, A1 and A2 astrocyte pairs may serve as an electrical drive participating in K+ buffering as well as synchronized or desynchronized astrocytic oscillations, although additional studies are needed to test this possibility. When GJC is attenuated during neuroinflammation, the productivity of “electrical drives” should be increased to fit the metabolic needs of the inflamed tissue. By extension, the amplitude and rate of astrocyte membrane currents should be markedly altered, which was observed in the present study at the height of the inflammatory response (i.e., postinfection day 3). The RMP of A1 and A2 populations were quite distinct under similar resting conductance (Table 1), probably reflecting their extreme transitional states that could occur during natural fluctuations. In particular, the amplitude of Gv was dramatically increased in both A1 and A2 astrocytes in closest proximity to the abscess margins. This reflects inward and outward current activation on a “passive” Vm range, which has been observed in other studies, although the entire Vc range was not examined by those investigators (Bordey et al. 2001; Schroder et al. 1999; Wang et al. 2008).
Both astrocytic RMP and Cm returned to values observed in uninfected slices at postinfection day 7, indicating that recovery processes have been initiated at this stage. This timing coincides with a decrease in inflammatory mediator expression within the abscess (Baldwin and Kielian 2004) and the genesis of the fibrotic capsule (Fig. 3B) that serves to sequester the lesion and protect surrounding parenchyma from further destruction (Liberto et al. 2004). A1 and A2 astrocytes displayed preferentially inward and outward voltage-dependent currents, respectively, and their frequencies were inversely correlated at postinfection days 3 and 7 (Fig. 6), which is in agreement with previous studies that utilized other models of brain injury (Anderova et al. 2004; Wang et al. 2008). Given the pronounced degree of parenchymal necrosis during abscess development, it is likely that regional neuronal loss and vascular damage affected A1 and A2 activation. However, at day 7 after infection, the major neuronal source of extracellular K+ and glutamate is expected to be dramatically diminished because of clearance in the extracellular space. The Gv (mainly K+ conductance) decrease observed near the inflammatory site at postinfection day 7 supports the interpretation that decreased extracellular [K+] ([K+]o) may provoke the reduction in voltage-dependent currents (Fig. 8). Accordingly, fewer A1 cells were observed at this time point in close proximity to the abscess (Fig. 6). At greater distances from the abscess site, the observed changes in A2 astrocytes during inflammation persisted until day 7 after bacterial challenge (i.e., +Gi increase), suggesting that membrane modification of ion channels had stabilized in concordance with changes in the abscess microenvironment. On the basis of our findings, we propose that A1 and A2 astrocytes exert different functional properties during neuroinflammation, namely, A1 astrocytes facilitate K+ clearance whereas A2 cells are critical for transporting or propagating ions/metabolites to the vasculature or surrounding brain regions. However, these possibilities remain speculative and should be examined in future studies. Another unresolved issue is whether A1 and A2 astrocytes represent unique populations or, alternatively, the same population that can express different complements of ion channels. We have not observed any evidence of astrocyte proliferation associated with brain abscesses in BrdU incorporation studies, whereas significant proliferation of Iba-1+ microglia was detected (unpublished observations). By extension, this supports the conclusion that the same astrocytes are present throughout the course of infection. Because the percentages of A1 and A2 astrocytes differed at days 3 and 7 after S. aureus exposure without any changes in the total astrocyte pool, we propose that some A1 cells may acquire A2 characteristics as the inflammatory milieu changes. Since there is currently no reliable way to discriminate between A1 and A2 astrocytes besides their electrophysiological properties, it would be challenging to isolate these subsets and study their responses in vitro. In addition, our data indicate that A1 and A2 phenotype is influenced by the local microenvironment, such that removal of cells would likely change the intrinsic electrophysiological parameters of A1/A2 cells from what is observed in intact slices.
Attenuation of GJC/HC activity by CBX application into the bath solution had no effect on the passive profile of astrocytes, and thus the voltage control should be not compromised by supposed “escaping” currents through GJC during whole cell patch-clamp recordings (Schools et al. 2006; Wallraff et al. 2006). Instead, we found that CBX application decreased Gv in A1 and A2 astrocytes similar to the effect of intracellular Cs+ concentration, which blocks most potassium currents. Our recent report demonstrated an inverse relationship between astrocytic GJC and HC activity (Karpuk et al. 2011). Both of these processes were observed in concordance with increased Gv in astrocytes surrounding the neuroinflammatory site. Here, we extend these findings to demonstrate that astrocytes, which display active HCs, also express voltage-dependent currents. In addition, preliminary experiments indicate that increasing [K+]o enhanced astrocyte HC activity in brain slices (data not shown). Taken together, since Gv was sensitive to K+ channel inhibitors and CBX, it is possible that K+ channels and connexin/pannexin channels/HCs interact by an intracellular mechanism similar to what has been shown for neurons (Kawamura et al. 2010; Thompson et al. 2008), although this remains to be tested.
In summary, these findings demonstrate the electrophysiological heterogeneity of striatal astrocyte populations, which experience distinct electrophysiological modifications in response to CNS inflammation. Delineating the changes in astrocytic functions that occur during inflammatory insults may uncover novel mechanisms whereby ion channels modulate cellular activity to promote wound healing and limit pathological inflammatory responses within the CNS.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant RO1 NS-053487 to T. Kielian.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.K., M.B., and T.K. conception and design of research; N.K. and M.B. performed experiments; N.K. and M.B. analyzed data; N.K., M.B., and T.K. interpreted results of experiments; N.K. and M.B. prepared figures; N.K. drafted manuscript; N.K., M.B., and T.K. edited and revised manuscript; N.K., M.B., and T.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Abdallah Hayar for intellectual discussions regarding the manuscript, Amanda Angle for excellent technical assistance, and Kari Nelson for editorial review of the manuscript.
REFERENCES
- Adermark L, Lovinger DM. Electrophysiological properties and gap junction coupling of striatal astrocytes. Neurochem Int 52: 1365–1372, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amzica F, Massimini M, Manfridi A. Spatial buffering during slow and paroxysmal sleep oscillations in cortical networks of glial cells in vivo. J Neurosci 22: 1042–1053, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderova M, Antonova T, Petrik D, Neprasova H, Chvatal A, Sykova E. Voltage-dependent potassium currents in hypertrophied rat astrocytes after a cortical stab wound. Glia 48: 311–326, 2004 [DOI] [PubMed] [Google Scholar]
- Baldwin AC, Kielian T. Persistent immune activation associated with a mouse model of Staphylococcus aureus-induced experimental brain abscess. J Neuroimmunol 151: 24–32, 2004 [DOI] [PubMed] [Google Scholar]
- Bordey A, Lyons SA, Hablitz JJ, Sontheimer H. Electrophysiological characteristics of reactive astrocytes in experimental cortical dysplasia. J Neurophysiol 85: 1719–1731, 2001 [DOI] [PubMed] [Google Scholar]
- Bordey A, Sontheimer H. Postnatal development of ionic currents in rat hippocampal astrocytes in situ. J Neurophysiol 78: 461–477, 1997 [DOI] [PubMed] [Google Scholar]
- Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol 72: 335–355, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci 11: 227–238, 2010 [DOI] [PubMed] [Google Scholar]
- Karpuk N, Burkovetskaya M, Fritz T, Angle A, Kielian T. Neuroinflammation leads to region-dependent alterations in astrocyte gap junction communication and hemichannel activity. J Neurosci 31: 414–425, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpuk N, Hayar A. Activation of postsynaptic GABAB receptors modulates the bursting pattern and synaptic activity of olfactory bulb juxtaglomerular neurons. J Neurophysiol 99: 308–319, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura M, Jr, Ruskin DN, Masino SA. Metabolic autocrine regulation of neurons involves cooperation among pannexin hemichannels, adenosine receptors, and KATP channels. J Neurosci 30: 3886–3895, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T, Barry B, Hickey WF. CXC chemokine receptor-2 ligands are required for neutrophil-mediated host defense in experimental brain abscesses. J Immunol 166: 4634–4643, 2001 [DOI] [PubMed] [Google Scholar]
- Kielian T, Esen N, Liu S, Phulwani NK, Syed MM, Phillips N, Nishina K, Cheung AL, Schwartzman JD, Ruhe JJ. Minocycline modulates neuroinflammation independently of its antimicrobial activity in Staphylococcus aureus-induced brain abscess. Am J Pathol 171: 1199–214, 2007a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T, Phulwani NK, Esen N, Syed MM, Haney AC, McCastlain K, Johnson J. MyD88-dependent signals are essential for the host immune response in experimental brain abscess. J Immunol 178: 4528–4537, 2007b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberto CM, Albrecht PJ, Herx LM, Yong VW, Levison SW. Pro-regenerative properties of cytokine-activated astrocytes. J Neurochem 89: 1092–1100, 2004 [DOI] [PubMed] [Google Scholar]
- MacVicar BA, Thompson RJ. Non-junction functions of pannexin-1 channels. Trends Neurosci 33: 93–102, 2010 [DOI] [PubMed] [Google Scholar]
- Matthias K, Kirchhoff F, Seifert G, Huttmann K, Matyash M, Kettenmann H, Steinhauser C. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci 23: 1750–1758, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima T, Hirase H. In vivo intracellular recording suggests that gray matter astrocytes in mature cerebral cortex and hippocampus are electrophysiologically homogeneous. J Neurosci 30: 3093–3100, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Kerr JN, Helmchen F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat Methods 1: 31–37, 2004 [DOI] [PubMed] [Google Scholar]
- Perillan PR, Li X, Simard JM. K+ inward rectifier currents in reactive astrocytes from adult rat brain. Glia 27: 213–225, 1999 [PubMed] [Google Scholar]
- Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science 322: 1551–1555, 2008 [DOI] [PubMed] [Google Scholar]
- Scemes E, Giaume C. Astrocyte calcium waves: what they are and what they do. Glia 54: 716–725, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schools GP, Zhou M, Kimelberg HK. Development of gap junctions in hippocampal astrocytes: evidence that whole cell electrophysiological phenotype is an intrinsic property of the individual cell. J Neurophysiol 96: 1383–1392, 2006 [DOI] [PubMed] [Google Scholar]
- Schroder W, Hager G, Kouprijanova E, Weber M, Schmitt AB, Seifert G, Steinhauser C. Lesion-induced changes of electrophysiological properties in astrocytes of the rat dentate gyrus. Glia 28: 166–174, 1999 [PubMed] [Google Scholar]
- Seifert G, Huttmann K, Binder DK, Hartmann C, Wyczynski A, Neusch C, Steinhauser C. Analysis of astroglial K+ channel expression in the developing hippocampus reveals a predominant role of the Kir4.1 subunit. J Neurosci 29: 7474–7488, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifri CD, Park J, Helm GA, Stemper ME, Shukla SK. Fatal brain abscess due to community-associated methicillin-resistant Staphylococcus aureus strain USA300. Clin Infect Dis 45: e113–e117, 2007 [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol (Berl) 119: 7–35, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi DK, Vargas JR, Wilcox KS. Increased coupling and altered glutamate transport currents in astrocytes following kainic-acid-induced status epilepticus. Neurobiol Dis 40: 573–585, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RJ, Jackson MF, Olah ME, Rungta RL, Hines DJ, Beazely MA, MacDonald JF, MacVicar BA. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science 322: 1555–1559, 2008 [DOI] [PubMed] [Google Scholar]
- Wallraff A, Kohling R, Heinemann U, Theis M, Willecke K, Steinhauser C. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J Neurosci 26: 5438–5447, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LP, Cheung G, Kronenberg G, Gertz K, Ji S, Kempermann G, Endres M, Kettenmann H. Mild brain ischemia induces unique physiological properties in striatal astrocytes. Glia 56: 925–934, 2008 [DOI] [PubMed] [Google Scholar]
- Zhou M, Xu G, Xie M, Zhang X, Schools GP, Ma L, Kimelberg HK, Chen H. TWIK-1 and TREK-1 are potassium channels contributing significantly to astrocyte passive conductance in rat hippocampal slices. J Neurosci 29: 8551–8564, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]