Abstract
Making decisions about future actions is a fundamental function of the nervous system. Classical theories hold that separate sets of brain regions are responsible for selecting and implementing an action. Traditionally, action selection has been considered the domain of high-level regions, such as the prefrontal cortex, whereas action generation is thought to be carried out by dedicated cortical and subcortical motor regions. However, increasing evidence suggests that the activity of individual neurons in cortical motor structures reflects abstract properties of “decision variables” rather than conveying simple motor commands. Less is known, though, about the role of subcortical structures in decision making. In particular, the superior colliculus (SC) is critical for planning and initiating visually guided, gaze-displacing movements and selecting visual targets, but whether and how it contributes more generally to sensorimotor decisions are unclear. Here, we show that the SC is intimately involved in orienting decisions based on odor cues, even though the SC does not explicitly process olfactory stimuli. Neurons were recorded from the intermediate and deep SC layers in rats trained to perform a delayed-response, odor-cued spatial choice task. SC neurons commonly fired well in advance of movement initiation, predicting the chosen direction nearly 1 s before movement. Moreover, under conditions of sensory uncertainty, SC activity varied with task difficulty and reward outcome, reflecting the influence of decision variables on the intercollicular competition thought to underlie orienting movements. These results indicate that the SC plays a more general role in decisions than previously appreciated, extending beyond visuomotor functions.
Keywords: olfactory-motor behavior, action selection, freely moving tetrode recording
an important question in cognitive neuroscience is how the motor output of perceptual decisions is controlled by the brain. Much cortical neurophysiological data suggest that selecting and generating actions may be complementary components of a single process (Carpenter et al. 1999; Cisek 2007; Cisek and Kalaska 2010; Gold and Shadlen 2000; Hernandez et al. 2002). However, less is known about the extent of this confluence of cognitive and motor representations beyond cortex (Ding and Gold 2010) and particularly whether it is limited to certain forms of sensorimotor transformations. We addressed these questions by focusing on the superior colliculus (SC), a midbrain structure known to be involved in planning, initiating, and coordinating gaze-displacing movements of the eyes, head, and body (Freedman et al. 1996; Gandhi and Katnani 2011; Sparks 1999) and in selecting among potential visuomotor targets (Basso and Wurtz 1997; Glimcher and Sparks 1992; Horwitz and Newsome 1999; Krauzlis et al. 2004; Kustov and Robinson 1996; McPeek and Keller 2002, 2004; Nummela and Krauzlis 2010; Port and Wurtz 2009; Thevarajah et al. 2009). In particular, many SC cells, which are selective for saccade direction, also represent the direction of a visual motion stimulus, suggesting that they may participate in linking stimulus input to motor output (Dorris et al. 2007; Horwitz and Newsome 2001a). However, this property may either reflect selectivity for motion direction itself, which we might expect to observe in visuomotor SC neurons during a visually guided saccade task (Krauzlis 2004), or some decision-related variable, such as the likelihood of a future reward.
In fact, several recent studies in primates have proposed that SC activity reflects decision-related variables, suggesting that it is directly involved in saccadic decisions (Horwitz et al. 2004; Kim and Basso 2008; Lee and Keller 2006). However, an outstanding question is whether the SC is involved only in motor decisions based on a modality that it explicitly represents—visual, auditory, or somatosensory—or whether it contributes to a wider class of decisions. To examine the latter possibility, we studied SC activity in the context of an odor-mixture categorization task in rats (Uchida and Mainen 2003). Since the SC is not known to receive direct olfactory input or represent odors explicitly, we would expect its activity, during our task, to reflect the stimulus only if it were to play a more general role in sensorimotor decisions.
We built upon our previous work on odor-cued orienting responses in the SC (Felsen and Mainen 2008), where we found that single neurons were selective for movement direction preceding and during the movement [see also Hirokawa et al. (2011)]. In our previous study, rats were free to initiate movement at any time during stimulus presentation; therefore, activity related to the execution of a motor plan could not be readily distinguished from activity underlying the decision itself (Schall 2005; Shadlen and Newsome 2001). To dissociate the activity related to each of these processes, we have modified the task to require the rat to wait for an auditory “go signal” before initiating its response. We found, as in saccadic tasks, that the activity of SC neurons could often predict the upcoming choice far in advance of movement initiation. More surprisingly, choice-predictive neurons were jointly modulated by discrimination difficulty and choice outcome, demonstrating that the critical sensory information necessary for constructing higher-order decision variables is carried in the SC along with the choice itself. Together, these observations suggest that the SC is involved in orienting decisions independent of the sensory modality on which those decisions are based.
EXPERIMENTAL PROCEDURES
Animal subjects.
Animal use procedures were submitted to and approved by the Cold Spring Harbor Laboratory Institutional Animal Care and Use Committee and carried out under a license obtained from the committee in accordance with National Institutes of Health standards. Four male Long-Evans hooded rats were used in these experiments. Rats had free access to food, but water was restricted to the behavioral session and ∼1 additional h/day.
Delayed-response, odor-cued spatial choice task.
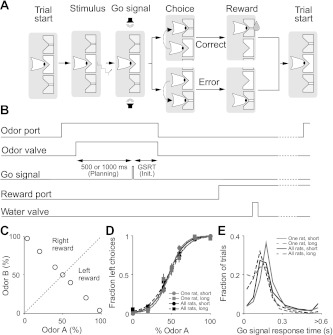
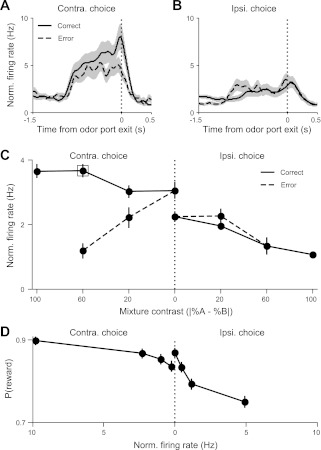
Rats were trained and tested on a delayed-response version of a two-alternative spatial choice task in which the identity of an odor was associated with the location of a water reward (Uchida and Mainen 2003). In each trial of the task, the rat first entered the odor port, triggering the delivery of an odor, waited 500 or 1,000 ms for the presentation of an auditory go signal (100-ms, 8-kHz tone, played from two lateral speakers), and then moved to one of the reward ports to harvest the reward, if any (Fig. 1, A and B). Premature odor port exit resulted in a 500-ms white noise burst and the absence of reward at either reward port. Odors were mixed with a pure air carrier and delivered at a flow rate of 1 liter/min using an olfactometer (Island Motion, Tappan, NY). To decorrelate the timing of port entry and the delivery of odor (or water), opening of the odor (or water) valve was delayed following entry into the odor (or reward) port by 200–500 ms (uniformly distributed). Probability of reward at each side as a function of odor identity is shown in Fig. 1C. 50/50 Trials were rewarded at both the left and right on 50% of randomly selected trials. Odors and delay lengths were interleaved randomly.
Fig. 1.
Behavioral performance on the delayed response odor-cued spatial choice task. A: schematic of trial events. In each trial, after entering the odor port, the rat receives an odor, waits for the go signal, exits the odor port, moves to 1 of the reward ports, and receives a water reward following correctly performed trials. B: timing of trial events and task epochs. GSRT, go signal response time; Init., initiation. The following panels focus on the planning and initiation epochs. C: relationship between fractions of (+)- and (−)-octanol (odors A and B, respectively) and reward side. 50/50 Trials were rewarded at both the left and right on 50% of randomly selected trials. On some trials, pure (+)- or (−)-carvone was presented, rewarded at the left and right, respectively (not shown). D: accuracy is independent of delay length. Lines show fits to p = 1/1 + e(−a − bx), where x is the proportion of odor A in the mixture ratio, p is the fraction of left choices, and a and b are free parameters. Error bars (sometimes smaller than symbols) show SE, based on binomial distribution. For this analysis and all others, only trials in which the rat successfully waited for the go signal before exiting the odor port are included. E: go signal response time (from go signal to odor port exit; B) was slightly shorter during the long-delay vs. short-delay trials.
The rats were initially naïve and were trained to perform the task within 6–8 wk (one session/day; ∼6 days/wk), using the following sequence of steps: 1) Entry into either of the two reward ports (left or right) was rewarded by water delivery to that port. 2) Entry into the central odor-sampling port, triggering the delivery of an odor, was required before water was available at either reward port. 3) Water was available only at the left reward port following delivery of some odors and mixtures and only at the right reward port following the delivery of other odors (Fig. 1C). 4) A go signal (tone) was introduced, such that exit from the odor port prior to the presentation of the signal resulted in a white noise burst and the unavailability of water at either reward port. The length of the delay preceding the go signal was increased gradually across sessions until reaching its maximum value of 1,000 ms.
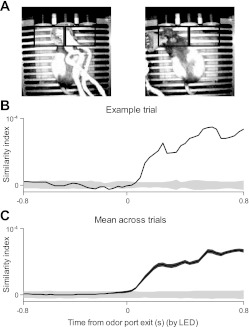
The timing of port entry and exit was measured using an infrared light-emitting diode (LED) transmitter/receiver pair placed on either side of the port: a “broken” LED beam indicated that the rat was in the port. Although this method of measurement allows small movements to be made while the beam remains broken, it provided as reliable a determinant of goal-directed movement initiation as an independent video analysis (see Fig. 10).
Fig. 10.
Trial-by-trial analysis of video recordings confirms the movement initiation time estimated using the light-emitting diode (LED) beam. A: example video frames in a trial in which the rat chose the left reward port. Black boxes show the left and right regions of interest for the analysis. B: similarity index (SI; see experimental procedures) calculated in each frame for an example trial. SI is expected to increase during movement. Gray shading, mean ± SE of the resampled distribution of SIs obtained after shuffling the left and right trials. Actual SI only becomes significantly different from resampled distribution after the time of odor port exit obtained using the integrity of the LED beam. C: as in B for the average SI across all trials for 1 session. Dark gray shading, ±SE across trials.
Surgery.
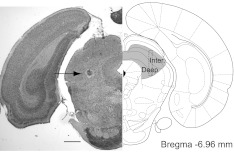
After initial training on the task, each rat was surgically implanted with a custom-made drive consisting of 14 independently adjustable tetrodes targeted to the intermediate and deep layers of the left SC (6.8 mm posterior to bregma and 1.7 mm lateral to the midline) (Felsen and Mainen 2008; Paxinos and Watson 2005). Anesthesia was induced with 3% isoflurane and maintained between 2% and 3% isoflurane throughout the procedure. Depth of anesthesia was monitored by tail and toe tail-pinch responses. Body temperature was maintained using a heating pad (HoMedics, Commerce Township, MI). The rat was placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA), and a small incision was made in the skin with a stainless-steel surgical blade. The skull was cleaned and dried, and a craniotomy was performed using a dental drill. The recording drive was placed over the craniotomy and attached to the skull, using several small skull screws (#0-80, J. I. Morris, Southbridge, MA) and dental acrylic (Lang, Wheeling, IL). One of the skull screws was used for providing electrical ground for the recording array. Following the surgery, rats were administered ketofen (Fort Dodge Animal Health, Overland Park, KS, now Pfizer Animal Health, Madison, NJ) as an analgesic (1 mg/kg) and rehydrated with sterile 0.9% saline (1 ml/kg), and the incision site was treated with a topical antibiotic. Rats were allowed to recover for 5 days before water restriction resumed and the recording sessions began. During that period, the tetrodes were gradually lowered to reach the intermediate layer of the SC.
Neural recording.
Individual tetrodes consisted of four twisted polyimide-coated nichrome wires (Kanthal Palm Coast, Palm Coast, FL; single-wire diameter, 12.5 μm), gold-plated to 0.2–0.4 MΩ impedance. Electrical signals were amplified and recorded using the NSpike multichannel acquisition system (L. Frank and J. MacArthur). Multiple single units were isolated offline by a combination of an automated expectation maximization algorithm (KlustaKwik, Ken D. Harris, Rutgers University, Newark, NJ) and by manually clustering spike features derived from the sampled waveforms using MClust software (A. David Redish, University of Minnesota, Minneapolis, MN). Isolation Distance, Lratio, autocorrelograms, and crosscorrelograms were used to quantify cluster quality (Schmitzer-Torbert et al. 2005). Tetrode depths were adjusted prior to each recording session to sample an independent population of cells across sessions, and their locations during each recording session were estimated based on their depth and later confirmed histologically based on electrolytic lesions and on the visible tetrode tracks (Fig. 2) (Felsen and Mainen 2008). Cells were not selected based on any criteria prior to beginning a recording session, and we therefore obtained recordings from many cells that were not selective for the movement directions we tested (see Fig. 4). Rats performed (i.e., successfully waited for the go signal) 226 ± 84 trials/session (mean ± SD), one session was performed/day, and a total of 34 recording sessions was obtained.
Fig. 2.
Histological verification of recording location. Following the final recording session, electrolytic lesions were made to identify the ventral-most extent of tetrode travel. A representative lesion (black arrow) marks the deep layers of the superior colliculus (SC). Schematic (right side) is from Paxinos and Watson (2005). Dotted box shows the typical extent of recordings for 1 tetrode across sessions. Original scale bar, 1 mm. Inter., intermediate.
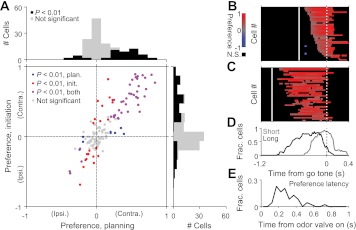
Fig. 4.
Timing of direction preference across the population. A: preferences calculated during planning (plan.) and initiation epochs (Fig. 1B) within each odor pair across the population of cells recorded [142 cells that met minimal criteria for trials and firing rate (see experimental procedures)]. Correlated values indicate that preference is similar between the 2 epochs. Marginal histograms show distribution of preferences during planning (top) and initiation (right) epochs. B: “preference curves” for short-delay trials for all cells significantly direction selective, both during the initiation epoch (A) and in at least 1 time window during the planning epoch in the long- and short-delay trials, sorted by time of the earliest significant bin. Preference curves were constructed by calculating the direction preference in a 200-ms window, translated by 20-ms increments. Trials are aligned to the go signal (solid line). Dotted line, odor valve open. Color scale shows significant preferences (P < 0.01, permutation test; positive values correspond to the preferred direction calculated during the entire planning epoch). Black boxes indicate bins with nonsignificant preferences (N.S.; P > 0.01, permutation test) or with <10 ipsiversive or contraversive trials. Note that for some cells, the preferred direction changed during the trial (corresponding to the blue boxes). C: preference curves for long-delay trials as in B. Cells are sorted in the same order as in B. D: fraction (Frac.) of cells selective at each time bin during long- and short-delay trials. Only the cells corresponding to the data shown in B and C are included. Abscissa is shared with B and C. E: fraction of cells for which 1st significant preference bin occurs relative to odor valve open.
Neural data analysis.
All data analysis was performed using Matlab (MathWorks, Natick, MA). To quantify the dependence of firing rate on the direction of upcoming movement, we used an algorithm based on receiver operating characteristic (ROC) analysis, which calculates the ability of an ideal observer to classify whether a given spike rate was recorded preceding leftward or rightward movement (Feierstein et al. 2006; Green and Swets 1966). We defined “preference” as 2(ROCarea − 0.5), a measure ranging from −1 to 1, where −1 signifies the strongest possible preference for leftward movement, and 1 signifies the strongest possible preference for rightward movement. Odor selectivity was calculated similarly, where trials were grouped by which odor was presented instead of the direction of movement. Odor selectivity was defined as 2(ROCarea − 0.5), ranging from 0 to 1, where 0 signifies minimal odor selectivity, and 1 signifies maximal odor selectivity. Note that selectivity is equivalent to the absolute value of the preference (we are not interested in which of the two odors was preferred). Statistical significance was determined with a permutation test: we recalculated the preference after randomly reassigning all firing rates to either of the two groups, repeated this procedure 500 times to obtain a distribution of values, and calculated the fraction of random values exceeding the actual value. For all analyses, we tested for significance at α = 0.01. This analysis is sensitive to both absolute and relative differences in firing rates and yields very similar results to another common metric of selectivity, (RateA − RateB)/(RateA + RateB) (Felsen and Mainen 2008). Only cells with a minimum number of four trials for each analyzed condition (e.g., leftward trials) and with a firing rate above two spikes/s for either of the analyzed conditions were included in that analysis. Our results were independent of the specific values selected for these criteria. Mean firing rates across the entire recording session, for all included cells, ranged from 1.0 to 52.9 spikes/s (median = 7.0 spikes/s; note that a cell can average greater than two spikes/s in a particular condition, whereas averaging less than two spikes/s across the entire session). Trials in which the rat exited the odor port before the go signal or movement time (from odor port exit to water port entry) was >1 s were excluded from all analyses.
Histology.
To verify the ultimate location of the tetrodes, electrolytic lesions were produced after the final recording session (Fig. 2). Rats were then deeply anesthetized with a cocktail of ketamine (Fort Dodge, now Pfizer Animal Health) and medetomidine (Pfizer, New York, NY) and perfused transcardially with 4% paraformaldehyde. The brain was removed and stored in 4% paraformaldehyde and was then sectioned at 50 μm and Nissl stained.
Video analysis.
To independently verify the time of movement initiation, as measured with the LED beam, we examined video recordings to determine when movement could be detected. Video of each session was recorded at 30 frames/s using an infrared camera (Supercircuits) installed above the ports. The video recording and behavior clocks were synchronized by flashing a light visible to the camera at the start of each trial.
For each frame of each trial, we examined how the luminance values of the pixels in left and right regions of interest (LROI and RROI, respectively; see Fig. 10A) depended on whether the rat chose the left or right reward port on that trial. We first found the absolute difference between luminance values in the LROIs of each frame and the LROIs of a background frame, recorded without the rat in the behavioral environment. We then normalized the power of the LROIs in each frame [i.e., time within the trial (t)] of each trial (i) as , where x is the pixel number, and D is the total number of pixels. We then calculated the mean-squared error between the LROI of a given frame of a trial and the average LROIs of all other trials in which the rat chose the same direction as , where n is the number of trials in which the rat chose the same direction. We expect this value to be relatively small during movement, since the luminance values on a given trial should, on average, resemble those on other trials in which the rat chose the same direction. We next calculated a similar relationship between the LROI of each frame and the average LROIs of all trials in which the rat chose the opposite direction, as , where m is the number of trials in which the rat chose the opposite direction. We expect this value to be relatively large during movement, since the luminance values on a given trial should not, on average, resemble those on trials in which the rat chose the opposite direction. We then calculated a similarity index (SI) with respect to the LROIs as SIL_ROIi,t = MSEL_ROI,diffi,t − MSEL_ROI,samei,t, which we expect to be relatively large during movement. MSER_ROI,samei,t, MSER_ROI,diffi,t and SIR_ROIi,t were calculated as above, with respect to the RROIs of each frame. Finally, we averaged SIL_ROIi,t and SIR_ROIi,t to compute the overall SI for each frame of each trial: (see Fig. 10, B and C). We expect this value to be large during movement. To estimate significance, we calculated the distribution of SI values expected by chance (see Fig. 10, B and C) by randomly shuffling the left and right trials and recomputing the resulting SI values 500 times. SI values falling within this range should not be considered significantly different from zero. Whereas this analysis may not detect small movements, due to the camera angle and resolution, it is useful for our purpose of determining when movement to the water port was initiated.
RESULTS
Rats were trained and tested on a delayed-response olfactory discrimination task (Fig. 1; see experimental procedures). In each trial of the task, the rat first inserted its snout into a central odor port, breaking a photobeam and triggering the delivery of an odor. After a short (500-ms) or long (1,000-ms) delay, during which odor delivery continued, a go signal (tone) was presented, indicating permission to exit the odor port and move to either the left or right reward port, where water was delivered following a correct response (Fig. 1, A and B). If the rat withdrew from the odor port prematurely, a brief white noise burst was presented immediately, and water was not delivered following entry into either reward port. Such aborted trials (25.7 ± 8.8% across sessions; mean ± SD) were discarded prior to all subsequent analyses. In each trial, the reward side was determined by the dominant component in a mixture of (+)-octanol and (−)-octanol (Fig. 1C).
Task performance, as measured by the fraction of trials in which the rat selected the correct reward port, was unaffected by the length of the delay period (Fig. 1D). Movement time (from odor port exit to reward port entry) was independent of delay length (P = 0.14). Go signal response time (from go signal to odor port exit) was shorter in long-delay trials (P < 10−5; Fig. 1E), perhaps reflecting heightened anticipation of the go signal following long delays.
We recorded from 197 well-isolated intermediate- and deep-layer neurons of the left SC during performance of the delayed-response task (Fig. 2; see experimental procedures), focusing on activity during odor sampling (Fig. 1, A and B), when the direction of movement is presumably selected, planned, and initiated. As reported previously (Felsen and Mainen 2008; Hirokawa et al. 2011), rat SC cells commonly showed selectivity for choice direction. Many of these cells exhibited choice selectivity at a short latency relative to stimulus onset, thus preceding movement initiation by a substantial interval that increased with the go signal delay. Figure 3 shows the activity of an example cell illustrating a typical SC response. The firing rate of this neuron increased soon after odor presentation began and remained elevated until movement initiation, independent of the length of the delay. The latency between the beginning of odor presentation and the increase in activity is consistent with typical odor sampling times observed during a self-paced (reaction time) version of this task (Feierstein et al. 2006; Felsen and Mainen 2008; Uchida and Mainen 2003), suggesting that the increase coincides with the time the rat has selected and can begin to plan its motor response. This activity profile is most prominent for trials in which the rat chose the preferred direction of the cell, which in this case was contraversive to the SC from which it was recorded.
Fig. 3.
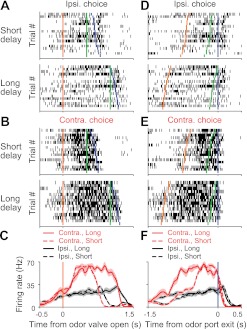
Rasters and perievent histograms for an example cell preferring contraversive movement. A: rasters for ipsiversive (Ipsi.; leftward) choices. Each row shows spikes (black ticks) in 1 trial, aligned to time of odor valve open (orange lines). Green lines, time of go signal; blue ticks, times of odor port exit. Short- and long-delay trials were interleaved during the session but are displayed separately here, sorted by odor sampling duration. Eighteen randomly selected trials are shown/group. B: as in A for contraversive (Contra; rightward) choices. C: perievent histograms showing average activity across trials, separately with respect to choice and delay length, aligned to odor port open. Shading, ±SE. Histograms are smoothed with a Gaussian filter (σ2 = 30 ms). D: trials ordered as in A, aligned to time of odor port exit (blue lines). Orange ticks, times of odor valve open; green ticks, times of go signal. E: as in D for contraversive trials, ordered as in B. F: as in C aligned to odor port exit.
Across the population of cells recorded, we calculated the preference of each cell for ipsiversive vs. contraversive choices during specific epochs of the trial (Fig. 4A; see experimental procedures) (Feierstein et al. 2006; Felsen and Mainen 2008). This measure ranges from −1 to 1; negative values indicate ipsiversive preference, positive indicate contraversive preference, and the magnitude reflects the strength of the preference. We defined the “planning” epoch as the time between the onset of odor presentation and the go signal presentation, when the rat can use the odor cue to plan whether to move to the left or right reward port but cannot yet initiate the movement (Fig. 1B). We defined the “initiation” epoch as the time between go signal presentation, when the rat is permitted to initiate movement, and the exit from the odor port, at which point execution begins (Fig. 1B). We found that during the planning epoch, more than one-third of SC neurons significantly preferred one direction of movement over the other [53 of 142 cells that satisfied our criteria for minimum trials and spike rate (see experimental procedures); P < 0.01, t-test]. Consistent with observations during the reaction-time version of the odor discrimination task (Felsen and Mainen 2008), a large fraction of cells preferred the contraversive over the ipsiversive choice (44/53 preferred contraversive; P < 10−5, χ2 test), and choice-selective neurons maintained their direction preference across epochs (Fig. 4A).
These data reveal that the activity of choice-selective SC neurons is not tied to the moment of response initiation but rather, can precede it by many hundreds of milliseconds. To examine this observation in more detail, we next studied how the time course of direction preference differed between short- and long-delay trials (Fig. 4, B–D). For each cell that significantly preferred a direction during the initiation epoch, we constructed “preference curves”, separately for short- and long-delay trials, by calculating preference in overlapping 200-ms windows after aligning trials to the go signal (Felsen and Mainen 2008). Whereas the time course over which choice preference developed varied considerably across the population of neurons (Fig. 4, B and C), it arose earlier, relative to the time of the go signal, during long-delay trials (Fig. 4D). These results indicate that the SC is involved in forming the motor plan and is not limited to the initiation of movements.
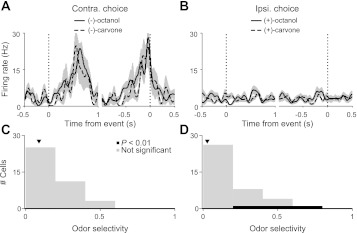
We next examined whether, in addition to representing the motor choice during the planning epoch, any features of the odor stimulus were also represented in the SC. An influence of the stimulus on the activity of SC cells selective for movement direction would further support the hypothesis that the SC is involved directly in the process of selecting the motor output in response to the stimulus. To address this question, we used the fact that in a subset of trials, pure (+)-carvone or (−)-carvone (rewarded at the left and right, respectively) was presented, allowing us to compare activity during the presentation of two different odors in which the same movement direction was correctly selected. We found minimal selectivity for odor identity during any trial epoch. Figure 5, A and B, shows activity for one cell during correct rightward and leftward trials, separately for each of the pure odors. Like most direction-selective neurons (Fig. 4A), this cell preferred upcoming contraversive (rightward) movement. However, firing rate was independent of the identity of the odor presented for either the preferred or antipreferred movement direction. For each cell in the population, we calculated odor preference exactly as we had calculated direction preference (see experimental procedures) and took selectivity to be the magnitude of the preference (Fig. 5, C and D). We found that very few cells exhibited selectivity for one pure odor over the other (0/39 for preferred direction; 3/39 for antipreferred direction; at P < 0.01).
Fig. 5.
SC activity does not depend on odor identity. A: perievent histograms for an example cell showing trials in which the rat correctly chose the contraversive (right and preferred) reward port when either pure (−)-octanol or (−)-carvone was presented, aligned to odor valve open (left dotted line) and odor port exit (right dotted line). Only short-delay trials are shown. Shading and smoothing as in Fig. 2B. B: as in A but for trials in which the rat correctly chose the ipsiversive (left and antipreferred) reward port when either pure (+)-octanol or (+)-carvone was presented. C: histogram of odor selectivity [(−)-octanol vs. (−)-carvone] during planning epoch in which the rat correctly chose the contraversive reward across the population of cells preferring contraversive choice during the initiation epoch and meeting the minimum trial number and firing-rate criteria (39 cells; see experimental procedures). Short- and long-delay trials are included. Arrowhead shows the selectivity for the example cell in A. D, and Figs. 6, 7, and 9, shows results from the same population. D: as in C but for selectivity for (+)-octanol vs. (+)-carvone in trials in which the rat correctly chose the ipsiversive (antipreferred) reward port. Arrowhead shows the preference for the example cell in B.
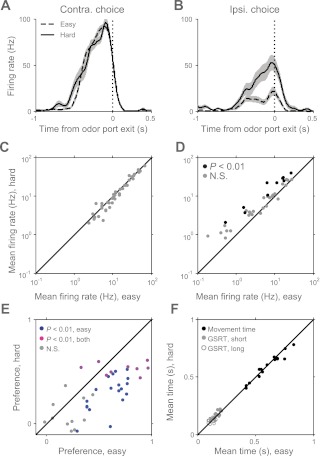
Although SC neurons showed no odor preferences during the formation of the motor plan, if they are involved in selecting the action, they might be expected to reflect decision variables associated with the stimulus, such as the likelihood of a future reward. To address this possibility, we first examined the dependence of SC activity on stimulus difficulty, which correlated with reward delivery (Fig. 1D). We defined “easy” discrimination trials as the 100/0, 80/20, 20/80, and 0/100 mixture ratios of the odors and “hard” discrimination trials as the 40/60, 50/50, and 60/40 ratios (Fig. 1C). When separated in this way, many SC neurons indeed showed difficulty dependence, primarily during trials in which the antipreferred direction was selected. Figure 6 shows an example cell that illustrates this phenomenon. Although this cell did not exhibit a difference in activity between easy and hard trials preceding movement in its preferred direction (contraversive; Fig. 6A), there is a clear difference preceding movement in the antipreferred direction (ipsiversive; Fig. 6B). In the population of cells preferring upcoming contraversive movement during the initiation epoch (Fig. 4A), although no individual cell showed a significantly different mean (P < 0.01, Wilcoxon rank-sum test), the population itself exhibited significant difficulty dependence preceding movement in the preferred direction (Fig. 6C; P < 0.05 across the population, t-test; mean difference = 1.01 spikes/s). In the antipreferred direction, difficulty dependence was much stronger, with many cells showing stronger firing on hard compared with easy trials [Fig. 6D; 10/39 cells individually significant (P < 0.01, Wilcoxon rank-sum test); P < 10−4 across population, t-test; mean difference = 3.40 spikes/s]. Consistent with these data, we found for nearly all neurons that direction preference was stronger during easy than hard trials (Fig. 6E). The dependence of firing rate and direction preference on mixture ratio was not accompanied by differences in movement speed or go signal reaction time, which were independent of discrimination difficulty (Fig. 6F; P = 0.22 for movement times, P = 0.095 for go signal response times on short-delay trials, and P = 0.69 on long-delay trials, t-tests). We did not observe difficulty dependence in the population of neurons that preferred upcoming ipsiversive movement [see Fig. 8, A and B; P > 0.7 across the population (t-test) for preferred and antipreferred direction; mean difference = 0.06 spikes/s (preferred direction) and 0.26 spikes/s (antipreferred direction)].
Fig. 6.
Difficulty dependence of SC activity during planning epoch. A: perievent histograms for a novel example cell showing short-delay trials in which the rat chose the contraversive (right and preferred) reward port, grouped by stimulus difficulty and aligned to odor port exit (dotted line). “Easy” trials are those in which the percent right odor [(−)-octanol] was 0, 20, 80, or 100. “Hard” trials are those in which the percent right odor was 40, 50, or 60. Trials in which the pure carvones were presented are excluded. Shading and smoothing as in Fig. 3C. B: as in A but for trials in which the ipsiversive (left and antipreferred) direction was selected. C and D: mean firing rate during the planning epoch of easy vs. hard correctly performed trials in which the preferred (C) or antipreferred (D) direction was selected for the contraversive-preferring population (as in Fig. 5C). Each marker represents 1 cell. All 50/50 trials were considered correct regardless of whether reward was delivered. E: direction preference calculated during the planning epoch of correctly performed easy vs. hard trials for the contraversive-preferring population (as in Fig. 5C). All 50/50 trials were considered correct. Preference is generally stronger during easy trials. F: mean go signal response times (from go signal to odor port exit; Fig. 1B) and movement times (from odor port exit to reward port entry) for easy vs. hard trials. One marker is shown/session for each time type (17 sessions). Times are independent of discrimination difficulty.
Fig. 8.
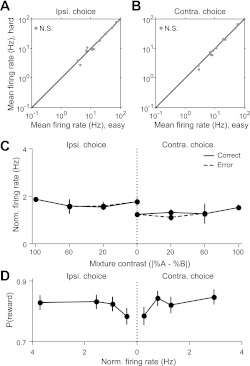
Difficulty and outcome dependence were not observed in the activity of SC cells that prefer upcoming ipsiversive movement. A and B: mean firing rate during the planning epoch of easy vs. hard correctly performed trials in which the preferred (A) or antipreferred (B) direction was selected for the ipsiversive-preferring population. Each marker represents 1 cell. All 50/50 trials were considered correct, regardless of whether reward was delivered. C: mean normalized firing rate during the planning epoch averaged across the ipsiversive-preferring population as a function of mixture contrast, separately for correct and error trials in each direction. Conventions as in Fig. 7C. D: reward probability as a function of normalized firing rate across the ipsiversive-preferring population, separately for each direction. Conventions as in Fig. 7D.
We next examined the dependence of activity on whether the planned movement was correct or incorrect, with which reward delivery was, by definition, associated. In the population of cells preferring upcoming contraversive movement (Fig. 4A), for each choice direction, we separated the trials of a given difficulty according to the odor cue presented. This analysis revealed an asymmetrical pattern of SC activity with respect to the choice. Preceding antipreferred choices, there was no difference in normalized firing rates between correct and incorrect trials (Fig. 7, B and C). However, preceding preferred choices, the level of activity on incorrect trials was reduced to that preceding antipreferred choices (Fig. 7, A and C). Finally, we also examined whether the probability of reward (i.e., choice accuracy) varied with SC activity. Preceding preferred choices, we found that accuracy was positively correlated with normalized firing rate, whereas preceding antipreferred choices, accuracy decreased with increasing firing rate (Fig. 7D). We did not observe these effects in other task epochs or in the population of neurons that preferred upcoming ipsiversive movement (Fig. 8, C and D).
Fig. 7.
SC activity depends on trial outcome and reflects reward probability. A: mean normalized perievent histogram for the contraversive-preferring population (as in Fig. 5C) on long-delay trials in which the preferred direction was selected, grouped by outcome, and aligned to odor port exit (dotted line). The activity of each cell was normalized to its mean rate over the duration of the recording session, here and for all other analyses of normalized (Norm.) firing rate, so that all neurons provide equal weight to the population means (alternative normalization factors did not affect the results). All 50/50 trials were considered correct. All panels include only the data from trials in which the octanols were presented. Smoothing and shading as in Fig. 3B. B: as in A but for trials in which the ipsiversive (left and antipreferred) direction was selected. C: mean normalized firing rate during the planning epoch averaged across the population as a function of mixture contrast, separately for correct and error trials in each direction. The firing rates during error trials at 100% odor contrast are not shown, because rats very infrequently chose the incorrect reward port on pure odor trials (Fig. 1D). Boxed marker indicates correspondence with markers in Fig. 9A. All 50/50 trials contribute to both correct and error trials. Normalization as in A. Error bars, ±SE. D: reward probability [P(reward)] as a function of normalized firing rate across the population, separately for each direction. Firing rates are binned such that an equal number of trials contribute to each bin.
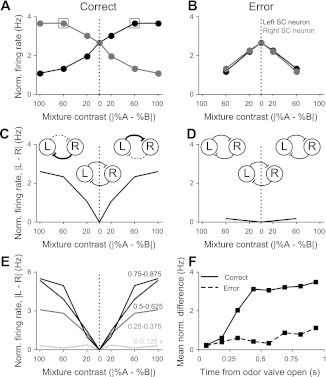
Together, the dependence of activity on trial outcome and discrimination difficulty suggests that activity in the SC may reflect decision variables critical for orienting choices. It is still unclear whether this information is conveyed to the SC from other brain structures or whether the SC itself may contribute to its production. To investigate the latter possibility, we used the data from our contraversive-preferring population to infer how activity across both SCs would depend on task difficulty and outcome. We assumed that right SC neurons would show a pattern of activity with respect to their preferred direction of movement that is symmetric to that of the left hemisphere neurons that we recorded (Fig. 4A) (Britten et al. 1992), as well as a similar dependence on difficulty (Fig. 6) and outcome (Fig. 7). The net activity of left and right SC neurons during easy trials then exhibits an interesting pattern: the activity differs depending on whether the rat is planning a correct or incorrect movement. Preceding a correct movement, activity in the two SCs diverges as discrimination difficulty decreases (i.e., as reward becomes more likely; Fig. 9A), whereas preceding an incorrect movement, left and right SC activity is similar across all discrimination difficulties (Fig. 9B). Note that the difference between easy and hard correct trials is more apparent in Fig. 9A than it appears in Fig. 6, because in Fig. 9A, we plot a normalized, average firing rate across the population in which we observe a higher firing rate during easy vs. hard trials (P < 0.05; Fig. 6C). Although this pattern of results does not rule out involvement of other regions that exhibit similar modulation of motor-related activity, such as premotor cortex (Pastor-Bernier and Cisek 2011) and lateral intraparietal cortex (Shadlen and Newsome 2001) in primates, it could be explained parsimoniously through competition in a mutually inhibitory SC circuit, as has been previously proposed to underlie movement generation (Lo and Wang 2006). For correct and, particularly, for easier trials, the net activity robustly favors one direction, reflecting a strongly imbalanced left–right interaction (Fig. 9C). For error trials, the left–right activity difference collapses, reflecting weaker competition in the circuit (Fig. 9D). Thus the degree of imbalance between activity in the left and right SC reflects the likelihood of an upcoming reward, which is highest for correctly performed easy trials. The difference in activity between the left and right SC on correctly performed easy trials emerges during the planning epoch, approaching its maximum ∼500 ms after the beginning of odor delivery (Fig. 9, E and F). Whereas the activity imbalance may be inherited from upstream brain regions that participate in the decision, our analysis suggests that intercollicular competition may contribute to producing, or perhaps maintaining throughout the delay period, the activity underlying the selection of orienting actions.
Fig. 9.
Inter-SC mutual inhibition is consistent with difficulty and outcome dependence in the contraversive-preferring population. A: normalized average activity of actual left and inferred right SC neurons as a function of mixture contrast, obtained from the data in Fig. 7C (e.g., the boxed markers here correspond to the boxed marker in Fig. 7C). Activity on 50/50 trials (mixture contrast = 0%) is averaged across left and right choices, because both choices were considered to be correct. Activity of a contraversive-preferring right SC neuron (gray) is assumed to mirror that of the recorded left SC neurons, such that its dependence on the percent left odor is the same as the dependence of the left neuron on percent right odor (i.e., the activity of a right neuron at 20% odor A is the same as that of a left neuron at 20% odor B; boxed symbols). B: as in A for error trials. Note that the activity on 50/50 trials is identical to that shown in A. C: magnitude of the difference between the left (L) and right (R) SC neurons as a function of odor contrast. Insets show schematics of strength of inhibition between left and right SC. Dotted lines, weaker inhibition; bold lines, stronger inhibition. D: as in C for error trials. E: as in C in different time windows during the planning epoch (labeled in figure from the time of odor valve open). Windows >500 ms show data from short-delay trials only. F: mean absolute difference across mixture contrasts between normalized left and right SC neurons in nonoverlapping, 125-ms windows.
DISCUSSION
This study examined the role played by the intermediate and deep layers of the rat SC, which have traditionally been associated with motor output, in perceptual decision making. We found that SC activity was direction selective nearly 1 s before movement initiation (Fig. 4). Although this activity was stimulus invariant with respect to pure odor cues (Fig. 5), when sensory evidence was ambiguous, the difficulty of the discrimination (Fig. 6) and the future trial outcome (Fig. 7) were reflected in SC firing. These results, along with a simple explanation for how the activity may be maintained in the SC (Fig. 9), add to recent evidence that the SC may contribute to higher-order decision-making functions (Horwitz et al. 2004; Kim and Basso 2008; Ratcliff et al. 2007; Song et al. 2011; Thevarajah et al. 2009).
Interpreting our results depends on an accurate determination of the time of movement initiation, defined here as when the LED beam at the opening of the port was no longer occluded by the snout of the rat (see experimental procedures). It is possible, however, that the rat began to move sooner than when the beam was broken or that SC activity reflects covert orienting movements that are not reflected in the LED signal. The activity of single cells in the rat prefrontal cortex has been shown to depend on shifts in body and head position (Cowen and McNaughton 2007), and some primate SC cells control neck muscle contractions (Corneil et al. 2002, 2008) and arm movements (Stuphorn et al. 1999; Werner et al. 1997), even in the absence of overt eye or head movement. SC activity may also reflect whisker-related sensory input or motor output (Drager and Hubel 1975; Hattox et al. 2002). However, for several reasons, we believe that the activity that we observed does not simply reflect covert movements. First, whereas it is impossible to rule out all such movements without exhaustively monitoring all muscle activity, a post hoc analysis of trial-by-trial video recordings failed to detect movement initiation any earlier than our operational definition (Fig. 10; see experimental procedures). Second, even if the animals made covert movements undetected by our video analysis (e.g., leaned toward the selected reward port) (Erlich et al. 2011), there is no reason to suspect that the magnitude of such movements would depend on discrimination difficulty, which modulates SC activity (Fig. 6). Finally, on long-delay trials, in which shorter response times suggest that rats anticipate the timing of the go signal (Fig. 1E), the activity of many cells decreased just prior to the go signal, before overt movements had begun (Fig. 4, C and D). These results indicate that some SC cells cease firing well before movement initiation, providing further support for the dissociation of SC activity and overt movement onset.
We have shown previously that the SC is necessary for initiating orienting movements in rats, functioning in a similar manner to that described for saccadic eye movements in monkeys (Felsen and Mainen 2008). If the initiation of previously selected actions were the extent of the involvement of the SC, we would expect its activity to reflect only the direction of movement and only near the time of initiation (Shadlen and Newsome 2001). Instead, the considerable temporal dissociation between SC activity and movement onset indicates that the SC does not simply initiate movements, in agreement with evidence obtained from the primate oculomotor system (Glimcher and Sparks 1992; Horwitz et al. 2004; Horwitz and Newsome 1999, 2001b; Kim and Basso 2008; Lee and Keller 2006; McPeek and Keller 2004; Port and Wurtz 2009; Ratcliff et al. 2007; Thevarajah et al. 2009).
In particular, the dependence of SC activity on the difficulty of the discrimination (Fig. 6) suggests that the patterns of SC activity observed should be considered part of the decision-making process itself (Kim and Basso 2008; McPeek and Keller 2004; Ratcliff et al. 2007; Shadlen and Newsome 2001; Thevarajah et al. 2009) and not simply the outcome of the decision. In fact, there is strong anatomical and physiological support for the idea that the SC participates in selecting the motor response most likely to lead to reward, given an arbitrary sensory stimulus. Across several species, the SC represents reward information (Ikeda and Hikosaka 2003; Isoda and Hikosaka 2008; Weldon et al. 2007, 2008) and directly projects to reward-related dopaminergic neurons in the substantia nigra pars compacta (Comoli et al. 2003; May et al. 2009; McHaffie et al. 2006). Whereas visual, auditory, and somatosensory information is known to be represented in the SC (King 2004), direct olfactory inputs have not been demonstrated; the association between odor and movement direction must instead occur upstream of the SC. Significantly, the absence of odor selectivity (Fig. 5) allows us to conclude that the SC activity that we recorded does not reflect stimulus parameters but may instead underlie decision variables. These results complement those of Song et al. (2011), who found that focal SC inactivation disrupts the selection of targets, not only for saccades but also for reaching movements. Thus our findings support the idea that the SC plays a role in sensorimotor decisions that is independent of explicit sensory representations and specific forms of motor output (Horwitz et al. 2004; Kim and Basso 2008; Song et al. 2011).
What is the specific nature of this role? We found that SC activity was modulated by discrimination difficulty (determined here by the odor mixture ratio; Fig. 6) but not by pure odor selectivity (Fig. 5). These data suggest that the activity may represent the value associated with a particular choice (Ikeda and Hikosaka 2003) but not the stimulus itself, as had been shown previously using a visuomotor task (Dorris et al. 2007; Horwitz and Newsome 2001a; Krauzlis 2004). Indeed, we found that firing rate preceding movement was correlated with reward probability in our population of cells preferring contraversive movement (Fig. 7D). However, we also observed a dependence of activity on whether planned contraversive choices were correct or incorrect (Fig. 7, A–C), which is not consistent with a representation of value alone. Instead, one possibility is that the activity may reflect the degree of confidence the animal has that its choice will lead to reward (Insabato et al. 2010; Kim and Basso 2008).
A previous study in primates similarly concluded that SC activity may reflect choice confidence (Kim and Basso 2008). During a task requiring oddball targets to be selected for saccades from among distractors, activity was shown to depend on whether choices were correct or incorrect. Furthermore, performance depended on the difference in activity between the neurons with targets and distractors in their response fields; specifically, more accurate performance was associated with a larger difference in activity between the “target” and “distractor” populations. Although we did not simultaneously record from left and right SC cells, we can infer the activity in a right SC cell preceding rightward movement from the recorded activity in a left SC cell preceding leftward movement (Britten et al. 1992) (Fig. 9). Given our observation that for easy trials (on which performance was more accurate than on hard trials), there was a greater difference between activity preceding contraversive and ipsiversive movements (Fig. 6E), we can infer that, for example, on difficult rightward trials, the difference in activity between the left SC [analogous to Kim and Basso's (2008) target population] and right SC (distractor population) would be small (Fig. 9, C and D). Therefore, consistent with the results of Kim and Basso (2008), more accurate performance was associated with a larger difference in activity between the left and right SC. This effect was largely due to a dependence of activity on task difficulty on antipreferred (i.e., ipsiversive) trials (Fig. 6, B and D), consistent with studies in the SC of primates performing a saccade task based on stimulus brightness (Ratcliff et al. 2007). Regardless of whether the difference between left and right SC activity is mediated by mutual inhibition or due to some other mechanism (Ratcliff et al. 2011), our results suggest a broad role for the SC in orienting decisions, independent of the type of movement or the modality of the sensory cue (Song et al. 2011). This similarity across species and tasks is somewhat surprising, given the structural and functional differences between the neural circuits controlling these classes of movements (Takakusaki et al. 2004). However, further experiments are needed to clarify whether, and precisely which, decision variables are represented in the rat SC, and we note that we focused only on the neurons that prefer contraversive movement and are therefore most similar to those examined in primates. The small population of neurons preferring ipsiversive movement, which we and others (Hirokawa et al. 2011) have observed in rats (Fig. 4A), may reflect differences between SC processing in rats and primates. It is also possible that the activity that we have described may reflect covert spatial attention, to which primate SC activity has been shown to be related (Ignashchenkova et al. 2004; Kustov and Robinson 1996; Muller et al. 2005).
Whereas it is conceptually straightforward to compartmentalize sensory decision making as a series of discrete stages from stimulus representation to motor output (Miller et al. 1960), analyses of neurophysiological data have increasingly suggested that activity in motor regions underlies decision-making processes (Cisek 2007). Our study adds to the mounting evidence that considerable overlap exists between the neural substrates for selecting, planning, and executing actions in the midbrain, extending previous results to freely moving rodents performing a nonvisual orienting task. Future studies can elucidate how the SC cooperates with interconnected cortical regions implicated in decisions, such as the lateral intraparietal cortex and frontal eye fields in primates (Gnadt and Andersen 1988; Schall and Hanes 1993) and the frontal orienting field in rats (Erlich et al. 2011), to provide a more complete description of the contributions of cortical and subcortical regions to sensorimotor decision making.
GRANTS
Support for this work was provided by the Swartz Center for Computational Neuroscience (G. Felsen), the Boettcher Foundation (G. Felsen), National Institute on Deafness and Other Communication Disorders (Z. F. Mainen), and the Center for the Neural Mechanisms of Cognition at Cold Spring Harbor Laboratory (Z. F. Mainen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: G.F. and Z.F.M. conception and design of research; G.F. performed experiments; G.F. analyzed data; G.F. and Z.F.M. interpreted results of experiments; G.F. prepared figures; G.F. drafted manuscript; G.F. and Z.F.M. edited and revised manuscript; G.F. and Z.F.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Barry Burbach for technical assistance, Joshua Cohen for assistance with behavioral training, and Ninglong Xu, Anne Churchland, Eric DeWitt, and members of the Mainen lab for helpful comments and discussions. We also thank several anonymous reviewers for constructive suggestions.
REFERENCES
- Basso MA, Wurtz RH. Modulation of neuronal activity by target uncertainty. Nature 389: 66–69, 1997 [DOI] [PubMed] [Google Scholar]
- Britten KH, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci 12: 4745–4765, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter AF, Georgopoulos AP, Pellizzer G. Motor cortical encoding of serial order in a context-recall task. Science 283: 1752–1757, 1999 [DOI] [PubMed] [Google Scholar]
- Cisek P. Cortical mechanisms of action selection: the affordance competition hypothesis. Philos Trans R Soc Lond B Biol Sci 362: 1585–1599, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci 33: 269–298, 2010 [DOI] [PubMed] [Google Scholar]
- Comoli E, Coizet V, Boyes J, Bolam JP, Canteras NS, Quirk RH, Overton PG, Redgrave P. A direct projection from superior colliculus to substantia nigra for detecting salient visual events. Nat Neurosci 6: 974–980, 2003 [DOI] [PubMed] [Google Scholar]
- Corneil BD, Munoz DP, Chapman BB, Admans T, Cushing SL. Neuromuscular consequences of reflexive covert orienting. Nat Neurosci 11: 13–15, 2008 [DOI] [PubMed] [Google Scholar]
- Corneil BD, Olivier E, Munoz DP. Neck muscle responses to stimulation of monkey superior colliculus. I. Topography and manipulation of stimulation parameters. J Neurophysiol 88: 1980–1999, 2002 [DOI] [PubMed] [Google Scholar]
- Cowen SL, McNaughton BL. Selective delay activity in the medial prefrontal cortex of the rat: contribution of sensorimotor information and contingency. J Neurophysiol 98: 303–316, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Gold JI. Caudate encodes multiple computations for perceptual decisions. J Neurosci 30: 15747–15759, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris MC, Olivier E, Munoz DP. Competitive integration of visual and preparatory signals in the superior colliculus during saccadic programming. J Neurosci 27: 5053–5062, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drager UC, Hubel DH. Responses to visual stimulation and relationship between visual, auditory, and somatosensory inputs in mouse superior colliculus. J Neurophysiol 38: 690–713, 1975 [DOI] [PubMed] [Google Scholar]
- Erlich JC, Bialek M, Brody CD. A cortical substrate for memory-guided orienting in the rat. Neuron 72: 330–343, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierstein CE, Quirk MC, Uchida N, Sosulski DL, Mainen ZF. Representation of spatial goals in rat orbitofrontal cortex. Neuron 51: 495–507, 2006 [DOI] [PubMed] [Google Scholar]
- Felsen G, Mainen ZF. Neural substrates of sensory-guided locomotor decisions in the rat superior colliculus. Neuron 60: 137–148, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman EG, Stanford TR, Sparks DL. Combined eye-head gaze shifts produced by electrical stimulation of the superior colliculus in rhesus monkeys. J Neurophysiol 76: 927–952, 1996 [DOI] [PubMed] [Google Scholar]
- Gandhi NJ, Katnani HA. Motor functions of the superior colliculus. Annu Rev Neurosci 34: 205–231, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher PW, Sparks DL. Movement selection in advance of action in the superior colliculus. Nature 355: 542–545, 1992 [DOI] [PubMed] [Google Scholar]
- Gnadt JW, Andersen RA. Memory related motor planning activity in posterior parietal cortex of macaque. Exp Brain Res 70: 216–220, 1988 [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. Representation of a perceptual decision in developing oculomotor commands. Nature 404: 390–394, 2000 [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: Wiley, 1966 [Google Scholar]
- Hattox AM, Priest CA, Keller A. Functional circuitry involved in the regulation of whisker movements. J Comp Neurol 442: 266–276, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A, Zainos A, Romo R. Temporal evolution of a decision-making process in medial premotor cortex. Neuron 33: 959–972, 2002 [DOI] [PubMed] [Google Scholar]
- Hirokawa J, Sadakane O, Sakata S, Bosch M, Sakurai Y, Yamamori T. Multisensory information facilitates reaction speed by enlarging activity difference between superior colliculus hemispheres in rats. PLoS One 6: e25283, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz GD, Batista AP, Newsome WT. Representation of an abstract perceptual decision in macaque superior colliculus. J Neurophysiol 91: 2281–2296, 2004 [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Newsome WT. Separate signals for target selection and movement specification in the superior colliculus. Science 284: 1158–1161, 1999 [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Newsome WT. Target selection for saccadic eye movements: direction-selective visual responses in the superior colliculus. J Neurophysiol 86: 2527–2542, 2001a [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Newsome WT. Target selection for saccadic eye movements: prelude activity in the superior colliculus during a direction-discrimination task. J Neurophysiol 86: 2543–2558, 2001b [DOI] [PubMed] [Google Scholar]
- Ignashchenkova A, Dicke PW, Haarmeier T, Thier P. Neuron-specific contribution of the superior colliculus to overt and covert shifts of attention. Nat Neurosci 7: 56–64, 2004 [DOI] [PubMed] [Google Scholar]
- Ikeda T, Hikosaka O. Reward-dependent gain and bias of visual responses in primate superior colliculus. Neuron 39: 693–700, 2003 [DOI] [PubMed] [Google Scholar]
- Insabato A, Pannunzi M, Rolls ET, Deco G. Confidence-related decision making. J Neurophysiol 104: 539–547, 2010 [DOI] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O. A neural correlate of motivational conflict in the superior colliculus of the macaque. J Neurophysiol 100: 1332–1342, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Basso MA. Saccade target selection in the superior colliculus: a signal detection theory approach. J Neurosci 28: 2991–3007, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ. The superior colliculus. Curr Biol 14: R335–R338, 2004 [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ. Activity of rostral superior colliculus neurons during passive and active viewing of motion. J Neurophysiol 92: 949–958, 2004 [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Liston D, Carello CD. Target selection and the superior colliculus: goals, choices and hypotheses. Vision Res 44: 1445–1451, 2004 [DOI] [PubMed] [Google Scholar]
- Kustov AA, Robinson DL. Shared neural control of attentional shifts and eye movements. Nature 384: 74–77, 1996 [DOI] [PubMed] [Google Scholar]
- Lee KM, Keller EL. Symbolic cue-driven activity in superior colliculus neurons in a peripheral visual choice task. J Neurophysiol 95: 3585–3595, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CC, Wang XJ. Cortico-basal ganglia circuit mechanism for a decision threshold in reaction time tasks. Nat Neurosci 9: 956–963, 2006 [DOI] [PubMed] [Google Scholar]
- May PJ, McHaffie JG, Stanford TR, Jiang H, Costello MG, Coizet V, Hayes LM, Haber SN, Redgrave P. Tectonigral projections in the primate: a pathway for pre-attentive sensory input to midbrain dopaminergic neurons. Eur J Neurosci 29: 575–587, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHaffie JG, Jiang H, May PJ, Coizet V, Overton PG, Stein BE, Redgrave P. A direct projection from superior colliculus to substantia nigra pars compacta in the cat. Neuroscience 138: 221–234, 2006 [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci 7: 757–763, 2004 [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Saccade target selection in the superior colliculus during a visual search task. J Neurophysiol 88: 2019–2034, 2002 [DOI] [PubMed] [Google Scholar]
- Miller GA, Galanter E, Pribram KH. Plans and the Structure of Behavior. New York: Holt, Rinehart and Winston, 1960 [Google Scholar]
- Muller JR, Philiastides MG, Newsome WT. Microstimulation of the superior colliculus focuses attention without moving the eyes. Proc Natl Acad Sci USA 102: 524–529, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummela SU, Krauzlis RJ. Inactivation of primate superior colliculus biases target choice for smooth pursuit, saccades, and button press responses. J Neurophysiol 104: 1538–1548, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Bernier A, Cisek P. Neural correlates of biased competition in premotor cortex. J Neurosci 31: 7083–7088, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic, 2005 [Google Scholar]
- Port NL, Wurtz RH. Target selection and saccade generation in monkey superior colliculus. Exp Brain Res 192: 465–477, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Hasegawa YT, Hasegawa RP, Childers R, Smith PL, Segraves MA. Inhibition in superior colliculus neurons in a brightness discrimination task? Neural Comput 23: 1790–1820, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Hasegawa YT, Hasegawa RP, Smith PL, Segraves MA. Dual diffusion model for single-cell recording data from the superior colliculus in a brightness-discrimination task. J Neurophysiol 97: 1756–1774, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD. Decision making. Curr Biol 15: R9–R11, 2005 [DOI] [PubMed] [Google Scholar]
- Schall JD, Hanes DP. Neural basis of saccade target selection in frontal eye field during visual search. Nature 366: 467–469, 1993 [DOI] [PubMed] [Google Scholar]
- Schmitzer-Torbert N, Jackson J, Henze D, Harris K, Redish AD. Quantitative measures of cluster quality for use in extracellular recordings. Neuroscience 131: 1–11, 2005 [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J Neurophysiol 86: 1916–1936, 2001 [DOI] [PubMed] [Google Scholar]
- Song JH, Rafal RD, McPeek RM. Deficits in reach target selection during inactivation of the midbrain superior colliculus. Proc Natl Acad Sci USA 108: E1433–E1440, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks DL. Conceptual issues related to the role of the superior colliculus in the control of gaze. Curr Opin Neurobiol 9: 698–707, 1999 [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Hoffmann KP, Miller LE. Correlation of primate superior colliculus and reticular formation discharge with proximal limb muscle activity. J Neurophysiol 81: 1978–1982, 1999 [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Saitoh K, Harada H, Kashiwayanagi M. Role of basal ganglia-brainstem pathways in the control of motor behaviors. Neurosci Res 50: 137–151, 2004 [DOI] [PubMed] [Google Scholar]
- Thevarajah D, Mikulic A, Dorris MC. Role of the superior colliculus in choosing mixed-strategy saccades. J Neurosci 29: 1998–2008, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Mainen ZF. Speed and accuracy of olfactory discrimination in the rat. Nat Neurosci 6: 1224–1229, 2003 [DOI] [PubMed] [Google Scholar]
- Weldon DA, DiNieri JA, Silver MR, Thomas AA, Wright RE. Reward-related neuronal activity in the rat superior colliculus. Behav Brain Res 177: 160–164, 2007 [DOI] [PubMed] [Google Scholar]
- Weldon DA, Patterson CA, Colligan EA, Nemeth CL, Rizio AA. Single unit activity in the rat superior colliculus during reward magnitude task performance. Behav Neurosci 122: 183–190, 2008 [DOI] [PubMed] [Google Scholar]
- Werner W, Dannenberg S, Hoffmann KP. Arm-movement-related neurons in the primate superior colliculus and underlying reticular formation: comparison of neuronal activity with EMGs of muscles of the shoulder, arm and trunk during reaching. Exp Brain Res 115: 191–205, 1997 [DOI] [PubMed] [Google Scholar]