Abstract
The molecular and physiological basis of the touch-unresponsive zebrafish mutant fakir has remained elusive. Here we report that the fakir phenotype is caused by a missense mutation in the gene encoding voltage-gated calcium channel 2.1b (CACNA1Ab). Injection of RNA encoding wild-type CaV2.1 restores touch responsiveness in fakir mutants, whereas knockdown of CACNA1Ab via morpholino oligonucleotides recapitulates the fakir mutant phenotype. Fakir mutants display normal current-evoked synaptic communication at the neuromuscular junction but have attenuated touch-evoked activation of motor neurons. NMDA-evoked fictive swimming is not affected by the loss of CaV2.1b, suggesting that this channel is not required for motor pattern generation. These results, coupled with the expression of CACNA1Ab by sensory neurons, suggest that CaV2.1b channel activity is necessary for touch-evoked activation of the locomotor network in zebrafish.
Keywords: motor, mutant, sensory, spinal cord
sensory-evoked motor behaviors, common to most animals, are typified by running or flight in terrestrial organisms and by swimming in aquatic organisms. Although these motor behaviors are seemingly different, much of the underlying neural circuitry is similar. Stimuli are first perceived by sensory neurons tuned to particular modalities. These sensory neurons relay inputs to interneurons, which then activate motor neurons and induce muscle contractions resulting in locomotion. Although much has been learned about the circuitry underlying sensory-evoked motor behaviors in vertebrates through comparative anatomy and in vivo electrophysiology, proportionally less is known about the contribution of specific genes to the formation and function of these circuits. To overcome this deficit within the context of touch-evoked motor behaviors, we and others have turned to the model organism zebrafish.
Zebrafish are ideally suited to address these questions because embryos develop externally and possess a relatively simple nervous system amenable to both in vivo electrophysiology (Drapeau et al. 1999) and optical techniques (McLean and Fetcho 2011). In addition, recent advances have rendered the zebrafish genome modifiable via forward and reverse genetic techniques (Lawson and Wolfe 2011). Finally, zebrafish mature quickly, going from a fertilized egg to an embryo capable of responding to tactile stimuli with highly stereotyped motor behaviors within the first days of life (Saint-Amant and Drapeau 1998). These features have fostered the use of zebrafish in large-scale mutagenesis screens aimed at identifying mutations that affect touch-evoked motor behaviors (Granato et al. 1996).
Subsequent work with zebrafish mutants isolated from forward genetic screens has identified genes necessary for touch-evoked motor behaviors at the level of skeletal muscle (Hirata et al. 2004, 2007, 2012; Ono et al. 2002; Schredelseker et al. 2005; Westerfield et al. 1990), premotor elements (Burgess et al. 2009; Cui et al. 2004; Hirata et al. 2005; Low et al. 2010b; McKeown et al. 2012), and sensory neurons (Low et al. 2011; Nakano et al. 2010). Here we report that the touch-unresponsive zebrafish mutant fakir results from a hypomorphic missense mutation in the gene encoding voltage-gated calcium channel 2.1b (CACNA1Ab). Our findings reveal that CACNA1Ab is expressed by sensory neurons and demonstrate that the locomotor network that underlies swimming in zebrafish is normal in fakir mutants. Collectively, these results suggest that normal CaV2.1b channel activity is required within mechanosensitive neurons for touch-evoked activation of the zebrafish locomotor network.
MATERIALS AND METHODS
Animal care and use.
Zebrafish were bred and raised according to approved guidelines set forth by the Animal Experimentation Ethics Committee, University of Montréal, and the Office of Animal Resources, Harvard University. Staging of embryos was performed as described previously (Kimmel et al. 1995). The fakir allele tm154 (fartm154) was identified in a screen for mutations affecting locomotor behaviors (Granato et al. 1996).
Mapping.
A mapping family for fakir was established by crossing a fakir carrier with a wild-type zebrafish from the WIK genetic background (Zebrafish International Resource Center, Eugene, OR). Offspring from this mapping family were subjected to high-resolution mapping using a previously described mapping procedure (Talbot and Schier 1999) and the following single-nucleotide polymorphism (SNP) primer sets: SNP-1, forward 5′-GCGCAACTCACTCAGTCATC-3′ and reverse 5′-AAGACGGACAAGCGGCTAC-3′; and SNP-2, forward 5′-TCGCTGTGGAGACTGAGACTT-3′ and reverse 5′-CGACTTGGTCCATGTTTCCT-3′.
Behavioral analysis.
Embryos beginning ∼17 h postfertilization (hpf) were dechorionated with pronase, placed individually into chambers of 24-well plates, and raised in a water bath at 28.5°C until the indicated time points. Spontaneous coiling in embryos was monitored using a custom-made acrylic 100-well dish (3 mm in diameter and 5 mm deep) over 2 min. Tactile stimuli were applied by striking the tail of embryos with a pair of no. 5 forceps up to three times. Zebrafish were scored according to their greatest response to touch at 48 hpf as either wild type (embryos exhibiting >5 body lengths of touch-evoked swimming), intermediate (embryos that responded to touch but swam <5 body lengths), or unresponsive (no visible response to touch). Behaviors were captured with PGR Flycap at 30 and 200 Hz using Flea2 (FL2–20S4M-C) and Grasshopper (GRAS-03K2M-C) cameras, respectively (Point Grey Research, Richmond, BC, Canada), mounted to an Olympus dissecting microscope (SZX7). Images were analyzed off-line using ImageJ (http://rsbweb.nih.gov/ij/).
Immunohistochemistry, α-bungarotoxin labeling, and in situ hybridization.
Labeling was performed at room temperature (22°C) on larvae 48–52 hpf, raised in 200 μM 1-phenyl-2-thiourea beginning at ∼20 hpf to prevent pigmentation, using the following procedures. For immunohistochemistry, larvae were fixed in 4% paraformaldehyde for 30 min and then washed 5 times (5×) for 10 min in washing buffer [phosphate-buffered saline (PBS) containing 0.1% Triton X-100] and 2× for 10 min in blocking buffer [wash buffer containing 2 mg/ml bovine serum albumin (BSA) and 5% heat-inactivated sheep serum]. Primary antibodies purchased from the Developmental Studies Hybridoma Bank (Iowa City, IA) were bound overnight in blocking buffer at the following dilutions: anti-Islet (1:100), which labels sensory neuron cell bodies; 3A10 (1:100), which labels Mauthner cells; and anti-SV2 (1:100), which labels synaptic vesicles within neurites. Thereafter, larvae were washed 5× for 10 min in washing buffer and 2× for 10 min in blocking buffer. Antibody staining was visualized using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA) and 0.15 mg/ml diaminobenzidine to produce a brown precipitate according to the manufacture's guidelines. Peripheral neurites were labeled using anti-acetylated tubulin antibody (1:1,000) obtained from Sigma-Aldrich (St. Louis, MO). These embryos were washed 5× for 10 min in washing buffer and 2× for 10 min in blocking buffer and were then incubated for 2 h at room temperature with anti-mouse Alexa-488 secondary antibody. All processed larvae were washed 6× for 10 min with washing buffer and then mounted in 70% glycerol in PBS for imaging.
Nicotinic acetylcholine receptors were labeled using Alexa-594-conjugated α-bungarotoxin (Invitrogen, Carlsbad, CA). Fixed embryos were washed 3× for 10 min with PBS, treated with collagenase at 1 mg/ml in PBS for 15 min, and then washed 3× for 10 min with PBS. Embryos were then preincubated in binding buffer (2% BSA, 0.5% Triton X-100, and 0.1% sodium azide in PBS) for 30 min, followed by incubation with Alexa-594-conjugated α-bungarotoxin at 10 μg/ml in binding buffer for 30 min. Thereafter, embryos were washed 3× for 10 min with binding buffer and mounted in 70% glycerol in PBS. Images were captured with a spinning disk confocal microscope (Olympus BX-51; Center Valley, PA) with a confocal light path (Quorum, Guelph, ON, Canada).
In situ hybridization was performed using variations of a previously described approach (Westerfield 2000). In brief, a cDNA fragment of CACNA1Ab was amplified using the following set of primers and subcloned into pGEM-T vector (Promega, Madison, WI): forward 5′-CCCAGGAGCGAAGCGAAGAACA-3′ and reverse 5′-GCTGGAGTCTGTCAGAATGAGACTGC-3′. Linearized plasmid (1 μg) was used as template to synthesize digoxigenin (DIG)-labeled sense and antisense riboprobes. Probe integrity was checked by gel electrophoresis.
Embryos were dehydrated with an increasing percentage of methanol in PBS and stored at −20°C for 30 min in methanol. Thereafter, embryos were rehydrated by decreasing the percentage of methanol in PBS containing 0.1% Tween, incubated at 37°C with proteinase K for 15 min to increase the penetration of riboprobes, and fixed again for 30 min at room temperature. Antisense and sense control riboprobes were hybridized for ∼16 h at 65°C. Anti-DIG antibody conjugated to alkaline phosphatase (Roche Applied Science, Indianapolis, IN) was bound at room temperature for 2 h, followed by chromogenic detection using a nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate solution (NBT/BCIP; Roche Applied Science). The chromogenic reaction was quenched at various time points for both antisense and sense control conditions. Staining for CACNA1Ab was considered specific when corresponding staining was absent in sense control.
Recording methods.
All reagents were obtained from Sigma-Aldrich unless otherwise noted. Electrophysiological recording from zebrafish (48–60 hpf) were obtained from axial skeletal muscle and neurons at room temperature using methods similar to those previously described (Buss and Drapeau 2000; Drapeau et al. 1999). In brief, larvae were anesthetized and dissected in Evans recording solution (in mM): 134 NaCl, 2.9 KCl, 2.1 CaCl2, 1.2 MgCl2, 10 glucose, and 10 HEPES, pH 7.5 with NaOH containing 0.02% (wt/vol) tricaine. The skin of a larva pinned laterally to a 35-mm Sylgard-coated dish was removed with a pair of no. 5 forceps. The solution was exchanged throughout the recording session at ∼1 ml/min with Evans containing curare at 3 and 15 μM for skeletal muscle and neuron recordings, respectively. To gain access to the spinal cord, the bath solution was replaced with recording solution containing 1 mg/ml collagenase type XI and incubated until the muscle started to separate at the somatic boundaries (∼10 min). Thereafter, the muscle was peeled away using suction applied to a broken pipette (∼50 μm). The internal recording solution contained (in mM) 116 K-gluconate, 16 KCl, 2 MgCl2, 10 HEPES, and 10 EGTA, pH 7.2 with KOH, and 0.1% sulforhodamine B for cell type identification. Borosilicate glass electrodes had resistances of 3–4 and 5–8 MΩ for muscle and neuron recordings, respectively, when filled with internal recording solution. Paired motor neuron-muscle recordings were performed in the absence of curare and in the presence of 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and 40 μM d-2-amino-5-phosphonovaleric acid (APV) to block excitatory inputs into motor neurons. Recordings were made with Axopatch 200B amplifiers (Axon Instruments, Union City, CA) low-pass filtered at 1–5 kHz, and sampled at 1–10 kHz. Tactile stimulation delivered as a jet of water to the tail (20–40 psi, 20 ms) and the application of acetylcholine to skeletal muscle (20 psi, 50–100 ms) were delivered through borosilicate glass pipettes using a Picospritzer III (Parker Hannifin, Cleveland, OH). Data acquisition was controlled by pClamp 10 software using a Digidata 1440A interface.
Electrophysiological recordings from the previously described stable human embryonic kidney (HEK293T) cell line (Piedras-Renteria et al. 2001) expressing the β1c- and α2δ-subunits were obtained at room temperature using methods similar to those previously described (Hamill et al. 1981). Flag-tagged human CaV2.1 (3 μg) was cotransfected with enhanced green fluorescent protein (1 μg) into cells growing in 60-mm-diameter dishes coated with Matrigel (BD Biosciences, Mississauga, ON, Canada) with Lipofectamine 2000 (Invitrogen, Burlington, ON, Canada). The following day, cells were split and reseeded on broken glass coverslips coated with Matrigel, and recordings were obtained 4–12 h later. Whole cell recordings were obtained using borosilicate glass electrodes with resistances of 5–8 MΩ when filled with internal recording solution (in mM): 145 Cs-aspartate, 10 EGTA, 10 HEPES, 5 NaCl, and 1 MgCl2, pH 7.4 with CsOH. The external recording solution contained (in mM) 140 NaCl, 2 KCl, 10 CaCl2, 1 MgCl2, and 10 HEPES, pH 7.5 with NaOH. Recordings were made with an Axopatch-1D amplifier (Axon Instruments) low-pass filtered at 5 kHz, and sampled at 10 kHz. Data acquisition was controlled by pClamp 8 software using a Digidata 1200B interface. The initial data analysis was done with Clampfit 10, and figures were prepared using SigmaPlot 11.0 and Adobe Illustrator CS2.
Site-directed mutagenesis.
The substitution of leucine 356 with a valine was performed through splice by overlap extension using the following primer sets: set 1, forward 5′-AGCTGGCTAGCGTTTAAACTTAAGCTTGGTACCGAGCTCGGATCCATG-3′ and reverse 5′-CTCCCCTGACAGCACACCCAGTACTACGTTCAGCATAAAA-3′; and set 2, forward 5′-TTTTATGCTGAACGTAGTACTGGGTGTGCTGTCAGGGGAG-3′ and reverse 5′-AAAGGGCGAAGACGACAATGAACAGGAAAAGGAGAAACAACAGGCTGAT-3′, where the underlined nucleotides indicate the base pair substitutions used to generate the missense mutation. PCR product was then digested and subcloned into wild-type cDNA using BsmBI. Mutagenesis was confirmed by DNA sequencing (Institute for Research in Immunology and Cancer Sequencing Core, University of Montréal) and is referred to as L356V, representing the substitution of leucine 356 with a valine.
Morpholino, RNA, and plasmid injections.
A splice-blocking morpholino (Gene Tools, Philomath, OR) was raised to cause the retention of intron 7 of CACNA1Ab: 5′-GATAGATCTTACCCTGAGAGAACAC-3′, resulting in a premature stop codon at position E358, which is equivalent to position E362 in the human sequence. Embryos for morpholino injections were of the TLAB genetic background (derived from adults from a TL × AB cross). The CACNA1Ab morpholino, diluted from a stock concentration to 250 μM in 1 nl, was injected into embryos between the one- and two-cell stage using a PicoPump (World Precision Instruments). For each injection condition, cDNA was synthesized using SuperScript II (Invitrogen, Carlsbad, CA) from total RNA extracted from 10 embryos at 48 hpf with TRIzol (Invitrogen, Carlsbad, CA) following the manufacturer's guidelines. Quantitative PCR of CACNA1Ab transcripts was performed using SYBR green (Qiagen, Valencia, CA) and normalized against an amplicon for zebrafish β-actin.
For RNA injections, capped RNA encoding wild-type human CaV2.1 (CaV2.1WT) and human CaV2.1 harboring the L356V substitution (CaV2.1L356V) were synthesized using a mMESSAGE mMACHINE T7 kit (Ambion, Austin, TX). A 1- to 2-nl solution of RNA diluted to 200 pg/nl in DEPC-H2O containing 0.1% fast green was injected into embryos at the one- to four-cell stage using a Picospritzer III (Parker Hannifin). Embryos were then sorted 6 h later for uptake of dye. Before the beginning of an experiment, embryos were dechorionated and staged as described above. Embryos were scored for their behavior at 48 h, and a chi-square analysis was performed on the results.
Statistical analysis.
Data were analyzed using Microsoft Excel. In text and all figures, data are averages ± SE. Asterisks in figures denote a Student's t-test P value <0.05.
RESULTS
Fakir mutants exhibit a progressive loss of touch-evoked motor behaviors.
The zebrafish mutant fakir was originally isolated in a screen for mutations affecting touch-evoked motor behaviors (Granato et al. 1996), due to its diminished ability to respond to tactile stimuli on the third day of development (Fig. 1). To expand on the initial characterization of the fakir mutant phenotype, we examined the two additional motor behaviors displayed by zebrafish embryos: spontaneous and touch-evoked coiling.
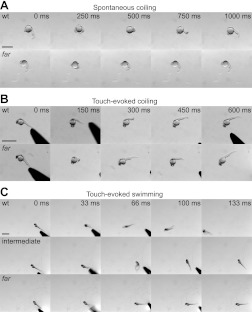
Fig. 1.
Fakir mutants exhibit a loss of touch-evoked swimming. A: time-lapse images of spontaneous coiling at 20-h postfertilization (hpf) wild-type (wt) and fakir (far) mutant embryos. Scale bar, 1 mm. B: time-lapse images of touch-evoked coiling from 26-hpf wt and far mutant embryos. Scale bar, 500 μm. C: time-lapse images of touch-evoked responses from 48-hpf wt, intermediate, and far mutant larvae. Scale bar, 1 mm.
Spontaneous coiling, the first motor behavior exhibited by zebrafish embryos, is characterized by slow, alternating contractions of the trunk and tail toward the head (Fig. 1A). Spontaneous coiling in zebrafish is neurogenic in nature (Westerfield et al. 1990) and intrinsic to the spinal cord, because coils persist following spinalization (Saint-Amant and Drapeau 1998). Compared with wild-type siblings, fakir mutants were found to exhibit a similar frequency of spontaneous coiling (Table 1).
Table 1.
Analysis of spontaneous and touch-evoked coiling in wild type and fakir mutants
| wt | far | P Value | |
|---|---|---|---|
| Spontaneous coiling frequency, Hz | 0.039 ± 0.005 | 0.052 ± 0.017 | 0.4 |
| Number of touch-evoked coils, % | |||
| 0 | 6.7 ± 6.7 | 22.2 ± 11.1 | 0.3 |
| 1 | 5.6 ± 5.6 | 6.7 ± 6.7 | 0.9 |
| 2 | 63.3 ± 18.6 | 58.9 ± 4.8 | 0.8 |
| 3 | 24.4 ± 12.4 | 12.2 ± 6.2 | 0.4 |
Values are means ± SE in wild-type (wt) and fakir (far) mutants (n = 18 for each, from 3 clutches).
At ∼21 hpf, tactile stimuli delivered along the body axis often evokes 1–3 coils of the trunk and tail (Fig. 1B). Touch-evoked coils are faster than spontaneous coils and require rostral elements of the spinal cord, because they are lost following lesions caudal to somite 10 (Downes and Granato 2006; Pietri et al. 2009). Compared with wild-type siblings, fakir mutants were also found to display a similar distribution of touch-evoked coils (Table 1). Thus spontaneous and touch-evoked coiling behaviors are normal in fakir mutants.
At 27 hpf, tactile stimuli begin to evoke bouts of swimming capable of propelling embryos at least one body length. Touch-evoked swimming increases such that at 48 hpf, embryos are capable of generating swimming bouts that cover multiple body lengths (Fig. 1C). In agreement with the initial report of the fakir mutant phenotype (Granato et al. 1996), a proportion of embryos consistent with a recessive mutation never produced episodes of swimming (23.0 ± 1.1%; P = 0.64, χ2 test, n = 224 total embryos from 3 clutches). However, a closer examination of the remaining touch-responsive embryos revealed that the majority of embryos initially responded to tactile stimuli but thereafter failed to display sustained bouts of swimming. Segregation of the touch-responsive embryos according to their extent of swimming (0–5 vs. >5 body lengths) revealed a ratio consistent with a 2:1 Mendelian inheritance of the mutant allele within the sibling population (49.7 ± 1.0 vs. 27.3 ± 0.2%, P = 0.73, χ2 test), suggesting that the reduced bouts of swimming represent a heterozygous “intermediate” phenotype, a finding further addressed below. Embryos displaying the intermediate phenotype were excluded from subsequent analysis unless otherwise indicated.
Elements of the touch-evoked circuit are present in fakir mutants.
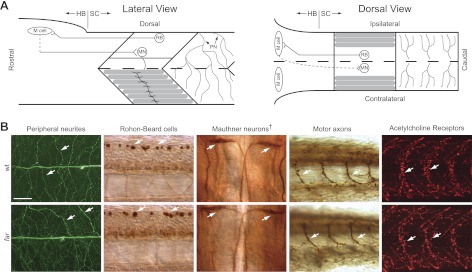
In an attempt to understand the ontogeny of the fakir phenotype, we first examined whether the fakir mutation caused gross disruptions in the morphology and distribution of neurons and proteins known to participate in zebrafish touch-evoked motor behaviors. In zebrafish, sensitivity to touch along the trunk and tail is conferred by Rohon-Beard (RB) neurons (Low et al. 2010a), a population of sensory neurons located within the dorsal spinal cord (Bernhardt et al. 1990). RBs extend peripheral neurites into the overlying skin and send a central process rostrally and caudally within the dorsal spinal cord. The rostral processes of RBs project ipsilaterally into the hindbrain (Bernhardt et al. 1990), where they lead to the activation of ∼90 bilateral pairs of reticulospinal neurons during motor behaviors (Gahtan et al. 2002). Of the ∼90 bilateral pairs of reticulospinal neurons activated during locomotion, the contribution of Mauthner cells in fish are best understood (Korn and Faber 2005). Mauthner cells receive input from mechanosensitive neurons (Zottoli and Faber 1979) and in turn make monosynaptic contacts with motor neurons (Jontes et al. 2000), which are most likely excitatory (Mongeon et al. 2008). Motor neurons drive activity within axial skeletal muscle via cholinergic neurotransmission (Westerfield et al. 1990), resulting in locomotion. Thus RBs, Mauthner cells, and motor neurons represent a minimal three-neuron circuit with the potential of translating touch into motion (Fig. 2A). When examined, fakir mutants were found to have RBs that extended peripheral neurites into the overlying skin (Fig. 2B). In addition, fakir mutants also possessed Mauthner cells whose axons crossed the midline and projected into the contralateral spinal cord (Fig. 2B) and motor neurons that extended axons into the axial skeletal musculature. Finally, labeling of nicotinic acetylcholine receptors (nAchRs) with α-bungarotoxin revealed a similar presence of nAchRs in fakir axial skeletal muscle (Fig. 2B). These findings indicate that the fakir mutant phenotype cannot be explained by a loss of one or more of these basic elements.
Fig. 2.
A minimal touch-evoked escape circuit is morphologically present in fakir mutants. A: lateral (left) and dorsal (right) schematics of the minimal touch-evoked escape circuit. HB, hindbrain; SC, spinal cord; PN, peripheral neurites; RB, Rohon-Beard cell; M cell, Mauthner cell; MN, motor neuron. B: immunohistochemical labeling of RB peripheral neurites innervating the skin (acetylated tubulin), RB cell bodies (Islet-1), Mauthner cells (3A10), motor axons (SV2), and nicotinic acetylcholine receptors (Alexa-594-conjugated α-bungarotoxin) highlighted by arrows in wt and far mutants. All images are lateral views with rostral to the left except for Mauthner cells, wherein the dagger denotes a dorsal view with rostral toward the top. Scale bar, 50 μm.
Fakir mutants lack touch-evoked synaptic drive to skeletal muscle.
Because components of the escape circuit were present and appeared normal in fakir mutants, we next examined activity within these elements in vivo, beginning with touch-evoked activation of axial skeletal muscle. In zebrafish, axial skeletal muscle comprises a single lateral layer of slow-twitch fibers and multiple medial layers of fast-twitch fibers (Devoto et al. 1996), both of which are recruited during swimming (Buss and Drapeau 2000). Whole cell current-clamp recordings from slow-twitch fibers in the presence of 3 μM curare, a concentration sufficient to attenuate membrane depolarizations below the level necessary to trigger excitation-contraction coupling, revealed that tactile stimuli routinely evoked a bout of fictive swimming in wild-type embryos (Fig. 3A; n = 5/5). The frequency of fictive swimming in wild-type embryos (36.3 ± 0.9 Hz) was consistent with previous reports of touch-evoked fictive swimming (Buss and Drapeau 2001). In contrast, similar tactile stimuli failed to evoke bouts of fictive swimming in fakir slow-twitch fibers (Fig. 3A; n = 0/5).
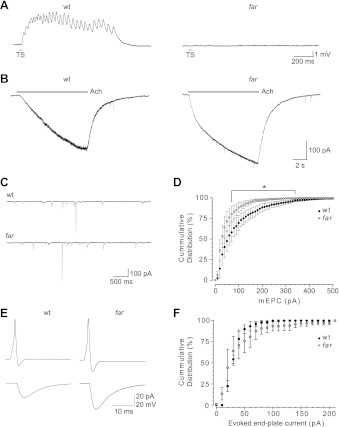
Fig. 3.
Synaptic transmission at the neuromuscular junction is present in fakir mutants. A: touch-evoked synaptic drive to wt and far mutant axial skeletal muscle (elicited by a water puff to the tail: 100 ms, 20 psi) under current clamp and in the presence of 3 μM curare. Gray bars here and in subsequent figures denote time and duration of stimulus. B: responsiveness of wt and far mutant slow-twitch skeletal muscle held at −60 mV to applied acetylcholine (ACh; 10 s, 100 μM). C: several seconds of miniature end-plate currents (mEPSCs) from wt and far mutant slow-twitch skeletal muscle held at −60 mV in the presence of tetrodotoxin (1 μM). D: a cumulative frequency distribution of mEPSC amplitudes from wt and far mutant slow-twitch skeletal muscle. Error bars represent SE. E: paired caudal primary (CaP) motor neuron–fast-twitch skeletal muscle recordings from wt and far mutants wherein a depolarizing current injection (2 ms) to the cell bodies triggered action potentials in motor neurons and evoked end-plate currents in wt and far mutant skeletal muscle. F: cumulative frequency distribution of evoked end-plate current amplitudes from skeletal muscle.
To determine whether the absence of touch-evoked responses in fakir axial skeletal muscle was the result of nonfunctional nAchRs, we examined the responsiveness of slow-twitch skeletal muscle to applied acetylcholine under whole cell voltage clamp. Slow-twitch fibers from both wild-type and fakir mutants responded to applied acetylcholine with pronounced inward currents (Fig. 3B; n = 5 for each), while neighboring fibers visibly contracted. When compared, the current responses were similar between wild-type and fakir mutants (26.5 ± 4.6 vs. 38.0 ± 11.8 pA/pF, respectively; P = 0.4), indicating that the nAchRs in both were functional and capable of causing muscle contractions when activated.
Because fakir skeletal muscle was capable of responding to acetylcholine, we next investigated whether the mutant phenotype was caused by a defect in transmitter release at the neuromuscular junction, starting with spontaneous transmitter release. In the presence of tetrodotoxin (Fig. 3C), which blocks spiking in zebrafish motor neurons (Cui et al. 2004), spontaneous miniature end-plate currents (mEPCs) were observed in slow-twitch axial skeletal muscle from both wild-type and fakir mutants. A comparison of mEPCs revealed that the overall frequency of mEPCs was indistinguishable between wild-type and fakir mutants (3.7 ± 0.2 vs. 3.0 ± 0.6 Hz, respectively; P = 0.3); however, events >70 pA were slightly reduced in fakir mutants (Fig. 3D). Thus fakir mutant motor neurons are capable of releasing acetylcholine spontaneously.
Finally, activity-dependent transmitter release at the neuromuscular junction was examined in fakir mutants through paired recordings between primary motor neurons and fast-twitch axial skeletal muscle. An initial analysis of membrane properties revealed that motor neurons from fakir mutants exhibited similar resting membrane potentials compared with motor neurons from wild-type embryos (Table 2). In addition, motor neurons from fakir mutants initiated action potentials in response to depolarizing current injections (Fig. 3E), with no significant difference in action potential threshold and amplitudes of over- and undershoot. Likewise, activation of motor neurons by current injection routinely evoked end-plate currents in both wild-type and fakir skeletal muscle (99.2 ± 0.8 vs. 95.8 ± 4.2%, respectively; P = 0.5). An analysis of the amplitude distribution of evoked end-plate currents failed to uncover a difference between wild-type and fakir mutants (Fig. 3F). These findings indicate that evoked synaptic communication at the neuromuscular junction is normal in fakir mutants, suggesting that the fakir phenotype is likely caused by a defect upstream of the neuromuscular junction.
Table 2.
Analysis of motor neuron membrane properties from wild type and fakir mutants
| Electrical Stimulation of Cell Body |
|||
|---|---|---|---|
| wt | far | P Value | |
| Resting membrane potential, mV | −60.5 ± 0.7 | −62.0 ± 1.3 | 0.3 |
| Action potential threshold, mV | −35.3 ± 1.0 | −36.3 ± 1.9 | 0.6 |
| Amplitude of overshoot, mV | 9.4 ± 2.7 | 4.6 ± 3.4 | 0.3 |
| Amplitude of undershoot, mV | −77.9 ± 3.0 | −73.9 ± 3.5 | 0.4 |
Values are means ± SE in wt (n = 18) and far mutants (n = 13).
Fakir motor neurons exhibit abbreviated responses.
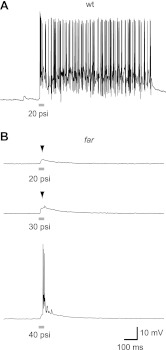
Because motor neurons in fakir mutants spiked and released neurotransmitter, we explored whether they were being activated in response to touch. In zebrafish embryos, a tactile stimulus evokes a bout of bursting in motor neurons (Drapeau et al. 1999), which is characterized by a prolonged train of action potentials atop a depolarized synaptic plateau. Touch-evoked bouts of bursting appear to be synaptically driven, rather than a consequence of intrinsic motor neuron membrane properties, because bouts of bursting in individual motor neurons are not terminated by hyperpolarizing current injections (Cui et al. 2004). We found that tactile stimuli, applied as a 20-psi puff of water to the tail of an embryo, reliably evoked sustained bouts of bursting in wild-type motor neurons (Fig. 4A; n = 9/10 trials, from 5 wild-type fish). In contrast, the same stimulus delivered to fakir embryos failed to evoke sustained bouts of bursting (n = 0/12 trials, from 6 mutants), often generating instead a very brief subthreshold synaptic event (Fig. 4B). When expressed as a number of spikes per stimulation, fakir mutants were found to exhibit 0.25 ± 0.2 spikes per stimulation at 20 psi (n = 12), compared with 37.1 ± 8.5 spikes per stimulation in wild-type embryos (n = 10).
Fig. 4.
Tactile stimuli triggers attenuated synaptic bouts in fakir motor neurons, which usually lacked action potentials (arrowheads). Touch-evoked bursting in motor neurons under current clamp from a wt (A) and a far mutant (B) caudal primary motor neuron in response to tactile stimuli (50 ms, 20–40 psi as indicated).
We next examined whether increasing the stimulation amplitude might increase the number of spikes per stimulation in fakir mutants. However, increasing the stimulation amplitude to 30 and 40 psi resulted in only a modest increase in the probability of observing action potentials in fakir mutants: 0.33 ± 0.3 and 0.70 ± 0.3 spikes per stimulation, respectively (Fig. 4B). These findings indicate that the fakir mutant phenotype arises from attenuated touch-evoked activation of motor neurons.
The fakir phenotype arises from a missense mutation in the gene encoding voltage-gated calcium channel 2.1b.
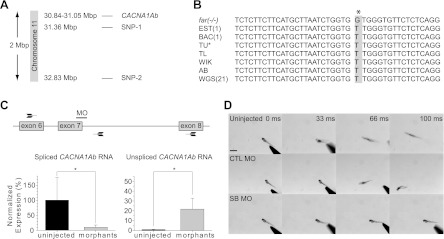
The fakir locus was previously mapped to chromosome 11 (Geisler et al. 2007). To further pinpoint the fakir locus, we performed high-resolution mapping from more than 5,000 meioses, which uncovered two tightly linked SNP markers (Fig. 5A). Assessment of the genes near these markers revealed the presence of a zebrafish paralog for the pore-forming (α) subunit of voltage-gated calcium channel 2.1 (CACNA1Ab), classically referred to as the P/Q-type calcium channel (Catterall et al. 2005). Considering that mutations in human CACNA1A have been linked to movement disorders (Pietrobon 2010), CACNA1Ab was identified as a candidate for fakir. Sequence analysis uncovered a thymine-to-guanine nucleotide substitution in fakir homozygous mutants (Fig. 5B), resulting in a leucine-to-valine missense mutation at amino acid residue 356 (L356V). Examination of all publicly available zebrafish sequences revealed a thymine to be the reported nucleotide at this position. In addition, sequencing of the corresponding region from several wild-type strains of zebrafish uncovered a thymine in each line, suggesting that the thymine-to-guanine nucleotide substitution was mutagenically induced.
Fig. 5.
Fakir mutants harbor a missense mutation in CACNA1Ab. A: location of the tightly linked single-nucleotide polymorphic markers (SNP) on chromosome 11 identified by meiotic mapping, and the gene encoding voltage-gated calcium channel 2.1b (CACNA1Ab). B: nucleotide sequence alignment of fakir homozygous mutant DNA (far−/−) highlighting the T > G nucleotide substitution, compared with DNA sequences from an expressed sequenced tag (EST), a bacterial artificial chromosome (BAC), the Tübingen genetic background (TU), the Tüpfel long fin genetic background (TL), the WIK and AB genetic backgrounds, and 21 whole genome sequences (WGS). C: target location of the splice-blocking morpholinos (MO) and its effect on the production of mature (spliced CACNA1Ab) RNA and the presence of immature (unspliced CACNA1Ab) RNA. Arrows are representative of the PCR primers used to assess extent of splice blocking. D: time-lapse images of touch-evoked motor behaviors from uninjected, control (CTL) MO-injected, or splice-blocking (SB) MO-injected embryos.
To determine whether the loss of CACNA1Ab was the cause of the fakir mutant phenotype, we performed RNA rescue and morpholino-mediated knockdown experiments. Injection of RNA encoding wild-type human CaV2.1 into three separate clutches of embryos obtained from incrosses of fakir carriers decreased the percentage of embryos exhibiting the homozygous fakir phenotype from 26.0 ± 2.0% in sham-injected controls (P = 0.97, χ2 test, n =39 embryos) to 7.3 ± 4.9% in RNA-injected embryos (P = 0.01, χ2 test, n = 55 embryos).
To determine whether knockdown of CaV2.1b would recapitulate the fakir mutant phenotype, we injected a splice-blocking morpholino predicted to truncate CaV2.1b at amino acid E362 into wild-type embryos. RT-PCR analysis of CACNA1Ab morphants revealed a significant reduction in mature CACNA1Ab transcripts and a corresponding increase in unspliced CACNA1Ab RNA (Fig. 5C). Behaviorally, the injection of the splice-blocking morpholino was found to eliminate touch-evoked motor behaviors (Fig. 5D). Collectively, these results indicate that CACNA1Ab is the causative gene in fakir.
L356V substitution alters CaV2.1 channel activity and exerts a dominant negative effect on zebrafish touch-evoked behaviors.
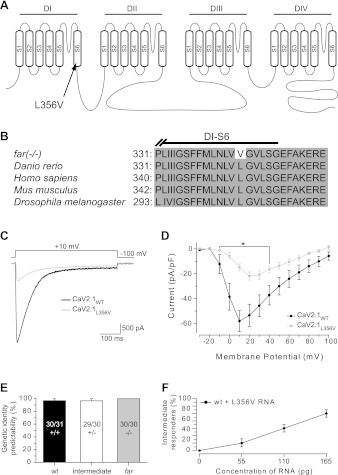
Leucine 356 is located within the sixth transmembrane segment of the first domain of CaV2.1 (Fig. 6A). Sequence analysis of CaV2.1 from several different species, including invertebrates, found leucine to be conserved at this position in CaV2.1 (Fig. 6B) and within the family of voltage-gated calcium channels from zebrafish as a whole (Flicek et al. 2012). The functional consequence of the valine substitution was examined by comparing whole cell voltage-clamp responses of CaV2.1L356V with responses of CaV2.1WT in a stable HEK293T cell line expressing the auxiliary β1c- and α2δ-subunits (Piedras-Renteria et al. 2001). In response to membrane depolarization, a rapidly activating inward current was observed in both CaV2.1WT (n = 19)- and CaV2.1L356V (n = 12)-transfected cells (Fig. 6C). Closer examination of the voltage-gated currents from HEK293T cells expressing CaV2.1L356V revealed a severe reduction in current density and an ∼10-mV depolarization of Vmax compared with CaV2.1WT (Fig. 6D). Thus the substitution of leucine 356 with a valine impairs CaV2.1 channel activity.
Fig. 6.
The fakir missense mutation reduces CaV2.1 channel activity and exerts a dominant negative effect on touch-evoked motor behaviors. A: a graphic representation of CaV2.1b indicating the location of the leucine 356-to-valine (L356V) missense mutation within segment 6 (S6), domain I (DI) found in fakir mutants. B: sequence comparison of zebrafish CaV2.1b to CaV2.1 from other organisms, highlighting the conservation of amino acids at and around position 356. C: typical currents evoked from HEK293T cells expressing either wild-type human CaV2.1 (CaV2.1WT) or human CaV2.1 harboring the L356V mutation (CaV2.1L356V) following membrane depolarization. D: average voltage-current density responses of CaV2.1L356V compared with CaV2.1WT. E: probability of predicting genetic identity from touch-evoked response in 3 phenotypically distinct groups of at least 30 embryos isolated from 3 different clutches. F: effect of RNA harboring the L356V substitution injected into wild-type embryos (n > 30 embryos each, from 3 clutches).
The observation that approximately one-half of the embryos from fakir carrier incrosses showed a partial reduction in touch responsiveness raised the possibility that embryos with reduced touch responsiveness were heterozygotes. Indeed, genotyping revealed that embryos displaying the intermediate touch-evoked motor phenotype were almost always heterozygotes (Fig. 6E), with a lone false positive originating from a wild-type embryo displaying reduced swimming. Considering this finding, we next examined whether reduced touch responsiveness in fakir heterozygotes was the cause of a dominant negative effect of the L356V mutation. To this end, we injected increasing doses of RNA encoding CaV2.1L356V into wild-type embryos, wherein we observed a concomitant increase in the percentage of wild-type embryos exhibiting the intermediate touch-evoked phenotype (Fig. 6F). These findings are consistent with the L356V missense mutation inducing the intermediate phenotype of heterozygotes via a dominant negative effect.
CACNA1Ab is expressed by sensory neurons and is dispensable for NMDA-evoked fictive swimming.
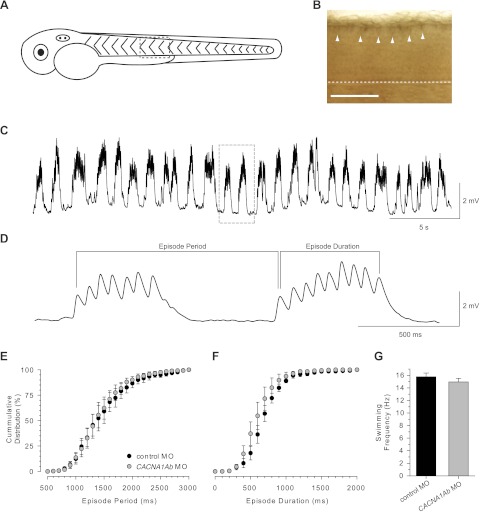
In an effort to identify the zebrafish neurons in which CaV2.1b is required for normal touch-evoked behaviors, we examined the expression pattern of CACNA1Ab at 48 hpf. Whole mount in situ hybridization revealed expression of CACNA1Ab by dorsal spinal cord neurons (Fig. 7B). The position and size of the dorsal spinal cord neurons is consistent with the reported location of RB sensory neurons (Bernhardt et al. 1990) and our previous staining of RBs (Fig. 2B), and thereby suggests that the fakir phenotype arises from a defect in sensory neurons.
Fig. 7.
CACNA1Ab is expressed by sensory neurons and is dispensable for NMDA-evoked fictive swimming. A: lateral representation of a 48-hpf zebrafish embryo. B: enlarged view of spinal cord, displaying antisense labeling of CACNA1Ab by RB sensory neurons (arrowheads). Dashed line indicates ventral edge of the spinal cord. Scale bar, 100 μm. C: a slow sweep of NMDA-evoked fictive swimming in the presence of 3 μM curare recorded under current clamp. Boxed area highlights enlarged area, shown in D. D: a faster sweep of NMDA-evoked fictive swimming from boxed area in C. Episode period and episode duration are demarcated by bracketed areas. E and F: cumulative distribution plots of episode period (E) and episode duration (F) from 2 min of NMDA-evoked fictive swimming (n = 5 fish for each; average episode period: wt 1,473 ± 110 vs. far 1,433 ± 100 ms, P = 0.80; average episode duration: wt 707 ± 49 vs. far 606 ± 66 ms, P = 0.25). G: fictive swimming frequency (frequency of end-plate potentials within an episode) from control and CACNA1Ab morphants.
Previous genetic and pharmacological studies have implicated CaV2.1 in a range of cellular activities ranging from neuronal excitability (Pineda et al. 1998) to gene expression (Sutton et al. 1999). However, the best characterized role for CaV2.1 within neurons is in the conduction of calcium into presynaptic terminals, an event necessary for activity-dependent neurotransmitter release. This contribution has been examined in detail in several naturally occurring CaV2.1 mutations in mice (Fletcher et al. 1996; Mori et al. 2000; Ophoff et al. 1996; Zwingman et al. 2001). Given the expression of CACNA1Ab by sensory neurons in zebrafish, we postulated that normal CaV2.1b channel activity is necessary for tactile stimuli to reach and activate neurons within the locomotor network. If true, then NMDA, which has been shown to drive patterned activity of the locomotor network similar to touch (Cui et al. 2004), might induce patterned activity in zebrafish lacking CaV2.1b. To address this point, touch-unresponsive CACNA1Ab morphants were chosen to remove any confounding effect of the L356V allele. From whole cell current-clamp recordings of skeletal muscle, we found that bath application of NMDA induced repetitive bouts of fictive swimming in both wild-type and CACNA1Ab morphants (Fig. 7C). A closer examination of NMDA-evoked fictive swimming (Fig. 7D) revealed that the distribution of episode periods, which reflect the propensity of the locomotor network to activate in response to NMDA, were similar between wild-type and CACNA1Ab morphants (Fig. 7E). Likewise, the distribution of episode durations, or the length of activity within the locomotor network once triggered, was also comparable between wild-type and CACNA1Ab morphants (Fig. 7F). Finally, the average fictive swimming frequency between CACNA1Ab morphants and controls was indistinguishable (Fig. 7G), indicating that the locomotor network that underlies swimming in zebrafish does not require CaV2.1b to generate normal patterns of fictive swimming. Collectively, these findings are consistent with a role for CaV2.1b in allowing tactile stimuli to activate the zebrafish locomotor network.
DISCUSSION
In this study we have demonstrated that the zebrafish mutant fakir harbors a missense mutation in the gene encoding for CaV2.1b (CACNA1Ab). When examined in vitro, the missense mutation was found to result in a hypomorphic CaV2.1 channel, a finding that suggests that the homozygous fakir mutant phenotype is caused by a reduction in CaV2.1b channel activity. Accordingly, injections of RNA encoding wild-type CaV2.1 into clutches of fakir incrosses reduced the number of fish displaying the homozygous touch-unresponsive phenotype, whereas the injection of a splice-blocking morpholino that truncates CaV2.1b prior to the second domain (E362stop) was found to induce the homozygous touch-unresponsive fakir phenotype. Unexpectedly, we also noticed a previously unreported “intermediate” phenotype in genetically identified fakir heterozygotes (Granato et al. 1996). Because the injection of RNA encoding the mutant CaV2.1 isoform into wild-type embryos induced the intermediate phenotype, we conclude that the intermediate fakir phenotype is most likely the result of a dominant negative effect by the mutant allele, rather than the consequence of haploinsufficiency. Collectively, these results indicate that CACNA1Ab is the causative gene in fakir and suggest that normal CaV2.1b channel activity is required for proper touch-evoked motor responses in zebrafish.
In an attempt to understand the physiological basis of the touch-unresponsive fakir phenotype, we examined the expression profile of CACNA1Ab and assessed electrophysiological activity within several cells belonging to the touch-evoked escape circuit. Our results indicate that CaV2.1b is not necessary within the locomotor network that underlies motor behaviors in zebrafish, as demonstrated by the ability to generate normal patterns of fictive swimming when the network is exogenously activated by bath application of NMDA. However, touch-evoked activation of these cells was either absent or severally diminished in homozygous fakir mutants. These results, coupled with the expression of CACNA1Ab by sensory neurons, suggest that CaV2.1b channel activity is required specifically in touch-sensitive neurons to trigger the zebrafish locomotor network.
Arguably, the best understood cellular role for CaV2.1 is in the conduction of calcium into presynaptic terminals during activity-dependent neurotransmitter release (Catterall et al. 2005). This raises the possibility that the fakir mutant phenotype might result from reduced synaptic communication at the RB-interneuron synapse; a hypothesis that could account for both the delayed onset of touch-unresponsiveness in homozygous fakir mutants and the presence of an intermediate heterozygous phenotype. In homozygous fakir mutants, a reduced amount of activity-dependent transmitter released from sensory neurons might be sufficient to activate downstream interneurons early in development, when neurons are small and therefore have high input resistance. However, as the input resistance of zebrafish neurons decreases during development (Saint-Amant and Drapeau 2000), the residual amount of transmitter released by sensory neurons in fakir homozygous mutants might become insufficient to activate the downstream interneurons necessary for transient and sustained activity within the zebrafish locomotor network. In a similar fashion, heterozygous RBs possessing a mixed population of wild-type and mutant CaV2.1b channels would be expected to release more transmitter than homozygous mutants but still less than wild-type embryos. This amount of transmitter released could be sufficient to trigger transient activity but insufficient for sustained activity in heterozygous embryos.
Interestingly, a recent pharmacologically mediated investigation into the identity of voltage-gated calcium channels expressed by zebrafish RBs was conducted (Won et al. 2011). This work found that RBs possessed voltage-gated calcium currents attributable to several members of the family, findings that raise questions regarding the apparent inability of other voltage-gated calcium channel family members to compensate for CaV2.1b in fakir mutants. One possible explanation for this phenomenon involves the calcium channel “slot” hypothesis (Cao et al. 2004), which postulates that although neurons may express several types of voltage-gated calcium channels, a particular subtype receives preferential inclusion at a fixed number of calcium channel slots. These calcium channel slots are presumably near active zones where calcium enters during activity-dependent transmitter release. Thus the presence of CaV2.1bL356V in fakir heterozygous and homozygous mutants would prevent other voltage-gated calcium channel family members expressed by RBs from compensating for mutated CaV2.1b. This hypothesis is supported by our finding that injection of RNA harboring the hypomorphic L356V mutation was capable of inducing the heterozygous intermediate fakir phenotype in wild-type zebrafish. Another possible explanation for the apparent lack of compensation by other calcium channels consistent with the results obtained from the pharmacological investigation (Won et al. 2011) could be the presence of a retention motif that precludes their movement away from the cell body, since these recordings were made from the cell bodies of RBs.
The ability to examine whether zebrafish RBs exhibit a slot preference for CaV2.1b, as well as to test whether the amplitude of activity-dependent transmitter release varies within fakir clutches in a predicted fashion (wild type > heterozygote > homozygote), will require the development of a paired recording preparation that facilitates whole cell voltage-clamp recordings from neurons directly postsynaptic to RBs. Development of such a recording preparation would also provide researchers with a genetically amenable in vivo model in which to examine the functional consequence of novel mutations in human CaV2.1, a channel linked to several neurological disorders in humans (Pietrobon 2010).
GRANTS
This work was supported by a grant from the National Science and Engineering Research Council of Canada (to L. Saint-Amant), an operating grant from the Canadian Institutes of Health Research (to L. Saint-Amant), a Chercheur Boursier award and the Groupe de Recherche sur le Système Nerveux Central from the Fond de Recherche en Santé du Québec (to L. Saint-Amant), an American Cancer Society Postdoctoral Fellowship (to I. G. Woods), and grants from the National Institutes of Health and the McKnight Endowment Fund for Neuroscience (to A. F. Schier).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.E.L., I.G.W., A.F.S., and L.S.-A. conception and design of research; S.E.L., I.G.W., M.L., J.R., and L.S.-A. performed experiments; S.E.L., I.G.W., M.L., J.R., A.F.S., and L.S.-A. analyzed data; S.E.L., I.G.W., A.F.S., and L.S.-A. interpreted results of experiments; S.E.L., I.G.W., A.F.S., and L.S.-A. prepared figures; S.E.L., I.G.W., A.F.S., and L.S.-A. drafted manuscript; S.E.L., I.G.W., A.F.S., and L.S.-A. edited and revised manuscript; A.F.S. and L.S.-A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank members of the Saint-Amant and Schier laboratories for helpful comments. We also thank Dr. Cao (Washington University, St. Louis, MO) for the human CaV2.1 construct and the stable HEK293T cell line expressing β1c- and α2δ-subunits.
REFERENCES
- Bernhardt RR, Chitnis AB, Lindamer L, Kuwada JY. Identification of spinal neurons in the embryonic and larval zebrafish. J Comp Neurol 302: 603–616, 1990 [DOI] [PubMed] [Google Scholar]
- Burgess HA, Johnson SL, Granato M. Unidirectional startle responses and disrupted left-right co-ordination of motor behaviors in robo3 mutant zebrafish. Genes Brain Behav 8: 500–511, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss RR, Drapeau P. Physiological properties of zebrafish embryonic red and white muscle fibers during early development. J Neurophysiol 84: 1545–1557, 2000 [DOI] [PubMed] [Google Scholar]
- Buss RR, Drapeau P. Synaptic drive to motoneurons during fictive swimming in the developing zebrafish. J Neurophysiol 86: 197–210, 2001 [DOI] [PubMed] [Google Scholar]
- Cao YQ, Piedras-Renteria ES, Smith GB, Chen G, Harata NC, Tsien RW. Presynaptic Ca2+ channels compete for channel type-preferring slots in altered neurotransmission arising from Ca2+ channelopathy. Neuron 43: 387–400, 2004 [DOI] [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev 57: 411–425, 2005 [DOI] [PubMed] [Google Scholar]
- Cui WW, Saint-Amant L, Kuwada JY. shocked Gene is required for the function of a premotor network in the zebrafish CNS. J Neurophysiol 92: 2898–2908, 2004 [DOI] [PubMed] [Google Scholar]
- Devoto SH, Melancon E, Eisen JS, Westerfield M. Identification of separate slow and fast muscle precursor cells in vivo, prior to somite formation. Development 122: 3371–3380, 1996 [DOI] [PubMed] [Google Scholar]
- Downes GB, Granato M. Supraspinal input is dispensable to generate glycine-mediated locomotive behaviors in the zebrafish embryo. J Neurobiol 66: 437–451, 2006 [DOI] [PubMed] [Google Scholar]
- Drapeau P, Ali DW, Buss RR, Saint-Amant L. In vivo recording from identifiable neurons of the locomotor network in the developing zebrafish. J Neurosci Methods 88: 1–13, 1999 [DOI] [PubMed] [Google Scholar]
- Fletcher CF, Lutz CM, O'Sullivan TN, Shaughnessy JD, Jr, Hawkes R, Frankel WN, Copeland NG, Jenkins NA. Absence epilepsy in tottering mutant mice is associated with calcium channel defects. Cell 87: 607–617, 1996 [DOI] [PubMed] [Google Scholar]
- Flicek P, Amode MR, Barrell D, Beal K, Brent S, Carvalho-Silva D, Clapham P, Coates G, Fairley S, Fitzgerald S, Gil L, Gordon L, Hendrix M, Hourlier T, Johnson N, Kahari AK, Keefe D, Keenan S, Kinsella R, Komorowska M, Koscielny G, Kulesha E, Larsson P, Longden I, McLaren W, Muffato M, Overduin B, Pignatelli M, Pritchard B, Riat HS, Ritchie GR, Ruffier M, Schuster M, Sobral D, Tang YA, Taylor K, Trevanion S, Vandrovcova J, White S, Wilson M, Wilder SP, Aken BL, Birney E, Cunningham F, Dunham I, Durbin R, Fernandez-Suarez XM, Harrow J, Herrero J, Hubbard TJ, Parker A, Proctor G, Spudich G, Vogel J, Yates A, Zadissa A, Searle SM. Ensembl 2012. Nucleic Acids Res 40: D84–D90, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahtan E, Sankrithi N, Campos JB, O'Malley DM. Evidence for a widespread brain stem escape network in larval zebrafish. J Neurophysiol 87: 608–614, 2002 [DOI] [PubMed] [Google Scholar]
- Geisler R, Rauch GJ, Geiger-Rudolph S, Albrecht A, van Bebber F, Berger A, Busch-Nentwich E, Dahm R, Dekens MP, Dooley C, Elli AF, Gehring I, Geiger H, Geisler M, Glaser S, Holley S, Huber M, Kerr A, Kirn A, Knirsch M, Konantz M, Kuchler AM, Maderspacher F, Neuhauss SC, Nicolson T, Ober EA, Praeg E, Ray R, Rentzsch B, Rick JM, Rief E, Schauerte HE, Schepp CP, Schonberger U, Schonthaler HB, Seiler C, Sidi S, Sollner C, Wehner A, Weiler C, Nusslein-Volhard C. Large-scale mapping of mutations affecting zebrafish development. BMC Genomics 8: 11, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato M, van Eeden FJ, Schach U, Trowe T, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Nusslein-Volhard C. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development 123: 399–413, 1996 [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- Hirata H, Saint-Amant L, Downes GB, Cui WW, Zhou W, Granato M, Kuwada JY. Zebrafish bandoneon mutants display behavioral defects due to a mutation in the glycine receptor beta-subunit. Proc Natl Acad Sci USA 102: 8345–8350, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Saint-Amant L, Waterbury J, Cui W, Zhou W, Li Q, Goldman D, Granato M, Kuwada JY. accordion, a zebrafish behavioral mutant, has a muscle relaxation defect due to a mutation in the ATPase Ca2+ pump SERCA1. Development 131: 5457–5468, 2004 [DOI] [PubMed] [Google Scholar]
- Hirata H, Watanabe T, Hatakeyama J, Sprague SM, Saint-Amant L, Nagashima A, Cui WW, Zhou W, Kuwada JY. Zebrafish relatively relaxed mutants have a ryanodine receptor defect, show slow swimming and provide a model of multi-minicore disease. Development 134: 2771–2781, 2007 [DOI] [PubMed] [Google Scholar]
- Hirata H, Wen H, Kawakami Y, Naganawa Y, Ogino K, Yamada K, Saint-Amant L, Low SE, Cui WW, Zhou W, Sprague SM, Asakawa K, Muto A, Kawakami K, Kuwada JY. Connexin 39.9 protein is necessary for coordinated activation of slow-twitch muscle and normal behavior in zebrafish. J Biol Chem 287: 1080–1089, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jontes JD, Buchanan J, Smith SJ. Growth cone and dendrite dynamics in zebrafish embryos: early events in synaptogenesis imaged in vivo. Nat Neurosci 3: 231–237, 2000 [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn 203: 253–310, 1995 [DOI] [PubMed] [Google Scholar]
- Korn H, Faber DS. The Mauthner cell half a century later: a neurobiological model for decision-making? Neuron 47: 13–28, 2005 [DOI] [PubMed] [Google Scholar]
- Lawson ND, Wolfe SA. Forward and reverse genetic approaches for the analysis of vertebrate development in the zebrafish. Dev Cell 21: 48–64, 2011 [DOI] [PubMed] [Google Scholar]
- Low SE, Amburgey K, Horstick E, Linsley J, Sprague SM, Cui WW, Zhou W, Hirata H, Saint-Amant L, Hume RI, Kuwada JY. TRPM7 is required within zebrafish sensory neurons for the activation of touch-evoked escape behaviors. J Neurosci 31: 11633–11644, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low SE, Ryan J, Sprague SM, Hirata H, Cui WW, Zhou W, Hume RI, Kuwada JY, Saint-Amant L. touché is required for touch-evoked generator potentials within vertebrate sensory neurons. J Neurosci 30: 9359–9367, 2010a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low SE, Zhou W, Choong I, Saint-Amant L, Sprague SM, Hirata H, Cui WW, Hume RI, Kuwada JY. NaV1.6a is required for normal activation of motor circuits normally excited by tactile stimulation. Dev Neurobiol 70: 508–522, 2010b [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown KA, Moreno R, Hall VL, Ribera AB, Downes GB. Disruption of Eaat2b, a glutamate transporter, results in abnormal motor behaviors in developing zebrafish. Dev Biol 362: 162–171, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean DL, Fetcho JR. Movement, technology and discovery in the zebrafish. Curr Opin Neurobiol 21: 110–115, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongeon R, Gleason MR, Masino MA, Fetcho JR, Mandel G, Brehm P, Dallman JE. Synaptic homeostasis in a zebrafish glial glycine transporter mutant. J Neurophysiol 100: 1716–1723, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori Y, Wakamori M, Oda S, Fletcher CF, Sekiguchi N, Mori E, Copeland NG, Jenkins NA, Matsushita K, Matsuyama Z, Imoto K. Reduced voltage sensitivity of activation of P/Q-type Ca2+ channels is associated with the ataxic mouse mutation rolling Nagoya (tgrol). J Neurosci 20: 5654–5662, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Fujita M, Ogino K, Saint-Amant L, Kinoshita T, Oda Y, Hirata H. Biogenesis of GPI-anchored proteins is essential for surface expression of sodium channels in zebrafish Rohon-Beard neurons to respond to mechanosensory stimulation. Development 137: 1689–1698, 2010 [DOI] [PubMed] [Google Scholar]
- Ono F, Shcherbatko A, Higashijima S, Mandel G, Brehm P. The zebrafish motility mutant twitch once reveals new roles for rapsyn in synaptic function. J Neurosci 22: 6491–6498, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophoff RA, Terwindt GM, Vergouwe MN, van Eijk R, Oefner PJ, Hoffman SM, Lamerdin JE, Mohrenweiser HW, Bulman DE, Ferrari M, Haan J, Lindhout D, van Ommen GJ, Hofker MH, Ferrari MD, Frants RR. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell 87: 543–552, 1996 [DOI] [PubMed] [Google Scholar]
- Piedras-Renteria ES, Watase K, Harata N, Zhuchenko O, Zoghbi HY, Lee CC, Tsien RW. Increased expression of alpha 1A Ca2+ channel currents arising from expanded trinucleotide repeats in spinocerebellar ataxia type 6. J Neurosci 21: 9185–9193, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietri T, Manalo E, Ryan J, Saint-Amant L, Washbourne P. Glutamate drives the touch response through a rostral loop in the spinal cord of zebrafish embryos. Dev Neurobiol 69: 780–795, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrobon D. CaV2.1 channelopathies. Pflügers Arch 460: 375–393, 2010 [DOI] [PubMed] [Google Scholar]
- Pineda JC, Waters RS, Foehring RC. Specificity in the interaction of HVA Ca2+ channel types with Ca2+-dependent AHPs and firing behavior in neocortical pyramidal neurons. J Neurophysiol 79: 2522–2534, 1998 [DOI] [PubMed] [Google Scholar]
- Saint-Amant L, Drapeau P. Motoneuron activity patterns related to the earliest behavior of the zebrafish embryo. J Neurosci 20: 3964–3972, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Amant L, Drapeau P. Time course of the development of motor behaviors in the zebrafish embryo. J Neurobiol 37: 622–632, 1998 [DOI] [PubMed] [Google Scholar]
- Schredelseker J, Di Biase V, Obermair GJ, Felder ET, Flucher BE, Franzini-Armstrong C, Grabner M. The beta 1a subunit is essential for the assembly of dihydropyridine-receptor arrays in skeletal muscle. Proc Natl Acad Sci USA 102: 17219–17224, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton KG, McRory JE, Guthrie H, Murphy TH, Snutch TP. P/Q-type calcium channels mediate the activity-dependent feedback of syntaxin-1A. Nature 401: 800–804, 1999 [DOI] [PubMed] [Google Scholar]
- Talbot WS, Schier AF. Positional cloning of mutated zebrafish genes. Methods Cell Biol 60: 259–286, 1999 [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio). Eugene, OR: Univ. of Oregon Press, 2000 [Google Scholar]
- Westerfield M, Liu DW, Kimmel CB, Walker C. Pathfinding and synapse formation in a zebrafish mutant lacking functional acetylcholine receptors. Neuron 4: 867–874, 1990 [DOI] [PubMed] [Google Scholar]
- Won YJ, Ono F, Ikeda SR. Identification and modulation of voltage-gated Ca2+ currents in zebrafish Rohon-Beard neurons. J Neurophysiol 105: 442–453, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zottoli SJ, Faber DS. Properties and distribution of anterior VIIIth nerve excitatory inputs to the goldfish Mauthner cell. Brain Res 174: 319–323, 1979 [DOI] [PubMed] [Google Scholar]
- Zwingman TA, Neumann PE, Noebels JL, Herrup K. Rocker is a new variant of the voltage-dependent calcium channel gene Cacna1a. J Neurosci 21: 1169–1178, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]