Abstract
Inner ear hair cells respond to mechanical stimuli with graded receptor potentials. These graded responses are modulated by a host of voltage-dependent currents that flow across the basolateral membrane. Here, we examine the molecular identity and the function of a class of voltage-dependent ion channels that carries the potassium-selective inward rectifier current known as IK1. IK1 has been identified in vestibular hair cells of various species, but its molecular composition and functional contributions remain obscure. We used quantitative RT-PCR to show that the inward rectifier gene, Kir2.1, is highly expressed in mouse utricle between embryonic day 15 and adulthood. We confirmed Kir2.1 protein expression in hair cells by immunolocalization. To examine the molecular composition of IK1, we recorded voltage-dependent currents from type II hair cells in response to 50-ms steps from −124 to −54 in 10-mV increments. Wild-type cells had rapidly activating inward currents with reversal potentials close to the K+ equilibrium potential and a whole-cell conductance of 4.8 ± 1.5 nS (n = 46). In utricle hair cells from Kir2.1-deficient (Kir2.1−/−) mice, IK1 was absent at all stages examined. To identify the functional contribution of Kir2.1, we recorded membrane responses in current-clamp mode. Hair cells from Kir2.1−/− mice had significantly (P < 0.001) more depolarized resting potentials and larger, slower membrane responses than those of wild-type cells. These data suggest that Kir2.1 is required for IK1 in type II utricle hair cells and contributes to hyperpolarized resting potentials and fast, small amplitude receptor potentials in response to current inputs, such as those evoked by hair bundle deflections.
Keywords: Kir2.1, KCNJ2, utricle, IK1
in the vestibular system, head movements are converted into sensory signals by mechanotransduction channels located at the apex of sensory hair bundles. The sensory signals then propagate as graded receptor potentials from the apex to the base of the hair cell. Small changes in the electrical properties of the basolateral membrane can modify the hair cell receptor potential and consequently, neurotransmitter release at the afferent synapse. These changes include the developmental acquisition of various ion channels required for proper modulation and transmission of the sensory signal, which continues until hair cells reach functional maturity, around postnatal day 8 (P8) (Rüsch et al. 1998), as well as modulation by various efferent mechanisms. To extend an understanding of the functional development and the molecular components required for hair cell signaling, we focus here on the Kir2 family of potassium inward rectifier channels and their contributions to mammalian vestibular hair cell function.
Kir2 channels play a physiological role in a variety of cells: they can affect resting potential, action potential firing rates, neurotransmitter release, insulin release, cell volume, and blood flow (Bichet et al. 2003; Doupnik et al. 1995; Isomoto et al. 1997; Nichols and Lopatin 1997). Kir2 channels are K+ selective and are active around the resting potential. They pass significantly larger inward currents at membrane potentials negative to the potassium reversal potential (EK = −82 mV) than outward currents at positive potentials (Lu 2004). However, unlike voltage-gated channels, Kir2 channels lack the voltage-sensing structure, S4, specific to those channels (Kurachi 2003) and thus are not gated by membrane voltage. Rather, their voltage dependence or rectification is derived from blockade of K+ efflux by intracellular Mg2+ (Matsuda et al. 1987) or polyamines (Lopatin et al. 1994) at depolarized potentials. These biophysical properties provide a negative-feedback mechanism that acts to repolarize the membrane potential following small deviations, which would otherwise drive the cell away from resting potential.
The Kir2 ion channel family includes four subunits in the mouse and two additional subunits, Kir2.5 and -2.6, which are present in the carp and human genomes, respectively (Hassinen et al. 2008; Ryan et al. 2010). Most Kir2 channels form functional homomeric tetramers, whereas some assemble into heteromeric tetramers (Preisig-Müller et al. 2002; Schram et al. 2002). All Kir2 channels have a descending P-loop, which includes the canonical GYG K+-selectivity sequence. Mutations in the GYG sequence render Kir2 channels nonfunctional and lead to disorders, such as Andersen-Tawil syndrome (ATS) (Plaster et al. 2001).
Expression of Kir2 channels has been reported in mouse auditory hair cells (Ruan et al. 2008) and avian vestibular hair cells (Correia et al. 2004; Navaratnam et al. 1995), but their physiological correlates and contributions have not been elucidated, particularly in mammals. In inner ear hair cells, the inward rectifier current, IK1, contributes to sensory signaling by maintaining resting potential at more negative values, lowering input resistance (Brichta et al. 2002; Géléoc et al. 2004; Holt and Eatock 1995; Rüsch et al. 1998) and enhancing receptor potentials by deactivation of their negative-feedback mechanism at depolarized membrane potentials (Goodman and Art 1996). Previously, Géléoc et al. (2004) showed a correlation between the acquisition of IK1 in mouse utricle in early development and more negative resting potentials.
Here, we used quantitative PCR (qPCR), a fluorescence-activated, cell-sorted database, and immunolocalization to examine the expression of Kir2 channels in the mouse vestibular system. Data were collected at different time points during development from embryonic day 15 (E15) through adulthood to identify temporal correlation between gene expression and physiological expression of IK1. We used the whole-cell, tight-seal recording technique to characterize the biophysical properties of IK1 in the postnatal mouse utricle. We show that IK1 can be blocked by BaCl2, a known blocker of Kir2 channels. In addition, we examined basolateral conductances in wild-type hair cells, hair cells transfected with a dominant-negative Kir2.1 construct, and hair cells excised from Kir2.1-deficient (Kir2.1−/−) mice. We found that cells that lack functional Kir2.1 do not express IK1 and have more depolarized resting potentials and slower but larger amplitude responses to hyperpolarizing current injections. These data indicate that Kir2.1 is essential for IK1 and plays a significant role in vestibular hair cell signaling.
MATERIALS AND METHODS
Tissue preparation.
Utricle epithelia were excised from mice between E15 and P386, according to a protocol approved by the Animal Care Committee at the University of Virginia (#3123) and at Children's Hospital Boston (#1959). Briefly, mice were killed by rapid decapitation. The temporal bone was excised and placed in MEM with Glutamax (Invitrogen, Carlsbad, CA) containing 10 mM HEPES (Sigma Aldrich, St. Louis, MO) and 0.05 mg/ml Ampicillin (pH 7.4). The utricle sensory epithelium was removed carefully and placed under two thin glass fibers on a glass coverslip to stabilize and flatten the tissue. We used tissue from mice of three different genotypes, all obtained from The Jackson Laboratory (Bar Harbor, ME). FVB/NJ wild-type mice served as a control, whereas Kir2.1+/− mice were used to breed heterozygous and homozygous mice lacking the Kir2.1 gene (Kir2.1−/−). Kir2.1−/− pups did not survive 12 h past birth, and all postnatal experiments were done on vestibular tissue, cultured for the indicated number of days. To confirm that our culture conditions did not alter patterns of ion channel gene expression, we compared data from acutely excised wild-type tissue with data from wild-type tissue maintained in culture for up to 20 days and found no electrophysiological differences (data not shown).
qPCR.
Utricles were acutely dissected from pre- and postnatal wild-type mice at six different ages: E15 (n = 20), E18 (n = 15), P0 (n = 24), P7 (n = 14), P25 (n = 30), and P180 (n = 14). RNA was isolated using an RNAqueous-Micro kit (Cat. #1931, Ambion, Austin, TX). RNA was purified using an RNA purification kit (DNA-Free RNA Kit; Cat. #R1013, Zymo Research, Irvine, CA). To eliminate genomic DNA and reverse transcribe isolated RNA into cDNA, we used the QuantiTect reverse transcription kit (Qiagen, Valencia, CA). RNA concentration was determined on a spectrophotometer (NanoDrop ND-1000, Thermo Fisher Scientific, Pittsburgh, PA). For quality assurance, samples from isolated RNA were analyzed for purity with a bioanalyzer (Agilent Technologies, Santa Clara, CA), then reverse transcribed into cDNA, and later, analyzed by qPCR. qPCR primers were designed with a melting temperature of 54°C for Kir2.1. The Kir2.1 qPCR primer sequences were: CACAGCTTCTCAAATCTAGGATCA and CTATTTCGTGAACGATAGTGATGG. For qPCR, iQ SYBR Green Supermix (#11761-100, Invitrogen) was used. We tested expression levels on the housekeeping gene, ribosomal 29S, with the following primers that had a melting temperature of 62°C: GGAGTCACCCACGGAAGTTCGG and GGAAGCACTGGCGGCACATG. To confirm primer specificity, we generated a plasmid that carried the Kir2.1 amplicon (130 base pairs). The size was confirmed by agarose gel electrophoresis, and the gel-purified product was sequenced to confirm Kir2.1 identity. To calculate expression ratios, we used the ΔΔ-comparative threshold method, normalizing cycle thresholds first to 29S and then to the E15 sample. The final analysis was performed using Origin 7.1 (OriginLab, Northampton, MA).
Generation of adenoviral vectors.
The coding sequence for murine (m)Kir2.1 in the pcDNA1/Amp vector was cloned previously and kindly provided by Dr. Lily Y. Jan (Kubo et al. 1993). Mutation from GYG to SYG in the pore loop region using RT-PCR was confirmed by sequencing the mutated gene, including the pore loop region, using the following primers with melting temperatures near 56°C: Kir2.1MutXhoIForw CTCGAGACTGTTTTCTAAAGCAGAAA and Kir2.1MutEcoRVRev GATATCTTTCCTTGAAACCTTTGGCT. The mutated gene, Kir2.1-G144S, was subcloned into the plasmid adenovirus (pAd)Track-cytomegalovirus (CMV)-green fluorescent protein (GFP) shuttle plasmid (He et al. 1998). The plasmid was linearized with the restriction endonuclease PmeI and transformed into BJ5183 competent cells (Agilent Technologies) with the adenoviral backbone plasmid, pAdEΔpol (Hodges et al. 2000). To confirm successful homologous recombination, kanamycin-resistant recombinants were digested with restriction endonuclease PacI and analyzed after running the product on agarose gels. We transfected C7 cells, a cell line used for adenovirus packaging (Amalfitano et al. 1998) with the linearized homologous recombinant plasmids. Following five rounds of serial passage, the crude lysate was filtered and purified with an AdenoX viral purification kit (BD Biosciences, Sparks, MD) to yield 2 ml Ad-CMV-GFP-CMV-Kir2.1-G144S at a titer of 2.2 × 108 viral particles/ml, which was aliquoted in 100 μl vials and stored at −80°C. Ad-GFP-human (h)Kir2.1 and pAd-vitellogenin (Vg)retinoid X receptor (RXR), at a titer of 4.3 × 1011 viral particles/ml, were generated, as described previously (Holt et al. 1999).
Immunohistochemistry.
Sensory epithelia were harvested at P0 from wild-type and Kir2.1−/− mice (The Jackson Laboratory) and from adult mice then placed in Glyo-Fixx (Thermo Scientific, Rockford, IL) overnight at 4°C. After permeabilization with 0.1% Triton X-100S (Sigma Aldrich), utricles were incubated with a Kir2.1 rabbit polyclonal antibody targeting the C-terminus (Prestige Antibodies, Sigma Aldrich) at a concentration of 1:600 in 1% polybutylene terephthalathe (PBT) at 4°C overnight. After incubation with 488-Alexa Fluor donkey anti-rabbit secondary antibodies and 647-phalloidin (Invitrogen) at 1:200 dilution in 1% PBT, sensory epithelia were mounted on coverslips using SlowFade Gold antifade reagent (Invitrogen). Adult vestibular tissue (see Fig. 3D) was prepared with an alternate procedure. Mice were perfused transcardially with 4% paraformaldehyde, 1% picric acid, 1% acrolein, and 5% sucrose in 0.1 M phosphate buffer. Sections were cut on a freezing, sliding microtome, digested with 2% Triton X-100 for 30 min; incubated for 2 days in a primary antibody cocktail of the same rabbit anti-Kir2.1 (1:200) and goat anticalretinin (1:400); and then rinsed and transferred to a secondary antibody cocktail of donkey anti-rabbit Alexa 594 and donkey anti-goat Alexa 488 (both diluted 1:200). Anti-calretinin was used to immunolabel extrastriolar type II hair cells and calyx afferents in the striolar region (Desai et al. 2005). All tissue was viewed through a 63× objective on a Zeiss 510 confocal laser-scanning microscope (Oberkochen, Germany). Images were analyzed using the Zeiss LSM Image Browser.
Fig. 3.
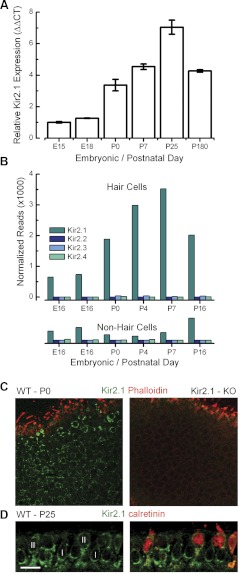
Expression of Kir2 subunits in mouse utricle. A: quantitative RT-PCR was used to estimate mRNA expression of Kir2.1 at various time points. The number of wild-type mouse utricles used to derive the template mRNA included: embryonic day 15 (E15) = 20, E18 = 15, P0 = 24, P7 = 14, P25 = 30, P180 = 14. Error bars represent SE for 3 replicates. CT, comparative threshold. B: normalized number of mRNA reads plotted as a function of age for hair cells and nonhair cells. Transcript counts are plotted for Kir2.1–Kir2.4. Two replicates are shown for the E16 sample. Data provided courtesy of Drs. Zheng-Yi Chen and David P. Corey (https://shield.hms.harvard.edu). C: confocal images of utricles at P0 from wild-type (left) and Kir2.1-deficient [Kir2.1−/−; knockout (KO)] mice (right). Projections through a series of focal planes through the epithelium show that Kir2.1 (green) is expressed in the basolateral membrane of hair cells but not in stereocilia stained with phalloidin (red). D: confocal image of a cross-section through an extrastriolar region of a wild-type P25 mouse utricle. The tissue was immunolabeled for Kir2.1 (green) and calretinin (red). Type I (I) and type II (II) hair cells are indicated in the figure. Original scale bar = 10 μm. Image provided courtesy of Dr. Anna Lysakowski and Mr. Steven Price.
Electrophysiology.
Organotypic cultures were generated from utricles, from wild-type, Kir2.1+/−, and Kir2.1−/− mice, ranging from age P0 to P386, P0 to P383, and P0 to P0 + 20 days in vitro, respectively. To remove the otolithic membrane, utricles were treated with Protease XXIV (Sigma Aldrich), added at 0.1 mg/ml for 20 min, and mounted on coverslips as described above. Epithelia were cultured in MEM with Glutamax (Invitrogen) containing 10 mM HEPES (Sigma Aldrich), 0.05 mg/ml Ampicillin (pH 7.4), and 2% FBS (Gemini Bio-Products, West Sacramento, CA). Current or voltage was recorded in standard artificial perilymph solution containing (in mM): 144 NaCl, 0.7 NaH2PO4, 5.8 KCl, 1.3 CaCl2, 0.9 MgCl2, 5.6 d-glucose, and 10 HEPES. Vitamins (1:50; Cat. #11120) and amino acids (1:100; Cat. #11130) were added from concentrates (Invitrogen). Final adjustment to pH 7.4 and ∼320 mOsmol/kg was done with NaOH. BaCl2 (Sigma Aldrich) was added to bath solution at 100 μM concentration. Hair cells were observed from the apical surface using an upright Axioskop FS microscope (Zeiss), equipped with a 63× water-immersion objective with differential interference contrast optics. Recording pipettes (3–5 MΩ), pulled from R6 capillary glass (King Precision Glass, Claremont, CA), were filled with intracellular solution containing (in mM): 135 KCl, 5 EGTA, 5 HEPES, 5 K2ATP, and 0.1 CaCl2, adjusted to pH 7.4 and ∼290 mOsmol/kg with KOH. Current or voltage was recorded in the whole-cell, voltage-clamp configuration at room temperature (22–24°C) with an Axopatch 200B (Molecular Devices, Palo Alto, CA), filtered at 1 kHz with a low-pass Bessel filter, digitized at ≥20 kHz with a 12-bit acquisition board (Digidata 1322) and pCLAMP 8.2 (Molecular Devices). Data were stored on disk for offline analysis using OriginPro 7.1 (OriginLab). ANOVA tests were applied to compare multiple means, and results are shown as means ± SD. Activation curves were fitted with a Boltzmann equation as follows: G(Vm) = Gmin + Gmax − Gmin/1 + exp[(Vm − V1/2)/s].
Gmax and Gmin are the maximum and minimum conductances, respectively. V1/2 is the membrane potential at which one-half of the conductance is activated, and S is the slope of the curve at the midpoint.
Type I and type II cells were identified based on the presence or absence, respectively, of the type I flask-shaped morphology, which was well preserved in the intact sensory epithelium. As a second indication, we used a voltage protocol designed to highlight the type I-specific current, IK,L, which is evident as early as E18 (Géléoc et al. 2004). Cells, which expressed IK,L, were excluded from the analysis.
RESULTS
Inward rectifier currents in utricle hair cells.
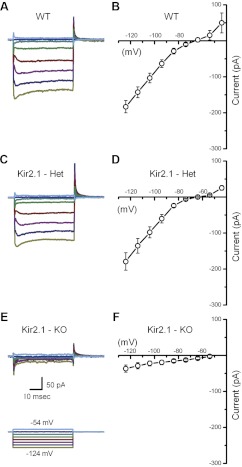
Consistent with previous studies (Géléoc et al. 2004; Rüsch et al. 1998), we observed strong inward rectifier currents throughout early development in mouse utricle type II hair cells. We used the whole-cell, tight-seal technique in voltage-clamp mode to record fast inward rectifier currents, which were evoked by a protocol that included hyperpolarizing voltage steps from a holding potential of −64 mV to potentials between −124 and −54 mV in increments of 10 mV. Figure 1, A and C, shows representative currents recorded from utricle type II hair cells at P2 and P25. These currents belong to the IK1 class of inward rectifiers as opposed to the slow inward rectifiers that belong to the Ih class (Géléoc et al. 2004; Horwitz et al. 2011; Rüsch et al. 1998). We did not record from type I hair cells, because they express the low voltage-activated outward current, IK,L, which is active at voltages similar to IK1, making it difficult to distinguish the latter. IK,L confers more hyperpolarized resting potentials to type I hair cells. However, Holt et al. (2007) concluded that loss of IK,L in type I hair cells did not depolarize the cells' resting potential to the extent expected, because in the absence of IK,L, persistent IK1 may compensate.
Fig. 1.
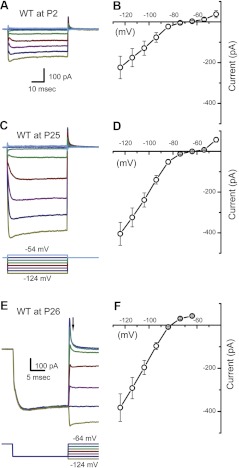
IK1 carries the potassium-selective inward rectifier current in mouse utricle hair cells. A: representative currents from a postnatal day 2 (P2) type II hair cell reveal voltage-dependent K+ inward rectifier currents of the IK1 variety. Currents were evoked by the protocol shown at the bottom of C, which included voltage steps in 10-mV increments between −54 and −124 mV from a holding potential of −64 mV. WT, wild-type. B: mean steady-state I(V) curve derived from current families, such as the one shown in A, for 5 P2 hair cells. C: a representative family of currents from a P25 type II hair cell. The scale bar shown in A also applies to C. D: mean steady-state I(V) curve derived from current families of 6 P25–P29 hair cells. E: a family of IK1 traces, evoked by the protocol (bottom), designed to facilitate estimation of the IK1 reversal potential. From the holding potential of −64 mV, the cell was hyperpolarized to −114 mV to fully activate IK1 and then stepped to a series of voltages between −124 and −54 mV. F: mean instantaneous I(V) generated from 9 P24–P31 hair cells. Currents were sampled at the time point indicated by the arrow in E. Reversal potential was taken as the 0 current potential of the instantaneous I(V) relation.
Figure 1, B and D, shows mean steady-state I(V) curves from type II cells at the same ages shown in Fig. 1, A and C, respectively. Typical of IK1 in other studies, the I(V) curves reveal small outward currents positive to −40 mV and large inward currents negative to the potassium equilibrium potential (EK = −82 mV). To confirm that the currents we recorded were K+ selective, we measured their reversal potential. The mean instantaneous I(V) curve recorded at P26 had a reversal potential of −81.8 mV, as shown in Fig. 1, E and F. The mean reversal potential for all wild-type cells was −83.1 ± 2.8 mV (n = 36), close to the K+ equilibrium potential. To extend previous studies (Géléoc et al. 2004; Rüsch et al. 1998), which examined IK1 up to P3 in mouse utricle type II hair cells, here, we describe IK1 throughout development and into adulthood, up to P386.
Voltage dependence of IK1.
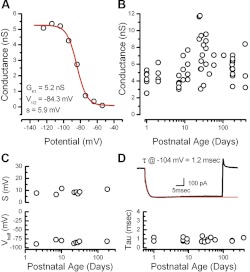
We plotted the activation curve for IK1 recorded from a representative wild-type hair cell at P9, as shown in Fig. 2A. The data revealed voltage dependence similar to that described for IK1 in a previous study by Rüsch et al. (1998). We fitted the data from wild-type cells with a first-order Boltzmann function. The Boltzmann fits revealed a mean of V1/2 = −83.5 ± 4.0 mV and a mean slope of 8.8 ± 1.8 mV (n = 8). The mean slope conductance was 4.8 ± 1.5 nS (n = 46). To examine developmental changes, we plotted the conductance as a function of postnatal age (Fig. 2B) but found no further increase in the maximal conductance up to 1 yr of age. Similarly, we plotted the activation curve parameters, V1/2 and slope, as a function of age and found that they were stable, up to 6 mo, the latest stage tested (Fig. 2C).
Fig. 2.
Activation range and developmental expression of IK1. A: a representative activation curve recorded from a P9 wild-type type II hair cell. The data were fit with a Boltzmann equation with a membrane potential at which 1/2 of the conductance is activated (V1/2) of −84 mV, a slope (s) of 5.9 mV, and a maximal conductance (GK1) of 5.2 nS. B: maximal inward rectifier conductances from type II hair cells plotted as function of age. The data were plotted on a log scale to help spread the data along the x-axis. C: slope and V1/2 values derived from 8 representative Boltzman fits plotted as a function of postnatal age. D: activation kinetics at −104 mV fitted with a single exponential (red trace). Bottom: the time constants (tau; τ) are plotted as a function of postnatal age for 20 representative cells.
IK1 activated within a few milliseconds with single exponential kinetics and was almost fully activated at −124 mV, consistent with previous characterizations (Holt and Eatock 1995; Rüsch et al. 1998). To quantify the activation kinetics, we fit traces evoked by steps to −104 mV with a single exponential function (Fig. 2D). The mean time constant of activation was 0.9 ± 0.2 ms (n = 20). Time constants from individual cells plotted as a function of age revealed little change through development and into adulthood (Fig. 2D). As such, the currents were similar in their voltage range of activation, activation kinetics, and K+ selectivity at all ages examined and were similar to those described previously as IK1 in prenatal and postnatal mouse utricle epithelia up to P3 (Géléoc et al. 2004; Rüsch et al. 1998). The data were also consistent with previous reports from acutely excised saccular hair cells from the leopard frog (Holt and Eatock 1995). The similar biophysical properties for IK1 in vestibular hair cells of various species and ages suggest that the currents may be carried by channels with a similar molecular identity.
Expression of Kir2 mRNA and protein.
To narrow in on the potassium channel genes that might underlie IK1 in mouse vestibular hair cells, we began with a qRT-PCR screen for Kir2.1 mRNA, since Kir2.1 expression was reported previously in avian vestibular hair cells (Correia et al. 2004; Navaratnam et al. 1995). We extracted mRNA from acutely excised sensory epithelia from pre- and postnatal mice ranging from E15 to P180 and used validated, specific primers designed for linear amplification of Kir2.1 mRNA (Fig. 3A). We found that Kir2.1 was highly expressed at all developmental ages tested and that there was a rise in Kir2.1 expression that paralleled the developmental acquisition of IK1 in mouse utricle type II hair cells, which begins as early as E15 (Géléoc et al. 2004). The increase in Kir2.1 mRNA expression during the 1st postnatal wk also coincided with the continued maturation of the vestibular system in mice (Rüsch et al. 1998). Furthermore, the total number of mature hair cells continues to increase until P16 (Kirkegaard and Nyengaard 2005; Li et al. 2008), which may contribute to the higher Kir2.1 mRNA expression level measured at P25 compared with P7.
As a second test for Kir2 gene expression, we screened a database, Shared Harvard Inner Ear Laboratory Database (SHIELD; https://shield.hms.harvard.edu), derived from RNA sequencing of a fluorescence-activated cell sorter (FACS). Vestibular hair cells that expressed GFP, driven by the hair cell promoter Pou4f3, were dissociated and FACS sorted. GFP-negative, nonhair cells were also collected. The experiment was repeated at developmental stages between E16 and P16, and the entire transcriptome for both GFP-positive hair cells and GFP-negative, nonhair cells was sequenced, quantified, and mapped against the National Center for Biotechnology Information (NCBI) build 37/mm9 mouse genome assembly. The number of mRNA transcript reads for Kir2.1–Kir2.4 is plotted as a function of development (Fig. 3B). The mRNA sequencing data show high Kir2.1 expression with a pattern that parallels both the qPCR data and the developmental acquisition of IK1. Importantly, the data also show little or no expression of Kir2.2, Kir2.3, or Kir2.4 and a very low level Kir2.1 expression in nonhair cells.
To confirm Kir2.1 protein expression in hair cells, we examined immunolocalization throughout utricles of organotypic cultures excised at P0 and incubated several days in vitro (Fig. 3C). Hair bundles were counterstained with AlexaFluor 647-phalloidin to illuminate actin and distinguish them from basolateral membranes. The immunohistochemistry data revealed that Kir2.1 localizes to the basolateral membranes of type I and type II hair cells but not hair bundles. We then confirmed the specificity of the Kir2.1 antibody in tissue excised from Kir2.1−/− mice that have a complete deletion of the open reading frame (Zaritsky et al. 2000, 2001). Utricles excised from Kir2.1−/− mice at the same age and stained using an identical protocol and identical image acquisition parameters revealed robust actin staining but no Kir2.1 protein expression in hair cells or elsewhere in the sensory epithelium (Fig. 3C). We also confirmed Kir2.1 protein expression in utricles harvested from young-adult wild-type mice. In this case, the tissue was sectioned and counterstained for calretinin, which labels 70–80% of extrastriolar type II hair cells (Desai et al. 2005). The image reveals robust Kir2.1 expression in the basolateral membranes of type I hair cells and in type II hair cells identified by colocalization with calretinin (Fig. 3D).
Dominant-negative suppression of IK1.
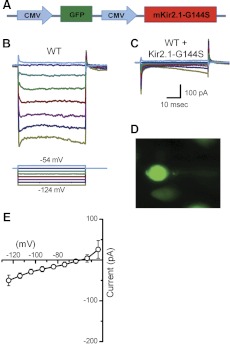
To examine the correlation between Kir2 channels and IK1, we transfected organotypic cultures generated from wild-type mouse utricle dissected at P0 with a dominant-negative construct that carried a point mutation in the Kir2.1 channel gene, which causes ATS, a hereditary cardiac disorder (Lu et al. 2006; Plaster et al. 2001; Tristani-Firouzi and Etheridge 2010). The point mutation (G144S) occurs in the Kir2.1 channel pore motif GYG and leads to irregular spiking activity and atrial fibrillation. To investigate the identity and function of IK1 in hair cells, we used the dominant-negative mutation and generated an adenoviral construct, Ad-mKir2.1-G144S. The bicistronic construct carried a CMV promoter to drive expression of GFP as a transfection marker and a second CMV promoter to drive expression of mKir2.1-G144S (Fig. 4A). Wild-type utricle epithelia were exposed to Ad-mKir2.1-G144S at titers of 2.2 × 108 viral particles/ml for 4 h and maintained in culture for up to 10 days. We recorded from control GFP-negative cells and transfected GFP-positive cells, which presumably also expressed the dominant-negative construct. The GFP-negative cells showed robust IK1 (Fig. 4B), whereas the GFP-positive cells (Fig. 4D) lacked IK1 entirely but retained Ih (Fig. 4C), evident as the small, slowly activating currents at the end of the step. The mean I(V) curve, taken near the end of the step from six wild-type cells transfected with the dominant-negative construct, revealed little or no residual IK1 (Fig. 4E). Since Kir2.1 can coassemble with all members of the Kir2 family to exert dominant-negative inhibition, these data suggest that the Kir2 family contributes to IK1 in vestibular hair cells but do not indicate precisely which Kir2 member is involved.
Fig. 4.
Adenoviral expression of dominant-negative Kir2.1 in wild-type utricle hair cells. A: adenoviral vector map for adenovirus (Ad)-murine (m)Kir2.1-G144S. The vector includes the coding sequence for Kir2.1 carrying a point mutation (G144S) in the conical GYG selectivity filter. Promoter sequences [cytomegalovirus (CMV)] are shown in blue, green fluorescent protein (GFP) in green, and the coding sequences for Kir2.1 in red. B: representative currents recorded from a wild-type cell at P7 (GFP-negative). The scale bar in C also applies to B. The stimulus protocol for recordings in B and C, shown at the bottom of B. C: data from a GFP-positive cell transfected with a dominant-negative Kir2.1 (G144S) construct. The data were recorded from the same tissue as the data shown in B (+3 days in culture). D: image of the GFP-positive cell recorded from C. E: mean I(V) curve sampled near the end of the voltage steps, derived from 6 GFP-positive cells transfected with Ad-Kir2.1-G144S.
Kir2.1−/− hair cells lack IK1.
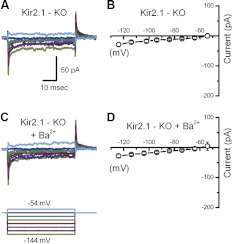
To examine which of the Kir2 subunits is necessary for IK1, we recorded from Kir2.1−/− mice. We excised utricles from Kir2.1−/− mice and recorded from type II hair cells in voltage-clamp mode to assay for physiologic expression of IK1. In all cells examined from Kir2.1−/− mice (n = 76), IK1 was entirely absent at every stage examined (Fig. 5, E and F), from P0 up to 20 days in vitro. Representative currents recorded from wild-type and heterozygous cells (Fig. 5, A–D) revealed robust IK1 with similar mean current. Furthermore, IK1 was similar in cells from early postnatal ages and in adulthood between wild-type and Kir2.1 heterozygous cells. These data strongly suggest that Kir2.1 is a molecular component necessary for IK1. Next, we wondered whether the small residual currents (<−25 pA) in Kir2.1−/− mice were due to any of the other Kir family subunits. Most Kir channels form functional homotetramers, whereas some form heterotetramers. Previous studies report that each of the four Kir2 channel subunits can coassemble into heteromers with all other Kir2 subunits in heterologous systems (Preisig-Müller et al. 2002; Schram et al. 2002). To investigate potential contributions from other Kir genes, we extracted utricles from Kir2.1−/− mice and recorded currents from type II cells before (Fig. 6A) and after (Fig. 6C) adding 100 μM BaCl2 to the external bath solution to block any residual IK1. The mean steady-state I(V) curves generated from both data sets did not reveal any differences that would suggest BaCl2 had inhibited residual inward rectifier currents in Kir2.1−/− mice (Fig. 6, B and D). These results confirm that none of the other Kir subunits contributed to IK1 in Kir2.1−/− mice. In other words, in the absence of the Kir2.1 gene in utricle type II hair cells, IK1 was abolished completely.
Fig. 5.
Inward rectifier currents recorded from Kir2.1 knockout mice. A and C: representative currents recorded from wild-type and heterozygous (Het) cells at P2. B: mean steady-state I(V) relation taken from 10 P0–P1 wild-type cells. D: mean steady-state I(V) relation taken from 6 P2 cells from heterozygous (Kir2.1+/−) mice. E: a family of representative currents recorded from a utricle type II hair cell excised from a Kir2.1−/− mouse at P0 and maintained in culture for 2 days. The stimulus protocol and scale bars shown below E also apply to A and C. F: mean steady-state I(V) relation taken from 8 P0 hair cells from Kir2.1−/− mice.
Fig. 6.
Current families recorded in the presence of BaCl2. A: current traces recorded from a Kir2.1−/− type II hair cell at P1 prior to application of BaCl2. The scale bar applies to A and C. B: mean steady-state I(V) relation taken from the 5 Kir2.1−/− cells prior to bath application of BaCl2. C: currents recorded from the same cell shown in A after bath application of 100 μM BaCl2, a pan-Kir2 blocker. The voltage protocol at the bottom applies to A and C. D: mean steady-state I(V) relation taken from the same 5 cells of B after application of 100 μM BaCl2.
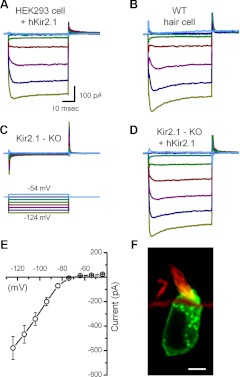
Because our results from Kir2.1−/− utricle hair cells showed that Kir2.1 channels are necessary to evoke IK1 in these cells, we wanted to test whether, conversely, Kir2.1 expression is sufficient to generate IK1. For this experiment, we used an adenoviral construct that carried the wild-type coding sequence of the hKir2.1 gene and transfected human embryonic kidney (HEK) cells, which do not express an endogenous IK1. We used an inducible expression system, which required transfection with two vectors, Ad-VgRXR and Ad-GFP-hKir2.1, the latter containing the wild-type Kir2.1 adenoviral construct, as described previously (Holt et al. 1999). Ad-VgRXR, which had a titer of 4.3 × 1011 viral particles/ml, contained the gene for the ecdysone receptor, controlled by a CMV promoter, and the gene for the RXR, controlled by a Rous sarcoma virus promoter. Ad-GFP::hKir2.1 contained the hKir2.1 gene fused to the GFP, under the control of the inducible ecdysone promoter. We transfected HEK cells with both constructs for 4 h, added 4 μM of the ecdysone analog muristerone A after 24 h to activate the ecdysone receptor, and incubated overnight. Muristerone A was reported to enhance gene expression driven by this promoter >30-fold (Johns et al. 1999). Therefore, we anticipated that cells infected with both adenoviral vectors and treated with muristerone A would express the GFP::hKir2.1 fusion protein. We observed membrane-restricted GFP expression as early as 24 h after transfection (Fig. 7F). HEK cells, which were GFP negative, did not have IK1 (data not shown), whereas data recorded from GFP-positive HEK cells revealed robust IK1 (Fig. 7A) with properties similar to IK1 in wild-type hair cells (Fig. 7B). The maximal conductance was 8.4 ± 2.6 nS, with a V1/2 of −86 ± 2.5 mV and slope factor of 5.9 ± 1.6 mV (n = 3). The time constant of activation, evoked by a step to −104 mV, was 1.6 ± 0.3 ms.
Fig. 7.
Adenoviral expression of wild-type human (h)Kir2.1 in human embryonic kidney (HEK) cells and Kir2.1−/− hair cells. A: representative traces recorded from a GFP-positive HEK cell transfected with hKir2.1. The scale bar applies to A–D. B: representative traces recorded from a wild-type type II hair cell excised at P0 and maintained in culture for 3 days. C: traces recorded from a GFP-negative Kir2.1−/− cell. The tissue was excised at P0, exposed to Ad-hKir2.1 for 24 h, and maintained in culture for 3 days. The voltage protocol applies to panels A–C. D: a representative family of currents recorded from a GFP-positive cell from the same tissue as described for C. E: mean I(V) relationship sampled near the end of the voltage steps derived from 8 GFP-positive Kir2.1−/− cells, transfected with hKir2.1. F: confocal image of a type II hair cell transfected with hKir2.1::GFP, counterstained with rhodamine-phalloidin. Original scale bar = 5 μm.
We then transfected wild-type hKir2.1 into organotypic cultures generated from mKir2.1−/− utricles extracted at P0. The tissue was exposed to the vector for 24 h and maintained in culture for 2 days. In contrast to GFP-negative Kir2.1−/− cells (Fig. 7C), transfected, GFP-positive cells (Fig. 7F) from Kir2.1−/− mice had robust IK1 (Fig. 7D). Steady-state I(V) curves from Kir2.1−/− cells transfected with the hKir2.1 displayed pronounced inward rectification, characteristic of IK1 (Fig. 7E). The maximal conductance was 21.7 ± 11.8 nS, with a V1/2 of −84 ± 3.2 mV and slope factor of 6.4 ± 2.0 mV. The time constant of activation, evoked by a step to −104 mV, was 1 ± 0.3 ms. Other than the maximal conductance—the result of overexpression of Kir2.1—there were no significant differences in the biophysical properties of exogenous Kir2.1 currents expressed in HEK cells and Kir2.1−/− hair cells and those of endogenous IK1 in type II hair cells. These data demonstrate that Kir2.1 alone is sufficient to produce robust inward rectifier currents with all of the properties of the native hair cell IK1.
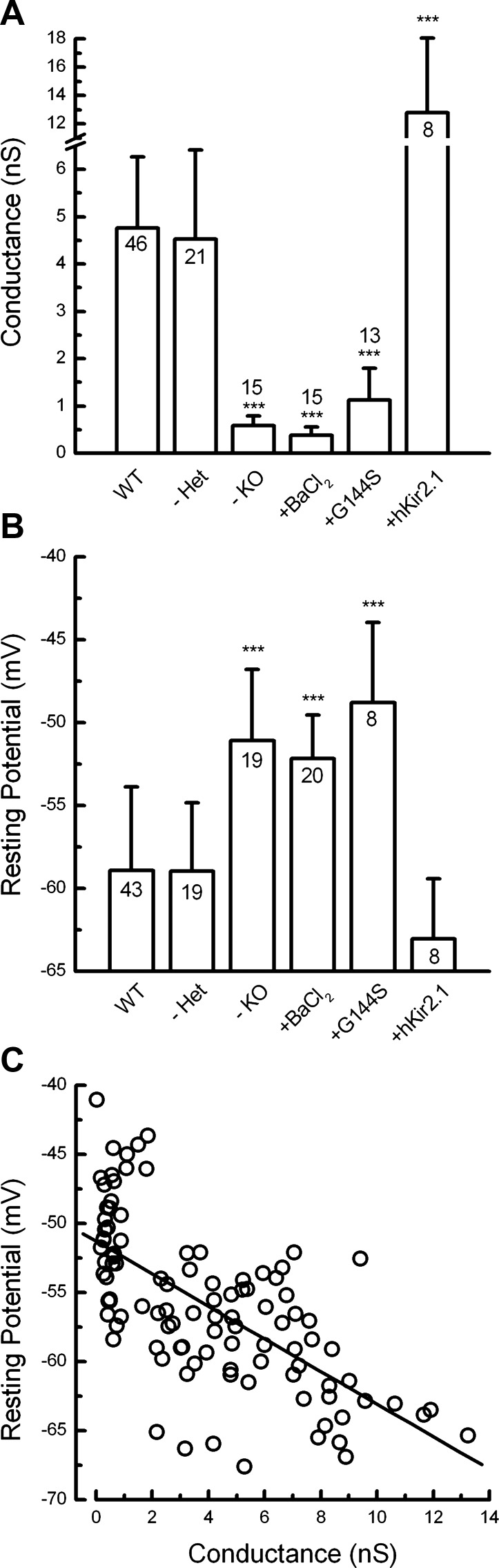
The mean whole-cell inward rectifier conductances, measured under the experimental conditions described in the previous sections, are summarized in Fig. 8A. Taken together, the data show that Kir2.1 is both necessary and sufficient to produce IK1 in type II hair cells of the mouse utricle.
Fig. 8.
Summary of conductance and resting potential data. A: mean maximal inward rectifier conductance measured from utricle type II hair cells under the experimental conditions labeled at the bottom of the x-axis: Kir2.1 heterozygotes (−Het), Kir2.1 homozygotes (−KO), wild-type + 100 μM BaCl2 (+BaCl2), wild-type transfected with Ad-mKir2.1-G144S (+G144S), and Kir2.1 homozygotes transfected with wild-type hKir2.1 (+hKir2.1). Error bars indicate SD. ***P < 0.001 relative to wild-type cells. The number of samples for each bar is indicated on the graph. B: mean resting potentials for the same experimental conditions indicated in A. Error bars indicate SD. ***P < 0.001 relative to wild-type cells. The number of samples for each bar is indicated on the graph. C: scatter plot of resting potential as a function of inward rectifier conductance derived from the data shown in A and B. All data under all conditions, for which we had both measurements from the same cell, are plotted (n = 103 cells). The data were fit with a linear regression (diagonal line), which had a slope of −1.2 mV/nS, a y-intercept of −51 mV, and a correlation coefficient of 0.66.
Functional contribution of Kir2.1.
Since the preceding data demonstrated that Kir2.1 is required for IK1, we were able to use the various IK1 blockers—BaCl2, Kir2.1 gene deletion, Kir2.1-G144S—and IK1 rescue to examine the functional contributions of Kir2.1 to vestibular hair cell signaling. Because Kir2.1 is potassium selective and active at the hair cell resting potential, we hypothesized that Kir2.1 may play a role in setting the membrane resting potential and regulating membrane excitability in vestibular hair cells. We predicted that the positive slope of the steady-state I(V) relationship should act through negative feedback to shift the resting membrane potential toward the K+ equilibrium potential. For small membrane-potential deviations around the K+ equilibrium potential, Kir2.1 may serve to stabilize the membrane response, driving the cell toward the K+ equilibrium potential. However, for larger depolarizations, we predicted rapid Kir2.1 deactivation, which would allow the cell to respond more freely without the stabilizing influences of Kir2.1. In other words, depolarizing inputs, sufficient to overcome the negative-feedback mechanism of Kir2.1, would tend to drive membrane potentials more positively, which would enhance Ca2+ influx (Bao et al. 2003; Rodriguez-Contreras and Yamoah 2001) and neurotransmitter release.
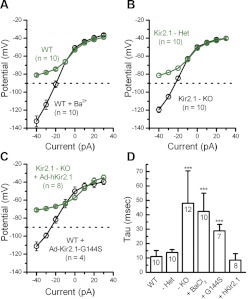
To test these hypotheses, we designed current-clamp protocols with small current injections, which ranged from −40 pA to 30 pA in 10-pA increments, and recorded the voltage responses of type II vestibular hair cells. We chose this range of current injections, because it permitted hyperpolarized voltage responses in wild-type cells, which remained positive to −90 mV, the most negative K+ equilibrium potential, given physiologically relevant K+ concentrations. In addition, 10–20% of the hair cells' transduction conductance is active at rest (Hudspeth 1989). Thus the hyperpolarizing current steps were designed to mimic the physiological current amplitudes (≤50 pA) evoked by negative hair bundle deflections and transduction channel closure, as well as small hyperpolarizing currents, which might result from efferent feedback onto type I and type II hair cells (Holt et al. 2006). With zero current injection, we found that Kir2.1 contributed to more hyperpolarized resting potentials with a mean resting potential of −59 ± 5.1 mV (n = 43; Fig. 8B). Data recorded from Kir2.1−/− cells showed significantly more depolarized resting potentials, by ∼10 mV on average, relative to wild-type cells. Regardless of experimental manipulation, inhibition of Kir2.1 activity led to depolarized resting potentials, whereas preservation or restoration of Kir2.1 activity conferred more negative potentials (Fig. 8B). To explore the correlation between the amplitude of the inward rectifier conductance and the resting membrane potential, we generated a scatter plot (Fig. 8C) of resting potential data as a function of inward rectifier conductance for each cell shown in Fig. 8, A and B. The data were fit with a linear regression that had a slope of −1.2 mV/nS (r = 0.66), which suggests that for every 0.83 nS of inward rectifier conductance, the hair cell resting potential hyperpolarizes by 1 mV.
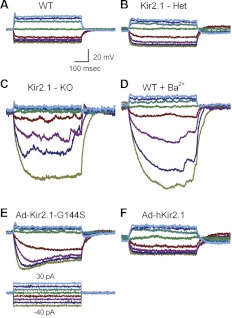
Figure 9 shows representative membrane responses under various conditions. In wild-type hair cells, hair cells from Kir2.1+/− mice, and in cells from Kir2.1−/− mice exposed to Ad-hKir2.1, we recorded fast, small amplitude membrane responses that followed the onset of the current step (Fig. 9, A, B, and F). In other words, cells that expressed either endogenous or exogenous Kir2.1 had similar membrane responses. In contrast, Kir2.1−/− hair cells, wild-type hair cells exposed to BaCl2, and cells exposed to Ad-Kir2.1-G144S had slow, large amplitude voltage responses (Fig. 9, C–E). To quantify the data, we plotted V(I) curves taken from the peak membrane responses as a function of the current injected from cells of various conditions. We saw a significant difference in the membrane-potential responses, particularly over the voltage range in which Kir2.1 was active (Fig. 10, A–C). Furthermore, wild-type cells exposed to BaCl2, cells that lacked Kir2.1, and cells transfected with mutant Kir2.1 displayed more hyperpolarized membrane-potential responses to current injections than cells that expressed Kir2.1 currents (Fig. 10, A–C).
Fig. 9.
Membrane responses recorded in current-clamp mode under various experimental conditions. Membrane potentials were evoked in type II hair cells by current steps that ranged from −40 to 30 pA in 10 pA. The voltage protocol shown at the bottom of E and the scale bars shown in A apply to all panels. A: representative responses recorded from wild-type cells at P2. B: data recorded from a Kir2.1+/− heterozygous cell at P1. C: membrane potentials recorded from a Kir2.1−/− homozygous cell at P0 + 7 days in culture. D: wild-type cell (P7) exposed to 100 μM BaCl2. E: data from a GFP-positive, wild-type cell transfected with Ad-Kir2.1-G144S for 24 h at P0 and maintained in culture for 12 days. F: data from a GFP-positive Kir2.1−/− hair cell excised at P0, transfected with wild-type Ad-hKir2.1 for 24 h, and maintained in culture for 3 days.
Fig. 10.
. Mean voltage-current relationships and membrane time constants measured under various experimental conditions. A: mean V(I) curves extracted from current-clamp responses to current steps between −40 and 30 pA. Responses from 10 cells were measured before (green) and after (black) application of 100 μM BaCl2. Error bars show SE. The dotted line represents −90 mV, the negative limit of the physiological range of membrane potentials. B: mean V(I) relations for 10 Kir2.1+/− cells (green) and 10 Kir2.1−/− cells (black). C: mean V(I) relations for 4 GFP-positive wild-type cells exposed to Ad-Kir2.1-G144S (black) and 8 GFP-positive Kir2.1−/− cells exposed to Ad-hKir2.1 (green). D: mean membrane time constants taken from exponential fits to voltage responses evoked by −40 pA current steps. The various experimental conditions are labeled at the bottom of the x-axis: wild-type (+/+; WT), Kir2.1 heterozygotes (+/−; −Het), Kir2.1 homozygotes (−/−; −KO), wild-type + 100 μM BaCl2 (+BaCl2), wild-type transfected with Ad-Kir2.1-G144S (+G144S), and Kir2.1 homozygotes transfected with wild-type hKir2.1 (+hKir2.1). The number of cells for each group is indicated on the graph. Error bars represent SD. ***P < 0.001 relative to wild-type cells.
We quantified the speed of the membrane response to −40 pA current injections (Fig. 10D) by fitting the change in membrane voltage with a single exponential equation. The time constant of the exponential fits was taken as the membrane time constant. Cells that lacked Kir2.1 had significantly slower responses (48 ± 22 ms, n = 12) than cells that expressed Kir2.1 currents (11 ± 4 ms, n = 10; Fig. 10D). The data confirmed that Kir2.1 currents help to speed the membrane-potential response to small, physiologically relevant current injections.
DISCUSSION
Kir2.1 is the molecular correlate of IK1.
Whereas previous studies have described IK1 in vestibular hair cells of goldfish (Sugihara and Furakawa 1996), frogs (Holt and Eatock 1995), turtles (Brichta et al. 2002; Goodman and Art 1996), pigeons (Masetto and Correia 1997), and mice (Géléoc et al. 2004; Rüsch et al. 1998), the molecular correlate has not been identified. Navaratnam et al. (1995) identified Kir2.1 mRNA, expressed in the apical one-half of the chick cochlear sensory epithelium, and Correia et al. (2004) identified Kir2.1 mRNA and cloned a Kir2.1 channel from pigeon vestibular hair cells. Based on their results, they hypothesized that Kir2.1 may contribute to IK1 in chick and pigeon hair cells, respectively. Whereas these studies did not test for expression of the other three members of the Kir2 channel subfamilies, Correia et al. (2004) suggested, based on phylogenetic analysis, that pigeon Kir2.1 does not combine with other Kir2 family subunits. However, previous data showed that Kir2.1 can combine into heteromers with other Kir2 channel subunits in other systems (Panama et al. 2010; Preisig-Müller et al. 2002; Schram et al. 2002).
In the present study, we showed that of the four Kir2 channel subfamilies described in the mouse genome, Kir2.1 mRNA was most highly expressed in mouse vestibular epithelia. In addition, we found a strong correlation between the temporal expression pattern of Kir2.1 mRNA and the physiological maturation of IK1 in the mouse vestibular system during embryonic stages between E15 and E18 (Géléoc et al. 2004) and during the first postnatal week (Rüsch et al. 1998). None of the other Kir2 family subunits displayed substantial expression at the developmental onset of IK1 or at any time point that we examined. Furthermore, Kirkegaard and Nyengaard (2005) found that hair cell numbers in mouse vestibular epithelia increase until P16. A larger number of hair cells expressing Kir2.1 are consistent with our data, which show an increase in Kir2.1 mRNA expression between P7 and P25.
Another result in support of Kir2.1 playing a major role in vestibular hair cells stemmed from our immunohistochemical data, in which we localized Kir2.1 protein to the basolateral membranes of utricle hair cells in wild-type mice but not in Kir2.1−/− mice. Kir2.1 was present in the basolateral membranes at both neonatal and adult stages. We also found that hair cells transfected with exogenous Kir2.1, which carried a dominant-negative mutation in the selectivity filter (G144S), lacked IK1, confirming the involvement of Kir2 family members. The strongest evidence in support of our hypothesis—that IK1 is composed of Kir2.1 channels—is based on our electrophysiological data from Kir2.1−/− mice. We showed that IK1 was present in wild-type and heterozygous hair cells that expressed Kir2.1, whereas it was abolished completely in hair cells that lacked Kir2.1 (Fig. 8). These data suggest that Kir2.1 channels are necessary for IK1 in mouse utricle hair cells.
To investigate the sufficiency of Kir2.1, we used an adenoviral vector, which expressed the wild-type gene to transfect HEK cells and hair cells of the cultured utricles excised from Kir2.1−/− mice. In both transfected cell types, we restored IK1 with all of the properties of the endogenous currents recorded from wild-type hair cells. Based on these data, we conclude that Kir2.1 is both necessary and sufficient to form the ion channels that carry IK1 in mouse vestibular hair cells.
Furthermore, we provide evidence that IK1 can be detected in mouse vestibular hair cells, not only in early development, as previous studies showed (Géléoc et al. 2004; Rüsch et al. 1998), but also into adulthood, past 1 yr of age (Fig. 2B). In contrast to cochlear hair cells, where IK1 is transiently expressed (Marcotti et al. 1999), IK1 expression in adult mouse utricle hair cells suggests that it plays a significant role in the mature vestibular system.
Function of Kir2.1 in vestibular hair cells.
Based on our electrophysiological data and the biophysical characteristics of IK1, we hypothesized that Kir2.1 channels provide several functional contributions to vestibular hair cell signaling. Previous studies in mice showed that IK1 is active at rest and has a reversal potential close to EK (−82 mV) (Géléoc et al. 2004; Rüsch et al. 1998). We corroborated these data with our results from wild-type utricle hair cells, which had a mean reversal potential of −83.1 mV ± 2.8 mV (n = 36). In addition, Géléoc et al. (2004) found a temporal correlation in utricle hair cells between the acquisition of IK1 at E15 and the cells' resting potential, which became increasingly more negative between E15 and E19. Based on these results, we predicted that Kir2.1 channels would contribute to sensory hair cell signaling by 1) keeping the resting potential more hyperpolarized, thus reducing cell excitability, 2) decreasing the amplitude of receptor potential responses, and 3) increasing the speed of receptor potentials due to lower input resistances and faster membrane time constants. We found that cells, in which Kir2.1 channels were blocked with BaCl2, and cells, which lacked functional Kir2.1 channel resting potentials, were significantly more depolarized, by 8–11 mV, respectively (Fig. 8B). Furthermore, when we measured membrane receptor potentials in response to current injections, which ranged from −40 pA to 30 pA, cells that expressed Kir2.1 displayed fast, small amplitude responses within a physiologically relevant range, positive to −90 mV (Figs. 9, A, D, and F, and 10). However, in cells that lacked functional Kir2.1 channels, responses to hyperpolarizing steps of −20 to −40 pA were slower (Fig. 9, B, C, and E), and amplitudes were larger, mostly exceeding physiological values. The slow responses were due to the increased membrane time constants, in turn, the result of higher membrane resistance in the cells that lacked Kir2.1 channels open at rest. The large amplitude responses in cells that lacked Kir2.1 channels were also the result of higher membrane resistance at rest. Thus for a given input current, the larger membrane resistance predicted larger, slower voltage responses, which was the case in all cells that lacked functional Kir2.1 channels. If we consider the contribution of Kir2.1 to signal processing in the frequency domain, we predict that Kir2.1 may extend the response range to higher frequencies. In wild-type type II hair cells, we measured mean membrane time constants of ∼10 ms, which is predicted to yield a low-pass corner frequency of ∼16 Hz (1/2πτ). Cells that lacked Kir2.1 had mean time constants of ∼50 ms or corner frequencies of ∼3 Hz. As these are both within the physiologic range of rodent head movements (up to 16 Hz) (Hullar et al. 2005), we suggest that Kir2.1 expression may also contribute to tuning of the type II hair cell response to physiologically relevant stimulus frequencies.
The large cell-to-cell variability in the amplitude of Kir2.1 expression may allow type II hair cells a mechanism to regulate their resting, K+-selective membrane resistance and therefore, their resting potential, the speed and amplitude of their response to small current input, and perhaps their frequency response in vivo. In heterologous cells, single-channel conductance of mKir2.1 ranged between 21 and 30 pS (Kubo et al. 1993; Panama et al. 2010; Takahashi et al. 1994), whereas Kir2.1 isolated from avian inner ear hair cells displayed single channel conductances between 13 and 29 pS (Correia et al. 2004; Navaratnam et al. 1995; Zampini et al. 2008). Our results showed an average whole-cell Kir2.1 conductance of 4.8 nS. Considering the single-channel conductance data from previous mKir2.1 studies, this suggests that vestibular type II hair cells express 160–229 functional channels/cell, on average. If we consider our most extreme measures of Kir2.1 whole-cell conductance, we estimate a range of Kir2.1 channel expression between ∼80 and 400 channels/cell. The exact number of Kir2.1 channels expressed in a given hair cell may be the result of position within the epithelium, number, and type of synaptic contacts, as well as other signaling requirements of the cell. For example, if hair cells use Kir2.1 channel expression as a mechanism to regulate their resting potential, we estimate that for every ∼33 Kir2.1 channels or 132 protein monomers expressed, the cell can hyperpolarize its resting membrane potential by 1 mV.
Other factors may regulate the activity and properties of Kir2.1 in hair cells, including intracellular polyamines and Mg2+. Kir2 channels have distinct binding sites along the lining of the channel pore, which allow Mg2+ and polyamines, such as spermine and spermidine, to interact and block K+ ion flux (Lopatin et al. 1994; Matsuda et al. 1987). Panama and Lopatin (2006) showed in heterologous cells that the magnitude of the block depends on polyamine concentration. Kir2 family members can also be inactivated by several kinases, such as src kinases and PKC, whereas others, such as PKA, increase Kir2 current (Zitron et al. 2004, 2008). Several Kir2 channel subunits can be activated by alkalization due to the pH sensitivity of histine residues in the linker region of the outer transmembrane helix and in the pore-forming loop (Coulter et al. 1995; Hughes et al. 2000; Qu et al. 2000; Zhu et al. 1999). Lastly, previous studies showed that Kir2.1 interacts strongly with phosphatidylinositol 4,5-bisphosphate (Du et al. 2004). These various signaling pathways may serve to further modulate the activity of Kir2.1 in hair cells, allowing the cells additional mechanisms to regulate their resting potentials and responses to small current inputs.
In summary, we have demonstrated that Kir2.1 is both necessary and sufficient to form the channels that carry IK1 in mouse vestibular type II hair cells. Our data show that Kir2.1 channels contribute to vestibular hair cell signaling by conferring more hyperpolarized resting potentials; by shaping hair cell receptor potentials in response to small, depolarizing and hyperpolarizing stimuli, such as those that occur due to mechanotransduction or efferent input; and by decreasing membrane resistance, allowing for faster responses. We also show that Kir2.1 is expressed, not just in early development but also throughout adulthood, during which its functional contributions persist. Further studies in mice with conditional deletion of Kir2.1 restricted to the vestibular system may shed light on the significance of IK1 activity for normal vestibular function in mice and humans. Lastly, since we have demonstrated that the dominant-negative G144S mutation in Kir2.1, which causes ATS in humans, also causes vestibular hair cell dysfunction, we suggest that patients with ATS may also suffer from vestibular abnormalities.
GRANTS
Support for this work was provided by National Institute of Deafness and Other Communication Disorders Grant DC05439 (J. R. Holt) and the Neurobiology and Development Training Program 5T32HD007323-24.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.R.H. conception and design of research; M.E.L. performed experiments; M.E.L. analyzed data; J.R.H. interpreted results of experiments; M.E.L. and J.R.H. prepared figures; M.E.L. drafted manuscript; J.R.H. edited and revised manuscript; M.E.L. and J.R.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the members of the Holt/Géléoc lab for helpful discussions, Anna Lysakowski for critical review of the manuscript, David P. Corey and Zheng-Yi Chen for sharing data from the SHIELD database, Gwenaelle Géléoc for training and helpful discussions, and the Journal of Neurophysiology reviewers for their careful and constructive review of the manuscript. We gratefully acknowledge the gift of the mKir2.1 clone from Dr. Lily Y. Jan.
REFERENCES
- Amalfitano A, Hauser MA, Hu H, Serra D, Begy CR, Chamberlain JS. Production and characterization of improved adenovirus vectors with the E1, E2b, and E3 genes deleted. J Virol 72: 926–933, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao H, Wong WH, Goldberg JM, Eatock RA. Voltage-gated calcium channel currents in type I and type II hair cells isolated from the rat crista. J Neurophysiol 90: 155–164, 2003 [DOI] [PubMed] [Google Scholar]
- Bichet D, Haass FA, Jan LY. Merging functional studies with structures of inward-rectifier K channels. Nat Rev Neurosci 4: 957–967, 2003 [DOI] [PubMed] [Google Scholar]
- Brichta AM, Aubert A, Eatock RA, Goldberg JM. Regional analysis of whole cell currents from hair cells of the turtle posterior crista. J Neurophysiol 88: 3259–3278, 2002 [DOI] [PubMed] [Google Scholar]
- Correia MJ, Wood TG, Prusak D, Weng T, Rennie KJ, Wang HQ. Molecular characterization of an inward rectifier channel (IKir) found in avian vestibular hair cells: cloning, and expression of pKir2.1. Physiol Genomics 19: 155–169, 2004 [DOI] [PubMed] [Google Scholar]
- Coulter KL, Perier F, Radeke CM, Vandenberg CA. Identification and molecular localization of a pH-sensing domain for the inward rectifier potassium channel HIR. Neuron 15: 1157–1168, 1995 [DOI] [PubMed] [Google Scholar]
- Desai SS, Zeh C, Lysakowski A. Comparative morphology of rodent vestibular periphery. I. Saccular and utricular maculae. J Neurophysiol 93: 251–266, 2005 [DOI] [PubMed] [Google Scholar]
- Doupnik CA, Davidson N, Lester HA. The inward rectifier potassium channel family. Curr Opin Neurobiol 5: 268–277, 1995 [DOI] [PubMed] [Google Scholar]
- Du X, Zhang H, Lopes C, Mirshahi T, Rohacs T, Logothetis DE. Characteristic interactions with phosphatidylinositol 4,5-bisphosphate determine regulation of Kir channels by diverse modulators. J Biol Chem 279: 37271–37281, 2004 [DOI] [PubMed] [Google Scholar]
- Géléoc GSG, Risner JR, Holt JR. Developmental acquisition of voltage-dependent conductances and sensory signaling in hair cells of the embryonic mouse inner ear. J Neurosci 24: 11148–11159, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman MB, Art JJ. Positive feedback by a potassium-selective inward rectifier enhances tuning in vertebrate hair cells. Biophys J 71: 430–442, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassinen M, Paajanen V, Vornanen M. A novel inwardly rectifying K+ channel, Kir2.5, is upregulated under chronic cold stress in fish cardiac myocytes. J Exp Biol 211: 2162–2171, 2008 [DOI] [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 95: 2509–2514, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges BL, Serra D, Hu H, Begy CA, Chamberlain JS, Amalfitano A. Multiply deleted [E1, polymerase-, and pTP-] adenovirus vector persists despite deletion of the preterminal protein. J Gene Med 2: 250–259, 2000 [DOI] [PubMed] [Google Scholar]
- Holt JC, Lysakowski A, Goldberg JM. Mechanisms of efferent-mediated responses in the turtle posterior crista. J Neurosci 26: 13180–13193, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JR, Eatock RA. Inwardly rectifying currents of saccular hair cells from the leopard frog. J Neurophysiol 73: 1484–1502, 1995 [DOI] [PubMed] [Google Scholar]
- Holt JR, Johns DC, Wang S, Chen ZY, Dunn RJ, Marban E, Corey DP. Functional expression of exogenous proteins in mammalian sensory hair cells infected with adenoviral vectors. J Neurophysiol 81: 1881–1888, 1999 [DOI] [PubMed] [Google Scholar]
- Holt JR, Stauffer EA, Abraham D, Géléoc GSG. Dominant-negative inhibition of M-like potassium conductances in hair cells of the mouse inner ear. J Neurosci 27: 8940–8951, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz GC, Risner-Janiczek JR, Jones SM, Holt JR. HCN channels expressed in the inner ear are necessary for normal balance function. J Neurosci 31: 16814–16825, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth AJ. How the ear's works work. Nature 341, 397–404, 1989 [DOI] [PubMed] [Google Scholar]
- Hughes BA, Kumar G, Yuan Y, Swaminathan A, Yan D, Sharma A, Plumley L, Yang-Feng TL, Swaroop A. Cloning and functional expression of human retinal Kir2.4, a pH-sensitive inwardly rectifying K+ channel. Am J Physiol Cell Physiol 279: C771–C784, 2000 [DOI] [PubMed] [Google Scholar]
- Hullar TE, Della Santina CC, Hirvonen T, Lasker DM, Carey JP, Minor LB. Responses of irregularly discharging chinchilla semicircular canal vestibular-nerve afferents during high-frequency head rotations. J Neurophysiol 93: 2777–2786, 2005 [DOI] [PubMed] [Google Scholar]
- Isomoto S, Kondo C, Kurachi Y. Inwardly rectifying potassium channels: their molecular heterogeneity and function. Jpn J Physiol 47: 11–39, 1997 [DOI] [PubMed] [Google Scholar]
- Johns DC, Marx R, Mains RE, O'Rourke B, Marbán E. Inducible genetic suppression of neuronal excitability. J Neurosci 9: 1691–1697, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard N, Nyengaard JR. Stereological study of postnatal development in the mouse utricular macula. J Comp Neurol 492: 132–144, 2005 [DOI] [PubMed] [Google Scholar]
- Kubo Y, Baldwin TJ, Nung Jan Y, Jan LY. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature 362: 127–133, 1993 [DOI] [PubMed] [Google Scholar]
- Kurachi Y. Voltage-dependent activation of the inward-rectifier potassium channel in the ventricular cell membrane of guinea-pig heart. J Physiol 366: 365–385, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Xue J, Peterson EH. Architecture of the mouse utricle: macular organization and hair bundle heights. J Neurophysiol 99: 718–733, 2008 [DOI] [PubMed] [Google Scholar]
- Lopatin AN, Makhina EN, Nichols CG. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature 372: 366–369, 1994 [DOI] [PubMed] [Google Scholar]
- Lu C, Lin J, Rajawat YS, Jerng H, Rami TG, Sanchez X, DeFreitas G, Carabello B, DeMayo F, Kearney DL, Miller G, Li H, Pfaffinger PJ, Bowles NE, Khoury DS, Towbin JA. Functional and clinical characterization of a mutation in KCNJ2 associated with Andersen-Tawil syndrome. J Med Genet 43: 653–659, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z. Mechanism of rectification in inward-rectifier K+ channels. Annu Rev Physiol 66: 103–129, 2004 [DOI] [PubMed] [Google Scholar]
- Marcotti W, Géléoc GS, Lennan GW, Kros CJ. Transient expression of an inwardly rectifying potassium conductance in developing inner and outer hair cells along the mouse cochlea. Pflugers Arch 439: 113–122, 1999 [DOI] [PubMed] [Google Scholar]
- Masetto S, Correia MJ. Electrophysiological properties of vestibular sensory and supporting cells in the labyrinth slice before and during regeneration. J Neurophysiol 78: 1913–1927, 1997 [DOI] [PubMed] [Google Scholar]
- Matsuda H, Saigusa A, Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+. Nature 325: 156–159, 1987 [DOI] [PubMed] [Google Scholar]
- Navaratnam DS, Escobar L, Covarrubias M, Oberholtzer JC. Permeation properties and differential expression across the auditory receptor epithelium of an inward rectifier K+ channel cloned from the chick inner ear. J Biol Chem 270: 19238–19245, 1995 [DOI] [PubMed] [Google Scholar]
- Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annu Rev Physiol 59: 171–191, 1997 [DOI] [PubMed] [Google Scholar]
- Panama BK, Lopatin AN. Differential polyamine sensitivity in inwardly rectifying Kir2 potassium channels. J Physiol 571: 287–302, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panama BK, McLerie M, Lopatin AN. Functional consequences of Kir2.1/Kir2.2 subunit heteromerization. Pflugers Arch 460: 839–849, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaster NM, Tawil R, Tristani-Firouzi M, Canún S, Bendahhou S, Tsunoda A, Donaldson MR, Iannaccone ST, Brunt E, Barohn R, Clark J, Deymeer F, George AL, Jr, Fish FA, Hahn A, Nitu A, Ozdemir C, Serdaroglu P, Subramony SH, Wolfe G, Fu YH, Ptácek LJ. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen's syndrome. Cell 105: 511–519, 2001 [DOI] [PubMed] [Google Scholar]
- Preisig-Müller R, Schlichthörl G, Goerge T, Heinen S, Brüggemann A, Rajan S, Derst C, Veh RW, Daut J. Heteromerization of Kir2.x potassium channels contributes to the phenotype of Andersen's syndrome. Proc Natl Acad Sci USA 99: 7774–7779, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Z, Yang Z, Cui N, Zhu G, Liu C, Xu H, Chanchevalap S, Shen W, Wu J, Li Y, Jiang C. Gating of inward rectifier K+ channels by proton-mediated interactions of N- and C-terminal domains. J Biol Chem 275: 31573–31580, 2000 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Contreras A, Yamoah EN. Direct measurement of single-channel Ca(2+) currents in bullfrog hair cells reveals two distinct channel subtypes. J Physiol 534: 669–689, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q, Chen D, Wang Z, Chi F, Yin S, Wang J. Topological and developmental expression gradients of Kir2.1, an inward rectifier K+ channel, in spiral ganglion and cochlear hair cells of mouse inner ear. Dev Neurosci 30: 374–388, 2008 [DOI] [PubMed] [Google Scholar]
- Rüsch A, Lysakowski A, Eatock RA. Postnatal development of type I and type II hair cells in the mouse utricle: acquisition of voltage-gated conductances and differentiated morphology. J Neurosci 18: 7487–7501, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan DP, da Silva MR, Soong TW, Fontaine B, Donaldson MR, Kung AW, Jongjaroenprasert W, Liang MC, Khoo DH, Cheah JS, Ho SC, Bernstein HS, Maciel RM, Brown RH, Jr, Ptácek LJ. Mutations in potassium channel Kir2.6 cause susceptibility to thyrotoxic hypokalemic periodic paralysis. Cell 140: 88–98, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schram G, Melnyk P, Pourrier M, Wang Z, Nattel S. Kir2.4, Kir2.1 K+ channel subunits co-assemble: a potential new contributor to inward rectifier current heterogeneity. J Physiol 544: 337–349, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara I, Furukawa T. Inwardly rectifying currents in hair cells and supporting cells in the goldfish sacculus. J Physiol 495: 665–679, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Morishige K, Jahangir A, Yamada M, Findlay I, Koyama H, Kurachi Y. Molecular cloning and functional expression of cDNA encoding a second class of inward rectifier potassium channels in the mouse brain. J Biol Chem 269: 23274–23279, 1994 [PubMed] [Google Scholar]
- Tristani-Firouzi M, Etheridge SP. Kir 2.1 channelopathies: the Andersen-Tawil syndrome. Pflugers Arch 460: 289–294, 2010 [DOI] [PubMed] [Google Scholar]
- Zampini V, Masetto S, Correia M. Elementary properties of Kir2.1, a strong inwardly rectifying K(+) channel expressed by pigeon vestibula type II hair cells. Neuroscience 155: 1250–1261, 2008 [DOI] [PubMed] [Google Scholar]
- Zaritsky JJ, Eckman DM, Wellman GC, Nelson MT, Schwarz TL. Targeted disruption of Kir2.1 and Kir22 genes reveals the essential role of the inwardly rectifying K+ current in K(+)-mediated vasodilation. Circ Res 87: 160–166, 2000 [DOI] [PubMed] [Google Scholar]
- Zaritsky JJ, Redell JB, Tempel BL, Schwarz TL. The consequences of disrupting cardiac inwardly rectifying K(+) current (I.(K.1)) as revealed by the targeted deletion of the murine Kir21 and Kir22 genes. J Physiol 533: 697–710, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Chanchevalap S, Cui N, Jiang C. Effects of intra- and extracellular acidifications on single channel Kir2.3 currents. J Physiol 516: 699–710, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitron E, Gunth M, Scherer D, Kiesecker C, Kulzer M, Bloehs R, Scholz EP, Thomas D, Weidenhammer C, Kathofer S, Bauer A, Katus HA, Karle CA. Kir2.x inward rectifier potassium channels are differentially regulated by adrenergic alpha1A receptors. J Mol Cell Cardiol 44: 84–94, 2008 [DOI] [PubMed] [Google Scholar]
- Zitron E, Kiesecker C, Luck S, Kathofer S, Thomas D, Kreye VA, Kiehn J, Katus HA, Schoels W, Karle CA. Human cardiac inwardly rectifying current IKir2.2 is upregulated by activation of protein kinase A. Cardiovasc Res 63: 520–527, 2004 [DOI] [PubMed] [Google Scholar]