Abstract
Local field potentials (LFPs) in primary motor cortex include significant information about reach target location and upper limb movement kinematics. Some evidence suggests that they may be a more robust, longer-lasting signal than action potentials (spikes). Here we assess whether LFPs can also be used to decode upper limb muscle activity, a complex movement-related signal. We record electromyograms from both proximal and distal upper limb muscles from monkeys performing a variety of reach-to-grasp and isometric wrist force tasks. We show that LFPs can be used to decode activity from both proximal and distal muscles with performance rivaling that of spikes. Thus, motor cortical LFPs include information about more aspects of movement than has been previously demonstrated. This provides further evidence suggesting that LFPs could provide a highly informative, long-lasting signal source for neural prostheses.
Keywords: electromyograms, brain-machine interfaces, action potentials, motor cortex, electrophysiology
action potentials from neurons in the primary motor cortex (M1) have been shown to correlate with both kinematic (Georgopoulos et al. 1982) and kinetic (Evarts 1968) movement parameters. In addition to providing insight into the neural control of movement, these correlations can be exploited to allow users to control brain-machine interfaces (BMIs). Kinematic BMIs based on spike decoders have allowed users to control a cursor (Carmena et al. 2003; Hochberg et al. 2006; Serruya et al. 2002; Taylor et al. 2002) or a robotic arm (Velliste et al. 2008). Alternatively, BMIs using electromyograms (EMGs) decoded from spikes offer the exciting possibility to restore natural movement by activating paralyzed muscles via functional electrical stimulation (FES) (Oby et al. 2010; Pohlmeyer et al. 2009).
Spikes can be difficult to record for the decades-long durations necessary for clinically viable BMI applications. Chronically implanted multielectrode arrays typically lose the ability to record spikes on most of their electrodes after several years (Krüger et al. 2010; Simeral et al. 2011). Thus, the feasibility of using spikes to control a BMI for decade-long periods is uncertain. LFPs from M1 also convey information about movement (Heldman et al. 2006; Murthy and Fetz 1996; Sanes and Donoghue 1993), and potentially could offer greater recording longevity than spikes, since they represent the combined activity of thousands of neurons (Andersen et al. 2004) and thus loss of activity from a few neurons will likely not cause an appreciable change. Therefore, LFPs offer an intriguing signal for complementing or perhaps replacing spikes as inputs to BMIs.
LFPs from M1 have been used to decode reach and grasp kinematics (Bansal et al. 2011, 2012; Mehring et al. 2003; Slutzky et al. 2010; Zhuang et al. 2010) and grasp type (Stark and Abeles 2007), often with an accuracy comparable to that using spikes (Mehring et al. 2003; Slutzky et al. 2010). However, LFPs have not been used to predict muscle activity. In this study, we used both LFPs and spikes recorded from M1 to decode the continuous time course of proximal and distal limb EMGs during both reach-to-grasp and isometric force-production tasks. A preliminary report of some of these data was published in 2011 (Slutzky et al. 2011b).
METHODS
All experiments and procedures were approved by the Northwestern University Institutional Animal Care and Use Committee. Four adult rhesus macaques (C, M, J, and T) were trained to perform the behavioral tasks for a liquid reward.
Behavioral Training
Reach-to-grasp task 1: multi-grasp.
Monkeys C and M performed the multi-grasp task, which began from a relaxed position with one hand resting on a touchpad, and the other arm restrained. Monkeys were cued to reach to one of three objects positioned ∼35 cm from the touchpad by illumination of an LED on the object and a simultaneous “go” tone. The monkeys executed one of three grasps (palmar, lateral, or precision) depending on the shape of the object. Force feedback, measured with a force-sensitive resistor embedded in each object, was given in the form of a circular cursor displayed on a monitor in front of the monkeys. Cursor movement was proportional to the amount of grasp force applied and was required to match one of two force targets. Grasp forces of 14 N and 20 N, 8 N and 12 N, and 0.6 and 1 N were required for the palmar, lateral, and pinch grasps, respectively. The grasp type and force target level of each trial was set in a pseudorandom order. Monkeys were required to maintain each grasp force within the target range for 0.5 s to receive a liquid reward. Monkeys had 5 s to achieve success in each trial.
Reach-to-grasp task 2: ball grasp.
Monkey J performed the ball grasp task with one arm, and the contralateral arm was restrained. The monkey was required to pick up a ball from a small tray and place it in a plastic tube with a 60-mm opening. The balls were of a variety of diameters and masses: 40 mm, 130 g; 40 mm, 95 g; and 24 mm, 60 g. The monkey began each trial by placing his hand on a touchpad for at least 0.2 s. A go tone, as well as illumination of an LED on the touchpad, indicated the beginning of a 5-s reach time period, during which the monkey attempted to pick up the ball from the tray. Removing the ball from the tray started another 5-s interval, during which the monkey was required to place the ball in the tube to receive a liquid reward.
Isometric wrist force task.
Monkey T performed the wrist force task with one arm, with the contralateral arm restrained. The monkey used isometric wrist force (in both the flexion-extension and radial-ulnar deviation axes) to move a computer cursor in a center-out task. The monkey was required to move the cursor from a central target (zero force) to one of eight peripheral targets, which were presented in random order. The monkey had 3 s to move the cursor into a target and hold it there for 0.5 s to obtain a liquid reward. The monkey's upper arm was restrained by a custom-fitted cast which maintained his elbow at a 90-degree angle. Force was measured by a 2-axis strain gauge mounted between casts on the monkey's hand and forearm. Cursor movement distance was proportional to the measured force along each axis. The targets corresponded to a force of 5 N, which was ∼35–50% of the monkey's maximum voluntary contraction.
Electrode Implantation
Following behavioral training, we surgically implanted a 96-channel silicon electrode array (Blackrock Microsystems) into the primary motor cortex of each monkey contralateral to the arm used in the behavior. All electrodes were 1.5 mm long. Monkeys were anesthetized with isoflurane and remifentanil and given the postoperative analgesics buprenorphine and meloxicam for 2 and 4 days, respectively. Monkeys M and C were implanted in arm motor areas, and monkeys J and T in hand motor areas of M1, as determined by stereotaxic coordinate, cortical topology, and intraoperative electrical stimulation using silver ball electrodes (2–5 mA, 200-μs pulses at 60 Hz). Further surgical details have been described elsewhere (Pohlmeyer et al. 2007b).
Recording Procedures
Recordings were performed using a Cerebus acquisition system (Blackrock), which analog band-pass filtered all signals from 0.3 Hz to 7.5 kHz before sampling at 30 kHz. Spikes were digitally high-pass filtered at 300 Hz and thresholded. Spike waveforms were sorted manually offline. Both single- and multi-unit spikes were included in the analysis. LFPs were digitally band-pass filtered from 0.3 to 250 Hz and resampled at 1 kHz (monkeys M, J, and T) or band-pass filtered from 0.3 to 500 Hz and resampled at 2 kHz (monkey C). Data sampled at 2 kHz were further downsampled to 1 kHz prior to analysis. Each 10-min recording file included in this analysis was obtained from a different day.
EMG signals were recorded using bipolar electrodes, either on the skin surface (monkeys M and C) or intramuscularly (monkeys J and T). We band-pass filtered the EMGs with cutoffs at 5 Hz and 500 Hz, and then sampled at 2 kHz. We then digitally processed the EMGs as follows: high-pass filtered at 50 Hz, full-wave rectified, then low-pass filtered at 5 Hz and downsampled to 20 Hz for decoding. We repeated our analysis with a low-pass filter cutoff of 10 Hz to evaluate whether even higher bandwidth signals could be decoded. All digital filtering was performed in both the forward and backward directions to avoid introducing phase delays in the output.
Decoding Methods and Performance Assessment
We selected six spectral features from each field potential signal: the local motor potential (LMP; a sliding window average of the raw LFP) (Mehring et al. 2004; Schalk et al. 2007), and the power in five different frequency bands (0–4, 7–20, 70–115, 130–200, and 200–300 Hz). We calculated both the LMP and the spectral power using 256-point windows that overlapped by 206 samples so as to provide one sample every 50 ms. We computed the power in each band by applying a Hanning window followed by a fast Fourier transform to each window. For each frequency band, we normalized the log of this power by subtracting the log of the mean power over the entire file. We chose the 150 features that were individually most strongly correlated with the EMG signal (as determined by the mean of the absolute values of the Pearson correlation coefficients, r, over all muscles for each file) to use for LFP decoding. This was done to reduce the dimensionality of the feature set from a potential total of up to 576 features (number of LFP channels times 6 features per channel). This feature reduction was performed only on the training, not the testing data. For decoding with spikes, we used spike counts in 50-ms bins for each neuron as features, and included all neurons in the analysis.
We used a Wiener cascade model, which has been described in detail elsewhere (Hunter and Korenberg 1986; Perreault et al. 1999; Pohlmeyer et al. 2007b), to decode the EMG signals. Briefly, we computed a set of causal linear filters of length 10 bins (500 ms) by fitting the input features to the outputs of a set of training data. We used a ridge regression technique (Nemati et al. 2007) to avoid ill-conditioned covariance matrices that otherwise result from the highly correlated inputs. The output of the Wiener filters was convolved with a static nonlinearity (Pohlmeyer et al. 2007b) implemented by fitting a second-order polynomial between the linear filter output and the EMG. This was done in a single iteration between linear and nonlinear components since, when we performed decoding using a second iteration, we found less than a 2% increase in prediction accuracy. For each file, we trained the Wiener cascade decoder on 9 min of data and tested it on the remaining minute. As a measure of performance, we calculated the mean fractional variance accounted for (VAF) between actual and decoded EMG over all 10 folds as follows (Fagg et al. 2009):
where M is the total number of samples in each fold, pj and p̂j are the actual and predicted samples for the fold, and p̄ is the mean EMG over the fold.
Spectral and Temporal Information Analysis
To determine the relative amount of EMG-related information in each frequency band, we decoded the EMGs using each frequency band separately. For completeness, we also included the 20–70 Hz frequency band, although it was not used in the overall ensemble decoding performance evaluation above. We used all electrodes in each file in this analysis (between 92 and 96 electrodes for the various monkeys). We performed a similar analysis to evaluate information content as a function of filter lag by decoding with a single lag ranging from 50 to 500 ms.
RESULTS
We analyzed data from a total of 10 files in four rhesus macaques (n = 2, 4, 2, and 2 files for monkeys C, M, J, and T, respectively). The mean number of trials per file was 171, 198, and 104 for the multi-grasp, ball-grasp, and isometric wrist force tasks, respectively. We recorded EMGs from 4 proximal arm muscles: biceps, triceps, and anterior and posterior deltoids in monkeys C and M during the multi-grasp task (Fig. 1A). We recorded from eight distal muscles in monkeys J and T during the ball-grasp task and isometric wrist force tasks, respectively (Fig. 1, A and B): extensor carpi radialis longus and brevis, extensor carpi ulnaris, extensor digitorum communis, flexor carpi radialis and ulnaris, and flexor digitorum superficialis and profundus. We placed two sets of electrodes in each of the digit flexor muscles (corresponding to fingers 2–3 and 4–5). We used at least 100 spike signals (one-half to three-quarters of which were single unit) and at least 92 LFP signals for decoding (see Table 1). For decoding using LFPs, we chose the top 150 features from a total of 552–576, as described in methods.
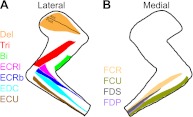
Fig. 1.
Schematic of recorded limb muscle locations. Both medial (A) and lateral (B) views of the arm are shown. Del, deltoids; Tri, triceps; Bi, biceps; ECRl and ECRb, extensor carpi radialis longus and brevis, respectively; EDC, extensor digitorum communis; ECU, extensor carpi ulnaris; FCR and FCU, flexor carpi radialis and ulnaris, respectively; FDS and FDP, flexor digitorum superficialis and profundus, respectively.
Table 1.
Number of units and LFPs used in decoding for each file of each monkey
| Monkey | Number of Units Used | % Single Units | Number of LFPs Used |
|---|---|---|---|
| M | 100, 110, 117, 120 | 53 | 95 |
| C | 110, 112 | 71 | 92 |
| J | 157, 163 | 70 | 94 |
| T | 128, 130 | 72 | 96 |
LFPs, local field potentials.
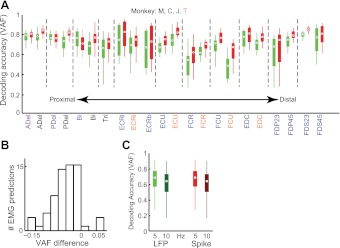
EMG predictions made using LFPs and spikes were very similar, and in most cases, highly accurate, for a wide variety of both proximal (Fig. 2A) and distal (Fig. 2B) muscles. We computed the mean decoding performance for each muscle using either LFPs or spikes (Fig. 3A). In the few cases with poor decoding performance, EMG was only poorly modulated by the behavior (e.g., opponens pollicis in the wrist force task). However, even in these cases, LFP and spike decoding performances were similar (these cases are not shown in Fig. 3A for clarity). Overall, the mean (±SD) VAF across all folds, muscles, and behavioral tasks (28 total muscle-file-task combinations) was 0.64 ± 0.16 for LFP decoding and 0.70 ± 0.14 for spike decoding. A histogram of the pairwise differences in decoding performance between spikes and LFPs (Fig. 3B) shows a small bias of 0.07 ± 0.04 toward better decoding using spikes (P = 0.003, paired t-test). Thus, EMG decoding performance using LFPs was only slightly inferior to that using spikes.
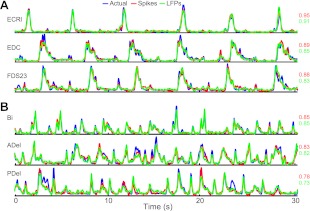
Fig. 2.
Examples of decoded limb electromyograms (EMGs). Shown are both distal (A; monkey J) and proximal (B; monkey C) EMG signals. Both spikes (red) and local field potentials (LFPs; green) accurately predicted actual EMG activity (blue). Numbers at right represent variance accounted for (VAF) for the test fold from which the examples were drawn.
Fig. 3.
Summary of EMG decoding. A: box plot summary of performance using spikes (red) and LFPs (green), showing VAF distributions over all data for each monkey. Black dots, medians; solid boxes, interquartile ranges; whiskers, overall ranges of nonoutlier data. Twenty-two of the 28 EMGs are shown, ordered from proximal to distal. Duplicated and task-irrelevant muscles are not included. Dashed lines separate different muscles, with labels color coded by monkey. B: histogram of pairwise differences (VAFLFP − VAFspike) across all muscles and monkeys. C: decoding performance of EMG with low-pass filter cutoffs of 5 Hz (light colors) and 10 Hz (dark colors) for spikes (red) and LFPs (green), averaged over all muscles and monkeys.
To explore the dependence of spike and LFP decoding accuracy on signal bandwidth, we repeated our analysis using an EMG low-pass filter cutoff of 10 Hz instead of 5 Hz, which caused decoding performance to decrease by ∼10% (Fig. 3C). Importantly, LFP and spike performances decreased by a similar amount, thus leaving their relation largely unaffected.
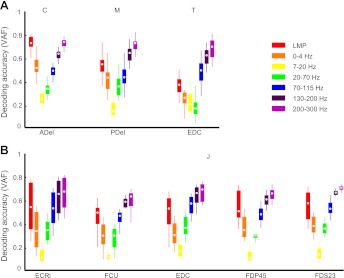
To understand which LFP frequency bands contained the most EMG-related information, we decoded EMG using each frequency band separately (Fig. 4). The three bands in the high-gamma range (70–300 Hz in this study) contributed more information (overall mean VAF = 0.61 ± 0.15) than the three bands centered at frequencies below 70 Hz (0.28 ± 0.15, P < 10−10, paired t-test), with the 130–200 Hz and 200–300 Hz bands contributing the most. This is consistent with prior kinematic decoding results using LFPs. The LMP also had substantial EMG-related information, slightly more than did the 70–110 Hz band (0.51 ± 0.2 vs. 0.48 ± 0.13, P = 0.01). Intriguingly, the LMP did not decode EMG nearly as well in the isometric case as it did in the movement cases, although the isometric data were from only one monkey, so the effect may be subject related. The delta band (0–4 Hz) had less information than the LMP (0.35 ± 0.15 vs. 0.51 ± 0.20, P < 10−10).
Fig. 4.
Relative information in different LFP frequency bands. Each box represents the decoding performance using either a single frequency band or the LMP, for one muscle over all files. A: representative examples of single muscles from monkeys C, M, and T. B: examples of 5 muscles from monkey J. Results from other muscles were consistent with these examples. Box symbols are the same as in Fig. 3.
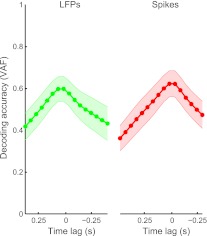
EMG-related information also varied as a function of the time lag between the M1 LFPs (or spikes) and EMGs. We found that the optimal lag for decoding EMGs was at 25 ± 86 ms (the center of the 0- to 50-ms bin) for LFPs and 2 ± 37 ms for spikes (Fig. 5). These differences were not statistically significant (P = 0.17, paired t-test). Time lags in Fig. 5 represent the centers of the bins. Spike times in Fig. 5 were shifted by 25 ms, and LFP times were shifted by 128 ms (to represent the centers of the 256-ms windows used for Fourier transforms).
Fig. 5.
Decoding performance using single time lags for spikes (red) and LFPs (green). Means (thick lines) and standard deviations (shaded areas) include data from all muscles and monkeys. Positive lags represent neural signals occurring before EMG activity.
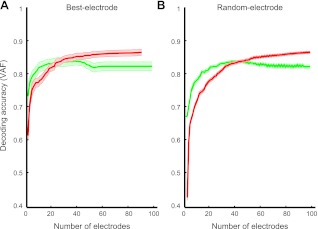
Finally, we examined decoding performance as a function of the number of electrodes for both LFPs and spikes, by incrementally adding electrodes and recomputing VAF. For spikes, we incremented by all the units recorded on a given electrode. Electrodes were selected in one of two ways: either by adding the most-informative electrodes first (i.e., those with highest r values, the best-electrode method), or in random order. For the random-electrode case, we used the mean of 10 different random electrode sequences. In the best-electrode case (Fig. 6A), LFPs slightly outperformed spikes when fewer than 14 electrodes were used in decoding (equivalence was defined as the point at which P exceeded 0.05 using a t-test at each number of electrodes). However, in the random-electrode case (Fig. 6B), LFPs substantially outperformed spikes even when up to 36 electrodes were used. In both cases, spikes outperformed LFPs with large numbers of electrodes. This corroborates the finding by Bansal et al. (2011) that the average LFP had more information than the average spike signal, but the most informative spikes had about as much information as the most informative LFPs.
Fig. 6.
Effects of number of electrodes on decoding performance. Changes in mean performance as a function of the number of electrodes for spikes (red) and LFPs (green), using the best-electrode selection method (A) and the random-electrode selection method (B). Shaded areas around thick lines represent standard errors. For the random-electrode selection method, these errors were computed over 10 random sequences.
DISCUSSION
These results clearly demonstrate for the first time that LFP signals recorded from a small area of motor cortex can be used to decode EMGs of multiple muscles from both the proximal and distal arm. Moreover, decoding accuracy was nearly as high as with spike signals. The performance level for both spikes and LFPs was comparable to prior studies that used spikes to decode EMG (Carmena et al. 2003; Oby et al. 2010; Pohlmeyer et al. 2007b). This extends the earlier observations of similar performance of LFPs and spikes that were noted for target discrimination and kinematic trajectory decoding (Mehring et al. 2003; Slutzky et al. 2010). It also adds to the previous demonstrations of high performance using posterior parietal lobe LFPs to decode saccadic and reach target (Pesaran et al. 2002; Scherberger et al. 2005). Since we did not record kinematic data in this study, it was not possible to determine the difference in bandwidth between EMG and kinematics in this particular task. However, prior studies have shown that muscles and the inertia of the limb act as low-pass filters (Bawa and Stein 1976; Mannard and Stein 1973) and thus EMG in general will have a higher bandwidth than kinematics.
The high-gamma frequency bands contained the most information about muscle activity. This adds to prior reach direction (Rickert et al. 2005) and trajectory decoding results using LFPs (Stark and Abeles 2007; Zhuang et al. 2010) as well as results using subdural field potentials, or ECoG (Chao et al. 2010). We also found a good deal of information in the LMP, and slightly less in the delta band, observations that are similar to previous human ECoG (Ball et al. 2009; Schalk et al. 2007) and monkey LFP studies (Bansal et al. 2011; Rickert et al. 2005). It is not clear why LMP and delta band decoding performance differed. It is possible that the removal of phase information from the delta band by the fast Fourier transform may be responsible. Interestingly, the LMP did not perform as well during the isometric task. The reason for this is also unclear, and we do not know if it will be a consistent observation across monkeys. To our knowledge, prior studies have not examined the LMP in isometric tasks. This question may merit further investigation.
The optimal time lag between LFPs and EMG was between 0 and 50 ms, as it was for spikes. This is similar to the peak lag at 50–75 ms for EMG prediction using singly recorded neurons (Cheney and Fetz 1980; Evarts 1966; Morrow and Miller 2003), and slightly shorter than the peak around 100 ms for force (Humphrey et al. 1970) and kinematics (Moran and Schwartz 1999).
The ability to decode the continuous time course of EMG suggests that LFPs could be used to control FES and reanimate paralyzed upper limb muscles. Implanted clinical FES systems such as the Freehand (Kilgore et al. 1997) are currently restricted to the use of residual movement and the activity of muscles in the shoulder or neck to control stimulation. In addition to adding cognitive burden, these methods limit the number of degrees of freedom that can be independently controlled, typically constraining the user to a few preprogrammed patterns of stimulation rather than direct control of individual muscles. A controller based on motor cortical signals could provide a more natural method and allow individual control of many muscles. Indeed, this approach has been successfully demonstrated in monkeys paralyzed by temporary nerve blocks, using spikes to control multi-channel stimulation (Moritz et al. 2008; Oby et al. 2010; Pohlmeyer et al. 2009; Pohlmeyer et al. 2007a).
We have shown previously that LFPs retain the same amount of movement-related information whether or not spike signals can be recorded from the same electrodes (Slutzky et al. 2011a). This provides evidence that LFPs may have greater longevity than spikes, which has been widely assumed but not proven (Andersen et al. 2004). The fact that LFPs performed as well as spikes with lower numbers of electrodes, but were gradually outperformed by spikes as more electrodes were added, is consistent with prior work with LFPs (Bansal et al. 2011). This result may reflect higher correlation among LFPs than spikes (Bansal et al. 2011; Stark and Abeles 2007), although correlations among LFP channels are lower in high-gamma bands (Slutzky, unpublished data; Bansal et al. 2012). Thus, LFPs could provide a high-performance, complementary or alternative BMI input signal to spikes, particularly in the long term after many spikes have been lost. The low sampling rate requirements, potential for greater longevity, and rich information content of LFPs make them attractive signal sources for BMIs.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants K08NS-060223 and R01NS-053603.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.D.F., C.E., and E.R.O. performed experiments; R.D.F. and M.W.S. analyzed data; R.D.F., L.E.M., and M.W.S. interpreted results of experiments; R.D.F. and M.W.S. prepared figures; R.D.F. and M.W.S. drafted manuscript; R.D.F., C.E., L.E.M., and M.W.S. edited and revised manuscript; R.D.F., L.E.M., and M.W.S. approved final version of manuscript; L.E.M. and M.W.S. conception and design of research.
ACKNOWLEDGMENTS
We thank Nicholas Hatsopoulos and Brian London for surgical assistance, and Eric Lindberg and Luke Jordan for assistance with behavioral training.
REFERENCES
- Andersen RA, Musallam S, Pesaran B. Selecting the signals for a brain-machine interface. Curr Opin Neurobiol 14: 720–726, 2004 [DOI] [PubMed] [Google Scholar]
- Ball T, Schulze-Bonhage A, Aertsen A, Mehring C. Differential representation of arm movement direction in relation to cortical anatomy and function. J Neural Eng 6: 16006, 2009 [DOI] [PubMed] [Google Scholar]
- Bansal AK, Truccolo W, Vargas-Irwin CE, Donoghue JP. Decoding 3-D reach and grasp from hybrid signals in motor and premotor cortices: spikes, multiunit activity and local field potentials. J Neurophysiol 107: 1337–1355, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal AK, Vargas-Irwin CE, Truccolo W, Donoghue JP. Relationships among low-frequency local field potentials, spiking activity, and three-dimensional reach and grasp kinematics in primary motor and ventral premotor cortices. J Neurophysiol 105: 1603–1619, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawa P, Stein R. Frequency response of human soleus muscle. J Neurophysiol 39: 788–793, 1976 [DOI] [PubMed] [Google Scholar]
- Carmena JM, Lebedev MA, Crist RE, O'Doherty JE, Santucci DM, Dimitrov DF, Patil PG, Henriquez CS, Nicolelis MA. Learning to control a brain-machine interface for reaching and grasping by primates. PLoS Biol 1: E42, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao ZC, Nagasaka Y, Fujii N. Long-term asynchronous decoding of arm motion using electrocorticographic signals in monkeys. Front Neuroeng 3: 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE. Functional classes of primate corticomotoneuronal cells and their relation to active force. J Neurophysiol 44: 773–791, 1980 [DOI] [PubMed] [Google Scholar]
- Evarts EV. Pyramidal tract activity associated with a conditioned hand movement in the monkey. J Neurophysiol 29: 1011–1027, 1966 [DOI] [PubMed] [Google Scholar]
- Evarts EV. Relation of pyramidal tract activity to force exerted during voluntary movement. J Neurophysiol 31: 14–27, 1968 [DOI] [PubMed] [Google Scholar]
- Fagg AH, Ojakangas GW, Miller LE, Hatsopoulos NG. Kinetic trajectory decoding using motor cortical ensembles. IEEE Trans Neural Syst Rehabil Eng 17: 487–496, 2009 [DOI] [PubMed] [Google Scholar]
- Georgopoulos A, Kalaska J, Caminiti R, Massey J. On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. J Neurosci 2: 1527–1537, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldman DA, Wang W, Chan SS, Moran DW. Local field potential spectral tuning in motor cortex during reaching. IEEE Trans Neural Syst Rehabil Eng 14: 180–183, 2006 [DOI] [PubMed] [Google Scholar]
- Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 442: 164–171, 2006 [DOI] [PubMed] [Google Scholar]
- Humphrey DR, Schmidt E, Thompson W. Predicting measures of motor performance from multiple cortical spike trains. Science 170: 758–762, 1970 [DOI] [PubMed] [Google Scholar]
- Hunter IW, Korenberg MJ. The identification of nonlinear biological-systems-Wiener and Hammerstein Cascade Models. Biol Cybern 55: 135–144, 1986 [DOI] [PubMed] [Google Scholar]
- Kilgore KL, Peckham PH, Keith MW, Thrope GB, Wuolle KS, Bryden AM, Hart RL. An implanted upper-extremity neuroprosthesis. Follow-up of five patients. J Bone Joint Surg 79: 533–541, 1997 [DOI] [PubMed] [Google Scholar]
- Krüger J, Caruana F, Dalla Volta R, Rizzolatti G. Seven years of recording from monkey cortex with a chronically implanted multiple microelectrode. Front Neuroeng 3: 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannard A, Stein RB. Determination of the frequency response of isometric soleus muscle in the cat using random nerve stimulation. J Physiol 229: 275–296, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehring C, Nawrot MP, de Oliveira SC, Vaadia E, Schulze-Bonhage A, Aertsen A, Ball T. Comparing information about arm movement direction in single channels of local and epicortical field potentials from monkey and human motor cortex. J Physiol (Paris) 98: 498–506, 2004 [DOI] [PubMed] [Google Scholar]
- Mehring C, Rickert J, Vaadia E, Cardosa de Oliveira S, Aertsen A, Rotter S. Inference of hand movements from local field potentials in monkey motor cortex. Nat Neurosci 6: 1253–1254, 2003 [DOI] [PubMed] [Google Scholar]
- Moran DW, Schwartz AB. Motor cortical representation of speed and direction during reaching. J Neurophysiol 82: 2676–2692, 1999 [DOI] [PubMed] [Google Scholar]
- Moritz CT, Perlmutter SI, Fetz EE. Direct control of paralysed muscles by cortical neurons. Nature 456: 639–642, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow MM, Miller LE. Prediction of muscle activity by populations of sequentially recorded primary motor cortex neurons. J Neurophysiol 89: 2279–2288, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Oscillatory activity in sensorimotor cortex of awake monkeys: synchronization of local field potentials and relation to behavior. J Neurophysiol 76: 3949–3967, 1996 [DOI] [PubMed] [Google Scholar]
- Nemati S, Fagg AH, Hatsopoulos NG, Miller LE. A comparison of linear and kalman filter models for arm motion prediction. Proc Ann Meeting Neural Control Movement, 2007 [Google Scholar]
- Oby ER, Ethier C, Bauman MJ, Perreault EJ, Ko J, Miller LE. Getting a grip on spinal cord injury: a novel application of a Brain Machine Interface. In: Statistical signal processing for neuroscience and neurotechnology, edited by Oweiss KG. Academic Press, Elsevier, 2010, p. 369–406 [Google Scholar]
- Perreault EJ, Kirsch RF, Acosta AM. Multiple-input, multiple-output system identification for characterization of limb stiffness dynamics. Biol Cybern 80: 327–337, 1999 [DOI] [PubMed] [Google Scholar]
- Pesaran B, Pezaris JS, Sahani M, Mitra PP, Andersen RA. Temporal structure in neuronal activity during working memory in macaque parietal cortex. Nat Neurosci 5: 805–811, 2002 [DOI] [PubMed] [Google Scholar]
- Pohlmeyer EA, Oby ER, Perreault EJ, Solla SA, Kilgore KL, Kirsch RF, Miller LE. Toward the restoration of hand use to a paralyzed monkey: brain-controlled functional electrical stimulation of forearm muscles. PloS One 4: e5924, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmeyer EA, Perreault EJ, Slutzky MW, Kilgore KL, Kirsch RF, Taylor DM, Miller LE. Use of intracortical recordings to control a hand neuroprosthesis. Proc 3rd International IEEE/EMBS Conf Neural Engineer, 2007a [Google Scholar]
- Pohlmeyer EA, Solla SA, Perreault EJ, Miller LE. Prediction of upper limb muscle activity from motor cortical discharge during reaching. J Neural Eng 4: 369–379, 2007b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickert J, Oliveira SC, Vaadia E, Aertsen A, Rotter S, Mehring C. Encoding of movement direction in different frequency ranges of motor cortical local field potentials. J Neurosci 25: 8815–8824, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Oscillations in local field potentials of the primate motor cortex during voluntary movement. PNAS 90: 4470–4474, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalk G, Kubanek J, Miller KJ, Anderson NR, Leuthardt EC, Ojemann JG, Limbrick D, Moran D, Gerhardt LA, Wolpaw JR. Decoding two-dimensional movement trajectories using electrocorticographic signals in humans. J Neural Eng 4: 264–275, 2007 [DOI] [PubMed] [Google Scholar]
- Scherberger H, Jarvis MR, Andersen RA. Cortical local field potential encodes movement intentions in the posterior parietal cortex. Neuron 46: 347–354, 2005 [DOI] [PubMed] [Google Scholar]
- Serruya MD, Hatsopoulos NG, Paninski L, Fellows MR, Donoghue JP. Instant neural control of a movement signal. Nature 416: 141–142, 2002 [DOI] [PubMed] [Google Scholar]
- Simeral JD, Kim SP, Black MJ, Donoghue JP, Hochberg LR. Neural control of cursor trajectory and click by a human with tetraplegia 1000 days after implant of an intracortical microelectrode array. J Neural Eng 8: 025027, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutzky MW, Lindberg E, Jordan LR, Miller LE. Decoding motor outputs with epidural and intracortical inputs: performance similarities and differences. Soc Neurosci Abstr 2010 [Google Scholar]
- Slutzky MW, Lindberg EW, Flint RD, Miller LE. Field potentials as brain machine interface inputs: evidence for greater longevity than spikes. Soc Neurosci Abstr 2011a [Google Scholar]
- Slutzky MW, Lindberg EW, Flint RD, Miller LE. Decoding muscle activity with field potentials. Proc 5th International IEEE/EMBS Conf Neural Engineer: 278–281, 2011b [Google Scholar]
- Stark E, Abeles M. Predicting movement from multiunit activity. J Neurosci 27: 8387–8394, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DM, Tillery SI, Schwartz AB. Direct cortical control of 3D neuroprosthetic devices. Science 296: 1829–1832, 2002 [DOI] [PubMed] [Google Scholar]
- Velliste M, Perel S, Spalding MC, Whitford AS, Schwartz AB. Cortical control of a prosthetic arm for self-feeding. Nature 453: 1098–1101, 2008 [DOI] [PubMed] [Google Scholar]
- Zhuang J, Truccolo W, Vargas-Irwin C, Donoghue JP. Decoding 3-D reach and grasp kinematics from high-frequency local field potentials in primate primary motor cortex. IEEE Trans Biomed Eng 57: 1774–1784, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]