Abstract
A high dose of sodium salicylate temporarily induces tinnitus, mild hearing loss, and possibly hyperacusis in humans and other animals. Salicylate has well-established effects on cochlear function, primarily resulting in the moderate reduction of auditory input to the brain. Despite decreased peripheral sensitivity and output, salicylate induces a paradoxical enhancement of the sound-evoked field potential at the level of the primary auditory cortex (A1). Previous electrophysiologic studies have begun to characterize changes in thalamorecipient layers of A1; however, A1 is a complex neural circuit with recurrent intracortical connections. To describe the effects of acute systemic salicylate treatment on both thalamic and intracortical sound-driven activity across layers of A1, we applied current-source density (CSD) analysis to field potentials sampled across cortical layers in the anesthetized rat. CSD maps were normally characterized by a large, short-latency, monosynaptic, thalamically driven sink in granular layers followed by a lower amplitude, longer latency, polysynaptic, intracortically driven sink in supragranular layers. Following systemic administration of salicylate, there was a near doubling of both granular and supragranular sink amplitudes at higher sound levels. The supragranular sink amplitude input/output function changed from becoming asymptotic at approximately 50 dB to sharply nonasymptotic, often dominating the granular sink amplitude at higher sound levels. The supragranular sink also exhibited a significant decrease in peak latency, reflecting an acceleration of intracortical processing of the sound-evoked response. Additionally, multiunit (MU) activity was altered by salicylate; the normally onset/sustained MU response type was transformed into a primarily onset response type in granular and infragranular layers. The results from CSD analysis indicate that salicylate significantly enhances sound-driven response via intracortical circuits.
subjective tinnitus is a disorder characterized by the perception of a phantom sound having no acoustic source in the environment. A recent assessment of the United States population indicates approximately 50 million adults have experienced tinnitus and roughly 16 million experienced frequent tinnitus (Shargorodsky et al. 2010). Although recent advances have been made toward developing treatment strategies for tinnitus sufferers (Dohrmann et al. 2007; Engineer et al. 2011; Londero et al. 2006; Schaette et al. 2010), no cure currently exists. The absence of a tinnitus cure is largely due to an incomplete understanding of the fundamental neural mechanisms underlying tinnitus perception.
Alterations in neural activity related to noise- or drug-induced tinnitus have been observed at the periphery (for review, see Cazals 2000), brain stem (Brozoski et al. 2002; Kaltenbach and Afman 2000), and midbrain (Chen and Jastreboff 1995; Longenecker and Galazyuk 2011; Ma et al. 2006); however, the auditory cortex is thought to play a key role in tinnitus perception (for review, see Eggermont 2008). Indeed, tinnitus-related alterations have been observed at the level of primary auditory cortex (A1) in both humans (Lockwood et al. 1999; Lorenz et al. 2009; Weisz et al. 2004; Wienbruch et al. 2006) and animal models (Lu et al. 2011; Stolzberg et al. 2011; Sun et al. 2009; Zhang et al. 2011). A1 is a complex circuit that dynamically integrates incoming thalamic signals with feedback from a recent history of activation (Happel et al. 2010; Kaur et al. 2005).
Recently, we reported that systemic salicylate administration in the rat results in a distortion of the normal tonotopic gradient of A1 (Stolzberg et al. 2011), similar to findings in humans with tinnitus (Wienbruch et al. 2006) and previous studies in the cat following sound trauma (Irvine et al. 2000; Kimura and Eggermont 1999; Seki and Eggermont 2002). The shifts in A1 excitatory frequency receptive fields (FRFs) observed in these studies have most often been attributed to a disinhibition of local circuits (Eggermont and Roberts 2004). Disinhibition in the central auditory system as a secondary effect of hearing loss (Scholl and Wehr 2008), or as a primary effect of tinnitus-inducing drugs such as salicylate (Su et al. 2009; Wang et al. 2006), may make the auditory cortex more permissive to shifting receptive field properties and increasing responsivity to normally suppressed neural activity. Animal models of salicylate-induced tinnitus have provided us with a greater understanding of the neurophysiologic changes that occur in subcortical or cortical auditory structures; however, no investigations to date have attempted to interpret electrophysiologic changes in the context of the neuronal circuitry of A1.
One established method of investigating interlaminar processing is by applying current-source density (CSD) analysis to local-field potential (LFP) activity sampled across cortical layers. CSD analysis reveals the net flow of ions into and out of neural tissue near the electrodes, thereby providing a detailed description of the activation pattern across cortical layers (Mitzdorf 1985). Recent evidence from CSD analysis of translaminar A1 response to sound following pharmacologically disrupted inhibition highlighted the importance of intracortical horizontal fibers residing in the superficial layers of the cortex to temporal and spectral integration (Happel et al. 2010; Moeller et al. 2010). Because horizontal intracortical fibers in A1 may play a critical role in altered FRFs and tinnitus (Eggermont and Roberts 2004), we predicted that excitatory horizontal intracortical projections in superficial layers of the A1 may be unmasked following salicylate-induced disinhibition in A1.
To investigate tinnitus-related changes in cortical processing, translaminar recordings were made from A1 using high-density linear array electrodes before and after systemic salicylate administration in anesthetized rats. Sound-evoked CSD and multiunit (MU) activity, reflecting postsynaptic potentials and suprathreshold output of local neurons, respectively, were sampled and analyzed. Understanding which aspects of the auditory cortex microcircuitry are altered during salicylate treatment is critical to understanding phantom sound perception and other auditory neurologic disorders such as hyperacusis (i.e., a marked intolerance to otherwise ordinary environmental sound).
MATERIALS AND METHODS
Surgery.
All procedures were approved by the Institutional Animal Care and Use Committee at the University at Buffalo, the State University of New York, and were carried out in accordance with National Institutes of Health guidelines. Seven young-adult (mean: 71.6 ± 3.7 d SE) male Sprague–Dawley rats (mean weight: 319.7 ± 22.4 g, SE; Charles River Laboratories, Inc., Wilmington, MA) were prepared for electrophysiology experiments as described in the following text. Surgeries and recordings were performed in a sound-attenuating booth (Acoustic Systems, Pelham, AL) under ketamine/xylazine anesthesia (60 mg/kg ketamine, 6 mg/kg xylazine; administered intraperitoneally [IP]). The absence of the toe-pinch withdrawal reflex was used as an indicator of anesthetic depth; the corneal reflex was tested and present during recordings. During the entire experiment, body temperature was maintained at 37°C by homeothermic heating pad (Harvard Apparatus, Holliston, MA).
Once anesthetized, the animal was fixed in a stereotactic frame using blunted ear bars. An incision was made along the midline of the skull, and then the ventral aspect of the skull was cleaned of any remaining tissue and fascia with a scalpel blade. The left temporalis muscle was reflected from the skull using a blunt dissection technique, to provide access to the temporal bone overlying the left A1. A stereotaxic manipulator was used to measure 5 mm caudal to bregma, the approximate stereotaxic coordinates of A1 (Polley et al. 2007; Rutkowski et al. 2003), and a mark was made on the skull for later drilling. A small hole was hand drilled and a stainless steel bone screw was inserted into the left parietal bone to serve as anchor for a headpost as well as electrical ground. To provide free field sound stimulation during the experiment, a small flathead aluminum rivet was used as a headpost. The aluminum rivet was glued (cyanoacrylic) onto the bregma and then cemented to the skull with dental acrylic. After the dental cement had hardened, the frame of a small cranial window was etched into the temporal bone (rostral–caudal × ventral–dorsal) with a small carbide drill bit, flushing with saline every few seconds to prevent overheating. The thin bone remaining in the etched frame was carefully perforated with a sharp scalpel blade and the thick center bone piece removed, exposing the dura-covered brain. The animal was subsequently removed from the stereotaxic frame and placed on a platform with a homeothermic heating pad and headpost holder for free field sound stimulation. To prevent movement during injection of the drug, a pediatric intravenous catheter holding either saline or sodium salicylate in saline (250 mg/kg; 50 mg/mL; n = 5), or saline alone as vehicle control (n = 2), was inserted into the peritoneal space and taped in place.
Electrophysiology.
Because the rat brain is lissencephalic and primary A1 cannot be reliably localized using anatomic features, we performed a brief mapping of sound-evoked LFP and multiunit (MU) responses to noise bursts and tone-driven receptive fields at the beginning of every experiment. For each animal, at least three penetrations with a single tungsten microelectrode (impedance ∼1 MΩ; FHC, Bowdoin, ME) were made, and these initial recordings helped to localize A1 prior to inserting the linear array electrode. Our criteria for identifying the rat A1 were: (1) a rostral–caudal, high-low frequency tonotopic gradient (Polley et al. 2007; Rutkowski et al. 2003), (2) canonical frequency receptive fields and short response latencies (∼10 ms) (Lakatos et al. 2005; Polley et al. 2007), and (3) sharp initial negative LFP peak (Di and Barth 1992).
Once the location of A1 was verified, a small slit in the dura was made, and a linear array electrode (model: A1x32–6mm-100–177-Z32; NeuroNexus Technologies, Ann Arbor, MI) was slowly inserted perpendicular to the cortical surface with a hydraulic microdrive (Trent Wells). The linear array consisted of 32 iridium electrodes (impedance ∼1 MΩ, area = 177 μm2), spaced 50 μm apart on a 15-μm-thick silicon substrate. Initially, the linear array was rapidly advanced to penetrate pia under manual control, then returned to the cortical surface, and subsequently slowly advanced (∼2 μm/s) by hydraulic microdrive until noise burst evoked LFP activity on the deepest channels exhibited a characteristic loss of sharp initial negative peak (typically 1,200–1,400 μm depth). Once at this depth, the linear array was allowed to settle in place for approximately 45 min before beginning the recording protocol; the array was maintained in this position for the duration of the experiment. Following the baseline recording protocol (see the following text), salicylate (n = 5) or saline (n = 2) was slowly injected via IP catheter, and the recording protocol was repeated each hour, beginning 0.5 h after injection. The postinjection results described in the following text are of the final recording protocol that started 3.5 h after injection.
Electrophysiologic signals were acquired with Tucker-Davis System 3 (TDT, Alachua, FL). MU activity was detected online (digitally sampled at approximately 25 kHz and bandpass filtered online at 300–3,500 Hz) using a voltage threshold manually set by the experimenter by visual inspection of the noise floor and spike amplitude for each channel prior to baseline recording. Spike detection thresholds set at baseline were maintained for the duration of the experiment. LFP activity was continuously acquired (digitally resampled at ∼610 Hz and bandpass filtered online at 2–300 Hz).
Sound stimulation.
Sound stimuli were generated by a TDT RX6 module (∼100 kHz sampling rate) and presented by a shielded dome tweeter (Fostex FT28D) placed approximately 10 cm from the animal's right ear (i.e., speaker positioned contralateral to the left A1 where recordings were performed). Sound stimuli were calibrated with custom MATLAB software (The MathWorks, Natick, MA) using a ¼-inch microphone (model 2520, Larson-Davis) and amplifier (800D, Larson-Davis); sound calibration data were acquired on RP2 processor (TDT). Sound levels are presented as dB sound pressure level (SPL; referencing 20 μPa).
Current-source density analysis.
CSD analysis provides a detailed spatial profile of ionic flow into (and out of) neural tissue (Mitzdorf 1985). One-dimensional CSD analysis was applied to mean poststimulus LFPs recorded simultaneously across cortical layers using the formula
| (1) |
where Φ is the LFP, z is the spatial coordinate, Δz is the interelectrode spacing (Δz = 50 μm), and n is the differentiation grid (n = 4). Briefly, the CSD equation estimates the second derivative of LFPs recorded at each time point across electrodes. A three-point Hamming filter was applied to smooth LFPs across channels prior to computing CSD.
To quantify the overall activation of A1 postsynaptic currents, the average of the rectified current (AVREC) measure was applied to the CSD analysis described earlier (Happel et al. 2010; Schroeder et al. 1997, 2001).
| (2) |
where CSD refers to Eq. 1, n refers to the number of channels of the CSD, and t refers to the time point index. CSD and AVREC analysis were performed with custom-written scripts in MATLAB (R2010b; The MathWorks).
Data analysis.
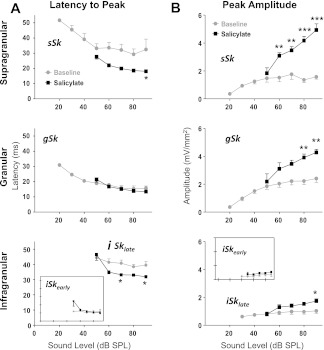
To assess laminar contributions to stimulus processing, noise burst input–output (IO) functions were generated using broadband sound stimuli (bandpass between 1 and 42 kHz) presented at levels from 0 to 90 dB SPL in 10 dB steps (50 repetitions; pseudorandomized presentation). CSD was applied to the mean noise burst evoked LFP at each sound level. CSD analysis reveals both current sinks and sources; sinks reflect net flow of positive ions into the neural tissue that corresponds to depolarization, whereas sources reflect the net repolarization and possibly inhibition of neighboring neural tissue. Sinks were identified here as being at least 3SDs above the mean voltage measured during 100 ms of prestimulus activity. Large and reliable sinks (Fig. 1B, red) appeared in supragranular (sSk) and granular (gSk) layers. Two additional sinks were identified in infragranular layers of A1 and were further identified as short (iSkearly) and long (iSklate) latency sinks. Peak amplitudes and latencies of these four sinks were used to compute noise burst IO functions for each of these sinks. The peak amplitude and latency of AVREC response were also generated.
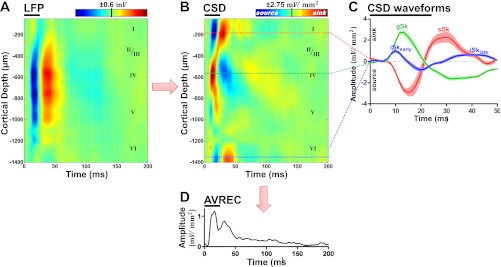
Fig. 1.
Noise burst evoked local-field potentials (LFPs) and current-source density (CSD) analysis in primary auditory cortex (A1). A: representative LFP profile recorded from electrode spanning the depth of A1 in response to 25-ms noise burst at 70 dB SPL (horizontal black bar) during baseline condition. Depth from the cortical surface is indicated on the left ordinate and approximate cortical layer is indicated on the right ordinate. B: CSD analysis on LFP profile in A. Red and blue coloring indicate current sinks and sources, respectively. C: cross-section waveforms extracted from the CSD profile in B. The supragranular (red), granular (green), and infragranular (blue) responses (mean ± SE; sinks are positive, sources are negative) were selected from the electrode with the peak amplitude within the region (see Fig. 2). Four sinks were identified within the first 50 ms of the response and were labeled: supragranular, sSk; granular, gSk; early infragranular, iSkearly; late infragranular, iSklate. D: averaged rectified current (AVREC) computed from CSD profile in B.
IO functions were also generated for noise burst evoked MU activity pooled by channels within supragranular (depth ≥ −350 μm), granular (−650 μm ≤ depth < −350 μm), upper infragranular (−950 μm ≤ depth < −650 μm), and lower infragranular (depth < −950 μm) cortical regions (see Fig. 2). Layer assignments were based on previous investigations of rat A1 (Kaur et al. 2005; Szymanski et al. 2009, 2011). MU IO functions were constructed for both the peak and mean (within 50 ms poststimulus window) of poststimulus time histograms (PSTHs). IO functions were subjected to two-way ANOVA and Bonferroni posttest comparisons for sound stimulus levels at 50 dB SPL and higher.
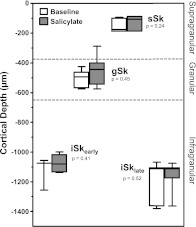
Fig. 2.
Distributions of CSD sink peak depths. There were no significant changes in depth of sink peaks following salicylate (two-tailed t-test).
Frequency receptive field (FRF) maps were generated for MU data using tone burst stimuli (25 ms; 5 ms rise/fall cos2 gate) between 1 and 42 kHz, with 30 logarithmic frequency steps. Stimuli were presented from 0 to 90 dB SPL in 10-dB steps (5 repetitions per frequency/level; pseudorandom order). MU FRF maps were generated by creating a matrix of the mean firing rate in response to each frequency/level pair over a 50-ms window following stimulus onset. Tuning was assessed using a graphical user interface written in MATLAB.
RESULTS
In this study we provide a first characterization of the laminar profile of sound-evoked neural activity in A1 following systemic treatment with sodium salicylate (250 mg/kg, IP), a reliable inducer of tinnitus in both humans and rats. After functional identification of A1 using a single tungsten microelectrode (see previous section), a 32-channel linear microelectrode array was inserted orthogonal to the cortical surface. LFP and MU activity were recorded simultaneously across cortical layers. Mean sound-evoked LFP activity was subjected to one-dimensional CSD analysis, revealing postsynaptic potentials reflecting local net depolarization of neural tissue near the electrode array.
Sound-evoked CSD profile.
The CSD of the mean LFP response to noise bursts exhibited a stereotyped activation profile for A1 (Barth and Di 1990; Happel et al. 2010; Kaur et al. 2005; Szymanski et al. 2009). A typical CSD profile recorded during a baseline condition is presented in Fig. 1B. The most robustly activated sink during the baseline recordings occurred in thalamorecipient granular layers (gSk). The granular sink (gSk) is attributed to inward currents evoked by monosynaptic activation of pyramidal cells residing in deep layer III and upper layer IV (Barth and Di 1990; Kaur et al. 2005; Szymanski et al. 2009) by tonotopically organized projections originating in the ventral medial geniculate body (vMGB) of the thalamus (Huang and Winer 2000). A source (Fig. 1B, blue) in the supragranular layers was consistently observed at the same time as gSk activation. This source reflects passive “return currents” balancing the gSk activation (Szymanski et al. 2009).
A small sink with slightly shorter latency than that of gSk was often observed in infragranular cortical layers (layers lower V/upper VI). This early infragranular sink (iSkearly) was present in most evoked responses; however, it did not always surpass the 3SD threshold to be detected for analysis (see previous section). The iSkearly sink is attributed to early monosynaptic activation of A1 by thalamic projections of nontonotopically organized medial MGB (Mitzdorf 1985; Prieto and Winer 1999; Szymanski et al. 2009) and likely plays a modulatory role in cortical signal processing.
Longer-latency sinks primarily reflect intracortical, polysynaptic connections (Happel et al. 2010; Mitzdorf 1985). A prominent supragranular sink (sSk) resided in the most superficial layers of the cortex and exhibited a longer-latency response to noise bursts than both gSk and iSkearly. This sink reflects activation of apical dendritic tufts of large pyramidal cells residing in layer V (Winer and Prieto 2001) and plays a critical role in spectral integration in A1 (Happel et al. 2010). The sSk sink was characterized by its protracted duration resulting from activation of fibers with high temporal jitter (Happel et al. 2010; Mitzdorf 1985). A corresponding source was observed in granular layers; however, the origins of this source are likely the combination of passive return currents and rebound inhibition following gSk response (Happel et al. 2010; Harding 1992).
Finally, a second infragranular sink (iSklate, layer V/VI) was observed at longer latency than sSk and presumably reflects the activation of intracortical and/or corticofugal projecting neurons residing in these layers (Prieto and Winer 1999; Szymanski et al. 2011; Winer and Prieto 2001). Figure 2 summarizes the distributions of these sink depths during baseline and salicylate recordings. Salicylate treatment did not have a significant effect on the depth of sink peaks (Fig. 2). Layer assignments are an approximation based on the translaminar activation pattern reported in previous CSD studies in rat A1 (Barth and Di 1990; Kaur et al. 2005; Szymanski et al. 2009, 2011). Qualitative changes in latency and magnitude were observed for sinks appearing later than 50 ms, but were not analyzed.
The averaged rectified current (AVREC; see materials and methods) summarizes the temporal evolution of the translaminar CSD response (Fig. 1D). AVREC is useful in the characterization of the overall activation of cortical tissue (Happel et al. 2010).
Effects of salicylate on sound-evoked CSD profile.
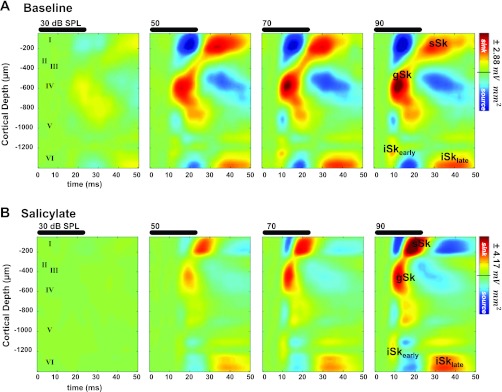
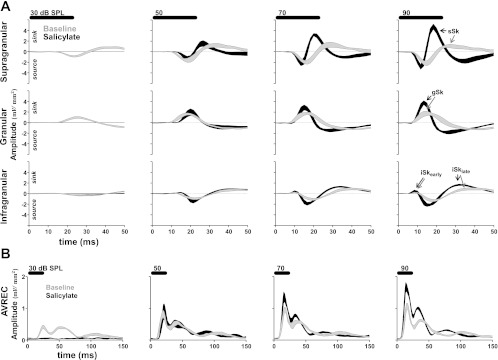
Systemic salicylate administration had pronounced effects on A1 responses to noise burst stimuli (Fig. 3). Normally, gSk exhibited the largest sink amplitude evoked by noise bursts (Figs. 3A, 4A, and 5B). The peak amplitude of sSk normally approached an asymptote in response to noise bursts greater than or equal to 50 dB SPL and always exhibited smaller amplitude responses than the gSk (Figs. 3A, 4A, and 5B). Following salicylate treatment, sinks did not reach the 3SD threshold for sound stimuli <50 dB; this result can most likely be explained by a reduced sensorineural output from the cochlea following systemic salicylate treatment (Brennan et al. 1996; Cazals 2000; Ralli et al. 2010; Stolzberg et al. 2011). This being the case, IO functions for sink amplitude and latency were evaluated only for 50 dB SPL and higher sound levels. Following salicylate administration, gSk and sSk peak response amplitudes were significantly enhanced [sSk F(1,40) = 104.01, P < 0.001; gSk F(1,40) = 37.18, P < 0.0001; Fig. 4A]. Furthermore, a significant interaction between the main effects of salicylate treatment and sound level was observed for sSk [F(4,40) = 7.38, P = 0.002], but not for the gSk amplitude [gSk F(4,40) = 1.77, P = 0.1537]. There was not a significant three-way interaction between sound level, sink, and condition [three-way ANOVA, F(12,143) = 1.68, P = 0.08]. Salicylate treatment significantly altered the normal laminar activation profile of A1 to noise bursts, resulting in sSk peak amplitudes often equal to or larger than gSk peak amplitudes at 80 and 90 dB SPL (Fig. 4A). Amplitudes of infragranular sinks were also significantly increased by the salicylate treatment, but to a lesser degree than gSk and sSk [iSkearly F(1,24), P = 0.0166, Fig. 4B; iSklate F(1,39), P < 0.001, Fig. 4B], and without a significant interaction between the main effects [iSkearly F(4,24) = 0.05, P = 0.9944; iSklate F(4,39) = 1.39, P = 0.2562].
Fig. 3.
Example of effects of salicylate on noise burst evoked CSD profile. A: CSD profile in response to broadband noise bursts with increasing sound levels. B: same recording experiment as in A following salicylate. Note increase in supragranular sink (sSk) amplitude relative to granular sink amplitude (gSk) as well as the shorter peak latency of sSk. Approximate layer assignments are indicated as numerals.
Fig. 4.
Effects of salicylate on supragranular, granular, and infragranular sinks and AVREC responses. A: plots show the mean supragranular, granular, and infragranular (rows) cross sections of CSDs before (dashed lines) and after salicylate treatment (solid lines) in response to 30, 50, 70, or 90 dB SPL noise bursts (25-ms duration, horizontal scale bars above each column). Current sinks and sources are positive and negative deflections, respectively. Sinks that were analyzed are indicated by arrows. B: plots show the AVREC response to noise burst stimuli. All plots are mean ± SE, n = 5 animals.
Fig. 5.
Salicylate's effects on peak latencies and amplitudes of sound-evoked CSD sinks. A: the sSk (top) and iSklate (bottom inset) peak latencies significantly decreased following salicylate, whereas gSk (middle) and iSkearly (bottom) peak latencies were not significantly altered. B: salicylate enhanced all sink peak amplitudes (see text for details), and enhanced sSk more than other sinks. (Error bars are SE; *P < 0.05, **P < 0.01, ***P < 0.001 from Bonferroni posttest on two-way ANOVA.)
The only significant effect observed in vehicle control animals was a small decrease in gSk sink amplitude 3.5 h following saline injection [gSk F(1,14) = 16.9, P = 0.0011]; however, this effect was opposite and smaller in magnitude than the experimental salicylate condition (largest effect at 90 dB: baseline M = 4.269 mv/mm2, SD = 3.223; saline M = 3.223 mv/mm2, SD = 0.218), no significant interaction was observed between the main effects of saline treatment and sound level [gSk F(6,14) = 0.71, P = 0.6508], and a post hoc test did not reveal significant changes at any sound level. It is likely that this small decrease in peak amplitude following vehicle control is due to nonspecific effects of the ketamine anesthesia.
During baseline recordings sSk exhibited a longer-latency response to sound stimulation than gSk and iSkearly (Happel et al. 2010; Szymanski et al. 2009), but had a consistently shorter peak latency than that of iSklate (Figs. 3, 4A, and 5A). The gSk and iSkearly sinks are activated via monosynaptic thalamocortical projections and were therefore expected to be at an absolute minimum synaptic latency at baseline [compare Fig. 3, A and B; see Figs. 4A and 5A; gSk F(1,40) = 0.53, P = 0.47; iSkearly F(1,24) = 3.84, P = 0.062]. The sSk latency was significantly shorter following salicylate treatment [F(1,40) = 30.25, P < 0.0001]. In contrast to gSk and iSkearly, sSk primarily reflects activation by horizontally projecting intracortical fibers important for spectral integration in A1 (Happel et al. 2010; Kaur et al. 2005). Despite the decreased peak latency of iSklate [F(1,39) = 19.97, P < 0.0001], this sink remained the longest-latency sink within the first 50 ms of the response. No significant interactions were found for the main effects of latency and sound level for any of the sinks [sSk F(4,40) = 0.55, P = 0.7013; gSk F(4,40) = 1.13, P = 0.3574; iSkearly F(4,24) = 2.38, P = 0.0795; iSklate F(4,39) = 2.46, P = 0.0615]. In saline control animals, no significant changes in peak latency were observed for any sinks.
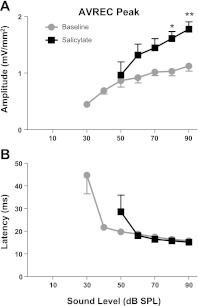
To quantify the overall responsiveness of the recorded region in A1 to sound stimulation, the AVREC (see Figs. 1D and 4B) was computed for each CSD profile at each noise burst sound level. The mean IO function for the peak of the AVREC response to broadband noise bursts significantly increased following salicylate [F(1,40) = 141.53, P < 0.0001; Fig. 5, top panel], whereas the latency of the largest AVREC peak was not altered [F(1,20) = 1.27, P = 0.27; Fig. 6B). Furthermore, a significant interaction was observed between the main effects of salicylate treatment and sound level for AVREC peak amplitude [F(4,40) = 4.29, P = 0.0055], and a smaller interaction between sound level and AVREC peak latency [F(4,40) = 2.98, P = 0.0305]. As discussed earlier, due to salicylate's effects on cochlear sensitivity, AVREC peak amplitude and latency IO functions were evaluated for significant changes at sound levels of 50 dB and higher.
Fig. 6.
AVREC peak amplitude input–output (IO) function. A: peak AVREC amplitude was significantly enhanced following salicylate. B: AVREC peak latency was unaltered by salicylate treatment.
A previous study conducted in our laboratory found a significant enhancement of sound-evoked surface potentials over auditory cortex following systemic salicylate treatment in both awake and ketamine-anesthetized rats (Sun et al. 2009), findings that were suggested to be a neural correlate of salicylate-induced hyperacusis. It is worth noting that since CSD sinks and sources are what drive surface potentials (Barth and Di 1990), the AVREC can be thought to bridge the gap between the enhanced sink responses described earlier (primarily sSk and gSk) with enhanced A1 sound-evoked response recorded at the cortical surface in our previous study.
Effects of salicylate on laminar profile of multiunit activity.
MU activity was recorded from each intracortical channel simultaneously with field potentials used for CSD analysis described earlier. Although the low-frequency CSD profile primarily reflects subthreshold currents near the electrode (Happel et al. 2010; Mitzdorf 1985; Schroeder et al. 1997), MU activity reflects suprathreshold spiking output (i.e., action potentials) of small groups of neurons near each electrode (Stark and Abeles 2007).
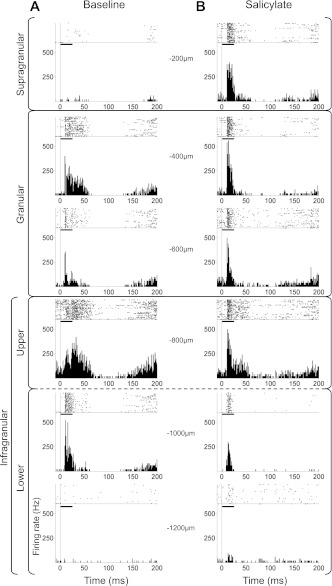
Figure 7 presents MU raster and histograms evoked by a 70 dB SPL noise burst from a representative experiment before (Fig. 7A) and after systemic salicylate administration (Fig. 7B). MUs were typically most responsive to the onset of the noise burst along with a somewhat sustained response.
Fig. 7.
Example of salicylate's effects on multiunit (MU) response to noise bursts across depth of A1. Top of each subpanel is a raster of spike times (each dot represents a spike) in response to 50 stimulus presentations (broadband noise burst at 70 dB SPL). Horizontal black line in each subplot represents duration of noise-burst stimulus. Bottom of each subpanel is a peristimulus time histogram (PSTH) of mean firing rates across 50 stimulus presentations. Depth from cortical surface of MU response is indicated to the side of each subpanel and corresponding layers are indicated on the left of the figure. A: baseline MU responses were greatest in granular and upper infragranular layers and typically exhibited onset and/or sustained response types. B: MU responses following salicylate were more responsive to the onset of the stimulus than baseline recordings. The supragranular layer that was relatively unresponsive during baseline recordings became responsive following salicylate.
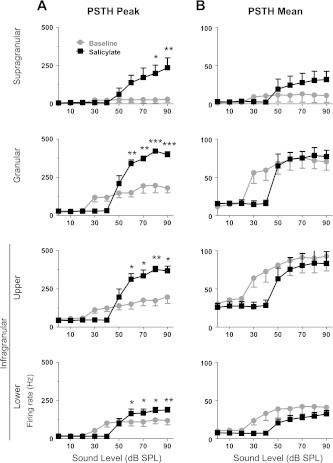
The spatial profile of MU activity was characterized by averaging the PSTH for each channel within supragranular, granular, upper infragranular, and lower infragranular layers. The phasic and sustained portions of the MU response were evaluated by measuring the peak and mean (within a 50-ms poststimulus window) firing rates, respectively. As mentioned earlier, systemic treatment with salicylate is known to decrease peripheral sensitivity and therefore the MU response measurements were tested for statistical significance at stimulus levels of 50 dB SPL and higher. The peak response IO functions (Fig. 8A) were significantly enhanced in all layers by salicylate treatment [supragranular F(1,40) = 26.87, P < 0.0001; granular F(1,40) = 66.96, P < 0.0001; upper infragranular F(1,40) = 43.74, P < 0.0001; lower infragranular F(1,40) = 7.49, P < 0.01]. In contrast to the greatly enhanced peak response following salicylate, the IO functions generated from the mean response (Fig. 8B) were enhanced in the supragranular layer [F(1,40) = 4.86, P = 0.033] and decreased in the lower infragranular layer [F(1,40) = 6.12, P = 0.017] to a small degree, whereas the mean firing rates of the granular [F(1,40) = 0.08, P = 0.78] and upper infragranular [F(1,40) = 1.02, P = 0.32] responses were not significantly affected by the salicylate treatment above 50 dB SPL. Despite a significantly enhanced peak response in the granular and upper infragranular layers, the mean response in these layers did not change. This indicates that spiking from the sustained phase of the normal response was reapportioned to the onset response. In other words, approximately the same number of action potentials was generated by granular and upper infragranular neurons following salicylate as during baseline recordings; however, the temporal evolution of the MU response was weighted toward stimulus onset following salicylate.
Fig. 8.
Salicylate alters MU laminar responses across layers of A1. Mean PSTHs were generated for supragranular, granular, upper infragranular, and lower infragranular channels, as indicated in Fig. 6. Both peak and mean firing rates were measured from the PSTHs to characterize the MU response. A: peak firing rate IO function of mean PSTHs was significantly enhanced following salicylate in all layers. B: mean firing rate within first 50 ms of response was less affected than peak response firing rate. (Repeated-measures two-way ANOVA with Bonferroni posttests, *P < 0.05, **P < 0.01, ***P < 0.001.)
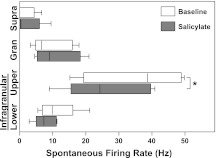
Laminar profile of spontaneous multiunit activity.
Spontaneous MU activity was recorded for 10 min in quiet during baseline and salicylate conditions. Figure 9 shows the distributions of mean spontaneous firing rates grouped into supragranular, granular, upper infragranular, and lower infragranular layers. The only significant change in MU spontaneous firing rates following salicylate treatment was a small decrease in upper infragranular layers [baseline: M = 35.00, SD = 15.19, salicylate: M = 26.91, SD = 13.01, one-way paired t-test: t(4) = 2.905, P = 0.0219]. This result agrees with the small effect observed by a previous study in cat A1 (Eggermont and Kenmochi 1998) and in the awake rat (Yang et al. 2007). No significant effects were observed in the other layers [supragranular t(4) = 0.4117, P = 0.3508; granular t(4) = 0.4681, P = 0.3321; lower infragranular t(4) = 0.9617, P = 0.1953]. No significant changes were observed for any layer in saline control experiments [supragranular t(2) = 0.1804, P = 0.4367; granular t(2) = 0.3119, P = 0.3923; upper infragranular t(2) = 0.7176, P = 0.2737; lower infragranular t(2) = 0.02310, P = 0.4918].
Fig. 9.
Effects of salicylate on spontaneous activity across cortical layers. A significant decrease in spontaneous firing rate was observed in upper infragranular layers following salicylate treatment. Supragranular, granular, and lower infragranular layers were not significantly altered. See Fig. 6 for layer designations. Boxplot whiskers represent data range, outer edge of box represents second and fourth quartiles of data, and midline represents median of data (*P < 0.05).
Laminar profile of multiunit frequency receptive fields.
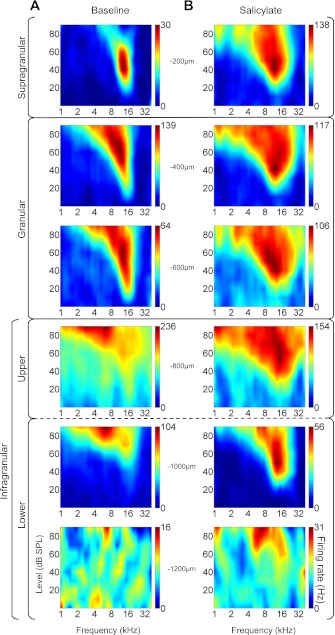
We have previously demonstrated that salicylate administration alters the tuning and/or FRF bandwidths (Stolzberg et al. 2011). To evaluate the laminar profile of excitatory FRFs in A1, maps were generated from MU responses on each channel before and after salicylate treatment. Maps representative of excitatory FRFs recorded in supragranular, granular, upper infragranular, and lower infragranular layers from a single experiment are presented in Fig. 10. Baseline maps (Fig. 10A) across layers were qualitatively similar to those reported previously for guinea pig A1 (Wallace and Palmer 2008).
Fig. 10.
MU frequency receptive fields (FRFs) sampled across depths of A1. A: MU FRFs during baseline recording of representative experiment in −200-μm steps starting at 200 μm below cortical surface (topmost plot). B: same experiment following systemic salicylate administration. Heat maps are calibrated to each electrode's maximal firing rate as indicated on the color bar to the right of each subplot. Electrode depth is indicated next to each subplot.
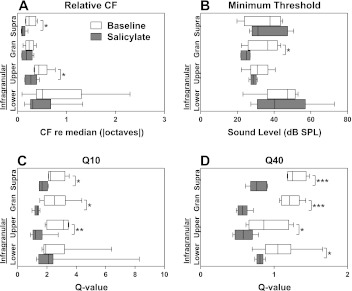
Tuning properties of FRFs were estimated for each channel and grouped into granular, upper infragranular, and lower infragranular layers. To evaluate the consistency of FRF tuning across cortical layers following salicylate, the characteristic frequencies (CFs; i.e., the stimulus frequency that elicits a response at the lowest sound level) were compared (in octaves) to the median CF across all layers within each recording (Fig. 11A). There was a significant decrease in the variability of CFs recorded from units in supragranular [t(9) = 3.046, P = 0.0139] and upper infragranular [t(10) = 2.434, P = 0.0352] layers indicating CFs were more similar across layers following salicylate. Minimum thresholds derived from FRF maps (Fig. 11B) significantly decreased for neurons in the granular layer [t(10) = 2.753, P = 0.0204] following salicylate compared with baseline; however, minimum thresholds were not significantly affected by salicylate treatment in other layers [supragranular t(9) = 0.3219, P = 0.7549; upper infragranular t(10) = 1.049, P = 0.3189; lower infragranular t(14) = 0.0282, P = 0.9779].
Fig. 11.
Effects of salicylate on MU FRF tuning properties across cortical layers. A: to quantify the variance in characteristic frequencies (CFs) across cortical depth, the absolute values of the CFs across layers were compared with the overall median CF within each recording. B: minimum thresholds of MU FRFs were significantly altered only in granular layers. Q-values were computed as C. Q10 values significantly decreased (broadened tuning) in supragranular, granular, and infragranular layers, but not in deep layers. D: Q40 values significantly decreased in all layers. Boxplot whiskers represent data range, outer edge of box represent second and fourth quartiles of data, and midline represents median of data. (Significance determined using standard t-test, *P < 0.05, ***P < 0.001.)
Q-values (Q = CF/bandwidth) are a useful indicator of the sharpness of tuning irrespective of frequency sensitivity. Q-values were computed for supragranular, granular, upper infragranular, and lower infragranular FRF tuning curves at 10 and 40 dB above minimum threshold (Fig. 11, C and D). Q10 values significantly decreased for the supragranular [t(9) = 2.911, P = 0.0173], granular [t(10) = 3.143, P = 0.0105], and upper infragranular [t(10) = 3.493, P = 0.0058] layers, but not for the lower infragranular layer [t(14) = 0.03565, P = 0.9721]. Q40 values significantly decreased for all layers [supragranular t(9) = 6.704, P < 0.0001; granular t(10) = 9.970, P < 0.0001; upper infragranular t(10) = 2.614, P = 0.0259; lower infragranular t(12) = 2.552, P = 0.0254]. These data indicate that FRFs broadened following salicylate treatment across all cortical layers, with the most significant effect occurring in the supragranular and granular layers.
DISCUSSION
The primary auditory cortex (A1) receives auditory signals already preprocessed based on temporal and spectral features along the auditory neuroaxis. A primary function of A1 is to reintegrate temporal and spectral features of sound into a representation of the acoustic environment (for a recent review, see Sharpee et al. 2011). Although previous investigations into salicylate's effects on the auditory brain have observed abnormal sound-evoked neural activity along the classical and nonclassical auditory pathway, the downstream positioning of A1 invokes the question as to whether abnormal neural activity observed in A1 is inherited or generated locally. To investigate this, we applied CSD analysis to translaminar LFP recorded simultaneously with MU activity across layers of A1. Our primary finding was that sound-evoked activation of the supragranular sink (sSk) in rat A1 exhibited a significantly shorter peak latency and enhanced peak amplitude following systemic administration of sodium salicylate (250 mg/kg, IP). The enhanced sSk peak, which was normally smaller than the thalamically driven granular sink (gSk), became equal to or greater in amplitude than gSk following salicylate at higher sound levels (Figs. 3, 4A, and 5B). Additionally, the MU response onset to noise bursts was significantly enhanced at the expense of the sustained phase of the MU response following salicylate. Taken together, we propose that systemically delivered salicylate at a dose known to reliably induce tinnitus behavior in rats (Lobarinas et al. 2004) results in intracortical circuits in A1 that are hyperresponsive to suprathreshold sound stimulation.
Several previous investigations have observed salicylate-related changes in neural activity in A1, secondary auditory cortex (A2), and anterior auditory field (Eggermont and Kenmochi 1998; Kenmochi and Eggermont 1997; Norena et al. 2010; Stolzberg et al. 2011; Sun et al. 2009; Yang et al. 2007; Zhang et al. 2011). To date, all of these recordings sampled neural activity isolated to thalamorecipient granular layers (layers III/IV) or from an undisclosed recording depth. Following thalamic activation of the granular layer, a cascade of intracortical synaptic connections propagate the signal for further processing by local networks in other layers within A1 or to other regions of the brain (Happel et al. 2010; Mitzdorf 1985; Szymanski et al. 2009, 2011).
CSD analysis applied to intracortically recorded translaminar LFPs is a common tool used in sensory neuroscience to reveal the net flow of ions into (sinks) and out of (sources) the neuropil. LFPs sampled by electrodes in close proximity (within a few hundred microns) overlap to a great extent due to volume conduction. CSD analysis applied across electrode sites results in a better resolved spatial profile of neural activity than simply observing the original LFPs (Barth and Di 1990; Mitzdorf 1985). We used this analytical tool to measure alterations in the laminar profile of sound-driven activation of A1 following systemic injection of salicylate.
High-dose systemic treatment with salicylate is well known to reduce peripheral sensitivity (for review, see Cazals 2000; Stolzberg et al. 2011), resulting in a significant reduction of auditory input to the brain (Sun et al. 2009). Despite this reduction, sound-evoked potentials have been observed to be significantly enhanced at the auditory cortex at suprathreshold intensities (Lobarinas et al. 2006; Sun et al. 2009). The primary result from CSD analysis on mean sound-driven LFPs recorded from A1 extends previous studies by identifying a significant enhancement of sink peak amplitude (Fig. 5B) and decreased peak latency (Fig. 5A) of the supragranular sink (sSk) following salicylate. The supragranular sink reflects the intracortical polysynaptic activation of dendritic tufts of medium-sized and large pyramidal neurons with cell bodies residing in layers III and V (Mitzdorf 1985; Winer 1984; Winer and Prieto 2001). This enhancement is particularly interesting in light of recent evidence that horizontal fibers within supragranular layers mediate intracortical spectral integration over large anatomic and spectral distances (Happel et al. 2010; Kaur et al. 2005; Metherate et al. 2005). Distortions of the normal tonotopic gradient in A1 have been observed following systemic salicylate administration (Stolzberg et al. 2011) and noise exposure (Eggermont and Komiya 2000; Engineer et al. 2011; Irvine et al. 2000; Robertson and Irvine 1989; Yang et al. 2011), as well as in humans experiencing tinnitus (Wienbruch et al. 2006). Our findings support the hypothesis (Eggermont and Roberts 2004) that intracortical horizontal fibers likely mediate the broad spectral integration required for abnormal A1 tonotopy during tinnitus.
It should be noted that in addition to intracortical horizontal fibers, supragranular layers also receive monosynaptic connections from medial and dorsal divisions of the medial geniculate thalamus (Mitani et al. 1987; Rubio-Garrido et al. 2009). However, because of the significant decrease in latency of the supragranular sink following salicylate (Fig. 5A), it is unlikely that the enhanced response reflects activation by thalamic fibers. Furthermore, Happel and colleagues (2010) showed that direct application of the γ-aminobutyric acid type A (GABAA) agonist muscimol or muscimol combined with the GABAB antagonist SCH-50911 to the surface of A1 abolished the supragranular sink driven by non-best-frequency sound stimuli, whereas the granular sink remained. Their result demonstrates that sSk predominantly represents activation by intracortical fibers. In contrast, both gSk and iSkearly sinks, reflecting monosynaptic thalamic activations, did not exhibit a decrease in latency following salicylate (Fig. 5A).
In the present study, the granular sink (gSk; layers III/IV), reflecting the strong principal thalamic drive received from vMGB, was also significantly enhanced following systemic salicylate administration (Fig. 5B). This enhancement is most likely the result of cortical disinhibition by the direct action of salicylate on cortical neurons. Evidence supporting this hypothesis is derived from studies of salicylate's effects in A1 in vitro. The application of salicylate during whole-cell patch clamp selectively hyperpolarized fast-spiking inhibitory interneurons (Su et al. 2009) and decreased the frequency and magnitude of evoked and spontaneous miniature postsynaptic currents in layer II/III pyramidal cells (effects of salicylate were not reported for other cortical layers; Wang et al. 2006). Cortical disinhibition by the direct action of salicylate on fast-spiking inhibitory interneurons would also be expected to play a role in the unmasking of intracortical excitatory horizontal fibers (Eggermont and Roberts 2004) within supragranular layers as described earlier. Furthermore, we previously found that despite a reduction in sound-evoked field potentials recorded from the cochlea (compound action potential) following systemic salicylate administration, a small increase in sound-evoked field potential was recorded from the inferior colliculus and a large increase recorded from the auditory cortex (Sun et al. 2009). This result differed from recordings following direct application of salicylate to the round window of the cochlea. Application of salicylate directly to the cochlea decreased sound-evoked activity recorded from each of these structures (Sun et al. 2009). Thus, despite reduced sensorineural output to the brain following salicylate, the auditory cortex was hyperresponsive to suprathreshold sounds. These findings are consistent with the hypothesis that in addition to decreased peripheral sensitivity (for a review on salicylate's peripheral effects, see Cazals 2000), systemic salicylate treatment directly affects the brain (Kenmochi and Eggermont 1997; Lu et al. 2011).
The late deep sink, iSklate, was the longest-latency sink consistently observed in our recordings. The iSklate sink most likely reflects polysynaptic intracortical perisomatic activation of large layer V/VI pyramidal neurons. Furthermore, iSklate may reflect, at least in part, a major output of initial processing by the A1 neural circuit. The infragranular layers contain a heterogeneous population of cells with a large number of descending projections (Prieto and Winer 1999; Winer and Prieto 2001). Because CSD analysis primarily reflects activation of neurons coextending the linear electrode array (which was inserted orthogonal to the cortical surface), we can speculate that iSklate primarily represents activation of vertically oriented pyramidal neurons. Following salicylate, the iSklate peak was significantly enhanced (Fig. 5B), possibly reflecting the result of intracortical signal amplification. If this is the case, then intracortical amplification may play an important role in modulating collicular and/or other subcortical sound processing. This interpretation of iSklate is based on the existence of subcortically projecting layer V/VI neurons (for review on corticofugal connections of auditory cortex, see Winer 2006) and the results from recent CSD experiments in rat A1 (Szymanski et al. 2011).
Strong sound-evoked MU activity was observed in granular and both upper and lower infragranular layers during baseline recordings, whereas supragranular MU activity was either not present or very low during baseline recordings (Fig. 7A). Salicylate treatment significantly increased the firing rate of the MU onset response in all cortical layers, most significantly in supragranular, granular, and upper infragranular layers (Figs. 7B and 8A); however, the mean MU response was unchanged at sound levels 50 dB or higher (Fig. 8B). This discrepancy between mean and peak firing rates is indicative of a reapportionment of spiking from the sustained phase of the MU response to the phasic onset phase of the response. In other words, the sound stimulus evoked approximately the same number of action potentials in granular and infragranular layers before and after salicylate treatment; however, the onset response of cortical neurons was significantly stronger than was observed during baseline recordings. The enhanced onset response may be explained by a salicylate-induced disinhibition as discussed earlier. Furthermore, the sustained phase of the MU response was suppressed following salicylate, which may be explained by a salicylate-protracted Ca2+-activated K+ conductance resulting in the increased refractory period of cortical neurons (Ochi and Eggermont 1996).
The spectral receptive fields of MUs varied across layers of A1, with a tendency for sharper frequency tuning in the granular layer, whereas infragranular FRFs tended to be broader (Figs. 10A and 11B). The laminar profile of MU FRFs presented here (Fig. 10A) shares similarities with single-unit FRFs recorded in guinea pig A1 (Wallace and Palmer 2008). Salicylate significantly broadened the frequency response of MUs in all layers (Fig. 11, C and D). Interestingly, the minimum threshold was significantly lower for granular layer MU FRFs following salicylate, whereas minimum thresholds of supragranular, upper and lower infragranular layer FRFs were not significantly affected (Fig. 11B). Since systemic salicylate reduces peripheral sensitivity, the reduction in minimum thresholds of FRFs observed in the granular layer was unexpected. One possible explanation of this result is that, as discussed earlier, a local (Su et al. 2009; Wang et al. 2006) or systemic (Lu et al. 2011) treatment with a high dose of salicylate directly disinhibits the auditory cortex, which has been shown to both expand the bandwidth and decrease the minimum threshold of FRFs of neurons in auditory cortex (Wang et al. 2000).
Spontaneous MU activity was unchanged in supragranular, granular, and lower infragranular layers, whereas a significant decrease was measured in upper infragranular layers (Fig. 9). This result corroborates previous MU recordings in the auditory cortex of awake rats following a similar systemic dose of salicylate (Yang et al. 2007). Eggermont and Kenmochi (1998) reported a similar trend toward decreased spontaneous firing rates in anesthetized cat A1 and increased spontaneous firing rates in the cat A2. They proposed that such a discordance between spontaneous firing rates in A1 and A2 following salicylate may be involved in the generation of the tinnitus perception (see discussion in Eggermont and Kenmochi 1998). However, previous studies observed an increase in c-fos immunoreactivity and 2-deoxyglucose uptake in A1 and other brain regions, possibly indicating an increase in spontaneous neural activity following acute salicylate-induced tinnitus compared with vehicle control animals (Mahlke and Wallhausser-Franke 2004; Wallhausser-Franke et al. 1996, 2003, 2006). The results of studies using protein and metabolic indicators of neural activity appear to be in conflict with our electrophysiologic findings as well as those of previous reports (Eggermont and Kenmochi 1998; Yang et al. 2007). More empirical data will be required to resolve this apparent discrepancy.
In considering the functional consequences of a hyperresponsive A1, it is worth noting that administration of salicylate at high doses has been suggested to induce hyperacusis-like behavior in rats, whereby the animals exhibit exaggerated startle reflex response to moderate to high intensity sounds (Sun et al. 2009). It is reasonable to propose that the intracortical gain enhancement we report here could provide the neural substrate for such hyperacusis-like behavior. Furthermore, altered intracortical processing within A1 circuits may indicate that this structure serves as a critical juncture in the auditory pathway gating the recruitment of other cortical and/or limbic structures in response to loud sounds. This may help to explain the high comorbidity (∼79%) of tinnitus and a decreased tolerance to sound experienced in hyperacusis (Dauman and Bouscau-Faure 2005). Ultimately, systemic salicylate administration in rats may be a useful model to investigate possible neural mechanisms underlying both tinnitus and hyperacusis (Sun et al. 2009).
Here we presented evidence that A1 circuitry abnormally amplifies sound-evoked neural activity primarily by way of intracortical circuits. Although aberrant neural activity has been observed in many brain regions following a high dose of salicylate, the amplification of auditory signals within A1 may result in recruitment of other cortical and limbic structures.
GRANTS
This research was supported in part by National Institute on Deafness and Other Communication Disorders Grants F31 DC-010931-02 (to D.S.), R01 DC-0090910 (to R.J.S.), R01 DC-009219 (to R.J.S.), and R03 DC-011374-01 (to B.L.A.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.S. and R.J.S. conception and design of research; D.S. performed experiments; D.S. analyzed data; D.S. and B.L.A. interpreted results of experiments; D.S. prepared figures; D.S. drafted manuscript; D.S., M.C., R.J.S., and B.L.A. edited and revised manuscript; D.S., M.C., R.J.S., and B.L.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Max Happel (the Leibniz-Institute for Neurobiology) for discussions regarding current-source density analysis, and the anonymous reviewers for valuable feedback on the manuscript.
REFERENCES
- Barth DS, Di S. Three-dimensional analysis of auditory-evoked potentials in rat neocortex. J Neurophysiol 64: 1527–1536, 1990 [DOI] [PubMed] [Google Scholar]
- Brennan J, Brown C, Jastreboff P. Salicylate-induced changes in auditory thresholds of adolescent and adult rats. Dev Psychobiol 29: 69–155, 1996 [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci 22: 2383–2390, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazals Y. Auditory sensori-neural alterations induced by salicylate. Prog Neurobiol 62: 583–631, 2000 [DOI] [PubMed] [Google Scholar]
- Chen GD, Jastreboff PJ. Salicylate-induced abnormal activity in the inferior colliculus of rats. Hear Res 82: 158–178, 1995 [DOI] [PubMed] [Google Scholar]
- Dauman R, Bouscau-Faure F. Assessment and amelioration of hyperacusis in tinnitus patients. Acta Otolaryngol 125: 503–509, 2005 [DOI] [PubMed] [Google Scholar]
- Di S, Barth DS. The functional anatomy of middle-latency auditory evoked potentials: thalamocortical connections. J Neurophysiol 68: 425–431, 1992 [DOI] [PubMed] [Google Scholar]
- Dohrmann K, Weisz N, Schlee W, Hartmann T, Elbert T. Neurofeedback for treating tinnitus. Prog Brain Res 166: 473–485, 2007 [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Role of auditory cortex in noise- and drug-induced tinnitus. Am J Audiol 17: S162–S169, 2008 [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Kenmochi M. Salicylate and quinine selectively increase spontaneous firing rates in secondary auditory cortex. Hear Res 117: 149–160, 1998 [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Komiya H. Moderate noise trauma in juvenile cats results in profound cortical topographic map changes in adulthood. Hear Res 142: 89–101, 2000 [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci 27: 676–682, 2004 [DOI] [PubMed] [Google Scholar]
- Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, Borland MS, Kilgard MP. Reversing pathological neural activity using targeted plasticity. Nature 470: 101–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel MF, Jeschke M, Ohl FW. Spectral integration in primary auditory cortex attributable to temporally precise convergence of thalamocortical and intracortical input. J Neurosci 30: 11114–11127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding GW. The currents that flow in the somatosensory cortex during the direct cortical response. Exp Brain Res 90: 29–39, 1992 [DOI] [PubMed] [Google Scholar]
- Huang CL, Winer JA. Auditory thalamocortical projections in the cat: laminar and areal patterns of input. J Comp Neurol 427: 302–331, 2000 [DOI] [PubMed] [Google Scholar]
- Irvine DR, Rajan R, McDermott HJ. Injury-induced reorganization in adult auditory cortex and its perceptual consequences. Hear Res 147: 188–199, 2000 [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Afman CE. Hyperactivity in the dorsal cochlear nucleus after intense sound exposure and its resemblance to tone-evoked activity: a physiological model for tinnitus. Hear Res 140: 165–172, 2000 [DOI] [PubMed] [Google Scholar]
- Kaur S, Rose HJ, Lazar R, Liang K, Metherate R. Spectral integration in primary auditory cortex: laminar processing of afferent input, in vivo and in vitro. Neuroscience 134: 1033–1045, 2005 [DOI] [PubMed] [Google Scholar]
- Kenmochi M, Eggermont JJ. Salicylate and quinine affect the central nervous system. Hear Res 113: 110–116, 1997 [DOI] [PubMed] [Google Scholar]
- Kimura M, Eggermont JJ. Effects of acute pure tone induced hearing loss on response properties in three auditory cortical fields in cat. Hear Res 135: 146–162, 1999 [DOI] [PubMed] [Google Scholar]
- Lakatos P, Pincze Z, Fu KM, Javitt DC, Karmos G, Schroeder CE. Timing of pure tone and noise-evoked responses in macaque auditory cortex. Neuroreport 16: 933–937, 2005 [DOI] [PubMed] [Google Scholar]
- Lobarinas E, Sun W, Cushing R, Salvi R. A novel behavioral paradigm for assessing tinnitus using schedule-induced polydipsia avoidance conditioning (SIP-AC). Hear Res 190: 109–114, 2004 [DOI] [PubMed] [Google Scholar]
- Lobarinas E, Yang G, Sun W, Ding D, Mirza N, Dalby-Brown W, Hilczmayer E, Fitzgerald S, Zhang L, Salvi R. Salicylate- and quinine-induced tinnitus and effects of memantine. Acta Otolaryngol Suppl 556: 13–19, 2006 [DOI] [PubMed] [Google Scholar]
- Lockwood AH, Salvi RJ, Burkard RF, Galantowicz PJ, Coad ML, Wack DS. Neuroanatomy of tinnitus. Scand Audiol Suppl 51: 47–52, 1999 [PubMed] [Google Scholar]
- Londero A, Langguth B, De Ridder D, Bonfils P, Lefaucheur JP. Repetitive transcranial magnetic stimulation (rTMS): a new therapeutic approach in subjective tinnitus? Neurophysiol Clin 36: 145–155, 2006 [DOI] [PubMed] [Google Scholar]
- Longenecker RJ, Galazyuk AV. Development of tinnitus in CBA/CaJ mice following sound exposure. J Assoc Res Otolaryngol 12: 647–658, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz I, Muller N, Schlee W, Hartmann T, Weisz N. Loss of alpha power is related to increased gamma synchronization: a marker of reduced inhibition in tinnitus? Neurosci Lett 453: 225–228, 2009 [DOI] [PubMed] [Google Scholar]
- Lu J, Lobarinas E, Deng A, Goodey R, Stolzberg D, Salvi RJ, Sun W. GABAergic neural activity involved in salicylate-induced auditory cortex gain enhancement. Neuroscience 189: 187–198, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WL, Hidaka H, May BJ. Spontaneous activity in the inferior colliculus of CBA/J mice after manipulations that induce tinnitus. Hear Res 212: 9–21, 2006 [DOI] [PubMed] [Google Scholar]
- Mahlke C, Wallhausser-Franke E. Evidence for tinnitus-related plasticity in the auditory and limbic system, demonstrated by arg31 and c-fos immunocytochemistry. Hear Res 195: 17–34, 2004 [DOI] [PubMed] [Google Scholar]
- Metherate R, Kaur S, Kawai H, Lazar R, Liang K, Rose HJ. Spectral integration in auditory cortex: mechanisms and modulation. Hear Res 206: 146–158, 2005 [DOI] [PubMed] [Google Scholar]
- Mitani A, Itoh K, Mizuno N. Distribution and size of thalamic neurons projecting to layer I of the auditory cortical fields of the cat compared to those projecting to layer IV. J Comp Neurol 257: 105–121, 1987 [DOI] [PubMed] [Google Scholar]
- Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev 65: 37–100, 1985 [DOI] [PubMed] [Google Scholar]
- Moeller CK, Kurt S, Happel MF, Schulze H. Long-range effects of GABAergic inhibition in gerbil primary auditory cortex. Eur J Neurosci 31: 49–59, 2010 [DOI] [PubMed] [Google Scholar]
- Norena AJ, Moffat G, Blanc JL, Pezard L, Cazals Y. Neural changes in the auditory cortex of awake guinea pigs after two tinnitus inducers: salicylate and acoustic trauma. Neuroscience 166: 1194–1209, 2010 [DOI] [PubMed] [Google Scholar]
- Ochi K, Eggermont JJ. Effects of salicylate on neural activity in cat primary auditory cortex. Hear Res 95: 63–76, 1996 [DOI] [PubMed] [Google Scholar]
- Polley DB, Read HL, Storace DA, Merzenich MM. Multiparametric auditory receptive field organization across five cortical fields in the albino rat. J Neurophysiol 97: 3621–3638, 2007 [DOI] [PubMed] [Google Scholar]
- Prieto JJ, Winer JA. Layer VI in cat primary auditory cortex: Golgi study and sublaminar origins of projection neurons. J Comp Neurol 404: 332–358, 1999 [DOI] [PubMed] [Google Scholar]
- Ralli M, Lobarinas E, Fetoni AR, Stolzberg D, Paludetti G, Salvi R. Comparison of salicylate- and quinine-induced tinnitus in rats: development, time course, and evaluation of audiologic correlates. Otol Neurotol 31: 823–831, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D, Irvine DR. Plasticity of frequency organization in auditory cortex of guinea pigs with partial unilateral deafness. J Comp Neurol 282: 456–471, 1989 [DOI] [PubMed] [Google Scholar]
- Rubio-Garrido P, Perez-de-Manzo F, Porrero C, Galazo MJ, Clasca F. Thalamic input to distal apical dendrites in neocortical layer 1 is massive and highly convergent. Cereb Cortex 19: 2380–2395, 2009 [DOI] [PubMed] [Google Scholar]
- Rutkowski RG, Miasnikov AA, Weinberger NM. Characterisation of multiple physiological fields within the anatomical core of rat auditory cortex. Hear Res 181: 116–130, 2003 [DOI] [PubMed] [Google Scholar]
- Schaette R, Konig O, Hornig D, Gross M, Kempter R. Acoustic stimulation treatments against tinnitus could be most effective when tinnitus pitch is within the stimulated frequency range. Hear Res 269: 95–101, 2010 [DOI] [PubMed] [Google Scholar]
- Scholl B, Wehr M. Disruption of balanced cortical excitation and inhibition by acoustic trauma. J Neurophysiol 100: 646–656, 2008 [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Javitt DC, Steinschneider M, Mehta AD, Givre SJ, Vaughan HG, Jr, Arezzo JC. N-Methyl-d-aspartate enhancement of phasic responses in primate neocortex. Exp Brain Res 114: 271–278, 1997 [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Lindsley RW, Specht C, Marcovici A, Smiley JF, Javitt DC. Somatosensory input to auditory association cortex in the macaque monkey. J Neurophysiol 85: 1322–1327, 2001 [DOI] [PubMed] [Google Scholar]
- Seki S, Eggermont JJ. Changes in cat primary auditory cortex after minor-to-moderate pure-tone induced hearing loss. Hear Res 173: 172–186, 2002 [DOI] [PubMed] [Google Scholar]
- Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med 123: 711–718, 2010 [DOI] [PubMed] [Google Scholar]
- Sharpee T, Atencio C, Schreiner C. Hierarchical representations in the auditory cortex. Curr Opin Neurobiol 21: 761–768, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark E, Abeles M. Predicting movement from multiunit activity. J Neurosci 27: 8387–8394, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzberg D, Chen GD, Allman BL, Salvi RJ. Salicylate-induced peripheral auditory changes and tonotopic reorganization of auditory cortex. Neuroscience 180: 157–164, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YY, Luo B, Wang HT, Chen L. Differential effects of sodium salicylate on current-evoked firing of pyramidal neurons and fast-spiking interneurons in slices of rat auditory cortex. Hear Res 253: 60–66, 2009 [DOI] [PubMed] [Google Scholar]
- Sun W, Lu J, Stolzberg D, Gray L, Deng A, Lobarinas E, Salvi RJ. Salicylate increases the gain of the central auditory system. Neuroscience 159: 325–334, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski F, Rabinowitz N, Magri C, Panzeri S, Schnupp J. The laminar and temporal structure of stimulus information in the phase of field potentials of auditory cortex. J Neurosci 31: 15787–16588, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski FD, Garcia-Lazaro JA, Schnupp JW. Current source density profiles of stimulus-specific adaptation in rat auditory cortex. J Neurophysiol 102: 1483–1490, 2009 [DOI] [PubMed] [Google Scholar]
- Wallace MN, Palmer AR. Laminar differences in the response properties of cells in the primary auditory cortex. Exp Brain Res 184: 179–191, 2008 [DOI] [PubMed] [Google Scholar]
- Wallhausser-Franke E, Braun S, Langner G. Salicylate alters 2-DG uptake in the auditory system: a model for tinnitus? Neuroreport 7: 1585–1588, 1996 [DOI] [PubMed] [Google Scholar]
- Wallhausser-Franke E, Cuautle-Heck B, Wenz G, Langner G, Mahlke C. Scopolamine attenuates tinnitus-related plasticity in the auditory cortex. Neuroreport 17: 1487–1491, 2006 [DOI] [PubMed] [Google Scholar]
- Wallhausser-Franke E, Mahlke C, Oliva R, Braun S, Wenz G, Langner G. Expression of c-fos in auditory and non-auditory brain regions of the gerbil after manipulations that induce tinnitus. Exp Brain Res 153: 649–654, 2003 [DOI] [PubMed] [Google Scholar]
- Wang HT, Luo B, Zhou KQ, Xu TL, Chen L. Sodium salicylate reduces inhibitory postsynaptic currents in neurons of rat auditory cortex. Hear Res 215: 77–83, 2006 [DOI] [PubMed] [Google Scholar]
- Wang J, Caspary D, Salvi RJ. GABA-A antagonist causes dramatic expansion of tuning in primary auditory cortex. Neuroreport 11: 1137–1140, 2000 [DOI] [PubMed] [Google Scholar]
- Weisz N, Voss S, Berg P, Elbert T. Abnormal auditory mismatch response in tinnitus sufferers with high-frequency hearing loss is associated with subjective distress level (Abstract). BMC Neurosci 5: 8, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienbruch C, Paul I, Weisz N, Elbert T, Roberts LE. Frequency organization of the 40-Hz auditory steady-state response in normal hearing and in tinnitus. NeuroImage 33: 180–194, 2006 [DOI] [PubMed] [Google Scholar]
- Winer JA. The pyramidal neurons in layer III of cat primary auditory cortex (AI). J Comp Neurol 229: 476–496, 1984 [DOI] [PubMed] [Google Scholar]
- Winer JA. Decoding the auditory corticofugal systems. Hear Res 212: 1–8, 2006 [DOI] [PubMed] [Google Scholar]
- Winer JA, Prieto JJ. Layer V in cat primary auditory cortex (AI): cellular architecture and identification of projection neurons. J Comp Neurol 434: 379–412, 2001 [DOI] [PubMed] [Google Scholar]
- Yang G, Lobarinas E, Zhang L, Turner J, Stolzberg D, Salvi R, Sun W. Salicylate induced tinnitus: behavioral measures and neural activity in auditory cortex of awake rats. Hear Res 226: 244–253, 2007 [DOI] [PubMed] [Google Scholar]
- Yang S, Weiner BD, Zhang LS, Cho SJ, Bao S. Homeostatic plasticity drives tinnitus perception in an animal model. Proc Natl Acad Sci USA 108: 14974–14979, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yang P, Cao Y, Qin L, Sato Y. Salicylate induced neural changes in the primary auditory cortex of awake cats. Neuroscience 172: 232–245, 2011 [DOI] [PubMed] [Google Scholar]