Abstract
Activation of the medial olivocochlear (MOC) efferents attenuates cochlear gain and reduces the amplitudes of mechanical, electrical, and neural cochlear outputs. The functional roles of the MOC efferents are not fully understood, especially in humans, despite postulations that they are involved in protection against acoustic trauma, facilitation of transient-sound perception, etc. Delineating the frequency tuning properties of the MOC efferents would provide critical evidence to support or refute these postulated functional roles. By utilizing spontaneous otoacoustic emissions (SOAEs), a cochlear measure sensitive to MOC modulation, we systematically demonstrate in humans that the contralateral MOC reflex is tuned to a fixed frequency band between 500 and 1,000 Hz independent of SOAE frequency. Our results question the role of the MOC reflex in protection against acoustic trauma or facilitation of transient-sound perception.
Keywords: spontaneous otoacoustic emissions, cochlear mechanics
the cochlea receives descending innervation from the superior olivary complex via the olivocochlear bundle. Olivocochlear fibers projecting from the medial parts of the superior olivary complex to outer hair cells (OHCs) in the cochlea constitute the medial olivocochlear (MOC) efferents. Activation of the MOC efferents attenuates the gain of the cochlear amplifier and reduces the magnitude of basilar membrane vibration (Murugasu and Russell 1996), auditory nerve activity (Galambos 1956; Wiederhold 1970; Wiederhold and Kiang 1970), and otoacoustic emissions (OAEs) (Mountain 1980). The MOC efferents have been suggested to be involved in protection against acoustic trauma, facilitation of transient-sound perception in noise, auditory attention, etc. (see Guinan 2006; Robles and Delano 2008 for reviews).
Acoustic activation of the MOC efferents is known as the MOC reflex. MOC efferents can be activated by acoustic stimuli presented to the ear being monitored (ipsilateral MOC reflex, mediated by the crossed olivocochlear bundle), to the opposite ear (contralateral MOC reflex, mediated by the uncrossed olivocochlear bundle), or to both ears simultaneously (bilateral MOC reflex, mediated by both crossed and uncrossed olivocochlear bundles) (Guinan 2006). In human subjects, ipsilateral and contralateral MOC reflexes may not differ significantly in strength (Guinan 2006). The ipsilateral MOC reflex, however, is confounded by the acoustical and mechanical interactions between the probe and the MOC elicitor. Hence the contralateral MOC reflex is often studied as an alternative, in a noninvasive manner through OAEs.
The MOC reflex exhibits tuning, i.e., acoustic elicitors at different frequencies elicit changes of varying magnitudes in cochlear measures (e.g., OAEs) and auditory nerve activity. Several groups have reported frequency-dependent MOC effects on spontaneous otoacoustic emissions (SOAEs) (Harrison and Burns 1993; Mott et al. 1989), stimulus-frequency otoacoustic emissions (SFOAEs) (Lilaonitkul and Guinan 2009a, 2012), transient-evoked otoacoustic emissions (TEOAEs) (Veuillet et al. 1991), and distortion-product otoacoustic emissions (DPOAEs) (Chery-Croze et al. 1993). However, no conclusive tuning pattern of the MOC reflex in humans has emerged. Differences in underlying generation mechanisms between OAE types, their different sensitivities to MOC influence, small sample sizes, as well as complications from the middle-ear muscle (MEM) reflex, may have contributed to this lack of consensus. A thorough understanding of the tuning properties of the MOC reflex would undoubtedly shed light on the functional roles of the MOC efferents.
We chose SOAEs to systematically study the tuning pattern of the contralateral MOC reflex. SOAEs are low-level sounds emitted from the cochlea without external stimulation (Kemp 1979). They are presumably generated within a restricted cochlear region (Martin and Hudspeth 1999; Shera 2003) and are more sensitive to MOC modulation than other OAE types (Backus and Guinan 2007; Harrison and Burns 1993; Moulin et al. 1993; Puel and Rebillard 1990). MOC activation consistently elevates SOAE frequency while reducing SOAE level (Harrison and Burns 1993; Mott et al. 1989; Zhao and Dhar 2010, 2011). Here we report the frequency tuning of contralateral MOC effects on SOAEs over wide frequency ranges for both the probe (SOAEs) and the MOC elicitor, while stringently controlling for contamination from the MEM reflex. The unexpected results provide new insight into the functional roles of the MOC efferents that are yet to be fully understood.
METHODS
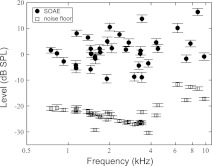
Eight subjects (7 women and 1 man) between 19 and 29 yr of age participated in this study. All subjects were prescreened for normal hearing thresholds (20 dB HL or better at octave frequencies between 250 and 8,000 Hz, measured by the Interacoustics Audio Traveller AA220) and normal middle ear functions (assessed by GSI TympStar Middle-Ear Analyzer) in both ears. All procedures were approved by the Northwestern University Institutional Review Board (Northwestern IRB #4X—Panel E, registration no. IRB00000736). Written informed consent was obtained from each subject before the tests. In each subject, the ear with more SOAEs of larger amplitudes was tested (1 left ear and 7 right ears). SOAEs above −10 dB SPL (standard deviations <2 dB over ∼1.5 h) were selected for this study, resulting in a total of thirty-five SOAEs (frequency: 749–9,722 Hz; level: −10–18 dB SPL; signal-to-noise ratio: 12–36 dB) (Fig. 1). Distribution of SOAEs among subjects is detailed in Table 1.
Fig. 1.
Scatterplot of all 35 spontaneous otoacoustic emissions (SOAEs) included in this study from 8 subjects. SOAEs lower than −10 dB SPL or with a SD over 2 dB during the course of the experiment (∼1.5 h) were rejected. Error bars represent ±1 SD.
Table 1.
Distribution of SOAEs among eight subjects
| Subject | SOAE Frequency, Hz |
|---|---|
| WTPM18 | 1,453,1,998 |
| WTPF25 | 831, 1,138, 1,576, 2,007, 2,318, 3,358, 4,376, 7,203 |
| WTPF53 | 3,134, 3,426, 4,361 |
| WTPF54 | 2,630, 3,341 |
| WTPF63 | 749, 1,465, 1,710, 1,908, 3,018, 3,426 |
| WTPF76 | 928, 1,142, 1,458, 1,693, 3,262 |
| WTPF84 | 1,325, 1,565, 1,894, 2,614, 3,818, 4,163, 4,967 |
| WTPF85 | 1,155, 1,394, 1,924, 2,075, 2,361, 2,758, 3,434, 4,484, 6,234, 7,727, 8,721, 9,712 |
SOAE, spontaneous otoacoustic emission.
Target SOAEs were monitored for 6 s, while a contralateral tone was presented from 3 to 6 s. Contralateral tones between 0.088 and 16 kHz were presented in half-octave steps at 55, 60, and 65 dB SPL to elicit MOC activity. Six trials were conducted for each condition. A short-time fast Fourier transform (3-s window) was performed on the first and last 3 s to yield estimates of SOAE level and frequency in the absence and presence of the contralateral tone, respectively. Contralateral elicitor-induced changes in SOAE level and frequency were then computed. Contralateral tones were calibrated by the depth-compensated simulator method (Lee et al. 2012).
MEM contraction was assessed by the phase-gradient delay method (for detailed description, see Zhao and Dhar 2011). SFOAEs were evoked by a 40 dB SPL probe tone swept from 0.8 to 1.8 kHz. The following triplet, a 40 dB SPL probe, a 60 dB SPL compressor, and the 40 dB SPL probe paired with a 65 dB SPL contralateral broadband noise (0.1–10 kHz), was repeated six times. SFOAEs and contralateral tone-induced vector change in the total ear canal pressure (ΔP) were extracted. A phase-gradient delay of ΔP around 10 ms near 1.5 kHz indicated MOC activation as the primary origin of ΔP. Since a 65 dB SPL contralateral broadband noise did not elicit MEM contraction that dominated OAE change in any of the subjects, it was unlikely that contralateral tones at 55, 60, or 65 dB SPL elicited MEM contraction that dominated OAE change. Thus we attributed the observed shifts in SOAE level and frequency to MOC activity.
RESULTS
Demonstration of frequency tuning of MOC reflex.
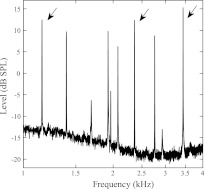
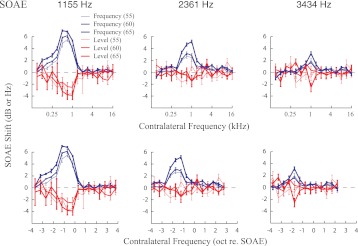
Contralateral tone-elicited, frequency-dependent MOC modulation of three SOAEs (1,155, 2,361, and 3,434 Hz) from subject WTPF85 (marked by arrows in Fig. 2) is displayed in Fig. 3. Red traces represent SOAE level changes, whereas blue traces represent SOAE frequency changes. Results obtained with three levels of contralateral MOC elicitor are displayed with different line widths. Contralateral elicitors increased SOAE frequency and reduced SOAE level. As the level of contralateral elicitors grew from 55 to 65 dB SPL in 5-dB steps the magnitude of SOAE change increased, but importantly the shapes of the tuning functions were preserved in most cases. Contralateral elicitors between 0.5 and 1 kHz produced the largest changes in all three SOAEs (Fig. 3, top), regardless of SOAE frequency. The frequency distance between the SOAE probe and the most effective contralateral elicitor increased with increasing SOAE frequency. The most effective contralateral elicitor was between 0.5 and 1 octave lower in frequency than the SOAE at 1,155 Hz, whereas the most effective contralateral elicitors were almost 2 octaves lower in frequency than the SOAEs at 3,434 Hz (Fig. 3, bottom).
Fig. 2.
SOAE spectrum of subject WTPF85 from a 60-s recording. The 3 SOAEs marked by arrows were examined in detail (results shown in Fig. 3).
Fig. 3.
Changes in SOAE level and frequency as a function of contralateral elicitor frequency. Tuning functions for 3 SOAEs (1,155, 2,361 and 3,434 Hz) from subject WTPF85 are displayed at left, center, and right, respectively, as a function of contralateral elicitor frequency in kHz (top) or octaves re. SOAE frequency (bottom). The tuning is skewed toward frequencies below the SOAE frequency. The skew grows with increasing SOAE frequency. Error bars represent ±1 SE. Results obtained with contralateral tones of 3 levels (55, 60, 65 dB SPL) are represented by different line widths.
Group results.
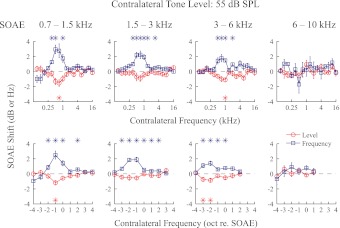
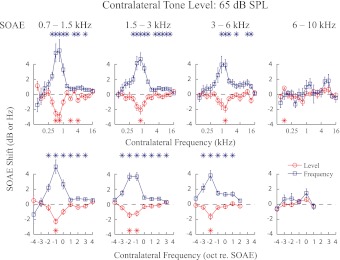
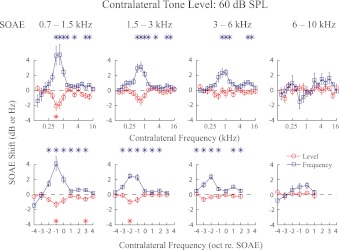
Thirty-five SOAEs recorded from eight ears (Fig. 1) were divided into four frequency bins (0.7–1.5 kHz, 1.5–3 kHz, 3–6 kHz, and 6–10 kHz), with 10, 11, 9, and 5 SOAEs in each bin, respectively. These frequency bins of similar widths were selected to yield approximately equal numbers of SOAEs in most bins for statistical analysis. Average changes in SOAE level and frequency for each bin with contralateral elicitor levels at 55, 60, and 65 dB SPL are presented in Figs. 4 through 6. While greater changes in SOAE level and frequency were seen with increasing contralateral elicitor level, the magnitudes of these changes decreased with increasing SOAE frequency (Figs. 4–6).
Fig. 4.
Effect of 55 dB SPL contralateral tones on SOAE level and frequency collapsed across all SOAEs included in this study. Thirty-five SOAEs from 8 subjects were divided into 4 frequency bins. The tuning functions for SOAE frequency and level changes appear to be mirror images of each other. Error bars represent ±1 SE. *Significant shifts (2-sided t-test, P < 0.01).
Fig. 6.
Same as Fig. 4 except with 65 dB SPL contralateral elicitors.
Fig. 5.
Same as Fig. 4 except with 60 dB SPL contralateral elicitors.
A consistent pattern of tuning was observed for changes in SOAE level and frequency. With the exception of SOAEs in the 6–10 kHz bin, where MOC effects were minimal and no tuning pattern could be observed, contralateral elicitors between 0.5 and 1 kHz were most effective in causing changes in SOAE level and frequency. Consequently, the most effective contralateral frequency was ∼1 octave below the SOAE frequency for the 0.7–1.5 kHz bin, 1–2 octaves below the SOAE frequency for the 1.5–3 kHz bin, and 2 octaves below the SOAE frequency for the 3–6 kHz bin (Figs. 4–6). Tuning patterns for SOAE frequency and level were roughly symmetrical. Sometimes another tuning peak can be observed at a higher frequency (e.g., 3–6 kHz bin, Fig. 5), although with a much smaller magnitude.
DISCUSSION
Contralateral acoustic stimulation is often preferred over ipsilateral acoustic stimulation for eliciting MOC activity in order to avoid complications related to intracochlear mechanical suppression. By utilizing fixed-level contralateral elicitors, we have observed frequency-dependent MOC effects on both SOAE frequency and level. Contralateral elicitors between 0.5 and 1 kHz produced the largest MOC effects, irrespective of SOAE frequency. The magnitude of changes in SOAE level and frequency decreased with increasing SOAE frequency. Below we compare our findings with previous studies on the frequency tuning of the MOC reflex. Possible physiological and anatomical roots that give rise to the frequency tuning as well as functional implications of the observed frequency dependence are also discussed.
SOAEs are particularly suitable for examining the MOC reflex as they do not require an external probe, are inherently low-level signals, and are sensitive to MOC influence. The frequency tuning of MOC effects on SOAEs has not been extensively studied, and the results of two published studies are not in agreement with each other. With contralateral tones at 80 dB SPL (15 dB higher than our loudest tones), Mott et al. (1989) observed frequency-dependent MOC effects on SOAEs in four human subjects (SOAE frequency ∼1,619–5,671 Hz). The most effective contralateral frequency was found to be 3/8 to 1/2 octave below the SOAE frequency for frequency shift and near the SOAE frequency for level shift. It is worth mentioning that their experiments were conducted only on stable SOAEs, varying less than 1 Hz or 1 dB over test sessions on a single day. However, Harrison and Burns (1993) failed to replicate frequency-dependent tuning on a group of 10 SOAEs. Crucially, the SOAEs studied were not selected to be stable in level and frequency as was done by Mott et al. (1989). In contrast to both reports, our results exhibit a strong frequency tuning of the contralateral MOC reflex with the most effective elicitors almost always between 0.5 and 1 kHz, irrespective of SOAE (probe) frequency. Two important differences between the present study and that of Mott et al. (1989) need to be considered. First, multiple observations of the same SOAE and the examination of 35 SOAEs allowed statistical comparisons and revealed a systematic frequency tuning of the MOC reflex independent of SOAE frequency. In comparison, the lack of error bars and the pronounced width of the tuning pattern prevented accurate estimation of the tuning tip frequency in previous work (Figs. 3 and 4, Mott et al. 1989). Second, the MEM reflex was stringently controlled in the results reported here, thereby isolating the tuning properties of the MOC reflex.
Results from tuning experiments of MOC inhibition of SFOAEs in humans are in general agreement with the results presented here on SOAEs. Contralateral inhibition of SFOAEs was skewed depending on the frequency of the probe: The most effective contralateral elicitor was greater in frequency than that of a 0.5-kHz probe, 1/2 octave lower than a 1.0-kHz probe, and at least 1.5 octaves lower than a 4.0-kHz probe (Lilaonitkul and Guinan 2009a, 2012). Similar skew has been observed on contralateral inhibition of TEOAEs (Veuillet et al. 1991) and DPOAEs (Chery-Croze et al. 1993) in humans and tone pip-evoked compound action potential (CAP) in cats (Liberman 1989), although the magnitude of the skew is smaller than that observed in SOAEs and SFOAEs.
Next we consider the possible anatomical and physiological underpinnings of the tuning patterns observed. Grossly defined, the contralateral MOC reflex is a neural network consisting of five layers: contralateral inner hair cells (IHCs) (sensors), contralateral spiral ganglion neurons, interneurons in the contralateral posteroventral cochlear nucleus (Brown et al. 2003; de Venecia et al. 2005), MOC neurons in the ipsilateral superior olivary complex, and ipsilateral OHCs (effectors). The frequency tuning of the system ought to be conferred by the frequency-selective connectivity between any two adjacent layers. That is, each neuron selectively receives input from the layer before and selectively sends output to the layer after. In this scenario, frequency-dependent tuning can originate from one or more of the layers. Below we evaluate the possibility of each of these layers being the primary contributor to the observed tuning.
Because innervations between contralateral IHCs and auditory nerve fibers have been demonstrated to be strictly tonotopically organized (Robertson 1984), they can be readily ruled out as the anatomical basis of the contralateral MOC reflex tuning pattern. Further down the reflex pathway, MOC neurons, the mediators of the reflex, display sharp tuning in response to contralateral sounds (Brown 1989; Liberman and Brown 1986; Robertson 1985) with characteristic frequencies (CFs) not confined to a narrow, 1-octave, range as observed here. However, an interesting feature unique to olivocochlear neurons is that they display a single tuning peak (at CF) at low input levels but two tuning peaks at moderate to high input levels: one peak at CF and an additional peak between 1.5 and 2 kHz in cats (Fig. 8 of Liberman 1988). This secondary peak may explain the tuning peak between 0.5 and 1 kHz in our results, considering the 1-octave shift in the cochlear map between cats and humans. However, it is intriguing that the primary tuning peak near the CF of the MOC neuron is not evident in our results. Also, the secondary peak in Liberman (1988) was elicited only by tone bursts at 70 dB SPL or above, levels higher than those used in this study. It is unknown whether these differences can be attributed to interspecies difference (cats vs. humans) or anesthesia (anesthetized vs. awake).
The CFs of MOC efferent neurons roughly match the CF of the cochlear region where the targeted OHCs reside (Brown 1989; Liberman and Brown 1986; Robertson 1985). Thus this innervation pattern should not be responsible for the tuning of the contralateral MOC reflex toward the frequency band between 0.5 and 1 kHz. Neither should the MOC innervation density in the ipsilateral cochlea be responsible (Guinan et al. 1984; Robertson et al. 1987). The reader is reminded that this knowledge about innervation pattern and density is inferred from animal models other than humans. Thus a species-specific pattern, unique to humans, cannot be ruled out. However, the MOC innervation of the OHCs is broad, spanning up to 2.8 mm along the organ of Corti (Liberman and Brown 1986), potentially enabling OHCs to be influenced by contralateral tones at frequencies away from the CF of the OHC residence.
Since MOC neurons project to cochlear regions with a similar CF, our results would be easy to interpret if all SOAEs (irrespective of frequency) were generated by OHCs in the cochlear region with CFs between 0.5 and 1 kHz. However, all evidence points to the contrary—SOAEs are generated by OHCs in cochlear locations tonotopically consistent with the SOAE frequency (Shera 2003).
SOAEs are thought to be generated in a localized region near the characteristic place of the SOAE frequency (Shera 2003). Similarly, auditory nerve fibers tonotopically innervate IHCs at the characteristic place of the fiber. If the generation locus of SOAE roughly matches the innervation region of an auditory nerve fiber with the same CF, then the tuning patterns of contralateral sound inhibition on SOAEs and auditory nerve fiber responses are expected to be similar. However, this does not appear to be the case. The discharge rates of auditory nerve fibers are most effectively inhibited by contralateral tones at CF in cats (Fig. 7 of Warren and Liberman 1989). In contrast, we observe the greatest alteration to SOAE level and frequency by contralateral tones between 0.5 and 1 kHz, irrespective of SOAE frequency. Perhaps this points to interspecies differences in contralateral MOC reflex tuning, differences between direct neural recording and indirect OAE measures, or differences between anesthetized cats and awake human subjects.
Occasionally, another tuning peak was observed at frequencies above the SOAE (e.g., 3–6 kHz bin, Fig. 5), but its magnitude was consistently smaller than the main tuning peak between 0.5 and 1 kHz. This tuning peak was not observed in a majority of the SOAEs studied, and its appearance in the group data likely represents the undue influence of outliers.
Considering existing anatomical and physiological evidence about SOAE generation and MOC innervation, the secondary tuning peak between 1.5 and 2 kHz observed in cat MOC neuron tuning functions in response to high-level contralateral tones appears to be the strongest candidate for a potential explanation of our results. However, why the primary peak near CF was not observed in our results is not known.
In closing, we speculate about the functional implications of the tuning pattern of the contralateral MOC reflex observed here. Real-world sounds are broadband in nature, and the MOC reflex integrates energy over a wide frequency range (Lilaonitkul and Guinan 2009b), lending support to the proposed functional roles of MOC efferents in protection against acoustic trauma and antimasking (Guinan 2006). However, if the MOC reflex evolved to protect hearing and/or to release masking, then it is counterintuitive that the reflex is most effectively elicited by sounds with energy concentrated mostly between 0.5 and 1 kHz. Our observation of narrow and fixed frequency tuning seems to detract from the currently held beliefs about the functional roles of the MOC efferents. It must be noted that the contralateral MOC reflex certainly does not operate in isolation from the ipsilateral reflex in the natural environment. Thus specific knowledge about the tuning pattern of the ipsilateral MOC reflex would complement the information presented here—and a complete picture of the operational dynamics of the MOC reflex would emerge. Although the functional implications of our unexpected results are not well understood, they may suggest that there are some unique properties of the human MOC system that are not fully discovered yet.
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders Grant DC-008420 and by Northwestern University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: W.Z. and S.D. conception and design of research; W.Z. performed experiments; W.Z. and S.D. analyzed data; W.Z. and S.D. interpreted results of experiments; W.Z. and S.D. prepared figures; W.Z. and S.D. drafted manuscript; W.Z. and S.D. edited and revised manuscript; W.Z. and S.D. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are grateful to Drs. Christopher Shera, Jonathan Siegel, Claus-Peter Richter, Mario Ruggero, and John Guinan for insightful discussions.
REFERENCES
- Backus BC, Guinan JJ., Jr Measurement of the distribution of medial olivocochlear acoustic reflex strengths across normal-hearing individuals via otoacoustic emissions. J Assoc Res Otolaryngol 8: 484–496, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC. Morphology and response properties of single olivocochlear fibers in the guinea pig. Hear Res 40: 93–109, 1989 [DOI] [PubMed] [Google Scholar]
- Brown MC, de Venecia RK, Guinan JJ., Jr Responses of medial olivocochlear neurons. Specifying the central pathways of the medial olivocochlear reflex. Exp Brain Res 153: 491–498, 2003 [DOI] [PubMed] [Google Scholar]
- Chery-Croze S, Moulin A, Collet L. Effect of contralateral sound stimulation on the distortion product 2f1-f2 in humans: evidence of a frequency specificity. Hear Res 68: 53–58, 1993 [DOI] [PubMed] [Google Scholar]
- de Venecia RK, Liberman MC, Guinan JJ, Jr, Brown MC. Medial olivocochlear reflex interneurons are located in the posteroventral cochlear nucleus: a kainic acid lesion study in guinea pigs. J Comp Neurol 487: 345–360, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos R. Suppression of auditory nerve activity by stimulation of efferent fibers to cochlea. J Neurophysiol 19: 424–437, 1956 [DOI] [PubMed] [Google Scholar]
- Guinan JJ., Jr Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear 27: 589–607, 2006 [DOI] [PubMed] [Google Scholar]
- Guinan JJ, Jr, Warr WB, Norris BE. Topographic organization of the olivocochlear projections from the lateral and medial zones of the superior olivary complex. J Comp Neurol 226: 21–27, 1984 [DOI] [PubMed] [Google Scholar]
- Harrison WA, Burns EM. Effects of contralateral acoustic stimulation on spontaneous otoacoustic emissions. J Acoust Soc Am 94: 2649–2658, 1993 [DOI] [PubMed] [Google Scholar]
- Kemp DT. Evidence of mechanical nonlinearity and frequency selective wave amplification in the cochlea. Arch Otorhinolaryngol 224: 37–45, 1979 [DOI] [PubMed] [Google Scholar]
- Lee J, Dhar S, Abel R, Banakis R, Grolley E, Zecker S, Siegel J. Behavioral hearing thresholds between 0.125 and 20 kHz using depth-compensated ear simulator calibration. Ear Hear (March 20, 2012). doi:10.1097/AUD.0b013e31823d7917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC. Rapid assessment of sound-evoked olivocochlear feedback: suppression of compound action potentials by contralateral sound. Hear Res 38: 47–56, 1989 [DOI] [PubMed] [Google Scholar]
- Liberman MC. Response properties of cochlear efferent neurons: monaural vs. binaural stimulation and the effects of noise. J Neurophysiol 60: 1779–1798, 1988 [DOI] [PubMed] [Google Scholar]
- Liberman MC, Brown MC. Physiology and anatomy of single olivocochlear neurons in the cat. Hear Res 24: 17–36, 1986 [DOI] [PubMed] [Google Scholar]
- Lilaonitkul W, Guinan JJ., Jr Frequency tuning of medial-olivocochlear-efferent acoustic reflexes in humans as functions of probe frequency. J Neurophysiol 107: 1598–1611, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilaonitkul W, Guinan JJ., Jr Reflex control of the human inner ear: a half-octave offset in medial efferent feedback that is consistent with an efferent role in the control of masking. J Neurophysiol 101: 1394–1406, 2009a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilaonitkul W, Guinan JJ., Jr Human medial olivocochlear reflex: effects as functions of contralateral, ipsilateral, and bilateral elicitor bandwidths. J Assoc Res Otolaryngol 10: 459–470, 2009b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Hudspeth AJ. Active hair-bundle movements can amplify a hair cell's response to oscillatory mechanical stimuli. Proc Natl Acad Sci USA 96: 14306–14311, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott JB, Norton SJ, Neely ST, Warr WB. Changes in spontaneous otoacoustic emissions produced by acoustic stimulation of the contralateral ear. Hear Res 38: 229–242, 1989 [DOI] [PubMed] [Google Scholar]
- Moulin A, Collet L, Duclaux R. Contralateral auditory stimulation alters acoustic distortion products in humans. Hear Res 65: 193–210, 1993 [DOI] [PubMed] [Google Scholar]
- Mountain DC. Changes in endolymphatic potential and crossed olivocochlear bundle stimulation alter cochlear mechanics. Science 210: 71–72, 1980 [DOI] [PubMed] [Google Scholar]
- Murugasu E, Russell IJ. The effect of efferent stimulation on basilar membrane displacement in the basal turn of the guinea pig cochlea. J Neurosci 16: 325–332, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel JL, Rebillard G. Effect of contralateral sound stimulation on the distortion product 2F1-F2: evidence that the medial efferent system is involved. J Acoust Soc Am 87: 1630–1635, 1990 [DOI] [PubMed] [Google Scholar]
- Robertson D. Brainstem location of efferent neurones projecting to the guinea pig cochlea. Hear Res 20: 79–84, 1985 [DOI] [PubMed] [Google Scholar]
- Robertson D. Horseradish peroxidase injection of physiologically characterized afferent and efferent neurones in the guinea pig spiral ganglion. Hear Res 15: 113–121, 1984 [DOI] [PubMed] [Google Scholar]
- Robertson D, Anderson CJ, Cole KS. Segregation of efferent projections to different turns of the guinea pig cochlea. Hear Res 25: 69–76, 1987 [DOI] [PubMed] [Google Scholar]
- Robles L, Delano PH. Efferent system. In: The Senses: A Comprehensive Reference, edited by Dallos P, Oertel D. San Diego, CA: Academic, 2008 [Google Scholar]
- Shera CA. Mammalian spontaneous otoacoustic emissions are amplitude-stabilized cochlear standing waves. J Acoust Soc Am 114: 244–262, 2003 [DOI] [PubMed] [Google Scholar]
- Veuillet E, Collet L, Duclaux R. Effect of contralateral acoustic stimulation on active cochlear micromechanical properties in human subjects: dependence on stimulus variables. J Neurophysiol 65: 724–735, 1991 [DOI] [PubMed] [Google Scholar]
- Warren EH, 3rd, Liberman MC. Effects of contralateral sound on auditory-nerve responses. II. Dependence on stimulus variables. Hear Res 37: 105–121, 1989 [DOI] [PubMed] [Google Scholar]
- Wiederhold ML. Variations in the effects of electric stimulation of the crossed olivocochlear bundle on cat single auditory-nerve-fiber responses to tone bursts. J Acoust Soc Am 48: 966–977, 1970 [DOI] [PubMed] [Google Scholar]
- Wiederhold ML, Kiang NY. Effects of electric stimulation of the crossed olivocochlear bundle on single auditory-nerve fibers in the cat. J Acoust Soc Am 48: 950–965, 1970 [DOI] [PubMed] [Google Scholar]
- Zhao W, Dhar S. The effect of contralateral acoustic stimulation on spontaneous otoacoustic emissions. J Assoc Res Otolaryngol 11: 53–67, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Dhar S. Fast and slow effects of medial olivocochlear efferent activity in humans. PLoS One 6: e18725, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]