Abstract
The dorsolateral prefrontal and posterior parietal cortices are two interconnected brain areas that are coactivated in tasks involving functions such as spatial attention and working memory. The response properties of neurons in the two areas are in many respects indistinguishable, yet only prefrontal neurons are able to resist interference by distracting stimuli when subjects are required to remember an initial stimulus. Several mechanisms have been proposed that could account for this functional difference, including the existence of specialized interneuron types, specific to the prefrontal cortex. Although such neurons with inverted tuning during the delay period of a working memory task have been described in the prefrontal cortex, no comparative data exist from other cortical areas that would establish a unique prefrontal role. To test this hypothesis, we analyzed a large database of recordings obtained in the dorsolateral prefrontal and posterior parietal cortex of the same monkeys as they performed working memory tasks. We found that in the prefrontal cortex, neurons with inverted tuning were more numerous and manifested unique properties. Our results give credence to the idea that a division of labor exists between separate neuron types in the prefrontal cortex and that this represents a functional specialization that is not present in its cortical afferents.
Keywords: computational neuroscience, intraparietal sulcus, monkey, neurophysiology, principal sulcus
the primate dorsolateral prefrontal and posterior parietal cortices are two cortical areas involved in working memory and a wide range of cognitive functions (Bisley and Goldberg 2010; Constantinidis and Procyk 2004). Neurophysiological studies have identified in the prefrontal cortex neural correlates of working memory in the form of sustained discharges during the delay intervals of working memory tasks (Constantinidis et al. 2001; Funahashi et al. 1989; Fuster and Alexander 1971; Miller et al. 1996). Very similar, sustained activity has also been observed in the posterior parietal cortex (Chafee and Goldman-Rakic 1998, 2000; Constantinidis and Steinmetz 1996). Indeed, human imaging studies almost invariably report concurrent prefrontal and parietal activation in attention and working memory tasks (Courtney et al. 1997; Jonides et al. 1993; Munk et al. 2002; Owen et al. 1998; Raye et al. 2002; Ungerleider et al. 1998). Despite this overall similarity, subtle but clear differences have also been described between the two areas in the context of working memory tasks. Prefrontal neurons are able to resist interference by distracting stimuli when subjects are required to remember the first of a sequence of stimuli and continue to represent the original stimulus after the presentation of distractors (di Pellegrino and Wise 1993; Qi et al. 2010). In contrast, posterior parietal neurons appear to represent the most recent stimulus, whether it is behaviorally relevant or not, in the context of the task (Constantinidis and Steinmetz 1996; Powell and Goldberg 2000).
Several mechanisms have been proposed that could account for this specialization, such as the effects of dopamine, which preferentially innervates the prefrontal cortex over its cortical afferents (Haber and Fudge 1997; Levitt et al. 1984). Computational modeling indicates that networks that incorporate dopamine inputs achieve a superior signal-to-noise ratio (Durstewitz et al. 1999, 2000). This occurs through dopamine's action on N-methyl-d-aspartate (NMDA) (Chen et al. 2004; Seamans et al. 2001; Wang 2001; Yang and Seamans 1996) and GABA receptors (Yang et al. 1999). However, dopaminergic innervation is denser at the medial prefrontal cortex and least pronounced in the lateral prefrontal cortex (Lewis et al. 1988). Factors specific to the intrinsic neural circuits of the two areas may also play a role in their unique functional properties. The existence of specialized interneuron types specific to the prefrontal cortex has been proposed as such an intrinsic mechanism (Wang et al. 2004). By some accounts, the prefrontal cortex contains an appreciable proportion of calbindin-containing interneurons (Conde et al. 1994; Krimer et al. 2005; Zaitsev et al. 2005), whereas such neurons are scarcer in other cortical areas (Elston and Gonzalez-Albo 2003). These neurons have been proposed to exhibit high tonic activity and to be suppressed by stimuli that activate neighboring pyramidal neurons (Wang et al. 2004). They could therefore serve to stabilize working memory by virtue of disinhibiting pyramidal neurons that have already been activated by a stimulus held in memory, while continuing to inhibit neurons representing stimuli away from the remembered stimulus (Wang et al. 2004). Although such neurons with “inverted tuning” during the delay period of a working memory task have been described in the prefrontal cortex, no comparative neurophysiological data exist from other cortical areas to support a unique prefrontal role. We were therefore motivated to compare the relative incidence and properties of physiologically identified neurons with inverted tuning in the dorsolateral prefrontal and posterior parietal cortex of the same monkeys performing working memory tasks.
METHODS
Three male rhesus monkeys (Macaca mulatta), weighing 5–12 kg, were used in this study. All surgical and animal use procedures in this study were reviewed and approved by the Wake Forest University Institutional Animal Care and Use Committee following National Institutes of Health guidelines.
Surgery and neurophysiology.
Experiments were performed as described previously in detail (Meyer et al. 2011; Qi et al. 2011). Briefly, 20 mm diameter recording cylinders were implanted over the prefrontal and the parietal cortex in each animal (Fig. 1). Penetrations analyzed here sampled areas 46 and 8a of the prefrontal cortex and areas 7a and lateral intraparietal cortex (LIP) of the posterior parietal cortex, determined based on structural MRI. Area LIP was determined anatomically, based on the depth of electrode penetration (>3 mm) from the surface of the cortex. Recordings were collected with either glass-coated Tungsten electrodes of 250 μm diameter and an impedance of 1 MΩ, measured at 1 kHz (Alpha Omega Engineering, Nazareth, Israel), or epoxylite-coated Tungsten electrodes with a diameter of 125 μm and an impedance of 4 MΩ at 1 KHz (FHC Bowdoin, ME). Epoxylite-coated electrodes were advanced into the brain by traversing the dura, glass coated through a dura-piercing guide tube. Electrical signals recorded from the brain were amplified, band-pass filtered between 500 Hz and 8 kHz, and stored via a modular data acquisition system at a temporal resolution of 25 μs (APM system, FHC).
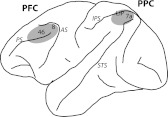
Fig. 1.
Schematic diagram of the monkey brain. The shaded areas indicate the recording sites in the dorsolateral prefrontal cortex (PFC) and posterior parietal cortex (PPC). AS, arcuate sulcus; IPS, intraparietal sulcus; LIP, lateral intraparietal area; PS, principal sulcus; STS, superior temporal sulcus.
Behavioral tasks.
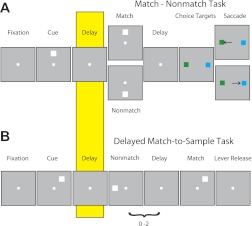
The monkeys performed tasks that required them to remember visual stimuli presented on a screen while maintaining fixation, monitored with an infrared eye-position tracking system (model RK-716; Iscan, Woburn, MA) and sampled at 240 Hz. One monkey was trained to perform a match-nonmatch task (Fig. 2A), and two monkeys were trained to perform a delayed match-to-sample task (Fig. 2B). In the match-nonmatch task (Fig. 2A), a white square of 2° size appeared for 0.5 s at one of nine possible locations arranged on a 3 × 3 grid with a 10° separation between adjacent stimuli. This cue was followed by a 1.5-s delay period. A second stimulus presentation and delay period followed, after which, two choice targets were presented. The monkey was required to saccade to a green target if the two successive stimuli matched and to a blue target if they did not (Meyer et al. 2011). In the delayed match-to-sample task (Fig. 2B), monkeys were trained to remember the location of the first stimulus and to release a lever when a subsequent stimulus appeared at the same location, ignoring nonmatching stimuli appearing at different locations. The stimuli analyzed here were 1.5° green or red squares and were displayed at one of nine locations along a 3 × 3 grid of either 10° or 15° separation between adjacent stimuli. Fixation was controlled for the entire duration of all tasks, and a trial was aborted if an eye deviation of >2° from the fixation target occurred at any point (including blinks). All monkeys were trained to perform additional types of trials, which are not analyzed here. The visual stimulus presentations were controlled by in-house software (Meyer and Constantinidis 2005), operated through Matlab (MathWorks, Natick, MA).
Fig. 2.
Behavioral tasks. Successive frames indicate the series of stimulus presentations. A: stimulus presentation in the match-nonmatch task. The monkey was required to remember the location of the 1st stimulus and saccade to 1 of 2 color-choice targets, depending on whether the 2nd stimulus appeared in the same location. B: stimulus presentations in the delayed match-to-sample task. Monkeys were required to remember the location of the 1st stimulus and release a lever when a subsequent stimulus appeared at the same location. Highlighted frame indicates the delay period analyzed for inverted tuning.
Data analysis.
Recorded spike waveforms were sorted into separate units using an automated cluster analysis method based on the KlustaKwik algorithm (Harris et al. 2000). A neuron's spike width was determined by calculating the distance between the two troughs of the average waveform, as described previously (Qi et al. 2011). Based on that classification, units were classified as fast spiking (FS; putative interneurons) if their spike width was ≤550 μs and regular spiking (RS; putative pyramidal neurons) if they exhibited spike widths >550 μs.
Firing rate of units was determined by averaging spikes in each task epoch. We identified neurons that had a significantly increased delay-period activity by comparing the discharge rates with the baseline fixation interval. We refer to these neurons as excited in the delay period. We similarly identified neurons that exhibited significantly decreased activity in the delay period. For this analysis, we compared the last 0.5 s of the delay-period activity following presentation of the stimulus at each location with the baseline fixation activity recorded from the same trials, using a paired t-test. Neurons with a significant decrease (P < 0.05) in firing rate in the delay period for at least one spatial location and no significantly elevated responses in the delay period for any other location were identified. This comparison, in practice, required a minimum firing rate of two spikes/s in the fixation period, below which decreases could be detected. Additionally, we identified a subgroup of neurons inhibited in the delay period, which also exhibited spatial selectivity for the delay period following stimuli at the nine different spatial locations (ANOVA, P < 0.05). This latter subgroup constitutes the inverted tuning neurons. To estimate the experiment-wise error rate of inverted tuning neuron identification, we performed a bootstrap test. Trials were first randomized with respect to the location of the stimulus; then, the selection criteria were applied as above. The randomization procedure was repeated 10 times for each neuron in our sample (1,909 neurons in total, across all areas), yielding a total of 19,090 tests, over which the false-positive rate was estimated.
To evaluate the spatial tuning of stimulus selectivity in the delay period, we rotated firing rates of each neuron for the eight peripheral stimuli, so that its peak response rate would always be at the same location. We then averaged responses for each location, relative to the peak location across all neurons, separately by cortical area and excited or inverted tuning neuron type. All data were expressed in units of stimulus separation from each other (which was either 10° or 15° of visual angle). The averaged data were then fitted to a Gaussian curve of the form
Here, r represents average population firing rate at each location x, B the baseline, A the amplitude, μ the peak, and σ the SD of the Gaussian. We fitted Gaussians separately to the inverted tuning neurons from the prefrontal and parietal cortex and also to all neurons pooled from both areas. To obtain an estimate of fit, we calculated the absolute value of the residuals from the Gaussian on a neuron-by-neuron basis. We then compared the residuals from the single and pooled population models using a paired t-test.
RESULTS
Database.
Our analysis involved two brain regions in the frontal and parietal lobe (Fig. 1), which are interconnected directly (Cavada and Goldman-Rakic 1989) and share very similar functional properties (Chafee and Goldman-Rakic 1998). A total of 1,227 neurons was analyzed in the posterior parietal cortex of three monkeys (monkey DA: 571, CA: 367, and EL: 289, respectively). Most of these neurons were collected in area 7a (n = 1,165); a small sample was collected from area LIP (n = 62). A total of 682 neurons was analyzed from the dorsolateral prefrontal cortex (DA: 230, CA: 2, and EL: 450, respectively). Most of these neurons were recorded in area 46 (n = 647); a smaller sample was recorded posterior to the principal sulcus and anterior to the arcuate, corresponding to area 8a (n = 35). Comparable quality of signals was obtained in the prefrontal and parietal cortex; there was no significant difference (t-test, P > 0.7) in the signal-to-noise ratio of average spike waveforms collected from the two areas (mean and SE for prefrontal cortex: 7.62 ± 0.10; posterior parietal cortex: 7.67 ± 0.13). The monkeys were trained to perform spatial working memory tasks; one of the monkeys (EL) performed the match-nonmatch task (Fig. 2A), and two of the monkeys (DA and CA) performed a delayed match-to-sample task (Fig. 2B). A total of 558 posterior parietal neurons responded to the task, evidenced by a significantly elevated firing rate during any of the stimulus presentations and delay periods in the task. Similarly, 437 prefrontal neurons exhibited a significantly elevated firing rate in one or more task periods.
Incidence of neurons with inverted tuning.
We identified neurons with inverted tuning during the delay period of the working memory tasks as those that exhibited three criteria, as defined previously (Wang et al. 2004): 1) average firing rates were significantly lower in the (first) delay period than the baseline fixation for at least one spatial stimulus, 2) no excited responses during the delay period following another stimulus, and 3) significant selectivity for the location of the preceding cue. Identical analysis methods were used for all areas and animals. We were thus able to compare the percentage of neurons with inverted tuning in the two areas. If inverted tuning is a characteristic of a special type of neurons (calbindin interneurons), as previously speculated (Wang et al. 2004), then a higher incidence of them would be expected in the prefrontal cortex.
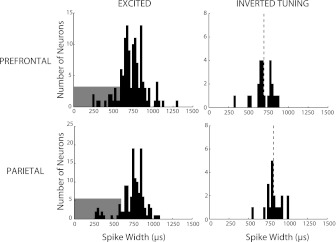
We encountered neurons with inverted tuning during the delay period in both the posterior parietal and dorsolateral prefrontal cortex (including areas LIP and 8a). An example neuron recorded from the dorsolateral prefrontal cortex is shown in Fig. 3 and an example from the posterior parietal cortex in Fig. 4. Across our entire sample, a total of 24/682 (3.5%) neurons exhibited inverted tuning in the prefrontal cortex and 20/1,227 (1.6%) in the parietal cortex. The respective proportion of inverted tuning neurons was significantly higher in the prefrontal than the parietal cortex (χ2 test, P < 0.05). No significant difference (χ2 test, P > 0.4) was observed in the percentage of inverted tuning neurons obtained from sessions in which epoxylite-coated and glass-coated electrodes were used (2.6% and 2.1%, respectively; 8/126 in the prefrontal cortex and 16/813 in the parietal compared with 16/556 in the prefrontal and 4/414 in the parietal), and the majority of prefrontal recordings was obtained with glass-coated electrodes (which yielded the overall lower percentage), whereas most parietal recordings were obtained with epoxylite-coated electrodes. Since the total percentage of neurons with inverted tuning was fairly small, we sought to estimate the expected error rate of inverted tuning neuron incidence by performing a bootstrap test, applying the selection criteria to neuronal responses after first randomizing trials with respect to stimulus location. The experiment-wise, false-positive rate was 0.6%. Essentially, identical, expected error rates were obtained for the sample of prefrontal (0.7%) and posterior parietal neurons (0.6%). The observed rate of inverted tuning neurons was significantly higher than the respective, expected error rates (χ2 test, P < 10−4 for the prefrontal cortex; P < 0.01 for the posterior parietal cortex). Expressed as a percentage of the total number of neurons modulated by the task [sum of the neurons with excitatory responses and those with inverted tuning, as in Wang et al. (2004)], inverted tuning neurons represented 5.2% of the prefrontal neurons and 3.5% of the parietal ones. The use of a more stringent significance criterion of α = 0.005 for the decrease in firing rate between the delay and fixation period resulted in an even greater disparity between areas, yielding 4.5% of prefrontal neurons and 1.4% of parietal neurons with inverted tuning. For comparison, 23.5% of dorsolateral prefrontal and 28.6% of posterior parietal neurons exhibited tuned, excited responses during the delay period.
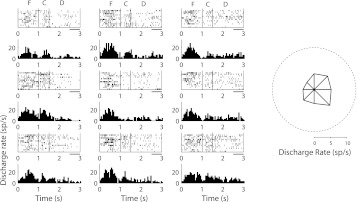
Fig. 3.
Rasters and peristimulus time histograms of a single inverted tuning neuron in the dorsal PFC. Rasters and histograms depict responses in spikes per second (sp/s) for the 9 cue locations, arranged as to indicate the spatial location of the corresponding cue. Responses during the fixation interval (F), cue presentation (C), and delay period (D) are shown. Horizontal bars represent interval of the delay period used to compute inverted tuning. The polar plot on the right denotes the average firing rate during the delay period for each location; the dotted circle represents the average firing rate during the baseline, fixation period.
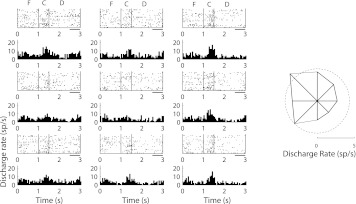
Fig. 4.
Rasters and peristimulus time histograms of a single inverted tuning neuron in the PPC. Conventions are the same as in Fig. 3.
Properties of neurons with inverted tuning.
We proceeded to examine whether the population of prefrontal neurons with inverted tuning displayed functionally unique properties vs. those identified in the parietal cortex. Neurons with inverted tuning, previously identified in the prefrontal cortex, were found to have average spike widths intermediate between neurons that were characterized as FS and RS (Wang et al. 2004). Spike width measured extracellularly is not a precise indicator of neuron type (Vigneswaran et al. 2011); however, it is notable that when we examined the spike widths of neurons identified as manifesting inverted tuning (Fig. 5), we observed a significant difference in average spike width between the prefrontal (692 μs) and parietal (820 μs) populations (Wilcoxon signed-rank test, P < 0.001). No such difference was present for the neurons with excited delay-period activity (Wilcoxon signed-rank test, P > 0.1). Had we wished to classify the inverted tuning neurons into FS and RS categories based on spike width, 12.5% of prefrontal neurons and 5% of parietal neurons would have been classified as FS. Within the group of neurons with increased delay-period activity, similar percentages of neurons were classified as FS and RS (11% and 89% in prefrontal; 8% and 92% in parietal cortex, respectively); these percentages represented no significant difference between areas (χ2 test, P > 0.4). The results indicated that prefrontal neurons with an inverted tuning curve had unique biophysical properties, consistent with the idea that they corresponded to calbindin interneurons.
Fig. 5.
Distribution of spike widths of neurons with excited delay-period activity and inverted tuning in the dorsal PFC and PPC. Shaded area in excited neurons indicates the spike widths corresponding to fast spiking neurons (putative interneurons); unshaded area represents regular spiking (putative pyramidal) neurons. Dotted lines represent mean spike widths of inverted tuning neurons in the 2 areas.
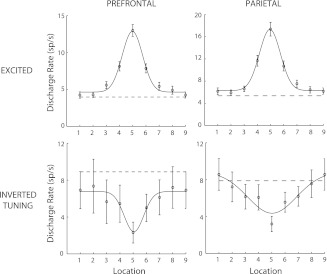
For neurons with inverted tuning to play a role in disinhibiting excitatory neurons, which have been activated by a remembered stimulus, the excited and inverted tuning populations must have comparable tuning widths. We therefore tested the tuning of neurons for the spatial stimuli and compared the posterior parietal and prefrontal populations. Overall, the population of prefrontal neurons displayed narrower tuning. The SD of the Gaussian curve that provided the best fit was 0.73 for the prefrontal population of inverted tuning neurons vs. 1.84 for the parietal population (in units of stimulus separation), more than twice as wide (Fig. 6). To test whether a single Gaussian curve fit the data equally well, we determined the optimal fit for the entire pool of neurons (σ = 1.04) and then compared the neuron-by-neuron residuals of the single- and two-Guassian models. Fitting the data to two curves resulted in a significant decrease of the fitting error (paired t-test, P < 0.05). Importantly, the SDs, which provided the best fits for the excitatory populations, were 0.77 and 0.79 for the prefrontal and parietal cortex, respectively (Fig. 6). In this case too, only the prefrontal population of inverted tuning neurons exhibited similar tuning with the population of excited neurons, as hypothesized for calbindin interneurons.
Fig. 6.
Population tuning curves for excited and inverted tuning neurons in the dorsal PFC and PPC. The arrangement of spatial locations has been rotated so that the best response is at location 5 for every neuron; points 1 and 9 represent the same location. Curve represents best Gaussian fit and dotted line the average firing rate in the baseline fixation period. Error bars represent SEs across neurons.
DISCUSSION
Our results demonstrate that neurons with inverted tuning during the delay periods of working memory tasks are much more numerous in the dorsolateral prefrontal than the posterior parietal cortex of monkeys. Furthermore, prefrontal neurons with inverted tuning exhibit unique biophysical and response properties consistent with the idea that a specialized type of prefrontal interneurons plays a special role in the prefrontal network during working memory. Computational models predict considerable benefits in the stability of persistent activity with the addition of a small percentage of neurons with inverted tuning in the same order (∼5%) of the neurons that we detected in the prefrontal cortex (Wang et al. 2004). Therefore, this population of neurons may be partly responsible for the ability of the prefrontal network to resist interference by distractors, although inverted tuning may not be the sole property that confers this functional specialization. Regardless of their ultimate functional implications, our current results add to a short list of subtle functional differences in the physiological properties of the prefrontal and posterior parietal cortex, two areas implicated in different cognitive functions but otherwise exhibiting very similar functional properties (Constantinidis and Procyk 2004).
Inverted tuning neurons.
Our study relied on the analysis methods of an earlier report of inverted tuning neurons in the prefrontal cortex (Wang et al. 2004) using monkeys trained in different spatial working memory tasks. Our present results in the prefrontal cortex essentially replicated the earlier findings. We find that a small but consistent population of prefrontal neurons (5.2% in the present study compared with 4.5% in the earlier study) exhibited delay-period activity, which was significantly below the fixation baseline rate, and was tuned for the spatial location of the stimuli (the definition of inverted tuning). Calbindin interneurons are reported to make up ∼5% of neurons in the prefrontal cortex (Gabbott and Bacon 1996). Spike width, measured extracellularly, is not a precise indicator of neuron type, as large, pyramidal motor neurons have been shown to exhibit narrow spikes (Vigneswaran et al. 2011). Nonetheless, we observed systematic differences between the two areas, although recordings were performed in the same monkeys, executing the same task, with the same recording techniques. The average spike width of prefrontal neurons with inverted tuning in our study (692 μs) fell between the means of the FS and RS distributions of the data set, from where the current data were derived [418 and 775 μs, respectively, in Qi et al. (2011)], also consistent with the biophysical properties of calbindin interneurons identified anatomically (Kawaguchi and Kubota 1993; Zaitsev et al. 2005). The prefrontal-inverted tuning neurons finally exhibited average tuning width that was very similar to the tuning width of neurons with elevated delay-period activity. This would be a critical property for inverted tuning neurons if they were to play a role in disinhibiting excited neurons with similar tuning (Wang et al. 2004).
In contrast, a significantly smaller population of posterior parietal neurons exhibited inverted tuning during the delay periods of the tasks. Even those neurons that fit the statistical criterion exhibited average spike width (820 μs), more consistent with RS neurons, differing significantly from that of prefrontal neurons. Furthermore, parietal neurons with inverted tuning were much more broadly tuned than parietal neurons with excited delay-period activity. For these reasons, they seem unsuitable to play a role in disinhibiting persistent delay-period activity in a manner that would decrease distractibility during the working memory task.
Neurophysiological specialization of the prefrontal cortex.
The dorsolateral prefrontal and posterior parietal cortices share multiple functional properties; similar percentages of neurons are active during equivalent epochs of behavioral tasks and exhibit levels of activity and temporal envelopes of responses that are virtually indistinguishable (Chafee and Goldman-Rakic 1998, 2000). The result is not particularly surprising, considering that the two areas are directly connected with each other (Cavada and Goldman-Rakic 1989). In our study too, similar percentages of dorsolateral prefrontal and posterior parietal neurons were observed with tuned, excitatory activity in the delay period of the tasks.
Subtle differences in the properties of the dorsolateral prefrontal and posterior parietal cortex have been identified in tasks requiring monkeys to remember the spatial location of a sample stimulus and to ignore intervening stimuli (Rawley and Constantinidis 2008). Initial studies comparing activity in the ventral prefrontal cortex and the inferior temporal (IT) cortex in object working memory tasks revealed that whereas IT neurons track the most recent stimulus of a sequence (Miller et al. 1993), prefrontal neurons are able to represent the stimulus actively retained in memory (Miller et al. 1996). Subsequently, an equivalent resistance to interfering stimuli was documented in spatial working memory tasks. In such tasks, neurons in posterior parietal areas 7a and LIP represent the location of the most recent stimulus, whether it is the remembered sample or the behaviorally irrelevant distractor (Constantinidis and Steinmetz 1996; Powell and Goldberg 2000). In contrast, prefrontal neurons can maintain the representation of the actively remembered sample (di Pellegrino and Wise 1993; Qi et al. 2010). The prefrontal representation of behaviorally relevant stimuli has been documented further in human imaging experiments (Cornette et al. 2002; Sakai et al. 2002), and it appears to represent a fundamental difference in cortical processing between the prefrontal cortex and its afferent inputs.
Intrinsic circuit differences between prefrontal and parietal cortex.
Computational models have been successful in replicating the properties of delay-period activity in the prefrontal cortex (Compte et al. 2000) and have been insightful in determining how resistance to interference may arise. The higher relative density of NMDA receptors compared with α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors and the role of dopamine have been identified as critical in this respect (Chen et al. 2004; Durstewitz et al. 2000; Seamans et al. 2001; Wang et al. 2008; Wang 2001; Yang and Seamans 1996). Other, more elemental differences between the prefrontal and parietal intrinsic network organization have also been suggested. It is notable, for example, that prefrontal neurons exhibit the most extensive dendritic trees and highest number of spines of any cortical neurons (Elston 2000, 2003), although the physiological implications of such anatomical specialization are yet unknown.
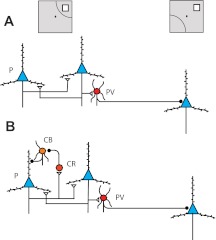
Differences in interneuron types in the prefrontal cortex provide another mechanistic explanation, which could contribute to the unique properties of the prefrontal cortex. Most interneurons in the cortex are parvalbumin-containing neurons (Fig. 7A), which physiologically make up the FS category of interneurons, and inhibit neurons with stimulus selectivity different than their own (Krimer et al. 2005; Zaitsev et al. 2005). In the prefrontal cortex, calbindin-containing interneurons are more numerous than in other cortical areas (Elston and Gonzalez-Albo 2003), although a direct, anatomical comparison with the parietal cortex is not available. Calbindin interneurons are inhibited by calretinin interneurons and in turn, inhibit the dendrites of pyramidal neurons in close vicinity, spatially restricted in vertical columns (Conde et al. 1994; Gabbott and Bacon 1996; Krimer et al. 2005; Zaitsev et al. 2005). The functional consequence of such a circuit in spatial working memory proposed by Wang et al. (2004) is illustrated in Fig. 7. After a stimulus has been presented and is maintained in memory, a population of pyramidal cells exhibits persistent activity. These neurons activate local parvalbumin interneurons, which in turn, inhibit pyramidal neurons with different stimulus preference. At the same time, the activated pyramidal neurons excite calretinin interneurons, which in turn, inhibit calbindin (inverted tuning) interneurons. The precisely localized axons of the calbindin interneurons then further disinhibit the pyramidal neurons that are already activated (Fig. 7B). Nonactivated, pyramidal neurons (selective for other stimuli) continue to be tonically inhibited by the combined action of parvalbumin interneurons and tonic inhibition of calbindin interneurons in their own microcolumns. As a result, once a stimulus is already held in memory in the circuit of Fig. 7B, a distracting stimulus appearing at a different location is less effective in suppressing the persistent activity of already-activated pyramidal neurons, which are both mutually excited and receive less inhibition by calbindin interneurons (Wang et al. 2004). Apart from resistance to the interference of actual distracting stimuli, the tonic action of calbindin interneurons during working memory suppresses the baseline activity of neurons tuned for stimuli away from the remembered stimulus, further sharpening the representation of the remembered stimulus in the population and essentially reducing the noise in the circuit.
Fig. 7.
Schematic diagram of the prototypical circuit network of (A) PPC and (B) dorsolateral PFC. Different types of neurons are indicated as follows: P, pyramidal neuron (blue triangles); PV, parvalbumin interneuron (red circles); CR, calretinin interneuron (light-red circle); CB, calbindin interneuron (orange circle). Open triangles denote excitatory synapses; black circles, inhibitory synapses. Insets on top are meant to illustrate that pyramidal neurons on the left side of the figure are driven by a stimulus in the receptive field (shown as an arc), whereas the same stimulus falls out of the receptive field of the neuron on the right side of the figure.
Calbindin interneurons may play other unique roles in the prefrontal cortex. Impairments in GABA neurotransmission among non-FS interneurons have been implicated in schizophrenia, a condition that compromises prefrontal function (Lewis et al. 2008). Anatomical studies also indicate that a considerable proportion of anterior cingulate projections terminates directly on calbindin interneurons, providing a means of controlling prefrontal excitability (Medalla and Barbas 2009, 2010). Our current results provide a clear instance of a specialization in the functional neuron types of the prefrontal cortex compared with that of its afferent cortical areas, which could account for its unique, functional qualities.
GRANTS
Support for this work was provided by the National Eye Institute Grants EY16773 and EY017077 and the Tab Williams Family Endowment Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.C. conception and design of research; F.K., X-L.Q., and C.C. performed experiments; X.Z., F.K., X-L.Q., and C.C. analyzed data; X.Z. and C.C. interpreted results of experiments; X.Z. and C.C. prepared figures; X.Z. and C.C. drafted manuscript; X.Z., F.K., X-L.Q., and C.C. edited and revised manuscript; X.Z., F.K., X-L.Q., and C.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Travis Meyer and Justin Rawley for their contributions to experiments that generated some of the data analyzed here, Kathini Palaninathan and Keith Roberts for technical help, and Ram Ramachandran for comments.
REFERENCES
- Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci 33: 1–21, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol 287: 422–445, 1989 [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Inactivation of parietal and prefrontal cortex reveals interdependence of neural activity during memory-guided saccades. J Neurophysiol 83: 1550–1566, 2000 [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J Neurophysiol 79: 2919–2940, 1998 [DOI] [PubMed] [Google Scholar]
- Chen G, Greengard P, Yan Z. Potentiation of NMDA receptor currents by dopamine D1 receptors in prefrontal cortex. Proc Natl Acad Sci USA 101: 2596–2600, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compte A, Brunel N, Goldman-Rakic PS, Wang XJ. Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb Cortex 10: 910–923, 2000 [DOI] [PubMed] [Google Scholar]
- Conde F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbumin in monkey prefrontal cortex: distribution and morphology. J Comp Neurol 341: 95–116, 1994 [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Franowicz MN, Goldman-Rakic PS. The sensory nature of mnemonic representation in the primate prefrontal cortex. Nat Neurosci 4: 311–316, 2001 [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Procyk E. The primate working memory networks. Cogn Affect Behav Neurosci 4: 444–465, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidis C, Steinmetz MA. Neuronal activity in posterior parietal area 7a during the delay periods of a spatial memory task. J Neurophysiol 76: 1352–1355, 1996 [DOI] [PubMed] [Google Scholar]
- Cornette L, Dupont P, Orban GA. The neural substrate of orientation short-term memory and resistance to distractor items. Eur J Neurosci 15: 165–175, 2002 [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV. Transient and sustained activity in a distributed neural system for human working memory. Nature 386: 608–611, 1997 [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Wise SP. Effects of attention on visuomotor activity in the premotor and prefrontal cortex of a primate. Somatosens Mot Res 10: 245–262, 1993 [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Kelc M, Gunturkun O. A neurocomputational theory of the dopaminergic modulation of working memory functions. J Neurosci 19: 2807–2822, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. J Neurophysiol 83: 1733–1750, 2000 [DOI] [PubMed] [Google Scholar]
- Elston GN. Pyramidal cells of the frontal lobe: all the more spinous to think with. J Neurosci 20: RC95, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston GN. The pyramidal neuron in occipital, temporal and prefrontal cortex of the owl monkey (Aotus trivirgatus): regional specialization in cell structure. Eur J Neurosci 17: 1313–1318, 2003 [DOI] [PubMed] [Google Scholar]
- Elston GN, Gonzalez-Albo MC. Parvalbumin-, calbindin-, and calretinin-immunoreactive neurons in the prefrontal cortex of the owl monkey (Aotus trivirgatus): a standardized quantitative comparison with sensory and motor areas. Brain Behav Evol 62: 19–30, 2003 [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol 61: 331–349, 1989 [DOI] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science 173: 652–654, 1971 [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Bacon SJ. Local circuit neurons in the medial prefrontal cortex (areas 24a,b,c, 25 and 32) in the monkey: I. Cell morphology and morphometrics. J Comp Neurol 364: 567–608, 1996 [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL. The primate substantia nigra and VTA: integrative circuitry and function. Crit Rev Neurobiol 11: 323–342, 1997 [DOI] [PubMed] [Google Scholar]
- Harris KD, Henze DA, Csicsvari J, Hirase H, Buzsaki G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J Neurophysiol 84: 401–414, 2000 [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, Mintun MA. Spatial working memory in humans as revealed by PET. Nature 363: 623–625, 1993 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Correlation of physiological subgroupings of nonpyramidal cells with parvalbumin- and calbindinD28k-immunoreactive neurons in layer V of rat frontal cortex. J Neurophysiol 70: 387–396, 1993 [DOI] [PubMed] [Google Scholar]
- Krimer LS, Zaitsev AV, Czanner G, Kroner S, Gonzalez-Burgos G, Povysheva NV, Iyengar S, Barrionuevo G, Lewis DA. Cluster analysis-based physiological classification and morphological properties of inhibitory neurons in layers 2–3 of monkey dorsolateral prefrontal cortex. J Neurophysiol 94: 3009–3022, 2005 [DOI] [PubMed] [Google Scholar]
- Levitt P, Rakic P, Goldman-Rakic P. Region-specific distribution of catecholamine afferents in primate cerebral cortex: a fluorescence histochemical analysis. J Comp Neurol 227: 23–36, 1984 [DOI] [PubMed] [Google Scholar]
- Lewis DA, Foote SL, Goldstein M, Morrison JH. The dopaminergic innervation of monkey prefrontal cortex: a tyrosine hydroxylase immunohistochemical study. Brain Res 449: 225–243, 1988 [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Morris HM. Cell and receptor type-specific alterations in markers of GABA neurotransmission in the prefrontal cortex of subjects with schizophrenia. Neurotox Res 14: 237–248, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalla M, Barbas H. Anterior cingulate synapses in prefrontal areas 10 and 46 suggest differential influence in cognitive control. J Neurosci 30: 16068–16081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalla M, Barbas H. Synapses with inhibitory neurons differentiate anterior cingulate from dorsolateral prefrontal pathways associated with cognitive control. Neuron 61: 609–620, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T, Constantinidis C. A software solution for the control of visual behavioral experimentation. J Neurosci Methods 142: 27–34, 2005 [DOI] [PubMed] [Google Scholar]
- Meyer T, Qi XL, Stanford TR, Constantinidis C. Stimulus selectivity in dorsal and ventral prefrontal cortex after training in working memory tasks. J Neurosci 31: 6266–6276, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci 16: 5154–5167, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Li L, Desimone R. Activity of neurons in anterior inferior temporal cortex during a short-term memory task. J Neurosci 13: 1460–1478, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk MH, Linden DE, Muckli L, Lanfermann H, Zanella FE, Singer W, Goebel R. Distributed cortical systems in visual short-term memory revealed by event-related functional magnetic resonance imaging. Cereb Cortex 12: 866–876, 2002 [DOI] [PubMed] [Google Scholar]
- Owen AM, Stern CE, Look RB, Tracey I, Rosen BR, Petrides M. Functional organization of spatial and nonspatial working memory processing within the human lateral frontal cortex. Proc Natl Acad Sci USA 95: 7721–7726, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell KD, Goldberg ME. Response of neurons in the lateral intraparietal area to a distractor flashed during the delay period of a memory-guided saccade. J Neurophysiol 84: 301–310, 2000 [DOI] [PubMed] [Google Scholar]
- Qi XL, Katsuki F, Meyer T, Rawley JB, Zhou X, Douglas KL, Constantinidis C. Comparison of neural activity related to working memory in primate dorsolateral prefrontal and posterior parietal cortex. Front Syst Neurosci 4: 12, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XL, Meyer T, Stanford TR, Constantinidis C. Changes in prefrontal neuronal activity after learning to perform a spatial working memory task. Cereb Cortex 21: 2722–2732, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawley JB, Constantinidis C. Neural correlates of learning and working memory in the primate posterior parietal cortex. Neurobiol Learn Mem 91: 129–138, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raye CL, Johnson MK, Mitchell KJ, Reeder JA, Greene EJ. Neuroimaging a single thought: dorsolateral PFC activity associated with refreshing just-activated information. Neuroimage 15: 447–453, 2002 [DOI] [PubMed] [Google Scholar]
- Sakai K, Rowe JB, Passingham RE. Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nat Neurosci 5: 479–484, 2002 [DOI] [PubMed] [Google Scholar]
- Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc Natl Acad Sci USA 98: 301–306, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Courtney SM, Haxby JV. A neural system for human visual working memory. Proc Natl Acad Sci USA 95: 883–890, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneswaran G, Kraskov A, Lemon RN. Large identified pyramidal cells in macaque motor and premotor cortex exhibit “thin spikes”: implications for cell type classification. J Neurosci 31: 14235–14242, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Stradtman GG, 3rd, Wang XJ, Gao WJ. A specialized NMDA receptor function in layer 5 recurrent microcircuitry of the adult rat prefrontal cortex. Proc Natl Acad Sci USA 105: 16791–16796, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ. Synaptic reverberation underlying mnemonic persistent activity. Trends Neurosci 24: 455–463, 2001 [DOI] [PubMed] [Google Scholar]
- Wang XJ, Tegner J, Constantinidis C, Goldman-Rakic PS. Division of labor among distinct subtypes of inhibitory neurons in a cortical microcircuit of working memory. Proc Natl Acad Sci USA 101: 1368–1373, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CR, Seamans JK. Dopamine D1 receptor actions in layers V–VI rat prefrontal cortex neurons in vitro: modulation of dendritic-somatic signal integration. J Neurosci 16: 1922–1935, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CR, Seamans JK, Gorelova N. Developing a neuronal model for the pathophysiology of schizophrenia based on the nature of electrophysiological actions of dopamine in the prefrontal cortex. Neuropsychopharmacology 21: 161–194, 1999 [DOI] [PubMed] [Google Scholar]
- Zaitsev AV, Gonzalez-Burgos G, Povysheva NV, Kroner S, Lewis DA, Krimer LS. Localization of calcium-binding proteins in physiologically and morphologically characterized interneurons of monkey dorsolateral prefrontal cortex. Cereb Cortex 15: 1178–1186, 2005 [DOI] [PubMed] [Google Scholar]