Abstract
Muscle sensory axons induce the development of specialized intrafusal muscle fibers in muscle spindles during development, but the role that the intrafusal fibers may play in the development of the central projections of these Ia sensory axons is unclear. In the present study, we assessed the influence of intrafusal fibers in muscle spindles on the formation of monosynaptic connections between Ia (muscle spindle) sensory axons and motoneurons (MNs) using two transgenic strains of mice. Deletion of the ErbB2 receptor from developing myotubes disrupts the formation of intrafusal muscle fibers and causes a nearly complete absence of functional synaptic connections between Ia axons and MNs. Monosynaptic connectivity can be fully restored by postnatal administration of neurotrophin-3 (NT-3), and the synaptic connections in NT-3-treated mice are as specific as in wild-type mice. Deletion of the Egr3 transcription factor also impairs the development of intrafusal muscle fibers and disrupts synaptic connectivity between Ia axons and MNs. Postnatal injections of NT-3 restore the normal strengths and specificity of Ia–motoneuronal connections in these mice as well. Severe deficits in intrafusal fiber development, therefore, do not disrupt the establishment of normal, selective patterns of connections between Ia axons and MNs, although these connections require the presence of NT-3, normally supplied by intrafusal fibers, to be functional.
Keywords: NT-3, ErbB2, Egr3, synaptic specificity, sensory-motor synapses
the spinal stretch reflex, which provides proprioceptive sensory feedback to regulate motor output, has been used as a model for neuroscientists to explore the mechanism underlying specific synaptic connections for many years. The development of the stretch-reflex circuit depends on interactions between its component parts (Chen et al. 2003; Ladle et al. 2007). A deficit in one component can influence the development of others. One example is the interaction between developing myotubes and proprioceptive sensory afferents. Differentiation and maturation of intrafusal muscle fibers in spindles require signals from afferent terminals; intrafusal fibers in mature spindles, on the other hand, support normal functions of muscle afferents by providing neurotrophic factors, such as neurotrophin-3 (NT-3) (Carr and Simpson 1978; Hamburger et al. 1981; Kucera and Walro 1987, 1988; Zelena 1994).
An important characteristic of the stretch reflex is the specificity of monosynaptic connections between muscle spindle sensory afferents (Ia axons) and motoneurons (MNs). Ia sensory neurons establish strong monosynaptic connections with homonymous and synergistic MNs but not with functionally antagonistic or unrelated MNs (Eccles et al. 1957; Frank 1983). Ia–MN connections appear to be specified from the outset in frogs, chickens, and rodents, because they are already specific during the period when these connections are still being made (Frank and Westerfield 1983; Lee and O'Donovan 1991; Mears and Frank 1997; Mendelson and Frank 1991). Previous experiments suggest that peripheral targets of developing Ia afferents influence their choice of synaptic targets in the spinal cord. Afferents forced to project to foreign muscles make synaptic contacts with MNs, projecting to those muscles rather than the MNs that they normally innervate (Smith and Frank 1987; Wenner and Frank 1995). One possibility is that this connectional specificity is determined by the intrafusal muscle fibers that Ia afferents contact. If this hypothesis were true, then blockade of the development of intrafusal fibers in spindles should result in inappropriate sensory-motor connections.
Immature myotubes are induced to form intrafusal fibers when sensory afferent axons contact primary myotubes during development (Kucera and Walro 1987; Zelena 1994). This induction relies on the neuregulin-1 tyrosine kinase receptor ErbB2 (Andrechek et al. 2002; Leu et al. 2003) and the zinc-finger transcription factor Egr3 (Tourtellotte et al. 2001; Tourtellotte and Milbrandt 1998). Selective elimination of either of these factors in skeletal muscle during embryonic development results in a major reduction in the number of spindles postnatally and an abnormal spindle morphology in the few spindles that are present (Leu et al. 2003; Shneider et al. 2009; Tourtellotte et al. 2001). If the development of a functionally appropriate stretch-reflex circuit depends on the presence of muscle spindles, then this circuit should be disrupted in ErbB2- and Egr3-deficient mutants, in which spindle numbers are drastically reduced. We therefore used these mutant strains to assess the importance of muscle spindles in the development of functional and specific synaptic connections between Ia axons and MNs.
In both mutant strains, the mice display abnormal hindlimb reflexes and ataxia, suggesting that some component of the stretch-reflex circuit may be abnormal. The disruption of normal reflex behavior in both Egr3 and ErbB2 mutant strains is not a direct consequence of the absence of sensory projections into the appropriate region of the spinal cord, however. Anatomical tracing of Ia afferent projections to the spinal cord reveals a normal projection of muscle sensory axons into the dendritic arbors of MNs (Chen et al. 2002; Leu et al. 2003). Ia axons project to their normal target region, and the axon terminals of these afferents, identified histochemically, make direct contact with motoneuronal dendrites (Shneider et al. 2009). It is unknown, however, if the functional pattern of sensory-motor connections is normal. Functional connectivity can be assessed most directly by making electrical recordings from identified MNs while stimulating identified Ia afferents.
A difficulty in using electrophysiology to assess functional sensory-motor connectivity, however, is that the amplitude of the excitatory postsynaptic potentials (EPSPs) in MNs, elicited by stimulation of Ia afferents, is reduced severely in these mutant strains. As shown in this report, homonymous Ia input to quadriceps (Q) MNs is reduced more than tenfold in both Egr3-deficient (Egr3−/−) and ErbB2-deficient (ErbB2−/−) mice. Postnatal systemic injection of the NT-3 restores functional Ia–MN connectivity, however [see also Chen et al. (2002) and Wang et al. (2007)]. We therefore used postnatal injections of NT-3 to augment Ia–MN synaptic potential amplitudes in both mutant strains, thereby enabling quantitative measurements of the specificity of these sensory-motor connections. In both strains, we found that the specificity of these connections was normal, providing strong evidence that the presence of normal muscle spindles is not required for the specification of the central connectivity of Ia sensory axons.
MATERIALS AND METHODS
Animals.
ErbB2flox/− and human skeletal α-actin (HSA)-Cre mice were obtained from Dr. Ulrich Müller (The Scripps Research Institute, La Jolla, CA). The two strains were crossed to generate ErbB2−/− mice, as described in Leu et al. (2003). Egr3 null mutant mice were obtained from Dr. Warren Tourtellotte (Northwestern University, Chicago, IL) (Tourtellotte et al. 2001). After experiments, each mouse was genotyped using the PCR procedure (Leu et al. 2003; Tourtellotte et al. 2001). For all mice, postnatal day (P)0 was defined as the first 24-h period after birth, and experiments were carried out using mice from P7 to P9. All of the procedures used in these experiments were approved by the Institutional Animal Care and Use Committee at Tufts University School of Medicine and conformed to National Institutes of Health guidelines. Experimenters were blinded to the genotypes of the mice during all recording and subsequent electrophysiological and morphological analyses.
Preparation.
An isolated spinal cord preparation was used for the experiments. Details of this preparation have been described previously (Mears and Frank 1997). Briefly, neonatal mice were anesthetized with hypothermia, perfused with cold saline, decapitated, skinned, and eviscerated. Dissection was performed in recirculating oxygenated (95% O2, 5% CO2) cold saline. The solution used for dissection and recording contained (mM): NaCl (127), KCl (1.9), KH2PO4 (1.2), CaCl2 (2), MgSO4 (1), NaHCO3 (25), and dextrose (20.5). The hindlimb nerves, including Q and hip adductors (Add), were dissected in continuity with the spinal cord. The cord was hemisected along the dorsoventral midline and positioned in the recording chamber with its cut medial surface uppermost. The preparation was maintained in rapidly circulating (∼35 ml/min) saline, which was gradually warmed to room temperature (22–25°C).
Electrophysiology.
For extracellular recordings, synaptic potentials elicited by stimulation of muscle nerves were recorded from the LS3 ventral root (VR) with a tight-fitting glass-suction electrode in close proximity to the cord. Signals (filtered with 0.1 Hz and 3 KHz high- and low-pass filters and digitized at 20 KHz) were acquired and stored for subsequent analysis.
For intracellular recordings, MNs were impaled with glass micropipettes (100–180 MΩ), filled with 2 M potassium methylsulfate, 500 mM QX-314 (to block antidromic action potentials) and 0.5% fast green (to facilitate placement of the micropipette). MNs were identified by antidromic action potentials, evoked by stimulation of the appropriate muscle nerve. QX-314, diffusing from the electrode tip, blocked somatic action potentials after several minutes, facilitating the measurement of homonymous synaptic inputs. A small, residual antidromic potential remained in some of the homonymous recordings (for example, the intracellular trace in Fig. 2B). Measurements only from those MNs with a resting potential more negative than −50 mV and in which synaptic potential amplitudes were stable for at least several minutes are included in this report.
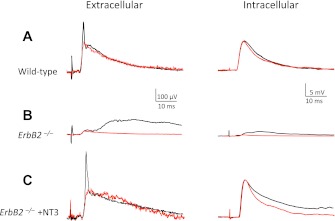
Fig. 2.
Transmission between Ia afferents and motoneurons (MNs) is abolished in ErbB2−/− mice but is restored by postnatal injections of neurotrophin-3 (NT-3; NT3). Monosynaptic excitatory postsynaptic potentials (EPSPs) were recorded extracellularly in P7–P9 mice from the L3 ventral root (left column) and intracellularly from Q MNs (right column), following stimulation of the Q nerve in wild-type (A), ErbB2−/− (B), and NT-3-treated ErbB2−/− (C) mutant mice. Notice that later, polysynaptic components, visible in the recordings, are not abolished in ErbB2−/− mice. Red traces in each recording in this and subsequent figures are the model traces used to measure monosynaptic EPSP amplitudes (see materials and methods for details).
Muscle nerves were stimulated via suction electrodes with square pulses of 0.1-ms duration at supramaximal levels (<7 V). The stimulation frequency was typically 0.33 Hz, which produced little or no synaptic fatigue. The resulting potentials, filtered at 3–10 KHz, were recorded digitally at 10–20 KHz. Individual traces (five to 15 traces, depending on the variability of the responses) were averaged online and stored for subsequent analysis using custom software (LabVIEW, National Instruments, Austin, TX).
The functional response of sensory axons to muscle stretch was assessed in wild-type and ErbB2−/− mice, which were not treated with NT-3, by recording from the Q nerve with a tight-fitting suction electrode while activating Ia afferents directly by tapping the muscle with a probe (a broom straw) attached to a piezoelectric bimorph (Chen et al. 2002; Lichtman and Frank 1984), or indirectly, by eliciting contraction of intrafusal muscle fibers with succinyl choline (SCh; refer to results for further explanation and references). For the tests with SCh, action potentials in sensory axons were recorded during 5-s periods, once/min, first in normal solution and then for 10–20 min following addition of 20 μM SCh to the perfusion medium. In wild-type mice, there was a marked increase in spike frequency beginning ∼5 min after the perfusion with SCh began and continuing for a few minutes before declining to background. The response was taken as the average firing frequency during the 5-s period in which the response frequency was maximal.
Data analysis.
To measure selectively the monosynaptic component of each synaptic potential, we created a “model” trace for each mouse of pure monosynaptic input, as used in several earlier studies from this laboratory (Arber et al. 2000; Mears and Frank 1997; Sah and Frank 1984). The model trace was elicited by a just-threshold stimulus to the homonymous muscle nerve. The resulting small synaptic potential was therefore unlikely to elicit action potentials in interneurons and therefore, minimized the activation of later, polysynaptic components. The smooth-falling phase of model traces provided an independent indication of the absence of polysynaptic components (see Figs. 2–4). We superimposed this model trace over traces to be analyzed and scaled it under software control so that its rising phase during the first few milliseconds matched that of the EPSP being measured. The amplitude of the scaled model then provided a measure of the amplitude of the monosynaptic portion of the EPSP.
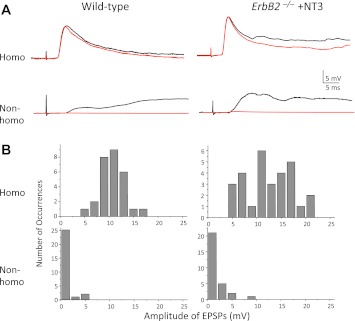
Fig. 3.
Monosynaptic Ia–MN synaptic connections in NT-3-treated ErbB2−/− mice are specific. A: representative intracellular traces in P7–P9 mice from Q MNs in response to stimulation of Q [homonymous (Homo)] and hip adductors [Add; nonhomonymous (Non-homo)] sensory axons. Note that polysynaptic nonhomonymous inputs are common in both wild-type and NT-3-treated mutant mice. B: amplitude frequency histograms of homonymous and nonhomonymous monosynaptic EPSP amplitudes in wild-type and NT-3-treated ErbB2−/− mice. All homonymous inputs are at least 5 mV in both wild-type and mutant mice, whereas nonhomonymous inputs are nearly all <5 mV. The specificity of homonymous vs. nonhomonymous Ia–MN connections is therefore preserved in NT-3-treated ErbB2−/− mice.
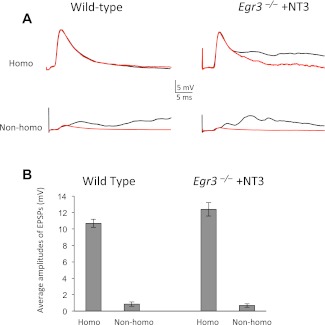
Fig. 4.
Monosynaptic Ia–MN connections are specific in NT-3-treated Egr3-deficient (Egr3−/−) mice. A: representative intracellularly recorded traces from Q MNs in response to stimulation of Q (homonymous) and Add (nonhomonymous) sensory axons. B: average amplitudes of monosynaptic Ia–MN EPSPs in wild-type and NT-3-treated Egr3−/− mice. In both types of mice, the average homonymous inputs are >10 times larger than nonhomonymous inputs.
The specificity of Ia synaptic connections with their target MNs was defined by comparing the amplitudes of homonymous EPSPs to that of non-homonymous EPSPs. A convenient measure of this specificity in individual mice is the specificity index (SI), defined here (see also Wang et al. 2007):
where EPSPhomo = average amplitude of monosynaptic inputs to homonymous MNs; EPSPnon-homo = average amplitude of monosynaptic inputs to non-homonymous MNs. This index is 1.0 when there are only homonymous inputs but falls to 0.0 when homonymous and non-homonymous inputs are of equal amplitude. As an example, if homonymous connections are ten times as large as non-homonymous ones, the specificity index is 0.82.
NT-3 administration.
Recombinant NT-3 (a gift from Regeneron Pharmaceuticals, Tarrytown, NY), diluted in PBS, was injected (5 μg/g) in a volume of 1–4 μl (depending on the weight of the mouse) into the right proximal hindlimb on P1, P3, P5, and P7.
RESULTS
Deficit of muscle spindle function in ErbB2 mutant mice.
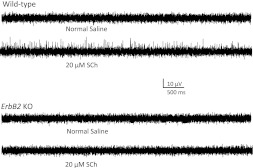
ErbB2 mutant mice displayed abnormal hindlimb extension reflexes and ataxia, as reported previously (Andrechek et al. 2002; Leu et al. 2003). The morphological development of spindles in these mice is blocked soon after sensory axons contact a single myotube, and mature muscle spindles never develop (Leu et al. 2003). We tested the ability of muscle sensory axons to respond to mechanical activation and conduct action potentials by tapping the muscle surface with a probe (see materials and methods) in ErbB2 mutant mice untreated with NT-3. When responses were elicited from mutant muscles, the sensory action potentials recorded in the Q muscle nerve had normal latencies, demonstrating functional axonal conduction. To test the function of spindles to muscle stretch in these mice, we initially attempted to record action potentials in the Q nerve elicited by maintained stretch of the Q muscle. Neonatal spindles adapt rapidly to maintained stretch even in wild-type mice, however, making these measurements problematic. Instead, we evoked spindle discharges in stretched muscles by addition of SCh to the bathing solution. SCh causes selective contraction of intrafusal but not extrafusal muscle fibers (Dutia 1980; Granit et al. 1953; Gregory and Proske 1987; Taylor et al. 1992). Both bag1 and bag2 fibers are activated (Boyd 1985; Gladden 1976), suggesting that SCh should cause contraction of intrafusal fibers, even in the severely atrophic spindles in ErbB2 mutant mice. Recordings were made before and after SCh application in both wild-type and ErbB2 mutant mice at P7–P9 (Fig. 1). In wild-type mice, application of 20 μM SCh evoked a robust discharge of action potentials, from 0 Hz before SCh to 12.9 ± 4.3 Hz (six mice) in the presence of SCh. In ErbB2 mutant mice, however, the maximal response in SCh was only 0.2 ± 0.14 Hz (seven mice). These results suggest that the functional development of intrafusal muscle fibers and/or their functional innervation by Ia sensory axons are blocked in ErbB2 mutant mice.
Fig. 1.
Loss of spindle responses to application of succinyl choline (SCh) in untreated ErbB2-deficient (ErbB2−/−) postnatal day (P)7–P9 mice. The response from isolated wild-type neonatal quadriceps (Q) muscles in normal solution adapted quickly during maintained stretch but was maintained for several minutes during perfusion with 20 μM SCh (2 top traces). In similar preparations from ErbB2−/− mice [knockout (KO); n = 7], however, there was virtually no response in either solution (2 bottom traces).
Transmission between Ia afferents and MNs is abolished in ErbB2 mutant mice.
Although the development of muscle spindles was blocked in ErbB2 mutant mice, central projections of Ia sensory axons appeared to be normal. In an earlier study using this mutant strain, Leu and coworkers (2003) reported the normal presence of parvalbumin-positive (PV+; a marker for myelinated muscle sensory neurons) cells in dorsal root (DR) ganglia, and PV+ central projections of Ia and Ib neurons were observed in Clarke's column and the ventral horn. In that study, it was not known if these projections were functional, however. We therefore assessed synaptic transmission between muscle sensory afferents and MNs by intracellular recordings from antidromically identified MNs. The amplitude of homonymous monosynaptic EPSPs (Q Ias to Q MNs and Add Ias to Add MNs) was reduced 20-fold in ErbB2 mutants (mutant: 0.52 ± 0.14 mV, n = 13; wild-type: 10.7 ± 0.5 mV, n = 28; P < 0.001; Fig. 2, A and B). To evaluate the average change in Ia afferents projecting to all L3 MNs, we recorded synaptic potentials extracellularly with a suction electrode from the cut L3 VR following stimulation of Q and Add afferents. Similar to the results for individual MNs, the amplitude of the composite VR EPSP was substantially smaller in mutant mice (mutant: 13.7 ± 3.1 μV, n = 4; wild-type: 104.0 ± 8.2 μV, n = 8; P < 0.001, Fig. 2, A and B). These results demonstrate that although Ia afferents still project robustly into the dendritic arbors of MNs in ErbB2 mutant mice, the functional synaptic input from Ia fibers to MNs is nearly abolished.
Synaptic function in ErbB2−/− mice is restored by postnatal injections of NT-3.
The deficits in spindle development and synaptic function but with normal anatomical central projections of Ia afferents in ErbB2 mutant mice are reminiscent of the situation in Egr3 mutant mice (Chen et al. 2002). In those mice, synaptic transmission could be restored by postnatal injections of NT-3. We therefore tested if the same procedure restored Ia–MN synaptic function in ErbB2 mutants. NT-3 was injected into hindlimb muscles of ErbB2 mutant mice on P1, P3, P5, and P7. We assessed the presence of Ia–MN synaptic connections between Ia afferents and MNs on P8, using both intracellular and extracellular recordings. Functional monosynaptic connections were restored completely (Fig. 2C). With intracellular recordings, the amplitudes of homonymous monosynaptic potentials were increased to levels slightly larger than normal, although the difference in average amplitude was not significant (P = 0.10). Average homonymous monosynaptic EPSPs from Q or Add afferents were 12.3 ± 0.9 mV (n = 29) compared with 10.7 ± 0.5 mV (n = 28) in normal mice. For extracellular recordings from L3 VRs, the average synaptic potential elicited by Q afferents was 116.8 ± 3.4 μV (n = 4) comparable with that in normal mice. Postnatal injections of NT-3 therefore restored the amplitude of Ia–MN monosynaptic EPSPs to normal levels, making it possible to assess the specificity of these synaptic connections.
Sensory-motor synaptic connections in ErbB2 mutant mice are specific.
The specificity of Ia–MN synaptic connections was assessed by measuring the amplitude of EPSPs in Q and Add MNs, elicited by stimulating Q and Add Ia afferents. If these connections were specific, then homonymous inputs would be much stronger than nonhomonymous inputs, as in normal mice. In contrast, if muscle spindles were required for these connections to be specific, then homonymous and nonhomonymous inputs would have similar strengths. The specificity of these connections in the NT-3-injected ErbB2−/− mice was nearly as high as in normal mice (Fig. 3, A and B). Homonymous EPSPs were 8.2 times larger than nonhomonymous EPSPs (homo: 12.3 ± 0.9 mV; nonhomo: 1.5 ± 0.4 mV; n = 29) compared with 12.6 times larger in normal mice (homo: 10.7 ± 0.5 mV; nonhomo: 0.85 ± 0.27 mV; n = 28).
Sensory-motor synaptic connections are specific in Egr3−/− mice treated with NT-3.
The specificity of Ia–MN synaptic connections in ErbB2 mutant mice suggests that the presence of normal muscle spindles is not required for the formation of specific connections between Ia afferents and MNs. To provide additional evidence, we assessed this specificity in a different strain of mice, in which spindle formation is also disrupted—Egr3−/− mice. In these mice, the absence of the transcription factor Egr3 results in an absence of the spindle-specific, slow-developmental myosin heavy chain; the number of nuclei in intrafusal fibers is decreased, and the spindles are small. Importantly, mutant spindles do not express mRNA for NT-3, as determined by in situ hybridization (Chen et al. 2002; Tourtellotte et al. 2001). Our earlier experiments, using extracellular recording from VRs, showed a 12-fold reduction in Ia–MN monosynaptic EPSPs in Egr3−/− mice by P8, but synaptic transmission could be restored by injection of NT-3 after birth (Chen et al. 2002). In the present study, using intracellular recordings from antidromically identified hindlimb MNs, we first confirmed that monosynaptic Ia input was largely blocked in P5–P7 Egr3−/− mice and that this input could be restored by postnatal NT-3 injections. There was a 12-fold reduction in the amplitude of homonymous Ia input to Q and Add MNs [10.7 ± 0.5 mV (n = 28) in wild-type mice; 0.9 ± 0.4 mV (n = 11) in Egr3−/− mice], similar to the large reduction seen earlier with the extracellular recording from VRs (Chen et al. 2002). Postnatal injections of NT-3 from P1 to P7 restored homonymous monosynaptic EPSPs in NT-3-treated Egr3−/− mice [NT-3-treated Egr3−/− mice: 12.4 ± 0.8 mV (n = 19); wild-type mice: 10.7 ± 0.5 (n = 28); Fig. 4], just as in NT-3-treated ErbB2−/− mice.
The restoration of Ia inputs to MNs by NT-3 injections made it possible to assess the specificity of these connections, as in ErbB2−/− mice. The monosynaptic connections in NT-3-treated Egr3 mutant mice are still highly specific. As in wild-type mice, monosynaptic homonymous EPSPs are more than tenfold larger than nonhomonymous connections [homo: 12.4 ± 0.8 mV (n = 19); nonhomo: 0.7 ± 0.2 mV (n = 19); Fig. 4B]. These results indicate that Egr3 and its downstream target proteins in intrafusal muscle fibers are not needed for the establishment of specific synaptic connections between Ia afferents and their target MNs.
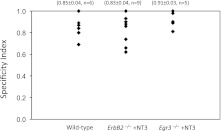
This specificity was apparent in individual mice, as shown by calculating an SI for each normal, NT-3-treated ErbB2−/− and NT-3-treated Egr3−/− mouse (Fig. 5). The SIs were high for all three groups of mice (wild-type: 0.85 ± 0.04, n = 6; NT-3-treated ErbB2 mutants: 0.83 ± 0.04, n = 9; NT-3-treated Egr3 mutants: 0.92 ± 0.03; n = 5), implying that homonymous connections were, on average, >10 times larger than nonhomonymous connections in normal and both strains of NT-3-treated mutant mice. Together, these data demonstrate that the highly specific patterns of monosynaptic connections in the stretch-reflex pathway do not depend on the presence of normal muscle spindles, although functional connectivity between Ia afferents and MNs is normally dependent on some factor (probably NT-3) expressed by normal spindles.
Fig. 5.
Monosynaptic Ia–MN synaptic connections are specific in individual mice. The specificity of these connections was measured by comparing homonymous and nonhomonymous EPSPs in the same mouse, expressed as a specificity index (SI), where a SI of 1 represents complete specificity (nonhomonymous EPSP amplitudes are 0), and a SI of 0 represents no specificity (homonymous and nonhomonymous EPSP amplitudes are equal). The average SIs for wild-type, NT-3-treated ErbB2−/−, and NT-3-treated Egr3−/− mice are similar, demonstrating that the specificity of these connections is preserved in both strains of mutant mice. The variability in SI values for individual mice is probably a consequence of the small number of Ia–MN connections assayed in each mouse.
DISCUSSION
The main goal of the present study was to determine whether normal muscle spindles are required for the formation of specific sets of synaptic connections between Ia afferents and MNs. Several lines of evidence suggest that during development, the peripheral targets of Ia sensory neurons determine which particular subsets of MNs these afferents contact (Chen et al. 2002, 2003; Wenner and Frank 1995). Ia sensory axons induce the formation of intrafusal muscle fibers before they establish their central connections with MNs, so some non-neuronal component of spindles, presumably the intrafusal muscle fibers, might be the source of peripheral factors that specify their target MNs. Disruption of the development of muscle spindles would then lead to aberrant monosynaptic connections between Ia afferents and MNs. We tested this idea in two lines of mice, in which spindle development is severely disturbed. In the ErbB2−/− mice used in this study, although the initial stage of spindle development occurs, a myotube initially contacted by a Ia fiber never develops normally and never recruits additional myotubes to form a normal muscle spindle (Leu et al. 2003). Furthermore, we found that Ia afferents in these mice are not activated by SCh, a selective activator of intrafusal fiber contraction, consistent with disruption of normal spindle function. Muscle sensory axons are still sensitive to mechanical stimuli and can still conduct action potentials, so this loss of function likely results from the absence of normal intrafusal muscle fibers or from loss of their normal Ia innervation. Although some isolated myotubes are still contacted by Ia afferents as late as P4, the nerve terminals are weakly stained with PV and have an irregular appearance (Leu et al. 2003). There is a similar, although less-severe, disruption of spindle development in Egr3−/− KO mice (Tourtellotte et al. 2001; Tourtellotte and Milbrandt 1998). Both strains of mice therefore are useful for testing the role of normal muscle spindles on the establishment of specific connections between Ia afferents and MNs.
A difficulty in assessing functional synaptic specificity in either of these strains is that there is a severe reduction in the amplitude of synaptic input from Ia afferents to MNs. One approach is to use a strain in which the reduction of these EPSPs is less severe. For example, in a study of a different ErbB2 mutant strain, monosynaptic inputs to MNs were reduced by only 80%, so it was possible to measure the specificity of only those synapses that remained functional (Shneider et al. 2009). Spindles expressing Egr3 still exist in these mice, however, so it is likely that the remaining functional contacts were made by Ia afferents innervating Egr3+ spindles, which might specify appropriate Ia–MN connections. Moreover, the small amplitude of the synaptic potentials in these mice required the investigators to base their assessment of specificity on the latencies of the EPSPs rather than on their amplitudes.
In the present study, the reduction of EPSP amplitudes was nearly complete, so postnatal injections of NT-3 were used to restore functional Ia–MN connectivity. This made it possible to measure the amplitudes of both homonymous and nonhomonymous EPSPs and therefore to determine synaptic specificity directly. As reported here, the monosynaptic connections in both ErbB2−/− and Egr3−/− mice, following postnatal administration of NT-3, are highly specific. These results provide strong evidence that although NT-3, or some other factor normally supplied by muscle spindles, is required for functional connectivity in this system, neither normal spindle function nor normal spindle structure is required for Ia afferents to make correct synaptic connections with their target MNs.
The loss of functional connectivity described for Egr3−/− and ErbB2−/− mice parallels a similar loss reported for another murine strain in which normal spindle development is disrupted. ShcA, which mediates ErbB2 signaling in various systems, is expressed in developing spindles, and the Shc3F−/− strain has morphologically abnormal spindles at birth, which eventually disappear. In Shc3F−/− mice, the amplitude of Ia–MN synaptic potentials is also reduced approximately tenfold (Hardy et al. 2007). The evidence from all of the mutant strains therefore provides strong support that some factor(s) produced by muscle spindles are required for functional synaptic transmission between spindle afferents and MNs.
Earlier evidence suggested that this factor was NT-3. NT-3 mRNA is not expressed in spindles in either the Egr3−/− or ErbB2−/− strain (Chen et al. 2002; Shneider et al. 2009). As shown in this report, injections of NT-3 into the proximal hindlimb during the first postnatal week restore functional synaptic connectivity, as do injections restricted to the second postnatal week (Chen et al. 2002). Transgenic expression of NT-3 in skeletal muscle from embryonic day 12 also restores functional Ia–MN connections when tested in Shc3F−/− and Egr3−/− P8–P9 pups (Hardy et al. 2007; Wang et al. 2007). The restoration of synaptic transmission by an increase in NT-3 in peripheral tissues in all of these murine strains with defective development of muscle spindles provides evidence that spindle-derived NT-3 is normally required for synaptic transmission to MNs.
An alternate view is suggested by Shneider et al. (2009). They created two new mouse strains, in which floxed NT-3 was deleted during development with Cre recombinase, expressed under the control of the Egr3 or myf5 promoter. In both strains, stimulation of the L5 DR elicited short-latency orthodromic action potentials in the L5 VR in P5 pups, despite the absence of detectable NT-3 mRNA in distal hindlimb muscle spindles. Short-latency DR–VR potentials are likely to be generated by monosynaptic connections between Ia afferents and MNs, which suggests that Ia–MN synaptic transmission may not depend on muscle-derived NT-3 at P5. A significant reduction in these potentials was seen at P14, however, consistent with earlier observations (Chen et al. 2002, 2003). The persistence of synaptic transmission at P5, despite the absence of NT-3 mRNA in hindlimb muscle spindles, led Shneider and coworkers (2009) to suggest that some factor(s) other than NT-3 but under the control of the Egr3 transcription factor may be sufficient to maintain synaptic transmission during the first postnatal week.
The results of Shneider et al. (2009) seem to be in conflict with those described in an earlier study of a double knockout of NT-3 and Bax (Patel et al. 2003). All Ia sensory neurons die in NT-3−/− mice, but they can be kept alive if Bax is knocked out as well. In these mice, Ia sensory neurons survive, and their axons reach the spinal cord, but they do not project into the ventral horn. As a result, they do not make anatomical contact with MNs. The presence of Ia–MN synaptic potentials in the conditional NT-3−/− mutants studied by Shneider et al. (2009) therefore indicates that there is likely to be another source of NT-3 that keeps the sensory neurons both alive and able to project into the ventral horn. Although NT-3 mRNA is largely restricted to intrafusal fibers shortly after birth, during prenatal development, NT-3 is expressed in extrafusal muscle fibers and nonmuscle tissue (Copray and Brouwer 1994; Ernfors et al. 1994). It is possible that in the conditional myf5-NT-3 deletion mutant examined by Shneider and coworkers (2009), tissues other than muscle spindles provided a sufficient source of NT-3 to keep Ia–MN synapses functional. NT-3 protein levels were not measured, and some NT-3 is likely to be present during prenatal development in those mutants, because many Ia afferents survive the period of normal cell death during which these sensory neurons are dependent on NT-3 (Ernfors et al. 1992; Huang and Reichardt 2001; Snider 1994). Moreover, DR stimulation activates all sensory afferents, not only those innervating limb muscles, so there may be sufficient NT-3 in central tissues during the first postnatal week to maintain functional Ia–MN synaptic transmission from afferents in more proximal muscles. In future experiments, to test whether NT-3 is required for functional transmission at Ia–MN synapses, it will be important to monitor the postnatal decline of NT-3 protein levels in skeletal muscle and other tissues directly rather than relying on expression patterns of NT-3 mRNA in limb muscles.
GRANTS
Support for this work was provided by a grant from the National Institute of Neurological Disorders and Stroke to E. Frank (NS-24373).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.W., L.L., and E.F. conception and design of research; Z.W. and L.L. performed experiments; Z.W., L.L., and E.F. analyzed data; Z.W. and E.F. interpreted results of experiments; Z.W. and E.F. prepared figures; Z.W. and E.F. drafted manuscript; Z.W. and E.F. edited and revised manuscript; Z.W. and E.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Claudia Barros and Ulrich Müller at The Scripps Research Institute for kindly providing ErbB2flox/− and HSA-Cre mice and Dr. Warren Tourtellotte at Northwestern University for kindly providing the Egr3 null mutant mouse line. We thank Regeneron Pharmaceuticals for its generosity for providing us recombinant NT-3. We also thank Bethany Kiernan and Danielle Gelfand for their help with the breeding and genotyping of animals.
REFERENCES
- Andrechek ER, Hardy WR, Girgis-Gabardo AA, Perry RL, Butler R, Graham FL, Kahn RC, Rudnicki MA, Muller WJ. ErbB2 is required for muscle spindle and myoblast cell survival. Mol Cell Biol 22: 4714–4722, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber S, Ladle DR, Lin JH, Frank E, Jessell TM. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell 101: 485–498, 2000 [DOI] [PubMed] [Google Scholar]
- Boyd IA. Intrafusal muscle fibres in the cat and their motor control. In: Feedback and Motor Control in Invertebrates and Vertebrates, edited by Barnes WJP, Gladden MH. London: Croom Helm, 1985, p. 123–144 [Google Scholar]
- Carr VM, Simpson SB., Jr Proliferative and degenerative events in the early development of chick dorsal root ganglia. I. Normal development. J Comp Neurol 182: 727–739, 1978 [DOI] [PubMed] [Google Scholar]
- Chen HH, Hippenmeyer S, Arber S, Frank E. Development of the monosynaptic stretch reflex circuit. Curr Opin Neurobiol 13: 96–102, 2003 [DOI] [PubMed] [Google Scholar]
- Chen HH, Tourtellotte WG, Frank E. Muscle spindle-derived neurotrophin 3 regulates synaptic connectivity between muscle sensory and motor neurons. J Neurosci 22: 3512–3519, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copray JC, Brouwer N. Selective expression of neurotrophin-3 messenger RNA in muscle spindles of the rat. Neuroscience 63: 1125–1135, 1994 [DOI] [PubMed] [Google Scholar]
- Dutia MB. Activation of cat muscle spindle primary, secondary and intermediate sensory endings by suxamethonium. J Physiol 304: 315–330, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol 137: 22–50, 1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Kucera J, Jaenisch R. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell 77: 503–512, 1994 [DOI] [PubMed] [Google Scholar]
- Ernfors P, Merlio JP, Persson H. Cells expressing mRNA for neurotrophins and their receptors during embryonic rat development. Eur J Neurosci 4: 1140–1158, 1992 [DOI] [PubMed] [Google Scholar]
- Frank E. Development of specific sensory-motor pathways in amphibians. Trends Neurosci 6: 463–467, 1983 [Google Scholar]
- Frank E, Westerfield M. Development of sensory-motor synapses in the spinal cord of the frog. J Physiol 343: 593–610, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladden MH. Structural features relative to the function of intrafusal muscle fibres in the cat. Prog Brain Res 44: 51–59, 1976 [DOI] [PubMed] [Google Scholar]
- Granit R, Skoglund S, Thesleff S. The action of succinylcholine and curare on the muscle spindles. Acta Physiol Scand 29: 86, 1953 [DOI] [PubMed] [Google Scholar]
- Gregory JE, Proske U. Responses of muscle receptors in the kitten to succinyl choline. Exp Brain Res 66: 167–174, 1987 [DOI] [PubMed] [Google Scholar]
- Hamburger V, Brunso-Bechtold JK, Yip JW. Neuronal death in the spinal ganglia of the chick embryo and its reduction by nerve growth factor. J Neurosci 1: 60–71, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy WR, Li L, Wang Z, Sedy J, Fawcett J, Frank E, Kucera J, Pawson T. Combinatorial ShcA docking interactions support diversity in tissue morphogenesis. Science 317: 251–256, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24: 677–736, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera J, Walro JM. Postnatal maturation of spindles in deafferented rat soleus muscles. Anat Embryol (Berl) 176: 449–461, 1987 [DOI] [PubMed] [Google Scholar]
- Kucera J, Walro JM. The effect of neonatal deafferentation or deefferentation on the immunocytochemistry of muscle spindles in the rat. Neurosci Lett 95: 88–92, 1988 [DOI] [PubMed] [Google Scholar]
- Ladle DR, Pecho-Vrieseling E, Arber S. Assembly of motor circuits in the spinal cord: driven to function by genetic and experience-dependent mechanisms. Neuron 56: 270–283, 2007 [DOI] [PubMed] [Google Scholar]
- Lee MT, O'Donovan MJ. Organization of hindlimb muscle afferent projections to lumbosacral motoneurons in the chick embryo. J Neurosci 11: 2564–2573, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu M, Bellmunt E, Schwander M, Farinas I, Brenner HR, Muller U. Erbb2 regulates neuromuscular synapse formation and is essential for muscle spindle development. Development 130: 2291–2301, 2003 [DOI] [PubMed] [Google Scholar]
- Lichtman JW, Frank E. Physiological evidence for specificity of synaptic connections between individual sensory and motor neurons in the brachial spinal cord of the bullfrog. J Neurosci 4: 1745–1753, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears SC, Frank E. Formation of specific monosynaptic connections between muscle spindle afferents and motoneurons in the mouse. J Neurosci 17: 3128–3135, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson B, Frank E. Specific monosynaptic sensory-motor connections in the chick embryonic spinal cord form in the absence of patterned neural activity and motoneuronal cell death. J Neurosci 11: 1390–1403, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel TD, Kramer I, Kucera J, Niederkofler V, Jessell TM, Arber S, Snider WD. Peripheral NT3 signaling is required for ETS protein expression and central patterning of proprioceptive sensory afferents. Neuron 38: 403–416, 2003 [DOI] [PubMed] [Google Scholar]
- Sah DW, Frank E. Regeneration of sensory-motor synapses in the spinal cord of the bullfrog. J Neurosci 4: 2784–2791, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shneider NA, Mentis GZ, Schustak J, O'Donovan MJ. Functionally reduced sensorimotor connections form with normal specificity despite abnormal muscle spindle development: the role of spindle-derived neurotrophin 3. J Neurosci 29: 4719–4735, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CL, Frank E. Peripheral specification of sensory neurons transplanted to novel locations along the neuraxis. J Neurosci 7: 1537–1579, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell 77: 627–638, 1994 [DOI] [PubMed] [Google Scholar]
- Taylor A, Rodgers JF, Fowle AJ, Durbaba R. The effect of succinylcholine on cat gastrocnemius muscle spindle afferents of different types. J Physiol 456: 629–644, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourtellotte WG, Keller-Peck C, Milbrandt J, Kucera J. The transcription factor Egr3 modulates sensory axon-myotube interactions during muscle spindle morphogenesis. Dev Biol 232: 388–399, 2001 [DOI] [PubMed] [Google Scholar]
- Tourtellotte WG, Milbrandt J. Sensory ataxia and muscle spindle agenesis in mice lacking the transcription factor Egr3. Nat Genet 20: 87–91, 1998 [DOI] [PubMed] [Google Scholar]
- Wang Z, Li LY, Taylor MD, Wright DE, Frank E. Prenatal exposure to elevated NT3 disrupts synaptic selectivity in the spinal cord. J Neurosci 27: 3686–3694, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenner P, Frank E. Peripheral target specification of synaptic connectivity of muscle spindle sensory neurons with spinal motoneurons. J Neurosci 15: 8191–8198, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelena J. Nerves and Mechanoreceptors: the Role of Innervation in the Development and Maintenance of Mammalian Mechanoreceptors. London: Chapman & Hall, 1994 [Google Scholar]