Abstract
Muscarinic receptors have long been known as crucial players in hippocampus-dependent learning and memory, but our understanding of the cellular underpinnings and the receptor subtypes involved lags well behind. This holds in particular for the hippocampal CA3 region, where the mechanisms of synaptic plasticity depend on the type of afferent input. Williams and Johnston (Williams S, Johnston D. Science 242: 84–87, 1988; Williams S, Johnston D. J Neurophysiol 64: 1089–1097, 1990) demonstrated muscarinic depression of mossy fiber (MF) long-term potentiation (LTP) through a presynaptic site of action and Maeda et al. (Maeda T, Kaneko S, Satoh M. Brain Res 619: 324–330, 1993) proposed a bidirectional modulation of MF LTP by muscarinic receptor subtypes. Since then, this issue, as well as muscarinic regulation of plasticity at associational/commissural (A/C) fiber-CA3 synapses has remained largely neglected, not least because of the lack of highly selective ligands for the different muscarinic receptor subtypes. In the present study, we performed field potential and whole cell recordings from the hippocampal CA3 region of M2 receptor knockout mice to determine the role of M2 receptors in short-term and long-term plasticity at A/C and MF inputs to CA3 pyramidal cells. At the A/C synapse, M2 receptors promoted short-term facilitation and LTP. Unexpectedly, M2 receptors mediated the opposite effect on LTP at the MF synapse, which was significantly reduced, most likely involving a depressant effect of M2 receptors on adenylyl cyclase activity in MF terminals. Our data demonstrate that cholinergic projections recruit M2 receptors to redistribute the gain of LTP in CA3 pyramidal cells in an input-specific manner.
Keywords: muscarinic receptors, hippocampus
muscarinic receptors play an essential role in various cognitive functions including attention, mental flexibility, and, in particular, learning and memory (Coyle et al. 1983; Hasselmo 2006; Sarter et al. 2005), but it was not until the advent of genetically engineered mice carrying single or double deletions of muscarinic receptor subtypes (M1–M5) that their specific contributions to cognitive processes could be directly elucidated (Wess et al. 2007). Regarding the relative significance of muscarinic receptor subtypes for hippocampus-dependent learning, behavioral studies suggest a preponderance of M2 receptors over M1 receptors. Disruption of the M2 receptor gene produced clear behavioral deficits in spatial learning and hippocampal memory tasks (Bainbridge et al. 2008; Seeger et al. 2004), whereas M1−/− mice, apart from selective cognitive deficits in tasks involving hippocampal-cortical interplay, showed intact hippocampal learning (Anagnostaras et al. 2003; Miyakawa et al. 2001). Furthermore, a novel selective M1 receptor antagonist that suppressed pilocarpine-induced seizures in mice did not impair hippocampus-dependent learning (Sheffler et al. 2009).
On the cellular level, both M2 and M1 receptors were found to promote synaptic plasticity at the Schaffer-CA1 synapse of the hippocampus. In slices from M2−/− mice, short-term plasticity in the CA1 region was abolished and long-term potentiation (LTP) was strongly reduced (Seeger et al. 2004). Moreover, lack of M2 receptors totally abrogated the so-called “muscarinic” LTP in CA1 pyramidal cells (Seeger et al. 2004), which refers to a long-lasting and pronounced enhancement of excitatory postsynaptic potentials (EPSPs) that is independent of high-frequency stimulation (HFS) and evoked by a transient application of a low concentration of a muscarinic agonist (Auerbach and Segal 1994; Markram and Segal 1990). In M1 receptor-deficient hippocampi, HFS produced normal LTP in area CA1 (Anagnostaras et al. 2003; Shinoe et al. 2005), but LTP was impaired when a theta burst pattern was used to induce LTP (Anagnostaras et al. 2003). Interestingly, M1 receptors were found to augment tetanus-induced LTP at the Schaffer-CA1 synapse when activated by endogenously released acetylcholine or a nanomolar concentration of the cholinergic agonist carbachol (Shinoe et al. 2005). Furthermore, two recent studies showed that M1 receptors enhance NMDA receptor function and facilitate LTP at the Schaffer-CA1 synapse by inhibition of small-conductance Ca2+-activated K+ (SK) channels (Buchanan et al. 2010; Giessel and Sabatini 2010).
Notwithstanding the good correlation between impaired (spatial) learning and reduced LTP in area CA1, a more comprehensive picture has to include muscarinic effects in area CA3. This region receives a dense cholinergic projection and exhibits the highest density of M2 receptors compared with other hippocampal regions of rat and mouse brain (Levey et al. 1995; Schwegler et al. 1996). CA3 pyramidal cells have two major excitatory inputs, one through associational/commissural (A/C) fibers and the other one through mossy fibers (MF) of dentate granule cells. In addition, fibers of the perforant path make synapses onto distal apical dendrites of CA3 pyramidal cells (Urban 2001). Whereas LTP at the A/C synapse displays “conventional” NMDA receptor dependence, the MF synapse is unusual in that LTP is predominantly NMDA receptor independent and mediated through a presynaptic mechanism (Nicoll and Schmitz 2005). Although muscarinic depression of MF LTP was first reported more than 20 years ago (Williams and Johnston 1988, 1990; Maeda et al. 1993), we still do not have a clear understanding of how muscarinic receptors adjust synaptic plasticity at the two excitatory synapses onto CA3 pyramidal cells. In the present study, we performed field potential and whole cell recordings in the CA3 region of hippocampal slices from wild-type (WT) and M2 receptor knockout mice. M2 receptors preferentially activate G proteins of the Gi family, leading to inhibition of adenylyl cyclase and, through a Gβγ-mediated, membrane-delimited pathway, to activation of G protein-regulated, inwardly rectifying K+ (GIRK) currents and inhibition of voltage-dependent Ca2+ currents (Wess et al. 2007). Our data demonstrate that, while promoting LTP at the A/C synapse, M2 receptors restrict synaptic plasticity at the MF synapse.
MATERIALS AND METHODS
Homozygous M2 receptor knockout mice (genetic background 129J1 × CF1) were generated as previously described (Gomeza et al. 1999). Age-matched M2+/+ mice were used in parallel as controls. Transverse hippocampal slices were prepared from the brain of adult mice (3–9 mo old) and maintained as described previously (Seeger et al. 2004). All procedures were carried out according to the guidelines and with the approval of the local German governments (Regierung von Schleswig-Holstein and Regierung von Mittelfranken). Artificial cerebrospinal fluid (aCSF) was gassed with 95% O2-5% CO2 and contained (in mM) 125 NaCl, 3 KCl, 2 CaCl2, 2 MgCl2, 1.25 NaH2PO4, 25 NaHCO3, and 10 d-glucose (pH 7.4). For whole cell recordings, CaCl2 was raised to 2.5 mM and MgCl2 was reduced to 1.5 mM.
Field excitatory postsynaptic potential (fEPSP) recordings from CA3 stratum radiatum were made using a multielectrode system (MED64/Performer 2.0 software; Panasonic, Osaka, Japan) superfused with warmed aCSF (30°C). Control stimulation frequency was 0.05 Hz, and strength was adjusted to 30–40% of maximum fEPSP amplitude. Stimulation electrodes were located in CA3 stratum radiatum to activate A/C synapses. LTP of A/C synapses was induced by HFS (3 × 100 Hz for 1 s at 10-s intervals) or three theta burst stimuli (TBS) with an interval of 10 s. Each TBS consists of 15 bursts of 4 pulses at 100 Hz, delivered at an interburst interval of 200 ms.
Whole cell patch pipettes for excitatory postsynaptic current (EPSC) recordings from CA3 pyramidal cells contained (in mM) 130 K-gluconate, 3 MgCl2, 5 EGTA, 5 HEPES, 2 Na2-ATP, and 0.3 Na3-GTP (pH 7.25, room temperature). Series resistance in whole cell configuration was 10–20 MΩ and compensated by 75–85%. Synaptic currents were recorded at −70 mV, after correcting for liquid junction potential (10 mV). Signals were filtered at 1 kHz and sampled at 10 kHz using the Multiclamp700B/Digidata1440A/pClamp10 system (Molecular Devices, Sunnyvale, CA). Constant-current pulses (width 0.1 ms) of 30–80 μA were delivered to a bipolar tungsten electrode at 0.1 Hz located in either the granule cell layer/hilus to evoke MF synaptic responses or in stratum radiatum of CA3 to evoke A/C synaptic responses, respectively. EPSCs were pharmacologically isolated by the GABAA receptor antagonist picrotoxin (100 μM). One hundred stimuli at 100 Hz (repeated 3 times at an interval of 10 s) were used to evoke MF LTP. The NMDA receptor antagonist d-2-amino-5-phosphonopentanoic acid (d-AP5; 50 μM) was present in all experiments on MF LTP to prevent contamination from A/C fibers. In addition, we used low stimulus intensities to minimize polysynaptic and/or A/C fiber activation. Data were included only when EPSCs were reduced >90% by the group II metabotropic glutamate receptor agonist (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG IV; 2.5 μM) at the end of the experiment. DCG IV and d-AP5 were obtained from Tocris (Bristol, UK). All other drugs were purchased from Sigma (Deisenhofen, Germany). Data are means ± SE. Statistical comparisons of data were performed using Student's t-test.
RESULTS
LTP at the A/C synapse is impaired in hippocampi from M2−/− mice.
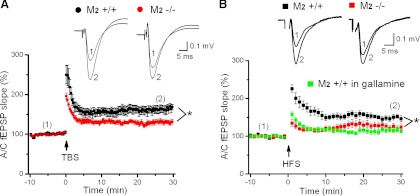
With the use of a multielectrode recording array, two independent A/C fiber pathways were stimulated and fEPSPs were recorded in stratum radiatum of the CA3 region. Measurements of input-output relationships, in which fEPSP amplitudes were plotted as a function of increasing stimulus intensities, did not reveal appreciable differences in the basic functioning of A/C synapses between hippocampi from M2+/+ and M2−/− mice (Table 1). Whereas the control pathway received only low-frequency stimulation (0.05 Hz) throughout the recording session to document stable recording conditions, the experimental pathway was subjected to tetanic stimulation using a TBS protocol. As illustrated in Fig. 1A, TBS reliably produced LTP of excitatory synaptic transmission in M2+/+ hippocampi. When determined 26–30 min after TBS, the slope of the fEPSP was enhanced to 163 ± 9% (n = 7 from 3 mice). By contrast, TBS produced substantially weaker LTP of A/C synapses in hippocampi from M2−/− mice (Fig. 1A), with the increase in fEPSP slope amounting to only 132 ± 5% (n = 11 from 3 mice; P = 0.005) 26–30 min after TBS. In addition to its effect on LTP, M2 receptor deficiency caused a similar reduction of the short-term potentiation (STP) immediately following TBS (Fig. 1A). When determined in the 1st minute post-TBS, fEPSP slope was increased to 246 ± 24% in M2+/+ hippocampi (n = 7 from 3 mice) but only to 179 ± 9% in M2−/− hippocampi (n = 11 from 3 mice; P = 0.006).
Table 1.
The input-output relationships of fEPSPs at A/C synapses were not altered in M2−/− mice
| fEPSP Amplitude, mV |
||||||
|---|---|---|---|---|---|---|
| 60 μA | 80 μA | 100 μA | 120 μA | 150 μA | 200 μA | |
| M2 +/+ | 0.45 ± 0.12 | 0.77 ± 0.19 | 1.31 ± 0.20 | 1.64 ± 0.17 | 1.96 ± 0.15 | 2.20 ± 0.12 |
| M2−/− | 0.37 ± 0.10 | 0.79 ± 0.12 | 1.18 ± 0.11 | 1.44 ± 0.12 | 1.66 ± 0.15 | 2.04 ± 0.25 |
| P | 0.60 | 0.91 | 0.52 | 0.35 | 0.20 | 0.58 |
Values are means ± SE of field excitatory postsynaptic potential (fEPSP) amplitude at associational/commissural (A/C) synapses as a function of increasing stimulus intensities in M2+/+ (n = 5) and M2−/− mice (n = 8).
Fig. 1.
M2 receptors enhance long-term synaptic plasticity at associational/commissural (A/C) synapses of CA3 pyramidal cells. A: theta burst stimulation (TBS) produced weaker long-term potentiation (LTP) of A/C field excitatory postsynaptic potentials (fEPSPs) in M2−/− hippocampi (red circles) than in M2+/+ hippocampi (black circles). Insets illustrate averaged traces of fEPSPs measured in CA3 stratum radiatum at the like-numbered time points before TBS (point 1, average of 30 traces) and after LTP had been introduced (point 2, average of 15 traces). B: a comparable decrease of LTP in M2-deficient hippocampi was observed when high-frequency stimulation (HFS) instead of TBS was used for induction and could be reproduced in M2+/+ hippocampi by the M2 receptor antagonist gallamine (green squares). Insets depict averaged traces at the like-numbered time points as indicated in A. *P < 0.05.
The significant reduction of both STP and LTP at A/C synapses of M2−/− hippocampi was reproduced when HFS was used in lieu of TBS for induction (Fig. 1B; STP: M2+/+, 226 ± 10%, n = 6 from 6 mice; M2−/−, 130 ± 7%, n = 7 from 5 mice, P = 0.001; LTP: M2+/+, 149 ± 5%, n = 6 from 6 mice; M2−/−, 124 ± 7%, n = 7 from 5 mice, P = 0.019). To dispel any concerns that the reduction of STP and LTP at A/C synapses of M2-deficient hippocampi was unrelated to the disruption of the M2 receptor gene or reflected some compensatory mechanisms, we repeated the above experiment in WT hippocampi in the presence of the allosteric M2 receptor antagonist gallamine (20 μM). As demonstrated in Fig. 1B (green data points), application of gallamine in WT hippocampi reduced STP and LTP in a fashion well comparable to that observed in the M2-deficient preparation (STP: 148 ± 6%, n = 5 from 3 mice, P = 0.001 vs. M2+/+; LTP: 116 ± 5%, n = 5 from 3 mice, P = 0.002 vs. M2+/+).
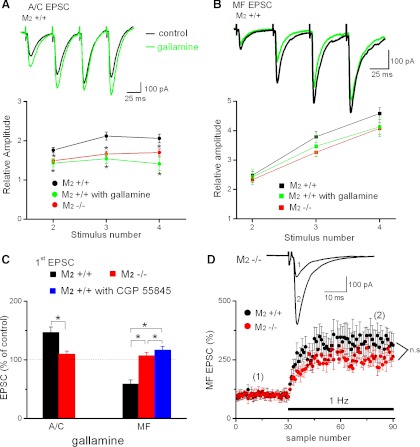
To gain insight into the role of M2 receptors during repetitive stimulation at the synaptic level of individual neurons, we performed whole cell recordings of pharmacologically isolated EPSCs from visually identified CA3 pyramidal cells in hippocampi from normal and mutant mice. As shown in Fig. 2A, trains of four stimuli (interpulse interval 50 ms) produced a facilitating response that was significantly weaker in M2−/− hippocampi (n = 9 from 5 mice) than in M2+/+ hippocampi (n = 11 from 5 mice). When examined in M2+/+ hippocampi, the allosteric M2 receptor antagonist gallamine (20 μM) mimicked the effect observed in M2−/− hippocampi (green traces in Fig. 2A, n = 6 from 4 mice). Notably, application of gallamine also caused a significant enhancement of the amplitude of the first EPSC in the train in normal hippocampi to 147 ± 9% of control (control, 127 ± 41 pA; gallamine, 175 ± 50 pA, n = 6 from 4 mice, P = 0.01, Fig. 2C). Gallamine did not affect EPSC kinetics (10–90% rise time: control, 4.9 ± 0.4 ms; gallamine, 4.7 ± 0.4 ms, n = 6, P = 0.50; decay time constant: control, 19.5 ± 2.7 ms; gallamine, 19.8 ± 2.7 ms, n = 6, P = 0.68). The increase of first EPSC amplitude in gallamine would be consistent with the notion that endogenously released acetylcholine (Descarries et al. 1997) inhibits glutamate release through M2 heteroceptors, as previously shown for the Schaffer-CA1 synapse (Seeger et al. 2004). Genetic disruption of M2 receptors relieves this inhibitory tonus, thereby reducing the degree of facilitation that this synapse can undergo during repetitive stimulation.
Fig. 2.
M2 receptors affect short-term plasticity at A/C synapses, but not at mossy fiber (MF) synapses, of CA3 pyramidal cells. A: reduced short-term plasticity of A/C excitatory postsynaptic currents (EPSCs) in M2−/− hippocampus. Traces at top are from a CA3 pyramidal cell of M2+/+ hippocampus, illustrating the facilitation of A/C EPSCs during a train of 4 stimuli before (control, black trace) and during (green trace) application of M2 receptor antagonist gallamine (20 μM). To compare quantitatively the degree of facilitation in M2+/+ hippocampi with and without gallamine (black and green circles, respectively) and in M2−/− hippocampi (red circles), the amplitudes of the 2nd to 4th EPSCs were normalized to that of the 1st EPSC. B: responses of MF EPSCs to 4 stimuli at 20 Hz in the absence (black trace) and presence of gallamine (green trace) in M2+/+ hippocampus. Amplitudes of the 2nd to 4th EPSCs during 20-Hz stimulation were normalized to amplitude of the 1st EPSC. Recordings from M2+/+ hippocampi in the absence (black squares) and presence of gallamine (20 μM; green squares) or from M2−/− hippocampi (red squares) showed that short-term plasticity was not sensitive to pharmacological suppression or genetic disruption of M2 receptors. C: in M2+/+ hippocampi, gallamine enhanced amplitude of the 1st EPSC at A/C synapses (left, black bar) but had the opposite effect at MF synapses (right, black bar). The inhibitory effect of gallamine on the 1st EPSC at MF synapses was reversed by the GABAB receptor antagonist CGP-55845 (blue bar). Effects of gallamine were absent in M2−/− hippocampi (red bars). D: comparison of normalized frequency facilitation during 1-Hz stimulation in M2+/+ and M2−/− hippocampi did not reveal a significant role of M2 receptors in this paradigm. Inset shows averaged traces from like-numbered time points. *P < 0.05.
Short term-plasticity at the MF synapse is not altered in hippocampi from M2−/− mice.
The MF synapse onto CA3 pyramidal cells is distinct from other excitatory synapses in the central nervous system in that it displays an unusually high degree of short-term plasticity. To determine whether the lack of M2 receptors affected the prominent short-term plasticity at this synapse, we performed whole cell recordings of pharmacologically isolated, AMPA receptor-mediated EPSCs. In the first set of experiments, we used the same train of four stimuli that we had applied previously at the A/C fiber input onto CA3 pyramidal cells. The input dependence of the response pattern becomes immediately obvious from a comparison of the EPSC sequences in Fig. 2, A and B. Whereas the quadruple-pulse facilitation attained a ratio of ∼2 at the A/C fiber synapse, the ratio was more than twice that large (>4) at the MF synapse (n = 53 from 23 mice). In contrast to the role of M2 receptors in short-term plasticity at the A/C fiber synapse, genetic disruption (n = 40 from 17 mice) or pharmacological inhibition (n = 19 from 13 mice) of M2 receptors did not cause a significant decrease in quadruple-pulse facilitation (Fig. 2B).
Interestingly, gallamine (20 μM) reduced the amplitude of the first EPSC in M2+/+ mice to 59 ± 7% of control (control, 87 ± 8 pA; gallamine, 49 ± 5 pA, n = 8 from 7 mice, P = 0.006), thus causing the exact opposite effect to what was seen at A/C fiber input (Fig. 2C). Endogenously released acetylcholine, acting on M2 receptors, is hence capable of exerting a reciprocal effect on the strength of excitatory transmission at A/C fiber vs. MF synapses during low-frequency stimulation. How do M2 receptors accomplish this remarkable pathway specificity? Inhibition of A/C fiber input presumably reflects direct inhibition of glutamate release through presynaptic M2 heteroceptors, as mentioned above. However, enhanced MF input via M2 receptor activation might be indirect in nature, involving interplay between neighboring GABAergic and glutamatergic terminals (Vogt and Regehr 2001). GABA spilling over from inhibitory terminals would act on inhibitory presynaptic GABAB receptors on nearby MF terminals, thereby depressing glutamate release. Activation of M2 heteroceptors on GABAergic terminals would reduce GABA release and thus alleviate the GABAB receptor-mediated inhibition of glutamate release. As a consequence, MF EPSC amplitude would rise, as observed. To test this hypothesis, we repeated the gallamine experiment in the presence of the GABAB receptor antagonist CGP-55845 (1–2 μM). In fact, the inhibitory effect of gallamine was abrogated by the GABAB receptor antagonist, producing even a small increase in MF EPSC amplitude (Fig. 2C, n = 5 slices from 3 mice).
A second, perhaps even more striking feature of MF short-term plasticity is the impressive ability to display frequency facilitation during a modest increase in stimulation frequency. A representative example of this phenomenon is illustrated in Fig. 2D, in which a change in stimulus frequency from 0.1 to 1 Hz gave rise to a dramatic growth in synaptic strength. As for quadruple-pulse facilitation, M2 receptor deficiency did not appreciably affect frequency facilitation (M2+/+, n = 9 from 7 mice; M2−/−, n = 6 from 4 mice).
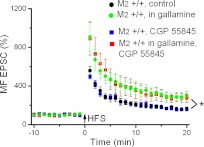
LTP is enhanced at the MF synapse in hippocampi from M2−/− mice.
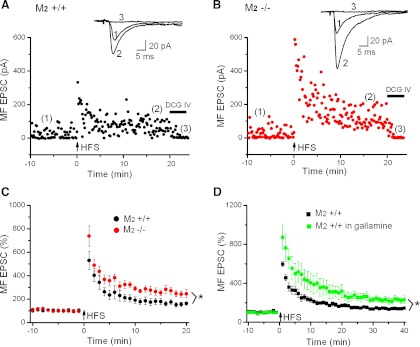
Although the lack of M2 receptors had no effect on short-term plasticity at the MF input onto CA3 pyramidal cells, M2 receptor deficiency significantly altered the extent of LTP this synapse can undergo. As illustrated in the representative recordings of Fig. 3, A and B, and summarized in Fig. 3C, HFS engendered significantly stronger LTP in M2−/− hippocampi than in M2+/+ hippocampi. When determined 16–20 min after HFS, the normalized MF EPSC amplitude was increased to 165 ± 9% in M2+/+ hippocampi (n = 12 from 9 mice), whereas the enhancement in M2−/− hippocampi attained 256 ± 27% (n = 9 from 7 mice; P = 0.04). To rule out contamination by inadvertent A/C fiber stimulation, we applied the presynaptic inhibitory group II metabotropic glutamate receptor agonist DCG IV (2.5 μM) at the end of each experiment (Fig. 3, A and B), which inhibits glutamate release from MF terminals, but not from A/C fiber terminals (Kamiya et al. 1996). The increase in long-term plasticity at the MF synapse was characteristically accompanied by a pronounced reduction in failure rate. A comparison between the two groups showed that the drop in synaptic failures was significantly stronger in M2-deficient hippocampi than in normal hippocampi (before HFS: M2+/+, 25.1 ± 7.0%, n = 7 from 5 mice; M2−/−, 23.0 ± 3.2%, n = 9 from 7 mice; P = 0.41; after HFS: M2+/+, 6.4 ± 2 .2%, n = 7 from 5 mice; M2−/−, 0.8 ± 0.5%, n = 9 from 7 mice; P = 0.02).
Fig. 3.
M2 receptors inhibit MF LTP. A and B: scatter plots of MF EPSCs before and after HFS to induce LTP in M2+/+ and M2−/− hippocampi. At the end of the recordings, EPSCs were suppressed by the group II metabotropic glutamate agonist DCG IV (2.5 μM), confirming that they were produced by MF activation. Each data point represents a single EPSC evoked at 0.1 Hz. Insets above plots depict averaged traces taken at the like-numbered time points. C: normalized time courses of MF LTP in M2+/+ hippocampi (black circles) and M2−/− hippocampi (red circles) indicate a suppressive effect of M2 receptors. D: normalized time courses of MF LTP in the absence (black squares) and presence of gallamine (green squares) show that the M2 receptor antagonist gallamine replicates the effect of M2 receptor knockout on MF LTP when examined in M2+/+ hippocampus. *P < 0.05.
To rule out the possibility that the enhanced LTP at the MF synapse in the mutant hippocampi was attributable to factors other than M2 receptor deficiency, we repeated the above experiment in normal hippocampi in the absence and presence of gallamine (20 μM). The M2 receptor antagonist reliably reproduced the effect of M2 receptor knockout, yielding a mean increase in normalized MF EPSC amplitude 16–20 min after HFS of 246 ± 35% (n = 7 from 6 mice, P = 0.03 compared with control M2+/+ hippocampi, P = 0.82 compared with M2−/− hippocampi). These data indicate that it is indeed the activation of M2 receptors by endogenously released acetylcholine that is responsible for the attenuation of LTP at this synapse. To exclude the possibility that the suppression of M2 receptors only augmented posttetanic potentiation and the early phase of LTP, we doubled the recording period and monitored MF EPSCs over 40 min after tetanic stimulation. When determined 36–40 min post-HFS, MF EPSCs obtained from gallamine-treated slices were still significantly more strongly potentiated (229 ± 29%, n = 7 from 6 mice) than those in control slices (142 ± 6%, n = 5 from 5 mice, P = 0.03), indicating the long-lasting nature of the M2 receptor-mediated effect on MF plasticity (Fig. 3D).
Disinhibition of adenylyl cyclase promotes MF LTP in M2-deficient hippocampi.
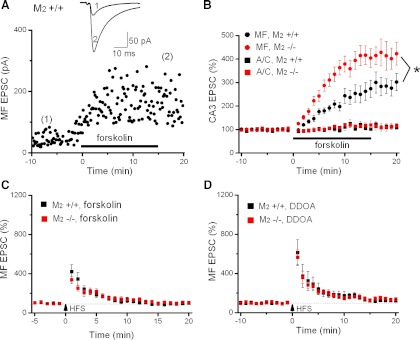
How do M2 receptors manage to control the strength of MF LTP? We first examined the hypothesis that M2 receptors might directly influence the presynaptic machinery producing MF LTP. It is widely accepted that a presynaptic rise in cAMP is an essential step in the underlying signaling cascade (Weisskopf et al. 1994). Consequently, activation of adenylyl cyclase by forskolin is capable of mimicking MF LTP, as shown previously (Weisskopf et al. 1994) and replicated in this study (Fig. 4, A and B). To examine whether M2 receptor activation interferes with presynaptic cAMP production, we measured and compared the strength of forskolin-induced LTP in hippocampi from M2+/+ and M2−/− mice. As depicted in Fig. 4B, the lack of M2 receptors significantly enhanced the ability of forskolin (50 μM) to produce MF LTP (mean increase in normalized MF EPSC amplitude 16–20 min after onset of forskolin application: M2+/+, 270 ± 33%, n = 10 from 7 mice; M2−/−, 407 ± 41%, n = 14 from 8 mice; P = 0.02). This finding strongly suggests that M2 receptor activation attenuates MF LTP through an inhibitory effect on cAMP-dependent signaling. Underscoring the pathway specificity of this effect (Weisskopf et al. 1994), application of forskolin did not appreciably affect EPSCs evoked by A/C fiber stimulation in either preparation (Fig. 4B, relative change in A/C EPSCs after 16–20 min of forskolin superfusion: M2+/+, 110 ± 10%, n = 5 from 3 mice; M2−/−, 112 ± 14%, n = 4 of 2 mice).
Fig. 4.
M2 receptors act on adenylyl cyclase to inhibit MF LTP A: scatter plot of potentiation of MF EPSCs by forskolin (50 μM) in M2+/+ hippocampus. Each data point represents a single EPSC evoked at 0.1 Hz. Inset above plots depicts averaged traces taken at the like-numbered time points. B: stronger potentiation of MF EPSCs by forskolin (50 μM) in M2 −/− hippocampi (red circles) than in M2+/+ hippocampi (black circles). By contrast, forskolin did not exhibit an appreciable effect at A/C synapses in either genotype (black and red squares) C: pretreatment with forskolin occluded subsequent induction of MF LTP by HFS regardless of genotype. D: pharmacological inhibition of adenylyl cyclase with DDOA reduced and equalized LTP in control (black squares) and M2-deficient hippocampi (red squares). *P < 0.05.
Two additional lines of evidence lend further support to the hypothesis that the disinhibition of adenylyl cyclase is pivotal to the enhanced MF LTP in M2-deficient hippocampi. In the first set of experiments, we found that in both preparations, forskolin (50 μM) occluded HFS-induced LTP when the electric stimulus trains were delivered 30 min after forskolin application (Fig. 4C). To avoid saturation of the EPSC responses that had been already potentiated by forskolin, stimulus intensities were reduced before HFS to obtain baseline responses. When determined 16–20 min post-HFS, relative EPSC amplitudes were 96 ± 8% of control for M2+/+ hippocampi (n = 7 from 5 mice) and 102 ± 8% of control for M2−/− hippocampi (n = 4 from 3 mice).
In the second set of experiments, hippocampal slices were preincubated with the cell-permeable adenylyl cyclase inhibitor dideoxyadenosine (DDOA; 10–15 μM) for 2–6 h before MF LTP was examined. As shown in Fig. 4D, inhibition of adenylyl cyclase did not affect STP but strongly diminished and equalized LTP in both preparations (relative increase in EPSC amplitude 16–20 min post-HFS in DDOA-containing vs. control solution: M2+/+, 126 ± 8%, n = 6 from 3 mice vs. 165 ± 9%, n = 12 from 9 mice, P = 0.008; M2−/−, 143 ± 13%, n = 5 from 3 mice vs. 256 ± 27%, n = 9 from 7 mice, P = 0.01; LTP in DDOA: P = 0.28, M2+/+ vs. M2−/−). These data indicate that once adenylyl cyclase activity is abrogated, HFS fails to elicit larger MF LTP in M2−/− than in M2+/+ hippocampi.
Lacking interaction between M2 and GABAB receptors in MF LTP.
Previous work by Vogt and Nicoll (1999) showed that activation of GABAB receptors increased the threshold for MF LTP when a relatively weak stimulation protocol (12 pulses at 25 Hz) was employed. Because we found that M2 receptors might exert an indirect control over MF EPSCs through GABAB receptor activation (Fig. 2C), we wondered whether M2 receptors might also modulate MF LTP through an interaction with GABAB receptors. If so, one might expect that in M2−/− hippocampi, lack of M2 heteroceptors on GABAergic terminals should strongly disinhibit the release of GABA during HFS, which in turn might act on GABAB receptors of MF terminals to attenuate LTP. In this scenario, M2 receptors would control MF LTP in a dual and counterbalancing fashion, directly decreasing LTP through attenuation of adenylyl cyclase activity and indirectly augmenting LTP through inhibition of GABA release. As shown below, however, our data strongly argue against such an interaction.
Because we used a much stronger protocol to evoke mossy fiber LTP than Vogt and Nicoll (1999) in their study, we first explored whether GABAB receptors by themselves would influence MF LTP under our experimental conditions. For this purpose, we compared the strength of MF LTP in the absence and presence of CGP-55845 (2 μM). At control stimulation before HFS, CGP-55845 enhanced EPSC amplitude in M2+/+ hippocampi to 121 ± 5% of control (control, 140 ± 15 pA; CGP-55845, 168 ± 16 pA, n = 5 from 3 mice, P = 0.01). With GABAB receptors blocked, MF LTP was 160 ± 5% (n = 6 from 5 mice), which was virtually identical to the potentiation in control recordings, in which GABAB receptors remained accessible (165 ± 9%, n = 12 from 9 mice, P = 0.60, Fig. 5). We then examined MF LTP in hippocampi that were superfused with both CGP-55845 and gallamine. Suppression of GABAB receptors did not influence the gallamine-induced increase in MF LTP, which was 287 ± 37% (n = 14 from 12 mice) in the presence of gallamine alone and 291 ± 63% (n = 6 from 4 mice, P = 0.59) when both antagonists were present. This observation suggests that GABAB receptors on MF terminals are not substantially involved in M2 receptor-mediated regulation of long-term plasticity.
Fig. 5.
GABAB receptors do not appear to be involved in muscarinic regulation of MF LTP. Application of the GABAB receptor antagonist CGP-55845 did not alter the time course or strength of MF LTP compared with control recordings (blue squares vs. black circles) and did not change enhancement of MF LTP by gallamine (green circles vs. red squares). *P < 0.05.
Role of nicotinic acetylcholine receptors.
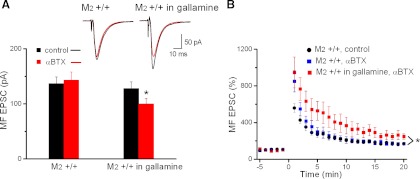
Finally, we explored the possibility that the presumed enhancement of acetylcholine release due to the lack of M2 autoreceptors on cholinergic terminals might promote LTP through activation of nicotinic acetylcholine receptors (nAChRs) on MF terminals. A recent electron microscopy study has indeed localized Ca2+-permeable α7 nAChRs on MF terminals (Bancila et al. 2009) Furthermore, Ca2+ imaging and electrophysiological studies in acute slices have underscored the functional significance of presynaptic α7 nAChRs at the MF-CA3 synapse (Grybko et al. 2010; Sharma et al. 2008). Specifically, presynaptic α7 nAChRs elevate intracellular Ca2+ concentration in MF boutons, leading to enhanced glutamate release. Most importantly in the context of our study, activation of α7 nAChRs was recently shown to rescue deficits in MF LTP of BACE1 knockout mice (Wang et al. 2010). It therefore seems conceivable that a stronger rise of endogenous acetylcholine during HFS in M2−/− hippocampi might produce sufficient activation of α7 nAChRs on MF terminals to promote LTP. We therefore used the specific α7 nAChR antagonist α-bungarotoxin (αBTX; 100 nM, preapplication for 10–15 min) to determine the contribution of α7 nAChRs to synaptic transmission and plasticity in normal and gallamine-treated slices. Whereas αBTX did not affect MF EPSCs when evoked at control stimulation frequency (0.1 Hz) under normal conditions, the antagonist reduced the amplitude of MF EPSCs during pharmacological suppression of M2 receptors (Fig. 6A). These data strongly suggest that the lack of M2 autoreceptors raises the level of endogenous acetylcholine without fully desensitizing α7 nAChRs, leading to a tonic enhancement of MF EPSCs.
Fig. 6.
Nicotinic receptor activation does not account for stronger MF LTP in M2-deficient hippocampi. A: the α7 nicotinic acetylcholine receptor antagonist α-bungarotoxin (αBTX; red bars) reduced MF EPSCs evoked at low stimulus frequency (0.1 Hz) when M2 receptors were pharmacologically suppressed by gallamine (20 μM; right) but not when M2 receptors were functional (left). B: αBTX did not impair the gallamine-induced augmentation of MF LTP. *P < 0.05.
Having identified a contribution of α7 nAChRs to basal synaptic transmission at the MF-CA3 synapse when acetylcholine release is not controlled by M2 autoreceptors, we were surprised to find that αBTX applied 10–15 min before HFS did not affect the enhancement of MF LTP that we typically observed when M2 receptors were genetically disrupted or pharmacologically suppressed (see above). In the presence of αBTX (100 nM), the augmenting effect of the M2 receptor antagonist gallamine (20 μM) on MF LTP was fully preserved (Fig. 6B). When determined 16–20 min post-HFS with M2 receptors intact and αBTX in the bathing solution, the normalized MF EPSC amplitude increased to 174 ± 13% (n = 6 from 5 mice), whereas MF EPSCs were enhanced to 254 ± 35% (n = 5 from 4 mice, P = 0.04) in the presence of gallamine and αBTX. Notably, αBTX did not shift the respective levels of LTP, indicating that the transient rise in endogenously released acetylcholine that should accompany tetanic stimulation fails to augment MF LTP through presynaptic α7 nAChRs, even when release-inhibiting M2 autoreceptors were suppressed.
DISCUSSION
In view of the behaviorally and clinically well-established significance of muscarinic acetylcholine receptors for various forms of learning and memory and the pivotal role of the hippocampus therein, it is quite astonishing that the effects and underlying mechanisms of muscarinic receptor activation on hippocampal synaptic plasticity, in particular when it comes to area CA3, are still not well understood. Our data indicate that activation of M2 receptors redistributes the weight between the two major glutamatergic inputs of CA3 pyramidal neurons in favor of the A/C synapse, where LTP is promoted, at the expense of the MF synapse, where LTP is curtailed. Given that the MF input might set off the built-in “detonator” of the CA3 network (Lawrence and McBain 2003), a general purpose of M2 receptors might be to counteract such a surge in the excitability of CA3 pyramidal cells, especially after highly frequent fiber activity.
In a previous study, application of muscarine was found to directly inhibit glutamate release from A/C terminals (Vogt and Regehr 2001), mostly likely through M2 receptor activation. Quite obviously, this would represent an unlikely target for M2 receptors to enhance LTP at the A/C synapse, as reported here. Instead, and analogous to the mechanism we have previously identified at the Schaffer-CA1 synapse (Seeger et al. 2004), presynaptic M2 receptors (heteroceptors) on nearby terminals of inhibitory interneurons might reduce concomitant GABA release that would interfere with NMDA receptor activation. Furthermore, postsynaptic M2 receptors might give rise to a long-lasting enhancement of glutamate responses (“muscarinic” LTP; Auerbach and Segal 1994). We have not reconfirmed that these mechanisms are also operative at the A/C synapse, but this assumption seems tenable, given the similarities in the upregulation of NMDA receptor-dependent LTP by M2 receptors in areas CA1 and CA3.
How can we explain the inhibitory effect of M2 receptors on MF LTP? As mentioned above, MF LTP is expressed presynaptically, manifested as an increase in transmitter release. The underlying events involve activation of Ca2+/calmodulin-sensitive adenylyl cyclase AC1 (and AC8) through presynaptic Ca2+ entry, enhanced cAMP levels, and subsequent activation of PKA (Nicoll and Schmitz 2005). As predicted by this signaling pathway, stimulation of AC1 by forskolin leads to a pronounced and lasting enhancement of MF EPSCs. Because M2 receptors inhibit adenylyl cyclases through the Gα-subunit of Gi/o proteins, they would be in prime position to constrain a crucial player of LTP induction. In fact, genetic disruption of M2 receptors significantly enhanced the forskolin response, strongly suggesting that M2 receptors normally impinge on the AC1 activity in MF terminals. That this effect is essential to account for the enhanced MF LTP in M2-deficient hippocampi was substantiated in two experimental paradigms, in which adenylyl cyclase activity was either maximally stimulated or pharmacologically suppressed before HFS was delivered. Under both conditions, M2 deficiency no longer yielded stronger HFS potentiation of EPSC responses.
Prima facie, these data strongly suggest a direct M2 receptor-mediated attenuation of adenylyl cyclase activity as the pivotal mechanism curtailing MF LTP. One might argue, however, that our findings do not per se rule out an indirect effect in which M2 receptors would reduce MF LTP through suppression of presynaptic Ca2+ influx. In other preparations, M2 receptors have been shown indeed to inhibit presynaptic Ca2+ channels via a membrane-delimited, Gβγ-mediated pathway (Brown 2010). However, two observations argue against a considerable contribution of this mechanism at the MF synapse. First, application of muscarine failed to alter presynaptic Ca2+ influx at MF terminals (Vogt and Regehr 2001). Second, presynaptic adenosine A1 receptors, which inhibit presynaptic Ca2+ entry independently of the cAMP-PKA cascade (Gundlfinger et al. 2007), are capable of controlling both short-term and long-term plasticity at the MF synapse (Moore et al. 2003). By contrast, M2 receptors exhibit a different inhibitory profile lacking appreciable effects on MF short-term plasticity, as demonstrated in this study. It seems therefore likely that A1 receptors and M2 receptors recruit separate or only partially overlapping pathways to modulate MF plasticity.
Because M2 receptors also serve as autoreceptors to control transmitter release from cholinergic terminals (Zhang et al. 2002), we wondered whether indirect mechanisms related to different levels of endogenous acetylcholine might contribute to the cholinergic modulation of MF LTP. If M2 receptor deficiency should have led to higher levels of endogenous acetylcholine, our data argue against the notion that stronger activation of α7 nAChRs was responsible for the enhanced MF LTP.
In a recent study, Ruiz et al. (2010) showed that presynaptic GABAA receptors enhance transmitter release and LTP induction at MF synapses. This finding offers the intriguing possibility that M2 heteroceptors on neighboring terminals of inhibitory interneurons might be able to gate MF LTP by controlling GABA release. In our study, all experiments were performed in the presence of the GABAA receptor antagonist picrotoxin to obtain uncontaminated MF EPSCs. The effect of M2 receptors on MF LTP reported here is therefore independent of this remote mechanism and can be best ascribed to M2 receptors located on MF terminals. Presynaptic M2 heteroceptors have been associated with glutamatergic terminals throughout the hippocampus (Rouse et al. 1997), and the overall density of M2 receptors in the hippocampus of various mouse strains is highest in area CA3 (Schwegler et al. 1996). Nevertheless, direct neuroanatomic evidence for their localization on MF terminals of mouse hippocampus remains to be established.
In a simplified view, the most influential model of how synaptic effects of muscarinic acetylcholine receptor activation facilitate hippocampal learning and memory posits the following scheme (Hasselmo et al. 1995; Hasselmo 2006): strong cholinergic activity suppresses the autoassociative network activity in the CA3 region to prevent it from interfering with the encoding of new information. Vice versa, weak cholinergic activity allows for stronger recurrent excitation in area CA3, thereby promoting autoassociative storage and recall. Experimentally, this model is based on the observation that the cholinergic agonist carbachol inhibits CA3 fEPSPs in stratum radiatum, where A/C fibers terminate, more efficiently than in stratum lacunosum-moleculare and in stratum lucidum, where fibers of the perforant path and MFs terminate, respectively (Kremin and Hasselmo 2007). Our findings add a new dimension to this model in that they allow incorporating muscarinic effects on synaptic plasticity in a pathway-specific fashion. During encoding, when acetylcholine levels are high, MF LTP would be attenuated, perhaps to avoid rapid saturation of excitatory synapses by the flow of afferent signals, whereas LTP at A/C synapses would be strengthened, perhaps to compensate for the presumed depression of the autoassociative network during this behavioral state. During retrieval, afferent input is weak and the intrinsic autoassociative network is busy with pattern completion and recall. The fall in acetylcholine levels accompanying this state would then redistribute the gain of synaptic plasticity to account for the altered activity at the two synapses. In this view, muscarinic facilitation or inhibition of synaptic plasticity in area CA3 (and perhaps elsewhere) might be seen as a means to scale plastic changes to the momentary strength of network activity during different mental states.
GRANTS
This work was supported by Deutsche Forschungsgemeinschaft Grant AL 294/9-1 (to C. Alzheimer).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: F.Z., J.W., and C.A. conception and design of research; F.Z. performed experiments; F.Z. analyzed data; F.Z. and C.A. interpreted results of experiments; F.Z. prepared figures; F.Z. and C.A. drafted manuscript; F.Z., J.W., and C.A. approved final version of manuscript; J.W. and C.A. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Didier Gremelle for technical assistance.
REFERENCES
- Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci 6: 51–58, 2003 [DOI] [PubMed] [Google Scholar]
- Auerbach JM, Segal M. A novel cholinergic induction of long-term potentiation in rat hippocampus. J Neurophysiol 72: 2034–2040, 1994 [DOI] [PubMed] [Google Scholar]
- Bainbridge NK, Koselke LR, Jeon J, Bailey KR, Wess J, Crawley JN, Wrenn CC. Learning and memory impairments in a congenic C57BL/6 strain of mice that lacks the M2 muscarinic acetylcholine receptor subtype. Behav Brain Res 190: 50–58, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancila V, Cordeiro JM, Bloc A, Dunant Y. Nicotine-induced and depolarisation-induced glutamate release from hippocampus mossy fibre synaptosomes: two distinct mechanisms. J Neurochem 110: 570–580, 2009 [DOI] [PubMed] [Google Scholar]
- Brown DA. Muscarinic acetylcholine receptors (mAChRs) in the nervous system: some functions and mechanisms. J Mol Neurosci 41: 340–346, 2010 [DOI] [PubMed] [Google Scholar]
- Buchanan KA, Petrovic MM, Chamberlain SE, Marrion NV, Mellor JR. Facilitation of long-term potentiation by muscarinic M1 receptors is mediated by inhibition of SK channels. Neuron 68: 948–963, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT, Price DL, DeLong MR. Alzheimer's disease: a disorder of cortical cholinergic innervation. Science 219: 1184–1190, 1983 [DOI] [PubMed] [Google Scholar]
- Descarries L, Gisiger V, Steriade M. Diffuse transmission by acetylcholine in the CNS. Prog Neurobiol 53: 603–625, 1997 [DOI] [PubMed] [Google Scholar]
- Giessel AJ, Sabatini BL. M1 muscarinic receptors boost synaptic potentials and calcium influx in dendritic spines by inhibiting postsynaptic SK channels. Neuron 68: 936–947, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomeza J, Shannon H, Kostenis E, Felder C, Zhang L, Brodkin J, Grinberg A, Sheng H, Wess J. Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA 96:1692–1697, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grybko M, Sharma G, Vijayaraghavan S. Functional distribution of nicotinic receptors in CA3 region of the hippocampus. J Mol Neurosci 40: 114–120, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlfinger A, Bischofberger J, Johenning FW, Torvinen M, Schmitz D, Breustedt J. Adenosine modulates transmission at the hippocampal mossy fibre synapse via direct inhibition of presynaptic calcium channels. J Physiol 582: 263–277, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. The role of acetylcholine in learning and memory. Curr Opin Neurobiol 16: 710–715, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Schnell E, Barkai E. Dynamics of learning and recall at excitatory recurrent synapses and cholinergic modulation in rat hippocampal region CA3. J Neurosci 15: 5249–5262, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H, Shinozaki H, Yamamoto C. Activation of metabotropic glutamate receptor type 2/3 suppresses transmission at rat hippocampal mossy fibre synapses. J Physiol 493: 447–455, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremin T, Hasselmo ME. Cholinergic suppression of glutamatergic synaptic transmission in hippocampal region CA3 exhibits laminar selectivity: implication for hippocampal network dynamics. Neuroscience 149: 760–767, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JJ, McBain CJ. Interneuron diversity series: containing the detonation-feedforward inhibition in the CA3 hippocampus. Trends Neurosci 26: 631–640, 2003 [DOI] [PubMed] [Google Scholar]
- Levey AI, Edmunds SM, Koliatsos V, Wiley RG, Heilman CJ. Expression of m1–m4 muscarinic acetylcholine receptor proteins in rat hippocampus and regulation by cholinergic innervation. J Neurosci 15: 4077–4092, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Kaneko S, Satoh M. Bidirectional modulation of long-term potentiation by carbachol via M1 and M2 muscarinic receptors in guinea pig hippocampal mossy fiber-CA3 synapses. Brain Res 619: 324–330, 1993 [DOI] [PubMed] [Google Scholar]
- Markram H, Segal M. Long-lasting facilitation of excitatory postsynaptic potentials in the rat hippocampus by acetylcholine. J Physiol 427: 381–393, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T, Yamada M, Duttaroy A, Wess J. Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J Neurosci 21: 5239–5250, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KA, Nicoll RA, Schmitz D. Adenosine gates synaptic plasticity at hippocampal mossy fiber synapses. Proc Natl Acad Sci USA 100: 14397–14402, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat Rev Neurosci 6: 863–876, 2005 [DOI] [PubMed] [Google Scholar]
- Rouse ST, Thomas TM, Levey AI. Muscarinic acetylcholine receptor subtype, m2: diverse functional implications of differential synaptic localization. Life Sci 60: 1031–1038, 1997 [DOI] [PubMed] [Google Scholar]
- Ruiz A, Campanac E, Scott RS, Rusakov DA, Kullmann DM. Presynaptic GABAA receptors enhance transmission and LTP induction at hippocampal mossy fiber synapses. Nat Neurosci 13: 431–438, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Brain Res Rev 48: 98–111, 2005 [DOI] [PubMed] [Google Scholar]
- Schwegler H, Boldyreva M, Pyrlik-Gohlmann M, Linke R, Wu J, Zilles K. Genetic variation in the morphology of the septo-hippocampal cholinergic and GABAergic system in mice. I. Cholinergic and GABAergic markers. Hippocampus 6: 136–148, 1996 [DOI] [PubMed] [Google Scholar]
- Seeger T, Fedorova I, Zheng F, Miyakawa T, Koustova E, Gomeza J, Basile AS, Alzheimer C, Wess J. M2 muscarinic acetylcholine receptor knock-out mice show deficits in behavioral flexibility, working memory, and hippocampal plasticity. J Neurosci 24: 10117–10127, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Grybko M, Vijayaraghavan S. Action potential-independent and nicotinic receptor-mediated concerted release of multiple quanta at hippocampal CA3-mossy fiber synapses. J Neurosci 28: 2563–2575, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffler DJ, Williams R, Bridges TM, Xiang Z, Kane AS, Byun NE, Jadhav S, Mock MM, Zheng F, Lewis LM, Jones CK, Niswender CM, Weaver CD, Lindsley CW, Conn PJ. A novel selective muscarinic acetylcholine receptor subtype 1 antagonist reduces seizures without impairing hippocampus-dependent learning. Mol Pharmacol 76: 356–368, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoe T, Matsui M, Taketo MM, Manabe T. Modulation of synaptic plasticity by physiological activation of M1 muscarinic acetylcholine receptors in the mouse hippocampus. J Neurosci 25: 11194–11200, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban NN. Revisiting the role of the hippocampal mossy fiber synapse. Hippocampus 11: 408–417, 2001 [DOI] [PubMed] [Google Scholar]
- Vogt KE, Nicoll RA. Glutamate and gamma-aminobutyric acid mediate a heterosynaptic depression at mossy fiber synapses in the hippocampus. Proc Natl Acad Sci USA 96: 1118–1122, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt KE, Regehr WG. Cholinergic modulation of excitatory synaptic transmission in the CA3 area of the hippocampus. J Neurosci 21: 75–83, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Song L, Lee A, Laird F, Wong PC, Lee HK. Mossy fiber long-term potentiation deficits in BACE1 knock-outs can be rescued by activation of alpha7 nicotinic acetylcholine receptors. J Neurosci 30: 13808–13813, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Castillo PE, Zalutsky RA, Nicoll RA. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science 265: 1878–1882, 1994 [DOI] [PubMed] [Google Scholar]
- Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov 6: 721–733, 2007 [DOI] [PubMed] [Google Scholar]
- Williams S, Johnston D. Muscarinic depression of long-term potentiation in CA3 hippocampal neurons. Science 242: 84–87, 1988 [DOI] [PubMed] [Google Scholar]
- Williams S, Johnston D. Muscarinic depression of synaptic transmission at the hippocampal mossy fiber synapse. J Neurophysiol 64: 1089–1097, 1990 [DOI] [PubMed] [Google Scholar]
- Zhang W, Basile AS, Gomeza J, Volpicelli LA, Levey AI, Wess J. Characterization of central inhibitory muscarinic autoreceptors by the use of muscarinic acetylcholine receptor knock-out mice. J Neurosci 22: 1709–1717, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]