Abstract
The sulfonylurea receptor 1 (Sur1)-regulated NCCa-ATP channel is a nonselective cation channel that is regulated by intracellular calcium and adenosine triphosphate. The channel is not constitutively expressed, but is transcriptionally upregulated de novo in all cells of the neurovascular unit, in many forms of central nervous system (CNS) injury, including cerebral ischemia, traumatic brain injury (TBI), spinal cord injury (SCI), and subarachnoid hemorrhage (SAH). The channel is linked to microvascular dysfunction that manifests as edema formation and delayed secondary hemorrhage. Also implicated in oncotic cell swelling and oncotic (necrotic) cell death, the channel is a major molecular mechanism of ‘accidental necrotic cell death' in the CNS. In animal models of SCI, pharmacological inhibition of Sur1 by glibenclamide, as well as gene suppression of Abcc8, prevents delayed capillary fragmentation and tissue necrosis. In models of stroke and TBI, glibenclamide ameliorates edema, secondary hemorrhage, and tissue damage. In a model of SAH, glibenclamide attenuates the inflammatory response due to extravasated blood. Clinical trials of an intravenous formulation of glibenclamide in TBI and stroke underscore the importance of recent advances in understanding the role of the Sur1-regulated NCCa-ATP channel in acute ischemic, traumatic, and inflammatory injury to the CNS.
Keywords: cerebral ischemia, glibenclamide, NCCa-ATP channel, necrotic cell death, spinal cord injury, subarachnoid hemorrhage, Sur1, traumatic brain injury
Introduction
The adenosine triphosphate (ATP) binding cassette (ABC) transporters constitute a large superfamily of integral membrane proteins, with >48 genes encoding human ABC transporters (Aguilar-Bryan et al, 1998; Shi et al, 2005; Aittoniemi et al, 2008; Lefer et al, 2009). The subfamily encoded by the Abcc genes comprises three classes: multidrug resistance-associated proteins (Abcc1–6 and Abcc10–13), the cystic fibrosis conductance regulator (Abcc7), and the sulfonylurea receptors (Abcc8 and Abcc9) (Deeley et al, 2006). Most ABC proteins couple ATP hydrolysis to the translocation of solutes, transporting endogenous substances, xenobiotics or drugs across biological membranes (Hollenstein et al, 2007). A small number of atypical ABC proteins mediate other processes, including DNA repair (bacterial MutS), mRNA trafficking (yeast Elf1p), and ion transport (Abcc7).
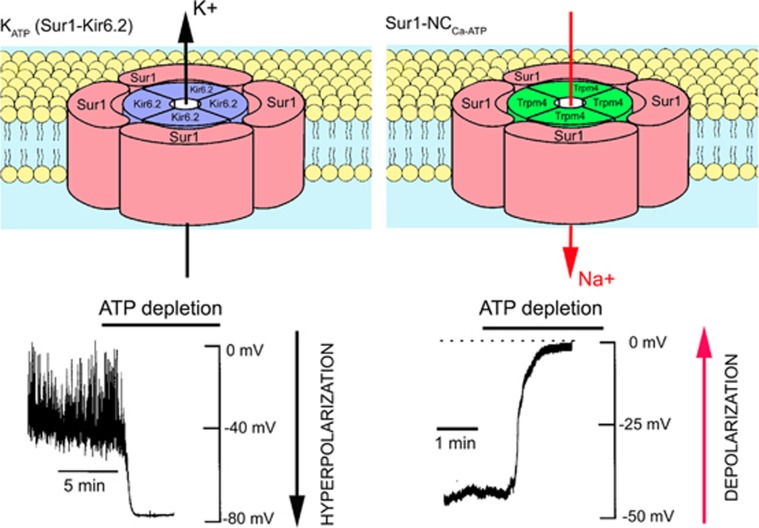
Arguably, the most atypical function thus far identified for members of the ABC superfamily involves the sulfonylurea receptors, Sur1/Abcc8 and Sur2/Abcc9 (Bryan et al, 2007; Burke et al, 2008; Aittoniemi et al, 2008). The Surs are not transporters and, by themselves, perform no recognized function. Instead, they undergo obligate association with heterologous pore-forming subunits to form ion channels. The best-recognized association for Sur1 is with the ATP-sensitive K+ channel, Kir6.2/Kcnj11, to form KATP channels in pancreatic β cells and neurons (Figure 1). Sur1 also can associate with Kir6.1/Kcnj8 (Ammala et al, 1996; Clement et al, 1997), but this combination apparently is not encountered in nature.
Figure 1.
Schematic diagrams of the KATP (Sur1-Kir6.2) and the Sur1-NCCa-ATP channels. The hetero-octameric structure comprising four Sur1 subunits and four Kir6.2 subunits depicted for KATP is widely accepted. The structure depicted for the Sur1-NCCa-ATP channel is hypothesized by analogy. Also shown are the principal physiological actions of the two channels when they are activated by ATP depletion: (1) outward flux of K+ via the K+-selective pore-forming subunit, Kir6.2, resulting in hyperpolarization with the KATP channel; (2) inward flux of Na+ via the nonselective monovalent cation pore-forming subunit, resulting in depolarization with the Sur1-NCCa-ATP channel. The channel schematic was adapted from Seino (1999); the recoding depicting hyperpolarization for KATP was adapted from Harvey et al (1999); the recoding depicting depolarization for NCCa-ATP was adapted from Chen and Simard (2001). ATP, adenosine triphosphate, Sur1, sulfonylurea receptor 1.
Sur1 contains two nucleotide-binding domains as well as high affinity binding sites for therapeutic sulfonylurea drugs and related compounds. Drugs such as glibenclamide (US adopted name, glyburide) and repaglinide bind with nanomolar or subnanomolar affinity, and are potent inhibitors of Sur1-regulated channel activity. Sur1 is the target of sulfonylurea drugs used to treat diabetes mellitus type 2, neonatal diabetes, and some forms of congenital hyperinsulinism.
Sur1 also associates with an ATP- and calcium-sensitive nonselective cation channel to form Sur1-regulated NCCa-ATP (henceforth, Sur1-NCCa-ATP) channels (Figure 1; Chen et al, 2003; Simard et al, 2007b). Although both KATP and Sur1-NCCa-ATP channels are regulated by Sur1, the two have opposite functional effects in central nervous system (CNS) injury—opening of KATP channels hyperpolarizes the cell and may be neuroprotective (Yamada and Inagaki, 2005), whereas opening of Sur1-NCCa-ATP channels depolarizes the cell and, if unchecked, is associated with oncotic (necrotic) cell death (Chen and Simard, 2001; Chen et al, 2003; Simard et al, 2006).
Here, we examine emerging evidence for the role of Sur1 and Sur1-NCCa-ATP channels in acute pathological processes associated with ischemic, traumatic, and inflammatory injury to the CNS.
Biophysical and Pharmacological Properties of the Sur1-NCCa-ATP Channel
The original patch-clamp experiments characterizing the Sur1-NCCa-ATP channel were performed on reactive astrocytes freshly isolated from the hypoxic gliotic capsule in vivo (henceforth, ‘gliotic capsule astrocytes') (Chen and Simard, 2001). These studies showed that the channel transports all inorganic monovalent cations (Na+, K+, Cs+, Li+, Rb+) with a single channel conductance of 25 to 35 pS, and is impermeable to Ca2+ and Mg2+ (Table 1). The fact that the channel readily conducts Cs+ makes it easy to distinguish from KATP and other potassium channels, a feature that has been exploited in all of the reports characterizing the properties of the Sur1-NCCa-ATP channel electrophysiologically. Studies using a series of organic monovalent cations of increasing size indicated that the channel has an equivalent pore radius of 0.41 nm. Channel opening requires physiological concentrations of calcium on the cytoplasmic side (10−8 to 10−5 mol/L). Channel opening is blocked by intracellular ATP (effective dose (EC)50, 0.79 μmol/L), but is unaffected by adenosine diphosphate or adenosine monophosphate. Patch-clamp recordings have since identified a channel with the same biophysical properties (and pharmacological properties; see below) in freshly isolated neurons after cerebral ischemia (Simard et al, 2006) and in cultured endothelial cells exposed to hypoxia or tumor necrosis factor α (TNFα) (Simard et al, 2007c, 2012c; Woo et al, 2012). Only two ion channels are known in the mammalian genome, transient receptor potential melastatin (Trpm) 4 and Trpm5, that possess similar properties of nonselective monovalent but not divalent cation transport, single channel conductance of 25 to 35 pS, and sensitivity to intracellular calcium and ATP (Vennekens and Nilius, 2007; Guinamard et al, 2011).
Table 1. Properties of the Sur1-NCCa-ATP and Trpm4 channels.
| Sur1-NCCa-ATP | Trpm4 | |
|---|---|---|
| Channel conductance | 25 to 35 pS | 25 pS |
| Divalent cation conductivity | No | No |
| Pore radius | 0.41 nm | — |
| Ca2+ activation (EC50) | 0.12 to 1.5 μmol/L | 1.3 μmol/L |
| ATP block (EC50) | 0.8 μmol/L | 0.13 to 1.7 μmol/L |
| ADP, AMP block | No | Yes |
| Voltage dependent | No | Yes |
| PIP2 activation | Yes* | Yes |
| PKC activation | No* | Yes |
| Glibenclamide block (EC50) | 48 nmol/L | 10 to 100 μmol/L |
| Riluzole block (EC50) | 31 μmol/L | 31 μmol/L |
EC, effective dose; AMP, adenosine monophosphate; ADP, adenosine diphosphate; ATP, adenosine triphosphate; PIP2, phosphatidylinositol 4,5-bisphosphate; PKC, protein kinase C; Sur1, sulfonylurea receptor 1; Trpm4, transient receptor potential melastatin 4.
Data for Sur1-NCCa-ATP channel are from published (Chen and Simard, 2001; Chen et al, 2003; Simard et al, 2012c) and unpublished observations (*Chen and Simard, unpublished). Data for Trpm4 channel (Nilius et al, 2003, 2004a,2004b, 2005a,2005b, 2006; Guinamard et al, 2004, 2006; Ullrich et al, 2005; Demion et al, 2007; Simard et al, 2012c) were selected to most closely approximate those of the Sur1-NCCa-ATP channel.
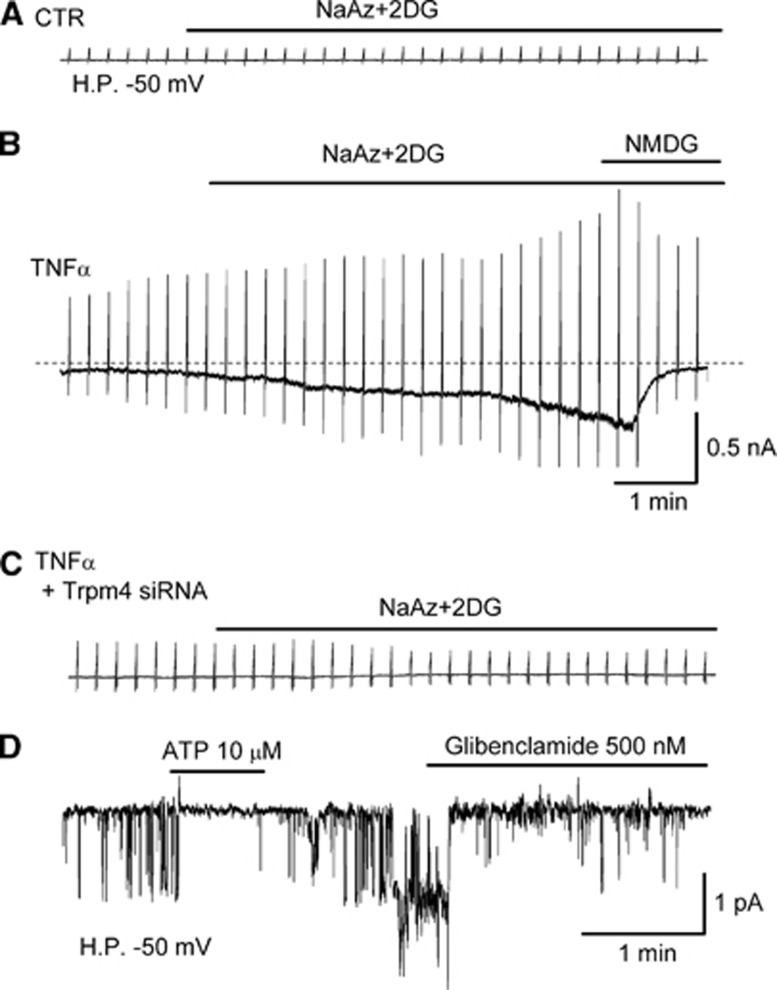
Certain pharmacological properties of the Sur1-NCCa-ATP channel are determined by the regulatory subunit, Sur1, and therefore approximate closely the pharmacological properties of KATP (Sur1-Kir6.2) channels (Chen et al, 2003). Studies on gliotic capsule astrocytes showed that the channel is blocked by first- and second-generation sulfonylureas, tolbutamide (EC50, 16.1 μmol/L at pH 7.4) and glibenclamide (EC50, 48 nmol/L at pH 7.4) (Chen et al, 2003). Block by sulfonylurea drugs is due to a prolongation of, and an increase in the probability of long closed states of the channel, with no effect on open channel dwell times or channel conductance. The inhibitory effect of glibenclamide on channel opening is prevented by an antibody directed against one of the cytoplasmic loops of Sur1 (Chen et al, 2003). The potency of block by glibenclamide is increased ∼8-fold at pH 6.8 (EC50, 6 nmol/L), compared with pH 7.4, consistent with the weak acid needing to enter the lipid phase of the membrane to cause block (Simard et al, 2006, 2008b), paralleling observations with KATP channels (Findlay, 1992). In the presence of ATP, the Sur1-NCCa-ATP channel is opened by the Sur1 activator (‘K+ channel opener'), diazoxide, but not by pinacidil or cromakalim, as expected for Sur1 but not for Sur2 (Chen et al, 2003). Glibenclamide block of Cs+ currents also is observed when Sur1-NCCa-ATP channels are induced in brain microvascular endothelial (bEnd.3) cells by exposure to TNFα (Figure 2).
Figure 2.
Sur1-NCCa-ATP channel currents in activated brain microvascular endothelial (bEnd.3) cells. (A to C) Macroscopic Cs+ currents recorded using a whole-cell nystatin-perforated patch technique during ramp pulses (±100 mV; 4/min; holding potential (H.P.), –50 mV) in bEnd.3 cells without (A) or with overnight exposure to 20 ng/mL tumor necrosis factor α (TNFα) (B, C), and without (A, B) or with coincubation with siRNA directed against transient receptor potential melastatin (Trpm4) (C); channel currents were activated by depleting adenosine triphosphate (ATP) using 1 mmol/L sodium azide (NaAz) plus 10 mmol/L 2-deoxyglucose (2DG); Cs+ was the principal cation in all solutions; note the block, predominantly of inward current, by bath application of 145 mmol/L N-methyl-D-glucamine (NMDG). (D) Single channel recordings of inside-out patches with Cs+ as the principal cation and with 1 μmol/L Ca2+ in the bath solution, showing 25 pS channel openings that were reversibly inhibited by application of ATP on the cytoplasmic side; the channel also was inhibited by glibenclamide. The recordings shown are representative of 5 to 7 recordings for each condition (Simard and colleagues, unpublished). Sur1, sulfonylurea receptor 1.
Other pharmacological properties of the channel are determined by the pore-forming subunit. When the channel is induced in bEnd.3 cells by exposure to TNFα, it can be blocked by riluzole (EC50, 31 μmol/L) (Simard et al, 2012c). Notably, Trpm4 channels heterologously expressed without Sur1 also are blocked by riluzole with the same concentration dependence (Simard et al, 2012c).
The Pore-Forming Subunit of the Sur1-NCCa-ATP Channel
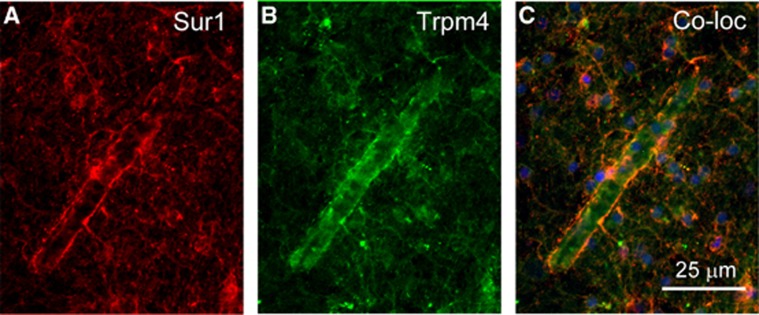
Like the KATP channel, the Sur1-NCCa-ATP channel is composed of pore-forming and regulatory subunits (Figure 1). The molecular identity of the regulatory subunit, Sur1, has been known for some time (Chen et al, 2003; Simard et al, 2006). It has been postulated that the pore-forming subunit is Trpm4 (Simard et al, 2007b). As noted above, both Trpm4 and Sur1-NCCa-ATP channels exhibit similar biophysical properties (Table 1). Exposure of bEnd.3 cells to TNFα normally induces expression of Sur1-NCCa-ATP channels, but not in the presence of siRNA directed against Trpm4 (Figure 2). After CNS injury, Sur1 and Trpm4 are upregulated de novo and colocalize in the same cells (Figure 3). Also, gene suppression of Abcc8 and Trpm4 results in exactly the same phenotype after spinal cord injury (SCI) (see below).
Figure 3.
Sulfonylurea receptor 1 (Sur1) and transient receptor potential melastatin (Trpm4) colocalize after central nervous system (CNS) injury in the human. (A to C) Human brain tissue freshly obtained during surgery to remove a blood clot due to rupture of an arteriovenous malformation shows prominent expression of Sur1 (red) and of Trpm4 (green) in a microvessel and in astroglial processes on the microvessel; the merged images also are shown (C); nuclei are labeled with 4′,6-diamidino-2-phenylindole dihydrochloride (blue). Same case as previously shown in Figure 2 of Simard et al (2008a).
To date, it has been difficult to show the simple coassociation and cofunction of Sur1 and Trpm4 in a heterologous expression system (Sala-Rabanal et al, 2012). This is a key experiment that is required for molecular characterization of the channel, and one that is readily performed with Sur1 and Kir6.2 (Aguilar-Bryan et al, 1998). One important difference between Trpm4 and Kir6.2 is that the latter requires assembly with Sur1 before it can be transported to the cell membrane, whereas Trpm4 does not. This property of Trpm4 makes it easy, in a heterologous expression system, to end up with Trpm4 dominating at the cell membrane. The difficulty in showing cofunction in a heterologous expression system may suggest that expression conditions as yet unidentified may need to be developed.
Function of the Sur1-NCCa-ATP Channel
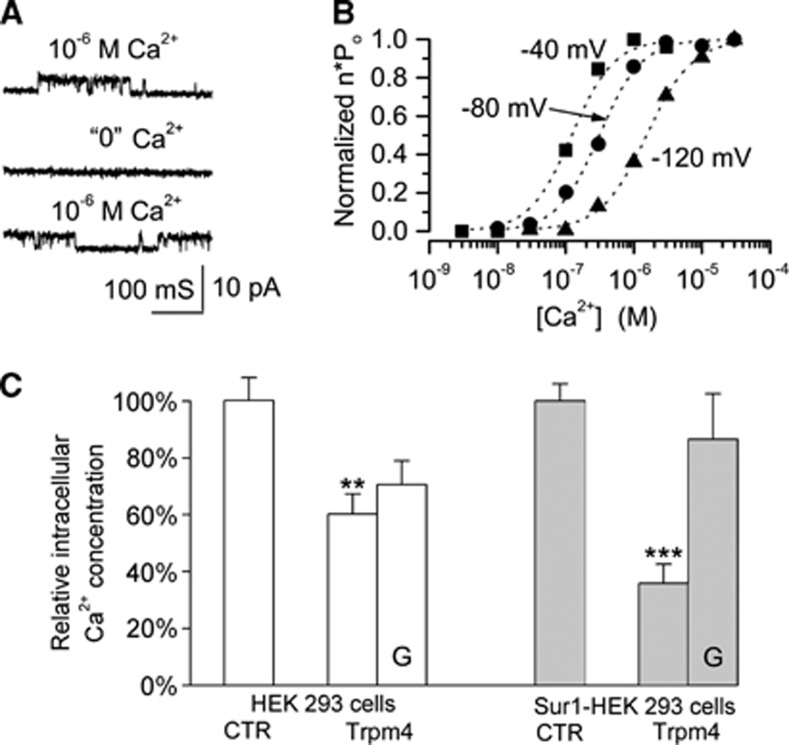
The main function of Trpm4 is the regulation of calcium influx (Vennekens and Nilius, 2007; Guinamard et al, 2011). The entry of calcium into a cell is governed by the electrochemical gradient for calcium, and the electrical portion of that gradient is determined by the cell membrane potential. Depolarization reduces the inward driving force for calcium, thereby decreasing its influx. Two properties of Trpm4 make it ideal for regulating calcium entry: (1) as a nonselective cation channel, its activation results in cell depolarization and (2) because it is activated by calcium, the cell membrane potential becomes directly linked to the intracellular calcium concentration. The same properties that make Trpm4 ideal for regulating calcium influx are shared by the Sur1-NCCa-ATP channel (Figure 4).
Figure 4.
The Sur1-NCCa-ATP channel is activated by intracellular Ca2+ and regulates Ca2+ influx. (A, B) Recordings of inside-out patches of freshly isolated reactive astrocytes showing: (1) single channel openings observed with 1 μmol/L Ca2+ on the cytoplasmic side that were reversibly inhibited in the absence of Ca2+; (2) the open channel probability as a function of Ca2+ concentration and membrane voltage; from Chen and Simard (2001). (C) Steady-state relative intracellular Ca2+ concentration in: (1) HEK293 cells (empty bars), without (CTR) and with transfection with Trpm4 plasmid (Trpm4); (2) HEK293 cells stably transfected to overexpress Sur1 (Sur1-HEK293; gray bars), without (CTR) and with transfection with Trpm4 plasmid (Trpm4); for both series, Trpm4-transfected cells also were studied in the presence of glibenclamide (10 μmol/L) (G); in all cases, the cells were exposed to the Ca2+ ionophore, A23187 (10 μmol/L), and the steady-state (∼2 minutes) intracellular Ca2+ signals were measured using the Fluo-4 NW calcium assay kit (F36206; Molecular Probes, Eugene, OR, USA); **P<0.01; ***P<0.001; (Simard and colleagues, unpublished). Sur1, sulfonylurea receptor 1; Trpm4, transient receptor potential melastatin 4.
These concepts are illustrated by an experiment in which cells were challenged with the calcium ionophore, A23187, which promotes a rise in intracellular calcium (Simard and colleagues, unpublished). Compared with control cells, cells that expressed Trpm4 accumulated significantly less intracellular calcium at steady state (Figure 4). When the foregoing experiment was repeated with cells that stably overexpress Sur1, again, transfection with Trpm4 resulted in less accumulation of intracellular calcium, compared with controls (Figure 4). Notably, the effect of Trpm4 transfection was greater in cells that also expressed Sur1, and in these cells, the effect was blocked by glibenclamide, consistent with a functional role for Sur1. These observations are consistent with the hypothesis that the Sur1-NCCa-ATP channel normally acts to protect against an excess influx of calcium during pathological conditions.
The Sur1-NCCa-ATP Channel and ‘Accidental Necrotic Cell Death'
During cell death, two types of blebs appear: dynamic blebs and larger stationary blebs (Charras, 2008). Dynamic blebbing is associated with the execution phase of apoptosis and appears closely related to blebbing in ‘healthy' cells; larger stationary blebs appear during cell necrosis and are a common feature of cells exposed to noxious stimuli such as hypoxia, oxidants, or ATP depletion.
In gliotic capsule astrocytes, depletion of ATP using the cytochrome oxidase inhibitor, sodium azide, causes activation Sur1-NCCa-ATP channels, resulting in rapid cell depolarization to 0 mV that is accompanied by progressive formation of large stationary blebs (Chen and Simard, 2001; Chen et al, 2003). This pathological process is referred to as ‘oncotic cell swelling' or ‘cytotoxic edema'. Oncotic cell swelling results in part from unchecked Na+ influx via Sur1-NCCa-ATP channels that is presumed to be accompanied by influx of Cl− and water. The formation of large stationary blebs, i.e., oncotic cell swelling, induced by sodium azide is blocked by glibenclamide and is reproduced without ATP depletion by opening the channel with the Sur1-activator, diazoxide (Chen et al, 2003).
Bleb formation of the same sort has been observed in vivo after a focal ischemic insult (see Figure 3 of Simard et al (2007a)). Notably, blebs that appear on neurons and microvascular endothelial cells in vivo show prominent immunolabeling for Sur1 (ibid.).
In cells that express the Sur1-NCCa-ATP channel, ATP depletion leads not only to oncotic cell swelling, but eventually to cell death. Cell demise is due predominantly to nonapoptotic, propidium iodide-positive, oncotic (necrotic) cell death (Simard et al, 2006). Oncotic cell death induced by ATP depletion is largely arrested by block of the Sur1-NCCa-ATP channel with glibenclamide (Simard et al, 2006) or by gene silencing of Sur1 (Simard et al, 2010c).
In cells that heterologously express Trpm4, ATP depletion with sodium azide also leads to the formation of large stationary blebs, oncotic cell swelling and propidium iodide-positive, oncotic (necrotic) cell death (Gerzanich et al, 2009), consistent with Trpm4, not Sur1, conveying sensitivity to ATP depletion.
The Sur1-NCCa-ATP channel is a major molecular mechanism of ‘accidental necrotic cell death' in the CNS. The only other identified mechanism, NMDA receptor-mediated excitotoxicity, is specific to neurons, whereas the mechanism mediated by the Sur1-NCCa-ATP channel involves all members of the neurovascular unit, including neurons, astrocytes, oligodendrocytes, and endothelial cells. Conceptually, ‘accidental' cell death is substantially different from ‘programmed' cell death (apoptosis, autophagy, and necroptosis). The designation ‘programmed' implies that a specific molecular sequence is initiated by an identified stimulus, with the explicit purpose of causing cell death. By contrast, ‘accidental' implies that molecular machinery that normally serves a role unrelated to death is inadvertently transformed into a cell executioner. In most types of CNS injury, including stroke and traumatic brain injury (TBI), the overwhelming form of cell death is not programmed, but is simply accidental necrosis, due to factors external to the cell or tissue.
The Sur1-NCCa-ATP channel is transcriptionally upregulated (see below), but its upregulation is not targeted specifically to inducing cell death. As reviewed above, evidence suggests that the normal function of Sur1-NCCa-ATP channels is to protect against a pathological rise in intracellular calcium, one of the hallmarks of CNS injury (Arundine and Tymianski, 2003; Bano and Nicotera, 2007). However, because Sur1-NCCa-ATP channels are also sensitive to the intracellular concentration of ATP, extreme depletion of ATP, as occurs in stroke and TBI, can result in persistent activation of the channels. Unchecked channel opening, in turn, leads to persistent sodium influx, resulting in oncotic cell swelling, formation of large stationary blebs, and eventual membrane rupture—oncotic (necrotic) cell death, or ‘accidental necrotic cell death'.
Upregulation of Sulfonylurea Receptor 1 Protein and mRNA in Central Nervous System Injury
The Sur1-NCCa-ATP channel has not been identified in normal tissues or cells, but is upregulated de novo after exposure to injurious stimuli (Figure 5). Sur1 is constitutively expressed in some but not in all neurons of the CNS, where it forms KATP channels exclusively (Liss and Roeper, 2001). Sur1 normally is absent in oligodendrocytes, astrocytes, endothelium, and many neurons (Sullivan and Harik, 1993). As detailed below, Sur1 protein and Abcc8 mRNA are progressively upregulated in all cells of the neurovascular unit, in many forms of CNS injury.
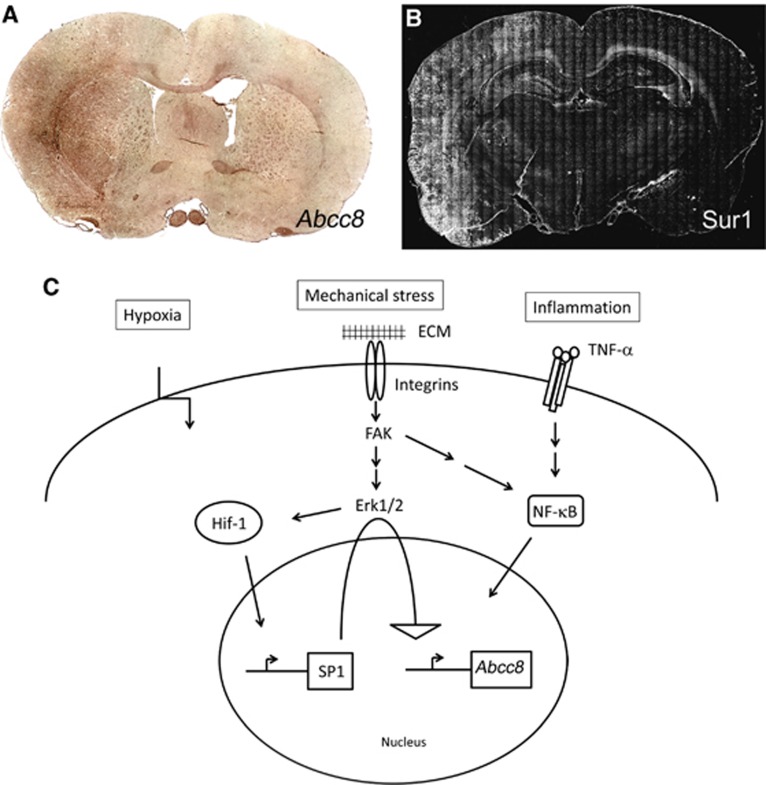
Figure 5.
Summary of Abcc8 gene regulation in central nervous system (CNS) injury. (A, B) In-situ hybridization for Abcc8 mRNA (A) and immunolabeling for sulfonylurea receptor 1 (Sur1) (B) in coronal sections of rat brain after 6-hour middle cerebral artery occlusion (MCAo) followed by 3 hours of reperfusion; from Simard et al (2010a). (C) Transcriptional activation of Abcc8, the gene that encodes Sur1, during hypoxia, mechanical trauma and inflammatory states involves Hif-1, Sp1, and NF-κB. The exact mechanism of activation of Sp1 and NF-κB during mechanical injury remains speculative at this time.
Animal models
The original work describing the channel identified Sur1 protein and Abcc8 mRNA in gliotic capsule astrocytes (Chen et al, 2003). In the same study, it was shown that Kir6.2 protein was absent. Notably, when gliotic capsule astrocytes were cultured under normoxic conditions, expression of Sur1-NCCa-ATP channels was lost (Chen and Simard, 2001).
Work with multiple models of cerebral ischemia has revealed that Sur1 is upregulated progressively during several hours after the onset of ischemia. After permanent mechanical middle cerebral artery occlusion (MCAo) in the rat, 3 hours are required before Abcc8 mRNA is increased 2.5-fold, and 8 hours are required before Sur1 protein is increased 2.5-fold (Simard et al, 2006). In a model with 105 minutes of mechanical MCAo followed by 1 hour of reperfusion, upregulation of Sur1 is observed only in microvascular endothelial cells (Woo et al, 2012). After 3 hours of reperfusion, upregulation becomes evident in neurons (Woo et al, 2012). By 24 hours, Sur1 is upregulated in all members of the neurovascular unit in the penumbra, including neurons, astrocytes, oligodendrocytes, and endothelium, whereas Sur1 expression is undetectable in the necrotic core (Simard et al, 2006, 2010b). In a model with 4.5 hours ischemia followed by a 30-minute infusion of recombinant tissue plasminogen activator (rtPA), Sur1 upregulation after infusion is apparent, but it increases 7- to 11-fold over the subsequent 19 hours, especially in watershed regions (Simard et al, 2012d). Expression of Sur1 protein in neurons and endothelial cells after ischemia has been corroborated by in-situ hybridization for Abcc8 mRNA (Simard et al, 2006, 2010b). In cerebral ischemia, upregulation of Sur1 takes place without upregulation of Kir6.2 protein or Kcnj11 mRNA (Simard et al, 2006).
In mechanical trauma to the CNS, upregulation of Sur1 is prominent in microvessels and neurons in both rats and mice. In TBI, upregulation of Sur1 was confirmed using immunohistochemistry, immunoblot analysis, and in-situ hybridization (Simard et al, 2009b; Patel et al, 2010). In a cortical impact model in rats that was calibrated to avoid primary contusion injury to the hippocampus, Sur1 was significantly upregulated in hippocampal neurons 6, 12, and 24 hours after injury, peaking at 12 hours (Patel et al, 2010). In the same study, quantitative RT-PCR of hippocampal tissues showed a doubling of the Abcc8 mRNA 6 hours after impact, with no change in mRNA for Kcnj8 (Kir6.1) or Kcnj11 (Kir6.2).
In SCI, upregulation of Sur1 in microvessels and neurons has been shown using immunohistochemistry, immunoblot analysis, and in-situ hybridization (Simard et al, 2007c, 2010c). In addition, in SCI involving rats and mice, upregulation of Trpm4 protein and mRNA has been shown in microvessels and neurons using immunohistochemistry, quantitative RT-PCR, and in-situ hybridization (Gerzanich et al, 2009).
In subarachnoid hemorrhage (SAH), upregulation of Sur1 in microvessels and neurons has been shown using immunohistochemistry, immunoisolation followed by immunoblot analysis, and in-situ hybridization (Simard et al, 2009a).
Sur1 also is upregulated in cultured cells under conditions that simulate injury. Exposing cultures of brain microvascular endothelial cells, both primary cultures and bEnd.3 cells, to hypoxia or TNFα results in upregulation of Sur1 protein and Abcc8 mRNA (Simard et al, 2007c, 2009a; Woo et al, 2012).
Human diseases
Two studies have analyzed systematically human tissues for Sur1 expression in the context of injury. Premature infants who die shortly after birth may be found to have a germinal matrix hemorrhage, either unrelated to or as the cause of death. Twelve such cases were examined for expression of Sur1 (Simard et al, 2008b). Regionally-specific upregulation of Sur1 protein and Abcc8 mRNA was found in the germinal matrix, compared with remote cortical tissues. Upregulation was prominent in progenitor cells in all cases. In veins, Sur1 was found predominantly in infants who had sustained germinal matrix hemorrhage, compared with controls without hemorrhage.
Seven cases of patients who died within 3 days of SCI were studied using immunohistochemistry and in-situ hybridization (Simard et al, 2010c). In these cases, the regional and cellular patterns of de-novo Abcc8/Sur1 transcription were similar to those observed in mice and rats. There was significant upregulation of Sur1 in both gray matter and white matter in the region of injury, compared with remote tissues, and upregulation was prominent in endothelium as well as in neurons.
Sporadic cases have been studied showing prominent expression of Sur1 after injury. Fresh biopsy specimens were obtained from patients undergoing brain surgery for resection of a metastatic tumor surrounded by a gliotic capsule, or for removal of an intracerebral hematoma secondary to rupture of an arteriovenous malformation (see Figure 2 of Simard et al (2008b)). Immunolabeling for Sur1 was prominent in these cases. In the gliotic capsule, astrocytes were labeled exclusively, whereas in tissues adjacent to the intracerebral hemorrhage, immunolabeling for Sur1 as well as in-situ hybridization for Abcc8 mRNA showed prominent upregulation in neurons and microvessels. New work with tissues from the second case confirmed expression of Sur1 and showed, in addition, colocalization of Trpm4 (Figure 3).
Transcriptional Regulation of Sulfonylurea Receptor 1 Expression
Recent investigations have begun to identify the transcriptional mechanisms involved in de-novo upregulation of Sur1/Abcc8 after exposure to injurious stimuli (Figure 5). Previously, transcriptional regulation of Abcc8 had been studied almost exclusively in the context of diabetes, since Sur1 is the regulatory subunit of the KATP channel involved in insulin secretion in pancreatic β cells (Ashfield and Ashcroft, 1998; Hernandez-Sanchez et al, 1999). Several transcription factors, including Sp1, FoxA2/HNF3β, Beta2/NeuroD, and STAT3, have been implicated in transcription of Abcc8 in pancreatic β cells (Ashfield and Ashcroft, 1998; Hernandez-Sanchez et al, 1999; Kim et al, 2002; Gorogawa et al, 2004; Lantz et al, 2004). Among these, Sp1 was found to have a critical role in basal expression of the gene (Ashfield and Ashcroft, 1998; Hernandez-Sanchez et al, 1999).
In the context of ischemia/hypoxia, Sur1/Abcc8 is upregulated by a complex mechanism of sequential gene activation that involves hypoxia-inducible factor 1α (Hif1α; Woo et al, 2012). Luciferase reporter activity driven by the Abcc8 promoter is increased by hypoxia and by coexpression of Hif1α but surprisingly, a series of luciferase reporter assays studying the Abcc8 promoter revealed that binding sites for Sp1, but not for Hif, were required for stimulation of Abcc8 transcription by Hif1α. Luciferase reporter assays studying the Sp1 promoter and chromatin immunoprecipitation analysis after cerebral ischemia indicated that Hif binds to Hif-binding sites on the Sp1 promoter to stimulate transcription of the Sp1 gene. These data, as well as previous data on cerebral ischemia (Simard et al, 2006), reaffirmed the critical role of Sp1 in expression of Abcc8 in rat, mouse, and human.
Sequential transcriptional activation is a process that amplifies and prolongs the original signal from a short-lived transcription factor such as Hif, and also delays transcription of the end-target gene, here Abcc8. This mechanism provides a novel molecular explanation for the extraordinarily long treatment window observed for inhibition of the end-target gene product, Sur1, by glibenclamide in cerebral ischemia (see below) (Simard et al, 2010b, 2012d).
Consistent with the role of Hif1 in upregulation of Sur1 in focal ischemia, pharmacological inhibition as well as gene suppression of Hif is protective in cerebral ischemia/hypoxia (Helton et al, 2005; Chen et al, 2007, 2010). Similarly, in the Rice-Vannucci model of neonatal hypoxia-ischemia, inhibiting Hif1 is protective (Chen et al, 2008a,2008b).
In the context of neuroinflammation, as occurs following SAH, Sur1/Abcc8 is upregulated by a mechanism that involves NF-κB (Simard et al, 2009a). Analysis of the 5′ flanking region of the Abcc8 promoter from rat and human showed the presence of at least two consensus NF-κB binding sites. Electrophoretic mobility shift assay showed that NF-κB physically interacts with the Abcc8 promoter. A luciferase promoter assay confirmed the functionality of the putative NF-κB binding sites in the Abcc8 promoter, with activity increasing ∼2-fold with TNFα (Simard et al, 2009a). Mutation of one of the consensus NF-κB binding sites completely abrogated the stimulatory effect of TNFα on Abcc8 promoter activity (Simard and colleagues, unpublished).
In the context of trauma, Sur1/Abcc8 is upregulated by a mechanism that may involve both Sp1 and NF-κB (Patel et al, 2010). The pattern of transcriptional activation correlates with the type of pathology that develops. In a cortical impact model of TBI calibrated to avoid contusion injury to the underlying hippocampus, both Sp1 and NF-κB were activated in the cortex overlying the hippocampus, where a hemorrhagic contusion was prominent. By contrast, in the hippocampus itself where contusion was absent, only Sp1, not NF-κB, was activated in neurons that also showed upregulation of Sur1 and cleavage of caspase-3 (Patel et al, 2010).
The mechanism for activation of Sp1 and NF-κB in trauma remains to be elucidated, but notably, both transcription factors are known to be mechanosensitive (Korenaga et al, 2001; Yun et al, 2002; Abumiya et al, 2002; Davis et al, 2004; Verstraeten et al, 2010). Mechanosensitive activation of these transcription factors likely occurs via integrins, the family of extracellular matrix receptors that has been widely implicated in a range of functions including gene expression, cell proliferation, migration and differentiation, and cell survival (Wu and Reddy, 2012). Ongoing in-vitro studies will help to elucidate the complex interplay of mechanical, hypoxic, and inflammatory signaling pathways responsible for Sur1 gene activation in various contexts of CNS injury (Figure 5).
Sulfonylurea Block and Gene Suppression of Sulfonylurea Receptor 1 in Spinal Cord Injury
Spinal cord injury caused by a blunt impact is associated with development of the autodestructive process termed as ‘progressive hemorrhagic necrosis' (PHN). It is characterized by progressive enlargement of the initial (hemorrhagic) contusion, often to twice its original volume, during the first 12 to 18 hours after injury. Microscopically, PHN appears as an advancing front of hemorrhagic necrosis that is due to the progressive catastrophic structural failure of microvessels in the penumbra surrounding the initial contusion. A unique model of impact SCI in the rat was developed specifically to permit study of this phenomenon (Simard et al, 2007c, 2012a). An impact is delivered to the cervical spinal cord of the rat in such a manner as to produce unilateral, but not bilateral, primary hemorrhagic injury. During the hours that follow, as PHN ensues, the hemorrhagic lesion expands to involve more and more of the contralateral spinal cord, converting a unilateral hemorrhagic injury into a bilateral hemorrhagic injury. It is evident that, if PHN could be halted, the hemorrhagic injury would be confined to the site of primary injury, sparing the contralateral spinal cord.
The progressive catastrophic structural failure of microvessels that characterizes PHN is associated with fragmentation of capillaries. Microvessels in the penumbra appear foreshortened, as small segments of nearly the same length and width (Simard et al, 2007c, 2010a). As noted above, microvessels in the penumbra upregulate Sur1 following impact.
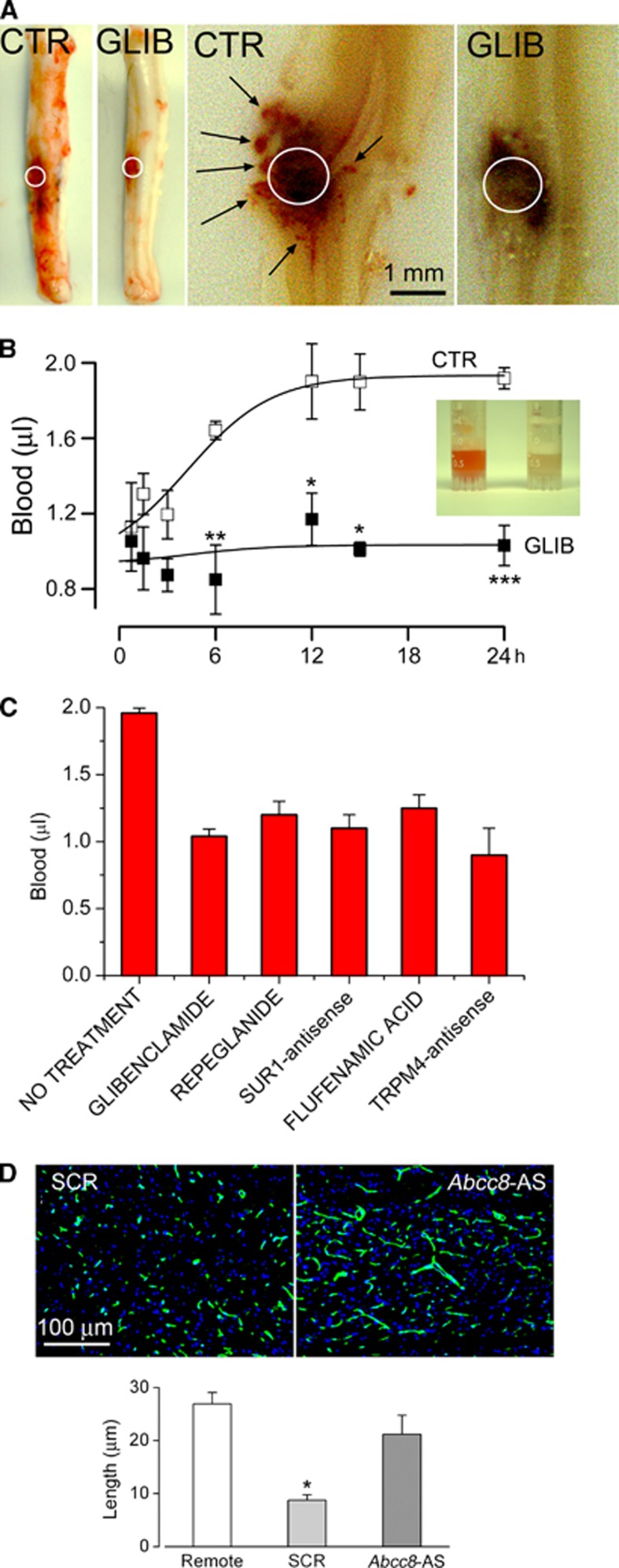
Pharmacological block of Sur1 using glibenclamide or repaglinide, administered as late as 3 hours after impact, protects the capillaries, prevents their fragmentation, halts PHN, and stops the spread of the hemorrhagic contusion to the contralateral side (Figure 6; Table 2; Simard et al, 2007c, 2010c, 2012a,2012c). To date, four separate series of rats treated with glibenclamide have been reported, all with similar results, including significant improvements in neurologic function, compared with vehicle-treated rats (ibid.). Independent experiments from another laboratory have replicated the beneficial effect of glibenclamide on PHN in SCI (Popovich et al, 2012).
Figure 6.
Inhibition of Sur1 or Trpm4, or gene suppression of Abcc8 or Trpm4 prevents secondary hemorrhage after spinal cord injury. (A) Whole spinal cords and spinal cord sections from rats administered vehicle (CTR) or glibenclamide (GLIB) after spinal cord injury, showing absence of petechial hemorrhages (arrows) with drug; white circle, region of impact. (B) Extravasated blood measured at various times after spinal cord injury in rats administered vehicle (CTR) or GLIB; both (A) and (B) are from Simard et al (2007c). (C) Extravasated blood measured 24 hours after spinal cord injury in rats administered vehicle, GLIB, repaglinide, Abcc8 antisense, flufenamic acid, or Trpm4 antisense; data compiled from Simard et al (2007c), Gerzanich et al (2009), and Simard et al (2010c). (D) Capillary fragmentation in penumbral vessels is prevented by administering Abcc8 antisense, compared with scrambled oligodeoxynucleotides (SCRs); from Simard et al (2010c). Sur1, sulfonylurea receptor 1; Trpm4, transient receptor potential melastatin 4.
Table 2. Effects of Sur1 block or Abcc8 suppression in CNS trauma.
| MODEL (Tx delay) | Na | Effects observed (all effects listed were statistically significant: 0.001⩽P< 0.05) |
|---|---|---|
| Rat lateral impact SCIb (none) | 4/5/3 6/6/3 | Glibenclamide: reduced hemorrhage and capillary fragmentation at 24 hours; improved function (angled plane, rearing) and reduced lesion volume to 1/3 of control at 7 days Repaglinide: reduced secondary hemorrhage and improved function (angled plane, rearing) at 24 hours Abcc8 antisense: reduced hemorrhage and improved function (angled plane, rearing) at 24 hours |
| Rat lateral impact SCIc (none) | 5/5 5/5 | Glibenclamide: improved function (rearing) and reduced lesion volume to 1/4 control at 6 weeks Abcc8 antisense: reduced hemorrhage and capillary fragmentation at 24 hours; improved function (trunk stability, angled plane, beam walk, rearing, BBB scores) and reduced lesion volume to 1/4 control at 6 weeks |
| Rat lateral impact SCId (none) | 8 9 | Glibenclamide: improved function (modified BBB scores, angled plane and rearing) and reduced lesion volume to 1/2 of control at 6 weeks |
| Rat medial impact SCId (none) | 8 9 | Glibenclamide: improved function (modified BBB scores, angled plane and rearing) and reduced lesion volume to 2/3 of control at 6 weeks |
| Rat lateral impact SCIe (none) | 4,4 4,4 | Glibenclamide: reduced hemorrhage and improved function (modified BBB scores) at 24 hours; improved function (angle plane) and reduced lesion volume to 2/3 of control at 7 days |
| Rat lethal lateral impact SCIf (3 hours) | 13 13 | Glibenclamide: compared with vehicle controls, reduced death due to spinal shock from 90% to 0% spared neurons at 16 hours; compared to riluzole, improved function (modified BBB scores, rearing and grip strength) and decreased lesion volume at 6 weeks |
| Mouse lateral impact SCIc (N/A) | 7 7 | Abcc8–/– mouse: reduced hemorrhage, capillary fragmentation and necrotic cells at 24 hours; improved function (BMS scores) and reduced lesion volume to 1/3 control at 1 week |
| Rat open contusion TBIg (none) | 7 7 | Glibenclamide: reduced hemorrhage and reduced capillary fragmentation at 24 hours; improved rearing and reduced lesion volume to 1/2 of control at 1 week Abcc8 antisense: reduced hemorrhage at 24 hours |
| Rat open noncontusion TBIh (none) | 5 5 | Glibenclamide: reduced hippocampal apoptosis and neurodegeneration, and improved rapid learning in Morris water maze task at 4 weeks |
SCI, spinal cord injury; TBI, traumatic brain injury; BBB, Basso, Beattie, Bresnahan rat scale for locomotion; BMS, Basso Mouse Scale for locomotion; Sur1, sulfonylurea receptor 1; CNS, central nervous system.
N, number of rats per group (upper cell, vehicle control; lower cell, glibenclamide); multiple series separated by ‘/' the numbers shown are those for functional outcomes.
Pharmacological block of Trpm4 using flufenamic acid or riluzole, administered as late as 3 hours after impact, also protects the capillaries, prevents their fragmentation, halts PHN, stops the spread of the hemorrhagic contusion to the contralateral side, and is associated with significant improvements in neurologic function, compared with vehicle-treated rats (Gerzanich et al, 2009; Simard et al, 2012c). The findings with riluzole are particularly important, since this agent currently is being considered for a clinical trial in SCI. As noted above, riluzole blocks heterologously expressed Trpm4 channels as well as Sur1-NCCa-ATP channels induced in endothelial cells. Although riluzole may exert multiple biological effects, including inhibition of excitotoxicity, it is likely that block of Sur1-NCCa-ATP channels is a central mechanism for its beneficial effect in SCI (Simard et al, 2012c).
Gene suppression of Abcc8 or of Trpm4 also prevents capillary fragmentation, halts PHN, and stops the spread of the hemorrhagic contusion to the contralateral side (Gerzanich et al, 2009; Simard et al, 2010c). Antisense oligodeoxynucleotides (AS-ODNs) do not normally enter the CNS (Wu et al, 1996; Pardridge, 1997), and they are excluded from endothelial cells of noninjured microvessels. However, experiments with fluorescent-tagged AS-ODNs show that after traumatic injury, they are taken up preferentially by penumbral microvessels of the spinal cord (and brain, see below). The mechanism involved in posttraumatic uptake of ODNs (∼7 kDa) by injured endothelium has not been determined, but could involve pannexin channels, which are large-pore, nonselective channels identified in CNS tissues including the vasculature (MacVicar and Thompson, 2010; Burns et al, 2012). Administration of AS-ODN directed against Abcc8 or against Trpm4 prevents capillary fragmentation and PHN (Gerzanich et al, 2009; Simard et al, 2010c). Importantly, AS-ODNs directed against Abcc8 and Trpm4 are equally effective, and are as effective as glibenclamide.
Gene suppression of Abcc8 or of Trpm4 obtained with knockout mice also is protective (Gerzanich et al, 2009; Simard et al, 2010c). For these experiments, the mice were studied using a unilateral impact to the thoracic spinal cord. As with AS-ODNs, knockout of Abcc8 or of Trpm4 prevents capillary fragmentation, halts PHN, and prevents spread of the hemorrhagic contusion to the contralateral side.
Sulfonylurea Block and Gene Suppression of Sulfonylurea Receptor 1 in Traumatic Brain Injury
As with SCI, TBI also may be associated with progressive expansion of a hemorrhagic contusion, but in this context a different term is used—hemorrhagic progression of a contusion (Kurland et al, 2012). As in SCI, the progressive catastrophic structural failure of microvessels that characterizes hemorrhagic progression of a contusion is associated with fragmentation of capillaries. Microvessels in the penumbra appear foreshortened, as small segments of nearly the same length and width (Simard et al, 2009b). As noted above, microvessels in the penumbra upregulate Sur1 following impact.
In a cortical impact model of severe injury, the hemorrhagic contusion visibly enlarges in all directions, and the amount of blood that accumulates in the tissues doubles during the first 12 hours after injury (Simard et al, 2009b). Pharmacological block of Sur1 using glibenclamide prevents capillary fragmentation, halts hemorrhagic progression of a contusion, and prevents the further accumulation of blood in the tissues (Table 2; Simard et al, 2009b). Preventing expansion of the hemorrhagic lesion is associated with significant improvements in neurologic function.
As in SCI, administration of AS-ODN directed against Abcc8 prevents capillary fragmentation and the further accumulation of blood (Simard et al, 2009b). Similarly, administration of AS-ODN directed against Trpm4 prevents capillary fragmentation and the further accumulation of blood (Gerzanich and colleagues, unpublished).
In a cortical impact model in rats that was calibrated to avoid primary contusion injury to the hippocampus, neuronal degeneration and apoptosis in the hippocampus were prominent and were associated with significant deficits in rapid spatial learning, a hippocampus-specific task (Patel et al, 2010). Pharmacological block of Sur1 using glibenclamide significantly reduced neuronal degeneration and apoptosis, and was highly effective in sparing rapid spatial learning.
At present, a prospective, multicenter, placebo-controlled, double-blind, Phase IIa trial of RP-1127 (glyburide for injection; Remedy Pharmaceuticals, Inc., New York, NY, USA) is underway to test the effect of RP-1127 in patients with moderate-to-severe TBI (ClinicalTrials.gov Identifier: NCT01454154).
Sulfonylurea Block of Sulfonylurea Receptor 1 in Cerebral Ischemia
Focal ischemic injury involving the brain is associated with progressive microvascular dysfunction that is manifested initially by the formation of ionic edema, which may be followed by formation of vasogenic edema and, if severe enough, may be followed by the catastrophic structural failure of capillaries—so-called, ‘hemorrhagic transformation' of an ischemic stroke (Simard et al, 2007a). Ionic and vasogenic edema, as well as hemorrhagic transformation, add mass to the brain that causes tissue swelling. Swollen tissues can compromise adjacent tissues and worsen the original insult. If severe, as in malignant cerebral edema after a large stroke, then death of the organism may ensue. The molecular events resulting in these various forms of microvascular dysfunction are complex, but they include the upregulation of Sur1 and subsequent Sur1-mediated microvascular dysfunction, as suggested by the salutary effects of inhibiting Sur1.
Animal models
The effect of glibenclamide has been studied in various rat models of stroke (Figure 7; Table 3). The experiments with mechanical MCAo reviewed below were performed using laser Doppler flowmetry to assure a reduction of >75% during monitoring for the initial 30 minutes after occlusion. Unless otherwise noted, a loading dose of 10 μg/mL was administered intraperitoneally at the time designated for treatment, and a constant infusion of 200 ng/h subcutaneously was started at the same time. This dosing regimen has been found repeatedly to be associated with physiologically unimportant reductions in serum glucose levels (Simard et al, 2006c, 2007c, 2009c).
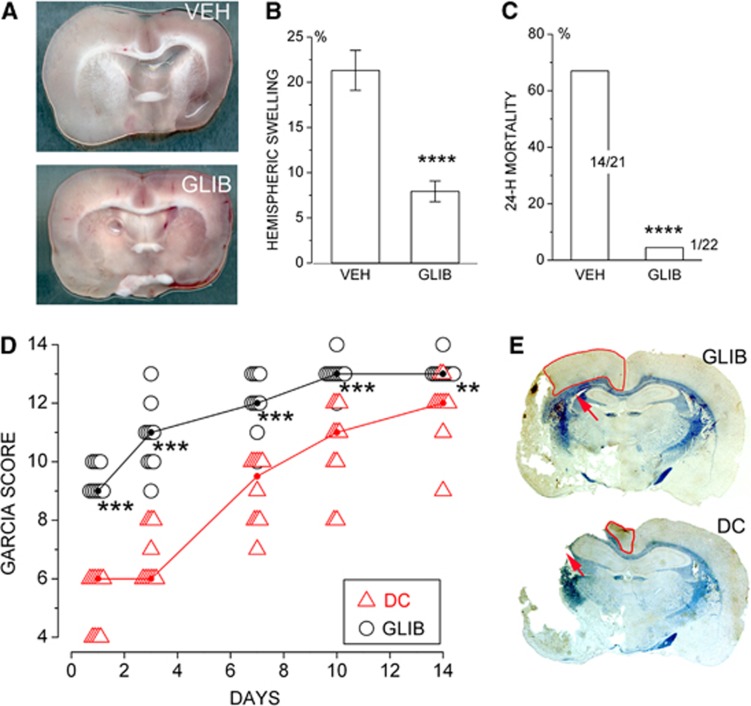
Figure 7.
Inhibition of sulfonylurea receptor 1 (Sur1) prevents brain swelling and death after malignant cerebral infarction. (A to C) Hemispheric swelling (A, B) and death (C) 24 hours after a 6-hour ischemic insult are significantly reduced by administering glibenclamide (GLIB) at 6 hours, at the time of recanalization, compared with vehicle (VEH). (D, E) After a 6-hour ischemic insult, neurologic function, measured using the Garcia scoring system (D), and tissue preservation (E) are significantly better in rats treated with GLIB compared with rats receiving decompressive craniectomy (DC) (both treatments administered at 6 hours, at the time of recanalization); all data from Simard et al (2010a).
Table 3. Effects of glibenclamide in preclinical rat models of stroke.
| MODEL (Tx delay) | Na | Effects observed (all effects listed were statistically significant: 0.001⩽P<0.05) |
|---|---|---|
| Thrombo-embolicb (none) | 7 9/9 | Reduced infarct volume by 53% to 57% at 48 hours and 7 days, respectively Improved laser Doppler flowmetry signals by 51% at 48 hours |
| Thrombo-embolicc (4 and 6 hours) | 9 5/5 | Reduced infarct volume by ∼50% at 48 hours |
| 60-Minute MCAod (7 hours) | 15 15,15,15 | Reduced subcortical (ventral pallidum) necrosis by 55% at 72 hours Reduced neuronal loss by 30% to 40% at 72 hours Reduced pathological calcium deposition by ∼50% at 72 hours |
| 105-Minute MCAoc (1¾ and 5¾ hours) | 11 8/7 | Reduced infarct volume by 41% at 48 hours |
| Permanent MCAoe (none) | 9 8 | Reduced infarct volume by 26% at 48 hours Reduced hemispheric swelling by >50% at 48 hours Improved neuroscores from 6.5 to 4.0, and grip strength from 41 to 61 |
| Permanent MCAoc (4 hours) | 12 14 | Reduced infarct volume by 51% at 48 hours |
| MCEb (none) | 27 27 | Reduced mortality from 65% to 24% at 7 days Reduced excess brain water by 42% at 8 hours |
| MCEf (6 hours) | 21/10 22/10 | Reduced mortality from 67% to 5% at 24 hours Reduced hemispheric swelling from 21% to 8% at 24 hours Better preservation of watershed cortex and white matter, better neuroscores, Garcia scores, and the trajectory of weight gain compared with decompressive craniectomy during 2 weeks after insult |
| MCE+rtPAg (4.5 and 10 hours) | 51 41/25 | Reduced mortality from 53% to 17% or 12% at 48 hours (vehicle versus drug @4.5 or 10 hours) Reduced swelling from 14.7% to 8.1% or 8.8% at 24 hours (same groups) Improved Garcia scores from 3.8 to 7.6 or 8.4 at 48 hours (same groups) |
| MCE+rtPAh (6 hours) | 7/9/22 8/13/20 | Reduced hemispheric swelling from 26% to 12% at 24 hours Reduced scores for hemorrhagic transformation from 2.4 to 0.6 at 24 hours Improved neuroscores from 7 to 3 at 2 weeks |
MCAo, middle cerebral artery occlusion; MCE, malignant cerebral edema; rtPA, recombinant tissue plasminogen activator.
N, number of rats per group (upper cell, vehicle control; lower cell, glibenclamide); multiple series separated by ‘/'.
The effect of glibenclamide was studied in three nonlethal rat models of ischemic stroke, including a thromboembolic model, a 105-minute temporary MCAo model, and a permanent MCAo model (Simard et al, 2006, 2009c). In these experiments, the administration of glibenclamide beginning as late as 5.75 hours after onset of ischemia was associated with 40% to 50% reduction in lesion volumes. In one study with thromboemboli, the use of glibenclamide was associated with cortical sparing that was attributed to improved leptomeningeal collateral blood flow due to reduced mass effect from edema (Simard et al, 2006). In the study with temporary MCAo, smaller lesions were associated with less hemispheric swelling (Simard et al, 2009c).
Independent experiments from other laboratories also have shown beneficial effects of glibenclamide in nonlethal rat models of stroke. One group studied a model with 60-minute MCAo and, beginning 6 hours after the onset of ischemia, administered three intravenous injections of glibenclamide during the first 24 hours (Ortega et al, 2012). Beneficial effects on neurologic outcome and neuronal preservation were observed at 3 days, including reduced subcortical (ventral pallidum) necrosis, reduced neuronal loss, and reduced pathological calcium deposition. A recent study in a model of permanent MCAo replicated the same dosing regimen of glibenclamide detailed in the report by Simard et al (2009c), and found that glibenclamide significantly reduced hemispheric swelling and lesion size, and significantly improved functional outcomes (Sayeed et al, 2011).
The effect of glibenclamide was examined in a rat model of malignant cerebral edema that used particle embolization (Simard et al, 2006). Malignant edema was manifested as an increase in tissue water from a normal value of 78% to 83% at 8 hours and was associated with 65% mortality at 7 days. Administration of glibenclamide (75 ng/h with no loading dose), beginning shortly after onset of ischemia, decreased tissue water to 80%, and reduced mortality at 7 days to 24%.
The effect of glibenclamide was examined in a rat model of malignant cerebral edema involving 6-hour temporary MCAo (Simard et al, 2010b). This insult was associated with thrombosis of proximal and distal middle cerebral artery branches. In some animals, spontaneous thrombolysis required 2 to 3 hours before laser Doppler flowmetry signals returned to normal. Malignant edema was manifested as 22% increase in hemisphere volume and was associated with 67% mortality at 24 hours. Administration of glibenclamide at 6 hours, at the time of recanalization, decreases hemispheric swelling to 7.5% and reduced mortality to 5%. Using this model, the effect of glibenclamide was compared with the effect of a large decompressive hemicraniectomy. Both treatments, administered at 6 hours, were equally effective in essentially eliminating mortality. However, neurologic function during the subsequent 2 weeks showed that glibenclamide was superior to decompressive craniectomy (Figure 7). The superior effect of glibenclamide on neurologic function was associated with better tissue preservation of the corpus callosum and of the watershed cortex of the anterior and middle cerebral arteries.
An important, albeit poorly studied outcome in animal models of stroke is the general health of the organism. Temporary MCAo in rats is associated with severe weight loss of unknown etiology that is not explained by dehydration, stress, or altered hormonal secretions (Petullo et al, 1999; Virtanen et al, 2003, 2004). In the foregoing study (Simard et al, 2010b), weight gain quickly resumed its normal trajectory in the rats treated with glibenclamide, but not in those treated with decompressive craniectomy. Several reasons potentially could account for this, including better sparing of cortico-striatal and hypothalamic tissues in the rats treated with glibenclamide.
At present, the only treatment for stroke that is approved by national medical regulatory agencies worldwide is rtPA, but this treatment, especially if used late and with large strokes, is associated with increased morbidity due to edema and hemorrhagic transformation (Lansberg et al, 2007). The effect of glibenclamide was examined in a rat model of malignant cerebral edema involving 4.5-hour MCAo plus administration of rtPA (0.9 mg/kg intravenously) at the time of occluder withdrawal (Simard et al, 2012d). In these experiments, the specific duration of MCAo and the dose of rtPA were chosen for their clinical relevance. Malignant edema was manifested as 8% and 15% increases in hemisphere volume measured at 10 and 24 hours, respectively, and was associated with 53% mortality at 48 hours. Administration of glibenclamide at 4.5 hours, at the time of recanalization and administration of rtPA, decrease hemispheric swelling to 4% and 8% at 10 and 24 hours, respectively, reduced mortality to 17%, and yielded significant improvements in neurologic function. Administration of glibenclamide at 10 hours after onset of ischemia (5.5 hours after recanalization plus administration of rtPA) decrease hemispheric swelling to 8% at 24 hours, reduced 48-hour mortality to 12%, and yielded significant improvements in neurologic function. In these experiments, lesion volumes were not changed by glibenclamide, consistent with 4.5-hour MCAo producing a maximum ischemic insult. However, examining the relationship between infarct volume and neurologic function was revealing. In the controls, a steep, highly significant negative correlation was found. By contrast, with glibenclamide, the association was much less steep and was not statistically significant, indicating that glibenclamide essentially dissociated functional outcome from infarct volume, presumably due to the significant reductions in edema and swelling.
The effect of glibenclamide was studied in a rat model of malignant cerebral edema involving 6-hour MCAo plus administration at the time of occluder withdrawal of a dose of rtPA (10 mg/kg intravenously) that is expected to be thrombolytic in rats (Simard et al, 2012b). Malignant edema was manifested as 25% increase in hemisphere volume and a high incidence of hemorrhagic transformation at 24 hours. Administration of glibenclamide at 6 hours, at the time of recanalization and administration of rtPA, decrease hemispheric swelling to 12% and significantly reduced scores measuring hemorrhagic transformation. Mortality and functional outcomes (neuroscores) were equally poor in rats without or with treatment with rtPA, with neuroscores of 7 to 8 in both groups (8 signifies death, 7 signifies coma). Glibenclamide significantly reduced mortality and improved neuroscores, both without or with treatment with rtPA, with neuroscores of 3 to 4. The best functional outcomes were observed in rats treated with a thrombolytic dose of rtPA plus glibenclamide. The beneficial effects of glibenclamide endured for the 2 weeks of observation.
Finally, protective effects of glibenclamide in the context of cerebral ischemia have been reported in two studies in which the effect was unlikely to be due to Sur1-NCCa-ATP channels, since there was no time for transcriptional upregulation of the channel. The first study involved a model of global ischemia/reperfusion (15 min/60 min), in which glibenclamide pretreatment was found to: (1) reduce neutrophil recruitment; (2) restore pro-oxidant/antioxidant balance; (3) reduce the inflammatory mediators, TNFα, and prostaglandin E2; (4) boost the antiinflammatory cytokine, interleukin-10 (Abdallah et al, 2011). Importantly, impairment of neutrophil recruitment by glibenclamide also has been reported in experiments with other organs subjected to ischemia/reperfusion (Da Silva-Santos et al, 2002; Pompermayer et al, 2005; Pompermayer et al, 2007; Figura et al, 2009).
The second study involved an in-vitro model of cerebral ischemia (hippocampal brain slices subjected to 14-minute oxygen/glucose deprivation), in which it was found that glibenclamide increased the number of viable hippocampal neurons (Nistico et al, 2007). This finding likely was due to inhibition of KATP (Sur1-Kir6.2) channels. Conventional thinking suggests that opening KATP channels should be protective, because the associated cell hyperpolarization would reduce influx of calcium via voltage-dependent Ca2+ channels (Yamada and Inagaki, 2005). However, this hypothesis discounts the fact that voltage-dependent Ca2+ channels undergo voltage-dependent inactivation, which diminishes the potential harm from prolonged cell depolarization. In addition, as Nistico et al point out opening of KATP channels might be harmful, if it results in an excess efflux of potassium that would then have to be compensated by ATP-consuming sodium-potassium pumps (Na+/K+-ATPase). Also, as discussed above, hyperpolarization increases the electrochemical driving force for calcium, increasing its influx via nonvoltage-dependent pathways. Thus, in some settings, neuronal depolarization is likely to be more protective than hyperpolariztion (Huang et al, 2001).
Humans
The effect of sulfonylureas on stroke in humans has been examined retrospectively in diabetic patients presenting with ischemic stroke, as well as prospectively in nondiabetic patients with large ischemic strokes. In the retrospective studies on diabetics, patients whose diabetes was managed without sulfonylurea drugs (controls) were compared with patients whose diabetes was managed with sulfonylurea drugs and who were maintained on sulfonylurea drugs after hospitalization for stroke.
The medical records of patients with diabetes mellitus admitted to the hospital within 24 hours of onset of acute ischemic stroke presenting to the Neurology Clinic, Charité Hospital, Berlin, Germany, during 1994 to 2000 were analyzed (Kunte et al, 2007). After exclusions, the cohort comprised 33 patients taking a sulfonylurea (glibenclamide, glimepiride, or glibornuride) at admission through discharge (treatment group) and 28 patients not on a sulfonylurea (control group). The primary outcome was a decrease in NIHSS (National Institutes of Health Stroke Scale) of 4 points or more from admission to discharge or a discharge NIHSS score=0, either of which is considered as a ‘major neurologic improvement'. The secondary outcome was a discharge modified Rankin Scale score of 2 or less, which signifies functional independence. No significant differences, other than stroke subtype, were observed among baseline variables between control and treatment groups. The primary outcome was reached by 36% of patients in the treatment group and 7% in the control group (odds ratio (OR)=7.4; 95% confidence interval (CI)=1.5 to 37; P=0.007). The secondary outcome was reached by 82% versus 57% (OR=3.4; CI=1.1 to 11; P=0.035). Subgroup analysis showed that the benefit of drug was observed only in patients with nonlacunar strokes, with 42% of patients on sulfonylureas who harbored a nonlacular stroke achieving the primary outcome, versus 0% of controls. Outcomes were independent of gender, previous transient ischemic attack, and blood glucose levels.
Another retrospective study of diabetic patients from the Registry of the Canadian Stroke Network was reported at the International Stroke Conference in 2009 (Silver et al, 2009; Frank Silver, personal communication). Of 15,814 patients screened, 2,448 with diabetes were admitted to hospital within 24 hours of symptom onset. For patients who had been on and continued on sulfonylurea drugs, compared with controls not on sulfonylureas, the likelihood of in-hospital mortality was less (OR=0.51; CI=0.37 to 0.71; P<0.05), and the likelihood of neurologic worsening was less (OR=0.70; CI=0.55 to 0.89; P<0.05). A subgroup of patients was analyzed that were treated with rtPA. For patients who had been on and continued on sulfonylurea drugs, compared with controls not on sulfonylureas, the likelihood of in-hospital mortality was less (OR=0.30; CI=0.12 to 0.73; P<0.05), and the likelihood of neurologic worsening was less (OR=0.52; CI=0.28 to 0.98; P<0.05). Overall, the available data on diabetic patients presenting with stroke suggest that, if a patient is on a sulfonylurea drug, then this drug should be continued, not stopped, unless a contraindication is present.
Recently, a multicenter, prospective, open label, Phase IIa trial of RP-1127 (glyburide for injection, Remedy Pharmaceuticals, Inc.) was completed (ClinicalTrials.gov Identifier: NCT01268683) (Kevin N Sheth and colleagues, personal communication). This clinical trial tested the effect of RP-1127 in 10 patients with a severe anterior circulation ischemic stroke (baseline infarct volume ⩾82 mL; mean, 102±23 mL; baseline NIHSS score, 19±8) at high risk for malignant cerebral edema. Of the 10 patients, 1 died, even after decompressive craniectomy. The incidence of malignant edema was 20%, compared with 88% in a prospective observational study of patients with infarct volumes ⩾82 mL (Thomalla et al, 2010). Moreover, 8/10 patients did not require osmotherapy, intubation, or decompressive craniectomy, and there were no clinically significant parenchymal hematomas (‘PH1/PH2'), in contrast to parenchymal hematoma rates of ∼30% in the DEFUSE and EPITHET trials in patients with a malignant profile (Mlynash et al, 2011). Most remarkable, the proportion of patients with 30-day modified Rankin Scale scores ⩽4 was 90%, compared with 24% (at 12 months) in control patients from a pooled analysis of decompressive craniectomy trials (Vahedi et al, 2007), or 29% (at 90 days) for patients with infarct volumes >70 mL (Sanak et al, 2006). A larger clinical trial studying the effect or RP-1127 in patients with large ischemic strokes is anticipated.
Sulfonylurea Block of Sulfonylurea Receptor 1 in Perinatal Cerebral Ischemia
The effect of glibenclamide was examined in a rat pup model of neonatal hypoxia-ischemia, induced by unilateral carotid ligation plus 2- or 2.5-hour exposure (moderate and severe insults, respectively) to 8% O2 in 10-day old pups (modified Rice-Vannucci model) (Zhou et al, 2009). Unlike the forgoing experiments in rats that used constant infusion of low-dose glibenclamide, in these studies, glibenclamide (10 μg/kg intraperitoneally) was administered first shortly after the insult and a second time at 24 hours. Brain edema, infarct volume, and brain tissue loss were not changed by treatment. However, in the moderate injury group, glibenclamide was associated with improvements of motor functions in the foot-fault test and in postural reflex test at 3 weeks after hypoxic-ischemic brain injury.
Sulfonylurea Block of Sulfonylurea Receptor 1 in Subarachnoid Hemorrhage
Subarachnoid hemorrhage initiates numerous injury cascades including oxidative stress, inflammation, and vasospasm (Macdonald et al, 2007; Simard et al, 2010a; Sehba et al, 2011).
The effect of glibenclamide was examined in a model of SAH induced by unilateral carotid filament puncture of the internal carotid artery (Simard et al, 2009a). Subarachnoid hemorrhage caused a large increase in barrier permeability (extravasation of immunoglobulin G) and disrupted the normal junctional localization of the tight junction protein, zona occludens 1. Glibenclamide significantly reduced both effects. In addition, SAH caused large increases in markers of inflammation, including TNFα and NF-κB, and markers of cell injury or cell death, including immunoglobulin G endocytosis and cleavage of caspase-3. Glibenclamide significantly reduced these effects as well.
Sulfonylureas and the Blood–Brain Barrier
As reviewed above, the treatment of acute CNS injury with glibenclamide may be highly beneficial. Normally, however, glibenclamide and other sulfonylureas do not accumulate in the brain in physiologically meaningful concentrations (Tomiyama et al, 1999). This accounts for the observation that, despite decades of clinical use in diabetic patients, there are no reports of side effects involving the CNS that are attributable directly to sulfonylureas (as distinct from CNS effects of hypoglycemia), even though the brain contains many regions with neurons that express KATP (Sur1-Kir6.2) channels (Liss and Roeper, 2001).
The net accumulation of sulfonylureas in the brain is governed by passive transport into, and active transport out of the brain. Transport across the blood–brain barrier (BBB) has been studied for the first-generation sulfonylurea, tolbutamide (Takanaga et al, 1998; Koyabu et al, 2004). The net accumulation of tolbutamide in the brain of rats is much lower than in other organs or tissues, and is almost nil compared with propranolol, a β-blocker with significant CNS side effects (Takanaga et al, 1998). Tolbutamide is actively extruded by an endothelial cell transporter that appears to be distinct from P-glycoprotein (Takanaga et al, 1998). Extrusion may use one of the organic anion transporters, of which organic anion transporter 3 has been found to have a prominent role in the efflux of acidic substances from brain to blood (Burckhardt and Burckhardt, 2011). Glibenclamide also is thought to be actively extruded by transporter(s) on the luminal side of the endothelial cells of the BBB.
Passive transport from the blood across an ‘intact' BBB into the brain is determined by lipid solubility. As weak acids, glibenclamide (pKa, 5.3) and tolbutamide (pKa, 5.4) exist in ionized (hydrophilic, impermeant) and nonionized (lipophylic, permeant) forms. According to the Henderson-Hasselbach relationship (a.k.a., the pH-partition hypothesis), an equilibrium is established between the ionized and nonionized forms. For a weak acid, the ratio of ionized to nonionized forms is 10(pH−pK):1. For a weak acid like glibenclamide with a pKa of 5.3, the ratio of ionized to nonionized at pH 7.3 will be 100:1, i.e., the ionized, impermeant form will dominate at neutral pH. Thus, poor lipid solubility at neutral pH, combined with active transport out of endothelial cells and into the blood, results in a very low net accumulation of sulfonylureas in the normal brain.
The situation is quite different in ischemic brain tissues. Ischemia is associated with high glycolytic activity resulting in lactic acidosis that can drive extracellular pH to values ∼6.5 (Nedergaard et al, 1991). The phenomenon termed as ‘ion trapping' occurs when there is a large permeability difference between ionized (impermeant) and nonionized (permeant) species of a drug across a membrane (Raghunand and Gillies, 2000). When extracellular pH is appreciably lower than intracellular pH, a weak acid will tend to concentrate in the more alkaline compartment, in our case, intracellularly in endothelial cells and neurons.
Apart from pH-dependent passive transport across an ‘intact' BBB, glibenclamide also may be passively transported into the brain after ‘breakdown' of the BBB. Breakdown of the BBB induced by ischemia leads to the passive transport of plasma across the compromised BBB, i.e., the formation of vasogenic edema. Glibenclamide, which is highly protein bound in the circulation (Skillman and Feldman, 1981), thus would be transported into the brain during the formation of vasogenic edema.
Empirical evidence indicates that glibenclamide indeed is transported from blood into the ischemic brain. Accumulation of the fluorescent analog, BODIPY-glibenclamide, in ischemic brain was reported after MCAo (Simard et al, 2006): in control tissues contralateral to the infarct, fluorescent drug was excluded, but in the ischemic tissues, the microvessels and neurons appeared bright with fluorescent drug (see Figure 6 of Simard et al (2006)). Independent confirmation of preferential uptake of glibenclamide into ischemic brain recently was published (Ortega et al, 2012): only 0.025% of injected [3H]glibenclamide entered the control brain, but after permanent MCAo (with the vessel still occluded), this value increased three-fold to 0.077% in the ischemic tissues.
The greater penetration of sulfonylureas into ischemic brain, due either to low pH or to breakdown of the BBB, has the effect that weak acids such as sulfonylurea drugs ‘selectively' target injured tissues over normal tissues. As a result, relatively low doses of drug can be used to obtain a favorable therapeutic effect, while minimally affecting insulin secretion in the pancreas.
Conclusion
Recent advances have broadened our understanding of the role of Sur1-NCCa-ATP channels in acute CNS injury, especially its role in oncotic cell swelling and oncotic (necrotic) cell death, and its role in microvascular dysfunction marked by edema formation and delayed secondary hemorrhage. The channel is transcriptionally upregulated de novo in all cells of the neurovascular unit, in numerous forms of CNS injury, bringing together in a single unifying theory many seemingly diverse types of CNS insult, including cerebral ischemia, TBI, SCI, and SAH. Growing evidence indicates that inhibiting this channel in all of the aforementioned conditions results in robust protection in animal models. Retrospective studies of diabetics, as well as a recent Phase IIa study in nondiabetic patients, suggest a highly promising translational potential for therapies targeting Sur1 in ischemic stroke. Ongoing or anticipated clinical trials in TBI and stroke underscore the importance of recent advances in understanding the role of the Sur1-NCCa-ATP channel in acute ischemic, traumatic, and inflammatory injury to the CNS.
Sur1 was discovered several decades ago and since has been the subject of intensive investigation due to its role in forming KATP channels, which are critically important in diabetes. As the present review suggests, the role of Sur1 in disease may be significantly larger than heretofore appreciated, due not to its association with Kir6.2, but to its involvement with a nonselective cation channel, possibly Trpm4. The lives of many patients already have been improved enormously by pharmacological manipulation of Sur1. If the preclinical and early phase clinical work reviewed here continues on its present trajectory, then it is likely that many more lives will be affected, perhaps even saved, by pharmacological manipulation of Sur1 in CNS injury.
Ethics statement
All of the experiments reviewed herein that were performed in the laboratory headed by the senior author adhered to the following criteria: (1) all experiments were performed in accordance with the relevant guidelines and regulations as stipulated in the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals; (2) in all cases, specific experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Maryland; and (3) all efforts were made to minimize the number of animals used and their suffering.
Acknowledgments
The authors acknowledge the enormous contributions made by the many scientists with whom we have been privileged to collaborate, and who coauthored the numerous publications describing this work during the last decade.
JMS holds a US patent (#7,285,574), ‘A novel nonselective cation channel in neural cells and methods for treating brain swelling', and is a member of the scientific advisory board and holds shares in Remedy Pharmaceuticals. No support was provided by Remedy Pharmaceuticals to JMS for this project.
Footnotes
This work was supported by grants to JMS from the Department of Veterans Affairs (Baltimore), the National Institute of Neurological Disorders and Stroke (NINDS) (NS060801; NS061808), the National Heart, Lung, and Blood Institute (HL082517), the Department of the Army (W81XWH 1010898) and the Christopher and Dana Reeve Foundation; to VG from NINDS (NS061934; NS072501); and to GS from the Neurosurgery Research Education Foundation of the American Association of Neurological Surgeons.
References
- Abdallah DM, Nassar NN, Abd-El-Salam RM. Glibenclamide ameliorates ischemia-reperfusion injury via modulating oxidative stress and inflammatory mediators in the rat hippocampus. Brain Res. 2011;1385:257–262. doi: 10.1016/j.brainres.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Abumiya T, Sasaguri T, Taba Y, Miwa Y, Miyagi M. Shear stress induces expression of vascular endothelial growth factor receptor Flk-1/KDR through the CT-rich Sp1 binding site. Arterioscler Thromb Vasc Biol. 2002;22:907–913. doi: 10.1161/01.atv.0000018300.43492.83. [DOI] [PubMed] [Google Scholar]
- Aguilar-Bryan L, Clement JP, Gonzalez G, Kunjilwar K, Babenko A, Bryan J. Toward understanding the assembly and structure of KATP channels. Physiol Rev. 1998;78:227–245. doi: 10.1152/physrev.1998.78.1.227. [DOI] [PubMed] [Google Scholar]
- Aittoniemi J, Fotinou C, Craig TJ, de WH, Proks P, Ashcroft FM. Review. SUR1: a unique ATP-binding cassette protein that functions as an ion channel regulator. Philos Trans R Soc Lond B Biol Sci. 2008;364:257–267. doi: 10.1098/rstb.2008.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammala C, Moorhouse A, Gribble F, Ashfield R, Proks P, Smith PA, Sakura H, Coles B, Ashcroft SJ, Ashcroft FM. Promiscuous coupling between the sulphonylurea receptor and inwardly rectifying potassium channels. Nature. 1996;379:545–548. doi: 10.1038/379545a0. [DOI] [PubMed] [Google Scholar]
- Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325–337. doi: 10.1016/s0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- Ashfield R, Ashcroft SJ. Cloning of the promoters for the beta-cell ATP-sensitive K-channel subunits Kir6.2 and SUR1. Diabetes. 1998;47:1274–1280. doi: 10.2337/diab.47.8.1274. [DOI] [PubMed] [Google Scholar]
- Bano D, Nicotera P. Ca2+ signals and neuronal death in brain ischemia. Stroke. 2007;38:674–676. doi: 10.1161/01.STR.0000256294.46009.29. [DOI] [PubMed] [Google Scholar]
- Bryan J, Munoz A, Zhang X, Dufer M, Drews G, Krippeit-Drews P, Aguilar-Bryan L. ABCC8 and ABCC9: ABC transporters that regulate K+ channels. Pflugers Arch. 2007;453:703–718. doi: 10.1007/s00424-006-0116-z. [DOI] [PubMed] [Google Scholar]
- Burckhardt G, Burckhardt BC. In vitro and in vivo evidence of the importance of organic anion transporters (OATs) in drug therapy. Handb Exp Pharmacol. 2011;201:29–104. doi: 10.1007/978-3-642-14541-4_2. [DOI] [PubMed] [Google Scholar]
- Burke MA, Mutharasan RK, Ardehali H. The sulfonylurea receptor, an atypical ATP-binding cassette protein, and its regulation of the KATP channel. Circ Res. 2008;102:164–176. doi: 10.1161/CIRCRESAHA.107.165324. [DOI] [PubMed] [Google Scholar]
- Burns AR, Phillips SC, Sokoya EM. Pannexin protein expression in the rat middle cerebral artery. J Vasc Res. 2012;49:101–110. doi: 10.1159/000332329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charras GT. A short history of blebbing. J Microsc. 2008;231:466–478. doi: 10.1111/j.1365-2818.2008.02059.x. [DOI] [PubMed] [Google Scholar]
- Chen C, Hu Q, Yan J, Lei J, Qin L, Shi X, Luan L, Yang L, Wang K, Han J, Nanda A, Zhou C. Multiple effects of 2ME2 and D609 on the cortical expression of HIF-1alpha and apoptotic genes in a middle cerebral artery occlusion-induced focal ischemia rat model. J Neurochem. 2007;102:1831–1841. doi: 10.1111/j.1471-4159.2007.04652.x. [DOI] [PubMed] [Google Scholar]
- Chen C, Ostrowski RP, Zhou C, Tang J, Zhang JH. Suppression of hypoxia-inducible factor-1alpha and its downstream genes reduces acute hyperglycemia-enhanced hemorrhagic transformation in a rat model of cerebral ischemia. J Neurosci Res. 2010;88:2046–2055. doi: 10.1002/jnr.22361. [DOI] [PubMed] [Google Scholar]
- Chen M, Simard JM. Cell swelling and a nonselective cation channel regulated by internal Ca2+ and ATP in native reactive astrocytes from adult rat brain. J Neurosci. 2001;21:6512–6521. doi: 10.1523/JNEUROSCI.21-17-06512.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Dong Y, Simard JM. Functional coupling between sulfonylurea receptor type 1 and a nonselective cation channel in reactive astrocytes from adult rat brain. J Neurosci. 2003;23:8568–8577. doi: 10.1523/JNEUROSCI.23-24-08568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Jadhav V, Tang J, Zhang JH. HIF-1alpha inhibition ameliorates neonatal brain injury in a rat pup hypoxic-ischemic model. Neurobiol Dis. 2008a;31:433–441. doi: 10.1016/j.nbd.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Jadhav V, Tang J, Zhang JH. HIF-1 alpha inhibition ameliorates neonatal brain damage after hypoxic-ischemic injury. Acta Neurochir Suppl. 2008b;102:395–399. doi: 10.1007/978-3-211-85578-2_77. [DOI] [PubMed] [Google Scholar]
- Clement JP, Kunjilwar K, Gonzalez G, Schwanstecher M, Panten U, Aguilar-Bryan L, Bryan J. Association and stoichiometry of K(ATP) channel subunits. Neuron. 1997;18:827–838. doi: 10.1016/s0896-6273(00)80321-9. [DOI] [PubMed] [Google Scholar]
- Da Silva-Santos JE, Santos-Silva MC, Cunha FQ, Assreuy J. The role of ATP-sensitive potassium channels in neutrophil migration and plasma exudation. J Pharmacol Exp Ther. 2002;300:946–951. doi: 10.1124/jpet.300.3.946. [DOI] [PubMed] [Google Scholar]
- Davis ME, Grumbach IM, Fukai T, Cutchins A, Harrison DG. Shear stress regulates endothelial nitric-oxide synthase promoter activity through nuclear factor kappaB binding. J Biol Chem. 2004;279:163–168. doi: 10.1074/jbc.M307528200. [DOI] [PubMed] [Google Scholar]
- Deeley RG, Westlake C, Cole SP. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev. 2006;86:849–899. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- Demion M, Bois P, Launay P, Guinamard R. TRPM4, a Ca2+-activated nonselective cation channel in mouse sino-atrial node cells. Cardiovasc Res. 2007;73:531–538. doi: 10.1016/j.cardiores.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Figura M, Chilton L, Liacini A, Viskovic MM, Phan V, Knight D, Millar TM, Patel K, Kubes P, Giles WR, Tibbles LA. Blockade of K(ATP) channels reduces endothelial hyperpolarization and leukocyte recruitment upon reperfusion after hypoxia. Am J Transplant. 2009;9:687–696. doi: 10.1111/j.1600-6143.2009.02553.x. [DOI] [PubMed] [Google Scholar]
- Findlay I. Effects of pH upon the inhibition by sulphonylurea drugs of ATP-sensitive K+ channels in cardiac muscle. J Pharmacol Exp Ther. 1992;262:71–79. [PubMed] [Google Scholar]
- Gerzanich V, Woo SK, Vennekens R, Tsymbalyuk O, Ivanova S, Ivanov A, Geng Z, Chen Z, Nilius B, Flockerzi V, Freichel M, Simard JM. De novo expression of Trpm4 initiates secondary hemorrhage in spinal cord injury. Nat Med. 2009;15:185–191. doi: 10.1038/nm.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorogawa S, Fujitani Y, Kaneto H, Hazama Y, Watada H, Miyamoto Y, Takeda K, Akira S, Magnuson MA, Yamasaki Y, Kajimoto Y, Hori M. Insulin secretory defects and impaired islet architecture in pancreatic beta-cell-specific STAT3 knockout mice. Biochem Biophys Res Commun. 2004;319:1159–1170. doi: 10.1016/j.bbrc.2004.05.095. [DOI] [PubMed] [Google Scholar]
- Guinamard R, Chatelier A, Demion M, Potreau D, Patri S, Rahmati M, Bois P. Functional characterization of a Ca(2+)-activated non-selective cation channel in human atrial cardiomyocytes. J Physiol. 2004;558:75–83. doi: 10.1113/jphysiol.2004.063974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinamard R, Demion M, Chatelier A, Bois P. Calcium-activated nonselective cation channels in mammalian cardiomyocytes. Trends Cardiovasc Med. 2006;16:245–250. doi: 10.1016/j.tcm.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Guinamard R, Salle L, Simard C. The non-selective monovalent cationic channels TRPM4 and TRPM5. Adv Exp Med Biol. 2011;704:147–171. doi: 10.1007/978-94-007-0265-3_8. [DOI] [PubMed] [Google Scholar]
- Harvey J, Hardy SC, Ashford ML. Dual actions of the metabolic inhibitor, sodium azide on K(ATP) channel currents in the rat CRI-G1 insulinoma cell line. Br J Pharmacol. 1999;126:51–60. doi: 10.1038/sj.bjp.0702267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helton R, Cui J, Scheel JR, Ellison JA, Ames C, Gibson C, Blouw B, Ouyang L, Dragatsis I, Zeitlin S, Johnson RS, Lipton SA, Barlow C. Brain-specific knock-out of hypoxia-inducible factor-1alpha reduces rather than increases hypoxic-ischemic damage. J Neurosci. 2005;25:4099–4107. doi: 10.1523/JNEUROSCI.4555-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Sanchez C, Ito Y, Ferrer J, Reitman M, LeRoith D. Characterization of the mouse sulfonylurea receptor 1 promoter and its regulation. J Biol Chem. 1999;274:18261–18270. doi: 10.1074/jbc.274.26.18261. [DOI] [PubMed] [Google Scholar]
- Hollenstein K, Dawson RJ, Locher KP. Structure and mechanism of ABC transporter proteins. Curr Opin Struct Biol. 2007;17:412–418. doi: 10.1016/j.sbi.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Huang H, Gao TM, Gong L, Zhuang Z, Li X. Potassium channel blocker TEA prevents CA1 hippocampal injury following transient forebrain ischemia in adult rats. Neurosci Lett. 2001;305:83–86. doi: 10.1016/s0304-3940(01)01821-3. [DOI] [PubMed] [Google Scholar]
- Kim JW, Seghers V, Cho JH, Kang Y, Kim S, Ryu Y, Baek K, guilar-Bryan L, Lee YD, Bryan J, Suh-Kim H. Transactivation of the mouse sulfonylurea receptor I gene by BETA2/NeuroD. Mol Endocrinol. 2002;16:1097–1107. doi: 10.1210/mend.16.5.0934. [DOI] [PubMed] [Google Scholar]
- Korenaga R, Yamamoto K, Ohura N, Sokabe T, Kamiya A, Ando J. Sp1-mediated downregulation of P2X4 receptor gene transcription in endothelial cells exposed to shear stress. Am J Physiol Heart Circ Physiol. 2001;280:H2214–H2221. doi: 10.1152/ajpheart.2001.280.5.H2214. [DOI] [PubMed] [Google Scholar]
- Koyabu N, Takanaga H, Matsuo H, Naito M, Tsuruo T, Ohtani H, Sawada Y. Tolbutamide uptake via pH- and membrane-potential-dependent transport mechanism in mouse brain capillary endothelial cell line. Drug Metab Pharmacokinet. 2004;19:270–279. doi: 10.2133/dmpk.19.270. [DOI] [PubMed] [Google Scholar]
- Kunte H, Schmidt S, Eliasziw M, del Zoppo GJ, Simard JM, Masuhr F, Weih M, Dirnagl U. Sulfonylureas improve outcome in patients with type 2 diabetes and acute ischemic stroke. Stroke. 2007;38:2526–2530. doi: 10.1161/STROKEAHA.107.482216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurland D, Hong C, Aarabi B, Gerzanich V, Simard JM. Hemorrhagic progression of a contusion after traumatic brain injury: a review. J Neurotrauma. 2012;29:19–31. doi: 10.1089/neu.2011.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansberg MG, Albers GW, Wijman CA. Symptomatic intracerebral hemorrhage following thrombolytic therapy for acute ischemic stroke: a review of the risk factors. Cerebrovasc Dis. 2007;24:1–10. doi: 10.1159/000103110. [DOI] [PubMed] [Google Scholar]
- Lantz KA, Vatamaniuk MZ, Brestelli JE, Friedman JR, Matschinsky FM, Kaestner KH. Foxa2 regulates multiple pathways of insulin secretion. J Clin Invest. 2004;114:512–520. doi: 10.1172/JCI21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefer DJ, Nichols CG, Coetzee WA. Sulfonylurea receptor 1 subunits of ATP-sensitive potassium channels and myocardial ischemia/reperfusion injury. Trends Cardiovasc Med. 2009;19:61–67. doi: 10.1016/j.tcm.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liss B, Roeper J. Molecular physiology of neuronal K-ATP channels (review) Mol Membr Biol. 2001;18:117–127. [PubMed] [Google Scholar]
- Macdonald RL, Pluta RM, Zhang JH. Cerebral vasospasm after subarachnoid hemorrhage: the emerging revolution. Nat Clin Pract Neurol. 2007;3:256–263. doi: 10.1038/ncpneuro0490. [DOI] [PubMed] [Google Scholar]
- MacVicar BA, Thompson RJ. Non-junction functions of pannexin-1 channels. Trends Neurosci. 2010;33:93–102. doi: 10.1016/j.tins.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Mlynash M, Lansberg MG, De Silva DA, Lee J, Christensen S, Straka M, Campbell BC, Bammer R, Olivot JM, Desmond P, Donnan GA, Davis SM, Albers GW. Refining the definition of the malignant profile: insights from the DEFUSE-EPITHET pooled data set. Stroke. 2011;42:1270–1275. doi: 10.1161/STROKEAHA.110.601609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M, Kraig RP, Tanabe J, Pulsinelli WA. Dynamics of interstitial and intracellular pH in evolving brain infarct. Am J Physiol. 1991;260:R581–R588. doi: 10.1152/ajpregu.1991.260.3.R581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Mahieu F, Prenen J, Janssens A, Owsianik G, Vennekens R, Voets T. The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate. EMBO J. 2006;25:467–478. doi: 10.1038/sj.emboj.7600963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Droogmans G, Voets T, Vennekens R, Freichel M, Wissenbach U, Flockerzi V. Voltage dependence of the Ca2+-activated cation channel TRPM4. J Biol Chem. 2003;278:30813–30820. doi: 10.1074/jbc.M305127200. [DOI] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Voets T, Droogmans G. Intracellular nucleotides and polyamines inhibit the Ca2+-activated cation channel TRPM4b. Pflugers Arch. 2004a;448:70–75. doi: 10.1007/s00424-003-1221-x. [DOI] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Janssens A, Voets T, Droogmans G. Decavanadate modulates gating of TRPM4 cation channels. J Physiol. 2004b;560:753–765. doi: 10.1113/jphysiol.2004.070839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Janssens A, Owsianik G, Wang C, Zhu MX, Voets T. The selectivity filter of the cation channel TRPM4. J Biol Chem. 2005a;280:22899–22906. doi: 10.1074/jbc.M501686200. [DOI] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Tang J, Wang C, Owsianik G, Janssens A, Voets T, Zhu MX. Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J Biol Chem. 2005b;280:6423–6433. doi: 10.1074/jbc.M411089200. [DOI] [PubMed] [Google Scholar]
- Nistico R, Piccirilli S, Sebastianelli L, Nistico G, Bernardi G, Mercuri NB. The blockade of K(+)-ATP channels has neuroprotective effects in an in vitro model of brain ischemia. Int Rev Neurobiol. 2007;82:383–395. doi: 10.1016/S0074-7742(07)82021-6. [DOI] [PubMed] [Google Scholar]
- Ortega FJ, Gimeno-Bayon J, Espinosa-Parrilla JF, Carrasco JL, Batlle M, Pugliese M, Mahy N, Rodriguez MJ. ATP-dependent potassium channel blockade strengthens microglial neuroprotection after hypoxia-ischemia in rats. Exp Neurol. 2012;235:282–296. doi: 10.1016/j.expneurol.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Drug delivery to the brain. J Cereb Blood Flow Metab. 1997;17:713–731. doi: 10.1097/00004647-199707000-00001. [DOI] [PubMed] [Google Scholar]
- Patel AD, Gerzanich V, Geng Z, Simard JM. Glibenclamide reduces hippocampal injury and preserves rapid spatial learning in a model of traumatic brain injury. J Neuropathol Exp Neurol. 2010;69:1177–1190. doi: 10.1097/NEN.0b013e3181fbf6d6. [DOI] [PubMed] [Google Scholar]
- Petullo D, Masonic K, Lincoln C, Wibberley L, Teliska M, Yao DL. Model development and behavioral assessment of focal cerebral ischemia in rats. Life Sci. 1999;64:1099–1108. doi: 10.1016/s0024-3205(99)00038-7. [DOI] [PubMed] [Google Scholar]
- Pompermayer K, Amaral FA, Fagundes CT, Vieira AT, Cunha FQ, Teixeira MM, Souza DG. Effects of the treatment with glibenclamide, an ATP-sensitive potassium channel blocker, on intestinal ischemia and reperfusion injury. Eur J Pharmacol. 2007;556:215–222. doi: 10.1016/j.ejphar.2006.10.065. [DOI] [PubMed] [Google Scholar]
- Pompermayer K, Souza DG, Lara GG, Silveira KD, Cassali GD, Andrade AA, Bonjardim CA, Passaglio KT, Assreuy J, Cunha FQ, Vieira MA, Teixeira MM. The ATP-sensitive potassium channel blocker glibenclamide prevents renal ischemia/reperfusion injury in rats. Kidney Int. 2005;67:1785–1796. doi: 10.1111/j.1523-1755.2005.00276.x. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Lemeshow S, Gensel JC, Tovar CA. Independent evaluation of the effects of glibenclamide on reducing progressive hemorrhagic necrosis after cervical spinal cord injury. Exp Neurol. 2012;233:615–622. doi: 10.1016/j.expneurol.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunand N, Gillies RJ. pH and drug resistance in tumors. Drug Resist Updat. 2000;3:39–47. doi: 10.1054/drup.2000.0119. [DOI] [PubMed] [Google Scholar]
- Sala-Rabanal M, Wang S, Nichols CG. On the potential interactions between Non-Selective cation channel TRPM4 and the sulfonylurea receptor SUR1. J Biol Chem. 2012;287:8746–8756. doi: 10.1074/jbc.M111.336131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanak D, Nosal' V, Horak D, Bartkova A, Zelenak K, Herzig R, Bucil J, Skoloudik D, Burval S, Cisarikova V, Vlachova I, Kocher M, Zapletalova J, Kurca E, Kanovsky P. Impact of diffusion-weighted MRI-measured initial cerebral infarction volume on clinical outcome in acute stroke patients with middle cerebral artery occlusion treated by thrombolysis. Neuroradiology. 2006;48:632–639. doi: 10.1007/s00234-006-0105-0. [DOI] [PubMed] [Google Scholar]
- Sayeed I, Wali B, Ishrat F, Hua F, Stein DG.2011Glybenclamide administration attenuates infarct volume, hemispheric swelling and functional deficits following permanent focal cerebral ischemia in rats Society of Neuroscience Meeting, Washington DCPoster # 62.05/FF11
- Sehba FA, Pluta RM, Zhang JH. Metamorphosis of subarachnoid hemorrhage research: from delayed vasospasm to early brain injury. Mol Neurobiol. 2011;43:27–40. doi: 10.1007/s12035-010-8155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino S. ATP-sensitive potassium channels: a model of heteromultimeric potassium channel/receptor assemblies. Annu Rev Physiol. 1999;61:337–362. doi: 10.1146/annurev.physiol.61.1.337. [DOI] [PubMed] [Google Scholar]
- Shi NQ, Ye B, Makielski JC. Function and distribution of the SUR isoforms and splice variants. J Mol Cell Cardiol. 2005;39:51–60. doi: 10.1016/j.yjmcc.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Silver FL, Fang J, Robertson AC, Casaubon L, Kapral MK. Possible neuroprotective effects of sulfonylureas in diabetic patients with acute ischemic stroke. Stroke. 2009;40:51. [Google Scholar]
- Simard JM, Chen M, Tarasov KV, Bhatta S, Ivanova S, Melnitchenko L, Tsymbalyuk N, West GA, Gerzanich V. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med. 2006;12:433–440. doi: 10.1038/nm1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Castellani RJ, Ivanova S, Koltz MT, Gerzanich V. Sulfonylurea receptor 1 in the germinal matrix of premature infants. Pediatr Res. 2008a;64:648–652. doi: 10.1203/PDR.0b013e318186e5a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Geng Z, Sheth K, Silver F.2012aDoes inhibiting SUR1 complement rtPA in cerebral ischemia Ann NY Acad Sci(in press) [DOI] [PMC free article] [PubMed]
- Simard JM, Geng Z, Woo SK, Ivanova S, Tosun C, Melnichenko L, Gerzanich V. Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2009a;29:317–330. doi: 10.1038/jcbfm.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007a;6:258–268. doi: 10.1016/S1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Kilbourne M, Tsymbalyuk O, Tosun C, Caridi J, Ivanova S, Keledjian K, Bochicchio G, Gerzanich V. Key role of sulfonylurea receptor 1 in progressive secondary hemorrhage after brain contusion. J Neurotrauma. 2009b;26:2257–2267. doi: 10.1089/neu.2009.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Popovich PG, Tsymbalyuk O, Gerzanich V. Spinal cord injury with unilateral versus bilateral primary hemorrhage—Effects of glibenclamide. Exp Neurol. 2012b;233:829–835. doi: 10.1016/j.expneurol.2011.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Schreibman D, Aldrich EF, Stallmeyer B, Le B, James RF, Beaty N. Unfractionated heparin: multitargeted therapy for delayed neurological deficits induced by subarachnoid hemorrhage. Neurocrit Care. 2010a;13:439–449. doi: 10.1007/s12028-010-9435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Tarasov KV, Gerzanich V. Non-selective cation channels, transient receptor potential channels and ischemic stroke. Biochim Biophys Acta. 2007b;1772:947–957. doi: 10.1016/j.bbadis.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Tsymbalyuk O, Ivanov A, Ivanova S, Bhatta S, Geng Z, Woo SK, Gerzanich V. Endothelial sulfonylurea receptor 1-regulated NC Ca-ATP channels mediate progressive hemorrhagic necrosis following spinal cord injury. J Clin Invest. 2007c;117:2105–2113. doi: 10.1172/JCI32041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Tsymbalyuk O, Keledjian K, Ivanov A, Ivanova S, Gerzanich V. Comparative effects of glibenclamide and riluzole in a rat model of severe cervical spinal cord injury. Exp Neurol. 2012c;233:566–574. doi: 10.1016/j.expneurol.2011.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Tsymbalyuk N, Tsymbalyuk O, Ivanova S, Yurovsky V, Gerzanich V. Glibenclamide is superior to decompressive craniectomy in a rat model of malignant stroke. Stroke. 2010b;41:531–537. doi: 10.1161/STROKEAHA.109.572644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Woo SK, Bhatta S, Gerzanich V. Drugs acting on SUR1 to treat CNS ischemia and trauma. Curr Opin Pharmacol. 2008b;8:42–49. doi: 10.1016/j.coph.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Woo SK, Norenberg MD, Tosun C, Chen Z, Ivanova S, Tsymbalyuk O, Bryan J, Landsman D, Gerzanich V. Brief suppression of Abcc8 prevents autodestruction of spinal cord after trauma. Sci Transl Med. 2010c;2:28ra29. doi: 10.1126/scitranslmed.3000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Woo SK, Tsymbalyuk N, Voloshyn O, Yurovsky V, Ivanova S, Lee R, Gerzanich V. Glibenclamide—10h treatment window in a clinically-relevant model of stroke. Transl Stroke Res. 2012d;3:286–295. doi: 10.1007/s12975-012-0149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Yurovsky V, Tsymbalyuk N, Melnichenko L, Ivanova S, Gerzanich V. Protective effect of delayed treatment with low-dose glibenclamide in three models of ischemic stroke. Stroke. 2009c;40:604–609. doi: 10.1161/STROKEAHA.108.522409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skillman TG, Feldman JM. The pharmacology of sulfonylureas. Am J Med. 1981;70:361–372. doi: 10.1016/0002-9343(81)90773-7. [DOI] [PubMed] [Google Scholar]
- Sullivan HC, Harik SI. ATP-sensitive potassium channels are not expressed in brain microvessels. Brain Res. 1993;612:336–338. doi: 10.1016/0006-8993(93)91682-i. [DOI] [PubMed] [Google Scholar]
- Takanaga H, Murakami H, Koyabu N, Matsuo H, Naito M, Tsuruo T, Sawada Y. Efflux transport of tolbutamide across the blood-brain barrier. J Pharm Pharmacol. 1998;50:1027–1033. doi: 10.1111/j.2042-7158.1998.tb06918.x. [DOI] [PubMed] [Google Scholar]
- Thomalla G, Hartmann F, Juettler E, Singer OC, Lehnhardt FG, Kohrmann M, Kersten JF, Krutzelmann A, Humpich MC, Sobesky J, Gerloff C, Villringer A, Fiehler J, Neumann-Haefelin T, Schellinger PD, Rother J. Prediction of malignant middle cerebral artery infarction by magnetic resonance imaging within 6 hours of symptom onset: a prospective multicenter observational study. Ann Neurol. 2010;68:435–445. doi: 10.1002/ana.22125. [DOI] [PubMed] [Google Scholar]
- Tomiyama Y, Brian JE, Jr, Todd MM. Cerebral blood flow during hemodilution and hypoxia in rats: role of ATP-sensitive potassium channels. Stroke. 1999;30:1942–1947. doi: 10.1161/01.str.30.9.1942. [DOI] [PubMed] [Google Scholar]
- Ullrich ND, Voets T, Prenen J, Vennekens R, Talavera K, Droogmans G, Nilius B. Comparison of functional properties of the Ca2+-activated cation channels TRPM4 and TRPM5 from mice. Cell Calcium. 2005;37:267–278. doi: 10.1016/j.ceca.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, Amelink GJ, Schmiedeck P, Schwab S, Rothwell PM, Bousser MG, van der Worp HB, Hacke W. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6:215–222. doi: 10.1016/S1474-4422(07)70036-4. [DOI] [PubMed] [Google Scholar]
- Vennekens R, Nilius B. Insights into TRPM4 function, regulation and physiological role. Handb Exp Pharmacol. 2007;179:269–285. doi: 10.1007/978-3-540-34891-7_16. [DOI] [PubMed] [Google Scholar]
- Verstraeten SV, Mackenzie GG, Oteiza PI. The plasma membrane plays a central role in cells response to mechanical stress. Biochim Biophys Acta. 2010;1798:1739–1749. doi: 10.1016/j.bbamem.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Virtanen T, Jolkkonen J, Sivenius J. Re: External carotid artery territory ischemia impairs outcome in the endovascular filament model of middle cerebral artery occlusion in rats. Stroke. 2004;35:e9–10. doi: 10.1161/01.STR.0000107295.05923.F4. [DOI] [PubMed] [Google Scholar]
- Virtanen T, Sivenius J, Jolkkonen J. Dehydration and stress do not explain severe weight loss after experimental stroke in rats. J Animal Veterinary Adv. 2003;2:247–252. [Google Scholar]
- Woo SK, Kwon MS, Geng Z, Chen Z, Ivanov A, Bhatta S, Gerzanich V, Simard JM. Sequential activation of hypoxia-inducible factor 1 and specificity protein 1 is required for hypoxia-induced transcriptional stimulation of Abcc8. J Cereb Blood Flow Metab. 2012;32:525–536. doi: 10.1038/jcbfm.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Boado RJ, Pardridge WM. Pharmacokinetics and blood-brain barrier transport of [3H]-biotinylated phosphorothioate oligodeoxynucleotide conjugated to a vector-mediated drug delivery system. J Pharmacol Exp Ther. 1996;276:206–211. [PubMed] [Google Scholar]
- Wu X, Reddy DS. Integrins as receptor targets for neurological disorders. Pharmacol Ther. 2012;134:68–81. doi: 10.1016/j.pharmthera.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Inagaki N. Neuroprotection by KATP channels. J Mol Cell Cardiol. 2005;38:945–949. doi: 10.1016/j.yjmcc.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Yun S, Dardik A, Haga M, Yamashita A, Yamaguchi S, Koh Y, Madri JA, Sumpio BE. Transcription factor Sp1 phosphorylation induced by shear stress inhibits membrane type 1-matrix metalloproteinase expression in endothelium. J Biol Chem. 2002;277:34808–34814. doi: 10.1074/jbc.M205417200. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Fathali N, Lekic T, Tang J, Zhang JH. Glibenclamide improves neurological function in neonatal hypoxia-ischemia in rats. Brain Res. 2009;1270:131–139. doi: 10.1016/j.brainres.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]