Abstract
Many neuroprotective agents have been effective in experimental stroke, yet few have translated into clinical application. One reason for this may be failure to consider clinical comorbidities/risk factors in experimental models. We have shown that a naturally occurring interleukin-1 receptor antagonist (IL-1Ra) is protective against ischemic brain damage in healthy animals. However, protective effects of IL-1Ra have not been determined in comorbid animals. Thus, we tested whether IL-1Ra protects against brain injury induced by experimental ischemia in aged JCR-LA (corpulent) rats, which have clinically relevant risk factors. Male, aged, lean, and corpulent rats exposed to transient (90 minutes) occlusion of the middle cerebral artery (tMCAO) were administered two doses of IL-1Ra (25 mg/kg, subcutaneously) during reperfusion. Brain injury and neuroinflammatory changes were assessed 24 hours after tMCAO. Our results show that IL-1Ra administered at reperfusion significantly reduced infarct volume measured by magnetic resonance imaging (50%, primary outcome) and blood–brain barrier disruption in these comorbid animals. Interleukin-1Ra also reduced microglial activation, neutrophil infiltration, and cytokines levels in the brain. These data are the first to indicate that IL-1Ra protects against ischemic brain injury when administered via a clinically relevant route and time window in animals with multiple risk factors for stroke.
Keywords: cerebral ischemia, inflammation, interleukin-1 receptor antagonist, neutroprotection, risk factors, therapy
Introduction
Stroke is a leading cause of death and disability in adults worldwide. Fibrinolysis with recombinant tissue plasminogen activator is the only licensed therapy for stroke, but is available to only a minority of patients due to the risk of hemorrhage. Numerous agents have shown efficacy in animal models of stroke, yet have failed to show benefit in clinical trials (Paul et al, 2007). Recent clinical failures have led the Stroke Therapy Academics Industry Roundtable (STAIR; Fisher et al, 2009; STAIR, 1999) to update their guidelines in order that better translation from bench-to-bedside might be made. These updated guidelines include the need to show effectiveness of any potential stroke drug in aged and comorbid animals.
Inflammation contributes to poor outcome after stroke (Denes et al, 2010a, 2010b; McColl et al, 2009), and the proinflammatory cytokine interleukin-1 (IL-1) is a key mediator of ischemic brain injury (Allan et al, 2005; Simi et al, 2007). The IL-1 receptor antagonist (IL-1Ra) is a naturally occurring, competitive inhibitor of IL-1 action, which is highly selective. There is considerable preclinical and clinical evidence showing that IL-1Ra is a promising candidate for the treatment of stroke and related disorders (Banwell et al, 2009; Emsley et al, 2005; Hannum et al, 1990; Rothwell, 2003). In experimental stroke, endogenous IL-1Ra is upregulated and protects the brain (Denes et al, 2008; Pinteaux et al, 2006), while administration of exogenous IL-1Ra reduces ischemic injury, even when administered 3 hours after the insult (Garcia et al, 1995; Loddick and Rothwell, 1996; Mulcahy et al, 2003; Relton et al, 1996; Stroemer and Rothwell, 1997). Subcutaneous IL-1Ra, administered at the time of occlusion, is neuroprotective in the rat and localizes to salvageable brain tissue (Greenhalgh et al, 2010), and IL-1Ra showed some beneficial effects in a small phase II clinical study in acute stroke (Emsley et al, 2005).
However, IL-1Ra has not yet been tested in comorbid animals after stroke, a key recommendation of the recent STAIR report. We have previously shown an elevated inflammatory burden in the brain of corpulent (Cp) rats in the absence of any acute injury, and activated microglia in patients at risk of stroke (Drake et al, 2011). Therefore, due to the neuroprotective effects of IL-1Ra demonstrated previously, we determined the effects of subcutaneous administration of IL-1Ra after experimental stroke in aged, Cp (Mangat et al, 2007) and lean rats.
Materials and methods
Animals
Studies were conducted on 15- and 16-month-old male, lean (JCR:LA-lean (cp/−); 400–500 g) and Cp (JCR:LA-cp (cp/cp); 900–1000 g) rats (Charles River, Wilmington, MA, USA). Cp rats are homozygous for the autosomal recessive cp gene (cp/cp), and spontaneously develop obesity, hyperlipidemia, insulin resistance, glomerular sclerosis, and atherosclerosis with enhanced vascular contractility and reduced vascular relaxation (Mangat et al, 2007). Animals were allowed free access to food and water and were maintained under temperature, humidity, and light-controlled conditions. All animal procedures were performed under the University of Manchester project license number (40/3076) and adhered to the UK Animals (Scientific Procedures) Act (1986).
Treatment Groups
A pilot study was undertaken to analyze the pharmacokinetic profile of subcutaneous human-IL-1Ra (r-met-huIL-1Ra: Kineret; Amgen, Thousand Oaks, CA, USA) or placebo (Amgen). Owing to the highly lipophobic formulation of the drugs, obese Cp rats received half the dose of IL-1Ra (50%) per body weight compared with lean rats. Plasma concentrations of IL-1Ra (measured by enzyme-linked immunosorbent assay) after subcutaneous administration reached levels that we believe are clinically therapeutic concentrations (∼8000 ng/ml; Emsley et al, 2005) and no differences in plasma concentrations were observed between lean or Cp rats (data not shown).
Animals were randomized for all the experiments, assessments were performed in a blinded manner and analysis was confirmed by two independent researchers. Lean and Cp rats received two doses (subcutaneous) of placebo or IL-1Ra (25 mg/kg and 12.5 mg/kg, respectively) at reperfusion and 6 hours later, and were allocated randomly to the following experimental groups: lean+tMCAO+placebo; lean+tMCAO+IL-1Ra; Cp+tMCAO+placebo; and Cp+tMCAO+IL-1Ra (n=6 per group). For the delayed administration study, animals were injected (subcutaneous, doses as above) with placebo or IL-1Ra at 3 hours of reperfusion (n=8 per group), and again, 3 hours later.
Group sizes were determined by power calculation (α=0.05, β=0.2) at http://www.stattools.net/SSizAOV_Pgm.php.
Focal Cerebral Ischemia
Focal cerebral ischemia was induced by 90 minutes transient occlusion of the middle cerebral artery (tMCAO) as described previously (Chen et al, 1986; Liu et al, 1989). Isofluorane (5% for induction and 1.5% during surgery) was used as anesthetic in a mixture of 70% N2O and 30% O2. Core body temperature was maintained at 37.0°C±0.5°C throughout the surgery by a heating blanket (Homeothermic Blanket Control Unit; Harvard Apparatus, Kent, UK) and monitored after recovery. Ischemia was induced by a transient ligature (90 minutes) of the left MCA trunk with a 10-0 suture (Prolene, Ethicon, Somerville, NJ, USA). Occlusion and reperfusion were confirmed visually under the surgical microscope. After surgery, animals were returned to their cages and allowed free access to water and food. It was decided, a priori, to exclude from the study those animals that showed brain hemorrhage at any time of the surgery or with no reperfusion (8% in total, corresponding to lean and Cp treated with IL-1Ra). The survival rate was 100%.
Magnetic Resonance Imaging
The primary outcome was infarct volume measured by magnetic resonance imaging (MRI) 23 hours after tMCAO. Animals anesthetized with isofluorane (5% for induction and 1.5% during scanning) were scanned using a Magnex 7-Tesla, horizontal-bore magnet (Agilent Technologies, Oxford Industrial Park, Yarnton, Oxford, UK) connected to a Bruker Biospec Avance III console (Bruker Biospin, Banner Lane, Coventry, UK) with a transmit/receive 2.5-cm surface coil. Rectal temperature and respiration rate were monitored. A T2-weighted fast spin-echo sequence based on RARE (Hennig et al, 1986) was used (repetition time=4.8 seconds, base echo time=20 ms, effective echo time=60 ms, number of echos=8, number of samples=256, number of views=128, number of averages=2 with 25 1 mm slices), which results in sufficient anatomical information to measure brain damage (Calamante et al, 1999; Wegener et al, 2006). Lesion volume was determined using Anatomist software (http://brainvisa.info/).
Stroke Outcome
Motor and behavioral outcomes and the adhesive removal test were assessed 24 hours before and 23 hours after tMCAO, as described previously (Hunter et al, 2000; Madrigal et al, 2003; Denes et al, 2008). Functional assessments were all performed by an observer blinded for treatments. Each test was conducted three times per trial, and the average taken to determine outcome.
Histology/Immunohistochemistry
Blood samples for pharmacokinetic analysis and cytokine assays were obtained from the tail vein before stroke (time 0), at reperfusion (time 1.5 hours), and at 6 hours, and from the right heart ventricle at 24 hours. Using isofluorane as anesthetic and after the final blood sample, animals were transcardially perfused with 0.9% saline followed by 4% paraformaldehyde (pH=7.4). Brains were removed, postfixed, cryoprotected in 30% sucrose and frozen at −20°C. Sections (30 μm) were cut on a sledge microtome (Bright series 8000; Bright Instruments, Huntingdon, UK) and stored at −20°C in antifreeze solution (30% ethylene glycol and 20% glycerol in phosphate-buffered saline) until processing.
Nissl staining was used to quantify the infarct volume on brain sections. Blood–brain barrier (BBB) damage was assessed by immunoglobin G immunostaining, as described previously (Greenhalgh et al, 2010). Double or triple immunofluorescence was performed on free-floating brain sections as previously described (Drake et al, 2011). The primary antibodies used were as follows: goat anti-rat metalloproteinase-9 (MMP-9; 1:100; R&D Systems, UK), rabbit anti-neutrophil serum (1:100; SJC; Anthony et al, 1998), rabbit anti-Iba1 (1:1000; Wako Chemicals, Germany), goat anti-Iba1 (1:1000; Abcam, Cambridge, UK), goat anti-chemokine (C-X-C motif) ligand (CXCL1; 1:100; R&D Systems, Abingdon, UK), rabbit polyclonal anti-IL-6 (1:100; Abcam). Biotinylated tomato lectin (1:100; Sigma-Aldrich, Poole, UK) was used to stain cerebral blood vessels. Antigens were visualized by using anti-goat or anti-rabbit fluorochrome-conjugated (Alexa Fluor 594 or Alexa Fluor 488, 1:500; Molecular Probes, Invitrogen, Paisley, UK) secondary antibodies and to visualize the lectin, streptavidin Alexa Fluor 350 conjugate, 1:500) was used. After mounting and applying coverslips, images from the sections were collected on an Olympus BX51 upright microscope and captured using a Coolsnap ES camera (Photometrics, Tucson, AZ, USA) through MetaVue Software (Molecular Devices, UK). Plasma cytokine levels were measured by cytometric bead array (R&D Systems; Denes et al, 2010a, 2010b).
Metalloproteinase-9/lectin-positive blood vessels, granulocytes, and Iba1/CXCL1-double-positive cells were counted in two and three random fields of view, respectively, inside the cortex, covering the infarct and penumbra, per section over three sections per animal (2 mm apart) covering the whole infarct in a rostro-caudal manner. Activated microglia were identified as showing: (1) increased Iba1 immunopositivity, (2) an enlarged and/or amenorrhea cell body, (3) complete or partial loss of thin, elongated processes. For Iba1/IL6-double-positive cells, all the sections per animal (14 sections) were analyzed.
Statistical Analysis
Data are expressed as mean±s.d. Statistical analysis was performed as follows: for two groups, Student's t-test (two-tailed), and for three or more groups, one-way or two-way analysis of variance followed by Bonferroni's post hoc multiple-comparison or paired comparison (n=6–8/experimental group).
Results
IL-1Ra Reduces Infarct Volume and BBB Breakdown in Aged Lean and Corpulent Rats
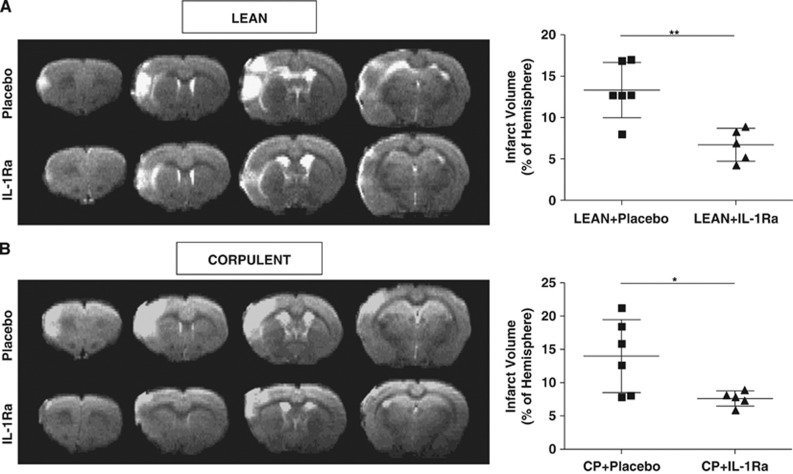
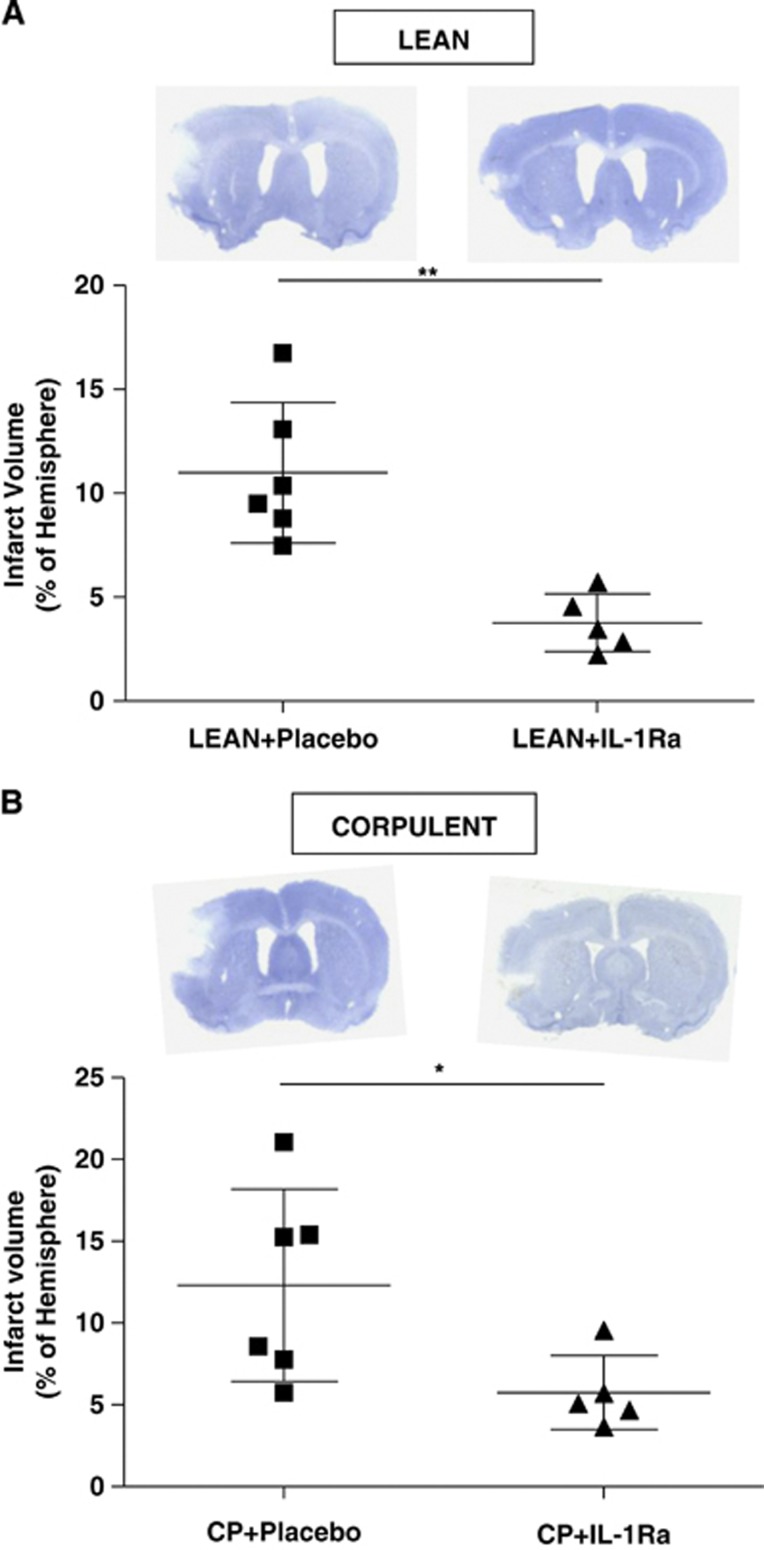
Interleukin-1Ra administration at reperfusion resulted in a significant (50%) reduction of infarct volume as measured by T2W images in lean and Cp when compared with placebo-treated animals (lean+placebo: 13.3%±3.3% versus lean+IL-1Ra: 6.7%±2% and Cp+placebo: 14%±5.5% versus Cp+IL-1Ra: 7.6%±1.1% P<0.05; Figures 1A and 1B) 23 hours after tMCAO. When measured by Nissl staining on post-mortem tissue at 24 hours after stroke, IL-1Ra significantly reduced infarct volume by 65% and 53% in leans and Cp, respectively (lean+placebo:11%±3.4% versus lean+IL-1Ra:3.7%±1.4% and Cp+placebo:12.3%±5.8% versus Cp+IL-1Ra:5.7%±2.3% P<0.05; Figures 2A and 2B), compared with placebo. Although ischemic brain damage was not different between Cp and lean animals (Figures 1 and 2), and despite changes in the inflammatory response in the brain due to IL-1Ra treatment (see below), of the inflammatory cytokines measured in plasma by cytometric bead array, only IL-6 was higher in Cp than lean rats before stroke (lean: 32.4±30.9 pg/ml versus Cp: 61.7±32 pg/ml; P<0.05), and none was affected by IL-1Ra.
Figure 1.
Effect of interleukin-1 receptor antagonist (IL-1Ra)/placebo on brain injury after transient occlusion of the middle cerebral artery (tMCAO), administered at reperfusion. Total infarct volume, expressed as percentage of ipsilateral hemisphere, was measured 23 hours after tMCAO on T2W images in lean (A) and in corpulent rats (Cp, B; n=6). *P<0.05; **P<0.01, Student's t-test.
Figure 2.
Effect of interleukin-1 receptor antagonist (IL-1Ra)/placebo on brain injury after transient occlusion of the middle cerebral artery (tMCAO), administered at reperfusion. Total infarct volume, expressed as percentage of ipsilateral hemisphere, was measured 24 hours after tMCAO in Nissl-stained brain sections in lean (A) and in corpulent rats (Cp, B; n=6). *P<0.05; **P<0.01, Student's t-test.
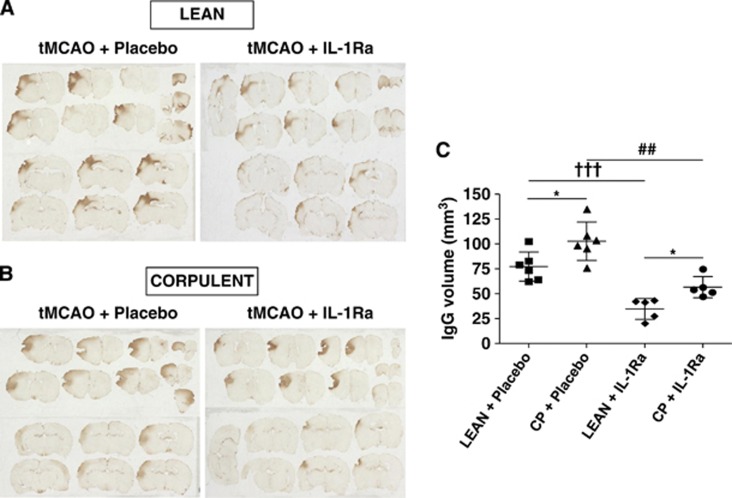
Interleukin-1Ra treatment significantly reduced immunoglobin G immunostaining in lean (55%) and Cp (45%) rats, compared with placebo, indicating a reduction of BBB damage (Figures 3A–C). Cp rats displayed significantly larger BBB damage compared with lean rats, both in placebo groups and with IL-1Ra treatment (Figure 3C).
Figure 3.
Effect of interleukin-1 receptor antagonist (IL-1Ra) on blood–brain barrier (BBB) damage after transient occlusion of the middle cerebral artery (tMCAO), administered at reperfusion. The volume of BBB damage was calculated on brain sections after immunoglobin G (IgG) staining 24 hours after tMCAO after administration of IL-1Ra/placebo to lean or corpulent (Cp) rats (A, B; n=6). *P<0.05 lean versus Cp with placebo or IL-1Ra, two-way analysis of variance with Bonferroni correction; †††P<0.001 lean+placebo versus lean+IL-1Ra; ##P<0.01 Cp+placebo versus Cp+IL-1Ra, Student's t-test (C).
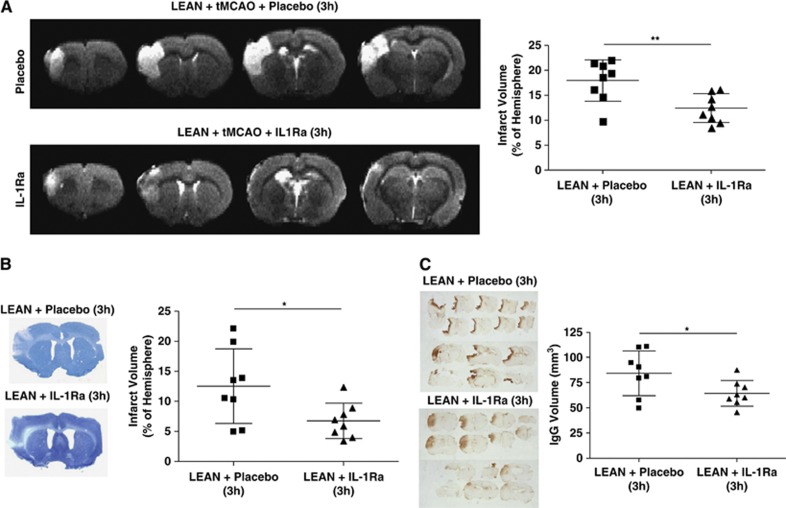
Delayed IL-1Ra administration in aged lean rats, starting 3 hours after the onset of reperfusion, induced a significant reduction of infarct volume (30%) as measured in T2W images (lean+placebo—3h: 18%±4% versus lean+IL-1Ra—3h: 12.4%±3% P<0.05; Figure 4A). When brain damage was measured by histology (Nissl-staining) 24 hours after stroke, also a significant reduction (46%) was observed in the IL-1Ra-treated group with delayed treatment (lean+placebo—3h: 12.5%±6.2% versus lean+IL-1Ra—3h: 6.7%±2.9% P<0.05; Figure 4B). Immunoglobin G staining revealed also a significant reduction in BBB damage (24%) in the delayed IL-1Ra-treated group when compared with the placebo group (Figure 4C).
Figure 4.
Effect of interleukin-1 receptor antagonist (IL-1Ra)/placebo on brain damage, administered at 3 hours of reperfusion to aged lean rats. Total infarct volume expressed as percentage of ipsilateral hemisphere and measured 23 hours after transient occlusion of the middle cerebral artery (tMCAO) on T2W images and 24 hours in Nissl-stained sections (A, B; n=8). Blood–brain barrier damage calculated on brain sections after immunoglobin G (IgG) staining 24 hours after tMCAO in aged leans treated with placebo or IL-1Ra (C; n=8). *P<0.05; **P<0.01, Student's t-test.
Interleukin-1Ra treatment did not improve functional outcomes in lean and Cp rats when administered at reperfusion, although a trend to improvement was observed in lean rats treated with IL-1Ra in both administration schemes (data not shown). It is important to note that due to the age and size of Cp rats, functional outcome measurements were difficult to perform.
Interleukin-1Ra Reduces Brain Inflammation, Microglial Activation, and Neutrophil Infiltration After Stroke in Comorbid Animals
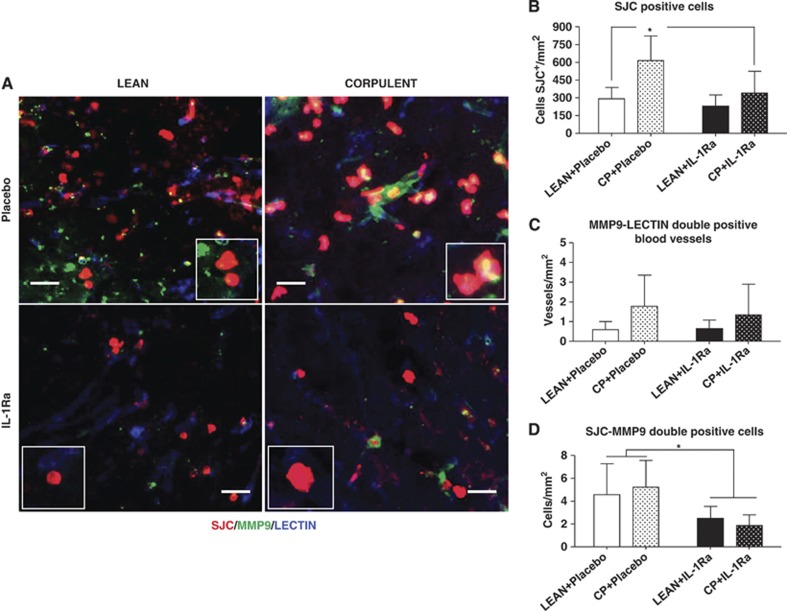
Immunofluorescence revealed that aged Cp rats had increased neutrophil infiltration in the brain after stroke compared with lean rats, which was reduced by IL-1Ra (Figures 5A and 5B). Although IL-1Ra treatment caused a significant reduction in the number of MMP-9-positive neutrophils when compared with placebo (Figure 5D), it did not change the number of MMP-9-positive blood vessels in either leans or Cp (Figure 5C).
Figure 5.
Effect of interleukin-1 receptor antagonist (IL-1Ra) on neutrophil infiltration and metalloproteinase-9 (MMP-9) expression in lean and corpulent (Cp) rats 24 hours after transient occlusion of the middle cerebral artery (tMCAO). (A, B) Number of neutrophils (SJC+ (red)); (A, C) number of blood vessels positive for MMP-9 (lectin+ (blue)/MMP-9+ (green)-double-positive vessels); and (A, D) SJC/MMP-9-double-positive cells (neutrophils) in the infarct and peri-infarct of lean and Cp rats receiving placebo or IL-1Ra. n=6/experimental group; *P<0.05, two-way analysis of variance with Bonferroni correction. Scale bar: 25 μm.
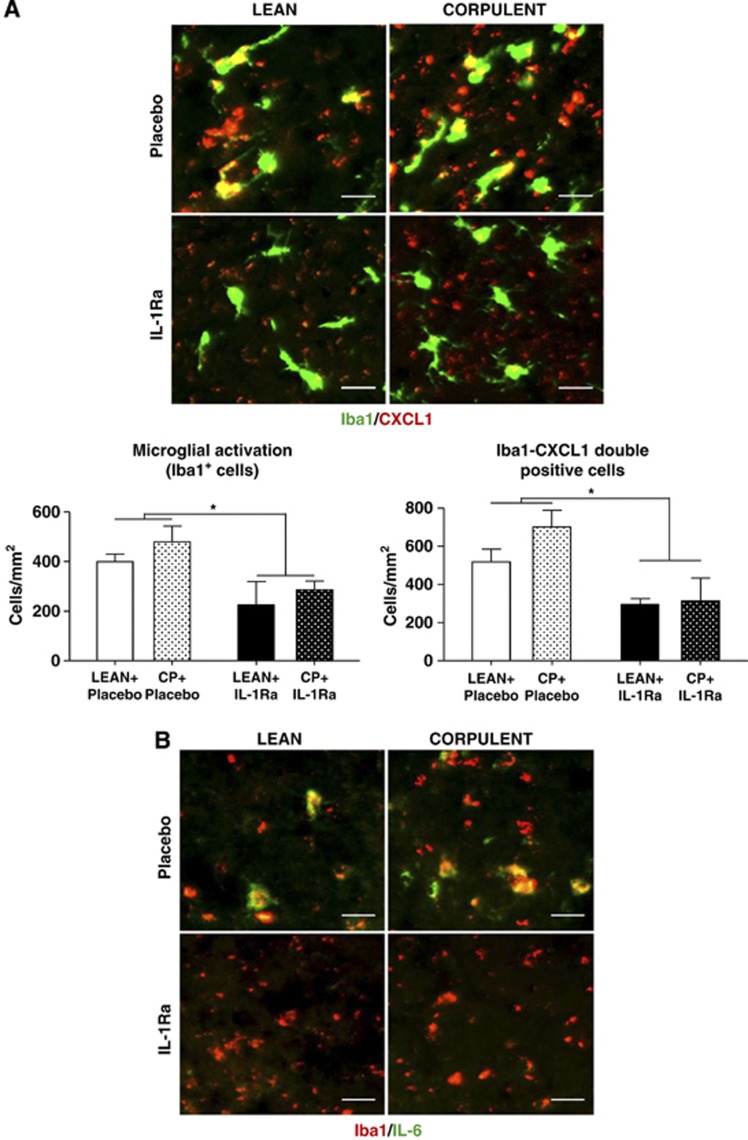
In both lean and Cp rats, IL-1Ra treatment resulted in a large (40%) reduction in microglial activation compared with the placebo-treated groups (Figure 6A). Immunofluorescence showed higher CXCL1 and IL-6 levels in the ipsilateral hemisphere in Cp than in lean rats. CXCL1 and IL-6 colocalized with Iba1-positive cells in the infarct and peri-infarct area and this immunoreactivity was reduced by IL-1Ra in Cp and lean animals (Figures 6A and 6B).
Figure 6.
Effect of interleukin-1 receptor antagonist (IL-1Ra) in CXCL1 and IL-6 expression and colocalization with microglia in lean and corpulent (Cp) rats 24 hours after transient occlusion of the middle cerebral artery (tMCAO). (A) Activated microglia as identified by increased Iba1 immunopositivity (green), thickened processes and irregular cell bodies and number of Iba1 (green)/CXCL1 (red)-double-positive cells (CXCL1+ microglial cells), and (B) microglial cells (Iba1+, red) expressing IL-6 (IL-6+, green) in the infarct and peri-infarct of lean and Cp rats. n=3/experimental group; *P<0.05, two-way analysis of variance with Bonferroni correction. Scale bar: 25 μm.
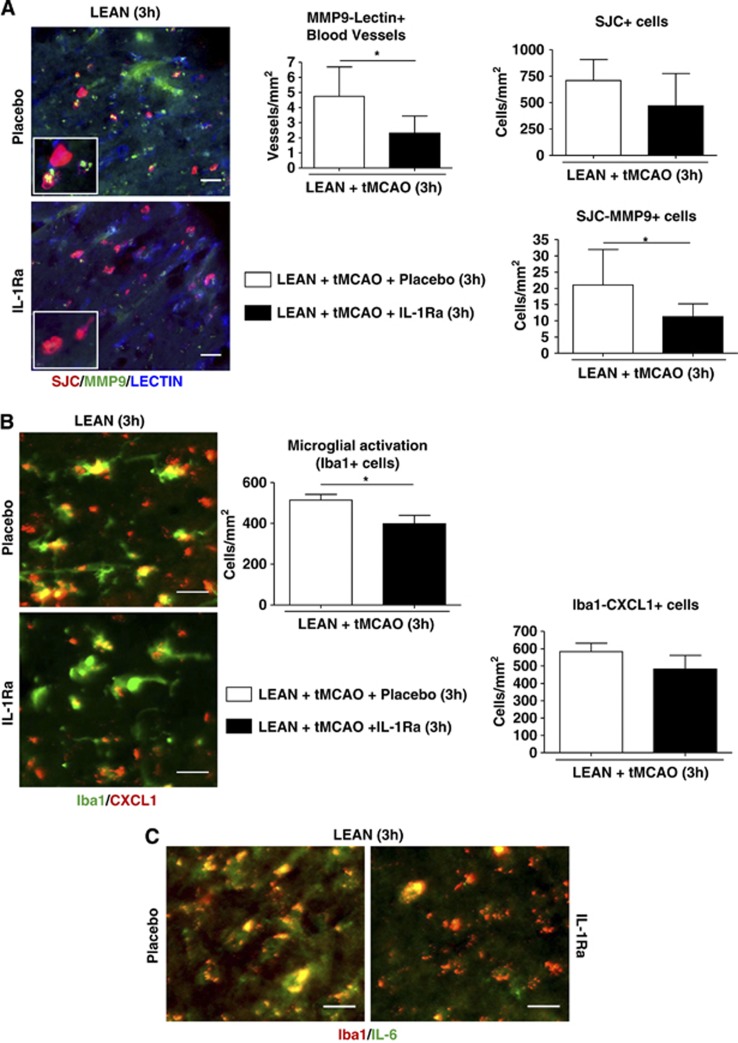
In 16-month-old lean rats, IL-1Ra administered at 3 hours of reperfusion significantly reduced the number of MMP-9-positive blood vessels and the number of MMP-9-positive neutrophils when compared with the placebo group, while neutrophil infiltration was unaffected (Figure 7A). Interleukin-1Ra also significantly reduced (22%) the number of activated Iba1-positive cells, although the number of Iba1-CXCL1-double-positive cells was not significantly decreased (Figure 7B). Delayed administration of IL-1Ra also reduced IL-6 expression in microglia (Figure 7C).
Figure 7.
Effect of delayed interleukin-1 receptor antagonist (IL-1Ra) treatment (3 hours of reperfusion) on neuroinflammatory changes in aged lean rats 24 hours after transient occlusion of the middle cerebral artery (tMCAO). (A) Number of blood vessels positive for metalloproteinase-9 (MMP-9; lectin+ (blue)/MMP-9+ (green)-double-positive vessels), total number of neutrophils SJC+ (red) and double SJC/MMP-9-positive cells (neutrophils) in aged lean rats treated with IL-1Ra or placebo. (B) Activated microglia identified by increased Iba1 immunopositivity (green), thickened processes and irregular cell bodies and double Iba1 (green)/CXCL1 (red)-positive cells (CXCL1+ microglial cells), and (C) microglial cells expressing IL-6 (Iba1+ (red)/IL-6+ (green)) in the infarct and peri-infarct of aged lean rats receiving IL-1Ra or placebo. n=3–8/experimental group; *P<0.05, Student's t-test. Scale bar: 25 μm.
Discussion
This study shows that subcutaneous administration of IL-1Ra is protective in aged animals with multiple risk factors for stroke, a key requirement in the most recent STAIR guidelines (Fisher et al, 2009) for the development of clinical candidates for the treatment of stroke. More specifically, our results show for the first time, that peripheral administration of IL-1Ra at time of reperfusion or 3 hours delayed reduces the ischemic brain damage in aged lean and Cp rats, as measured by MRI (T2W images), a clinically relevant primary outcome. The MRI outcome was confirmed by post-mortem histological analysis using Nissl staining, which is used widely in experimental stroke studies.
Our data also show that although 15-month-old Cp rats have multiple risk factors for stroke and evidence of increased systemic inflammation, infarct volume was similar to lean animals of around the same age. This is in contrast to previously published work that reports increased ischemic injury with chronic systemic infection or obesity (Denes et al, 2010a, 2010b; McColl et al, 2009; McColl et al, 2010). However, in our study it is likely that the extent of ischemic damage is close to maximal due to the duration of MCAO, thus no further increase was possible (Ginsberg and Busto, 1989; Mohamed et al, 1985). This is supported by our own unpublished observation that an increase in infarct volume is seen in lean rats between the ages of 13 and 15 months, with no further increase between 15 and 16 months of age.
It is well known that disruption of the BBB damage markedly influences the pathogenesis of stroke. Although no differences were observed in infarct volume between lean and Cp rats, the latter showed increased BBB damage after stroke. This difference in BBB damage may be explained by increased systemic and cerebrovascular inflammation in the presence of comorbidity (Drake et al, 2011). Indeed, peripheral IL-6 is implicated in atherosclerosis (Schuett et al, 2009), which, together with increased neuroinflammation, may lead to alterations in vascular permeability before stroke and/or influence BBB breakdown after stroke. This is supported by our recent data indicating that an acute systemic inflammatory challenge alters the kinetics of BBB disruption after experimental stroke, an effect that is both neutrophil and MMP-9 dependent (McColl et al, 2007; McColl et al, 2008), and by previous studies confirming the role of MMP-9 in BBB disruption and hemorrhagic transformation experimentally and clinically (Castellanos et al, 2003; Gasche et al, 1999; McColl et al, 2010). An important role for neutrophils and MMP-9 in ischemic injury in the presence of systemic inflammation is confirmed in this study, more neutrophils being seen in Cp rats. Importantly, IL-1Ra treatment reduces the expression of IL-6 in microglia, reduces the increase in neutrophils number, and the expression of neutrophil associated MMP-9, which may explain the reduction in BBB breakdown with IL-1Ra in lean and Cp animals. Our results also show a reduction in the expression of vascular- and neutrophil-associated MMP-9 and a reduction in BBB damage with a delayed administration of IL-1Ra (3 hours of reperfusion) in older lean animals. Increases in neutrophil infiltration after stroke could be driven by microglial-derived IL-6 and CXCL1 (Chapman et al, 2009; McColl et al, 2007; Rodriguez-Yanez and Castillo, 2008), both of which are increased by systemic inflammation. This is also demonstrated by our results, where aged and comorbid animals show higher expression of microglial-associated IL-6 and CXCL1 after stroke, an effect again reversed centrally but not peripherally by IL-1Ra in this study.
In this study, functional (sensori-motor) outcome was assessed before stroke, and at a single acute time-point poststroke, using conventional scales. Despite IL-1Ra treatment having previously been shown to improve stroke outcome using other models of stroke (Garcia et al, 1995), no significant effect of IL-1Ra was observed on behavior in either lean or Cp rats using this approach. It is important to note that the age and size of the Cp rats determined the experimental model (distal MCA occlusion) used to induce transient ischemia in this study, an approach that only produced damage in the somatosensory cortex with no injury in the basal ganglia (Chen et al, 1986). Hence, functional deficits may be minimal and/or acute assessment may not be the optimal approach. Therefore, future studies in comorbid animals and in female and/or male animals when possible will determine longer-term effects of IL-1Ra, with delayed treatment and with an exhaustive assessment of the outcome.
In summary, our results show that IL-1Ra protects against brain injury and reduces neuroinflammation when administered peripherally to aged and comorbid animals at reperfusion or 3 hours later. These findings address concerns raised in a recent systematic review on IL-1Ra in stroke (Banwell et al, 2009), and provide further supporting evidence for IL-1Ra as a lead candidate for the treatment of ischemic stroke.
Acknowledgments
The authors wish to thank Prof S Williams and K Davies (Imaging Sciences and Biomedical Engineering, University of Manchester) for their support in MRI scanning. Special thanks go to P March, J Kott and R Fernandez and the Bioimaging Facility within the Faculty of Life Sciences, and to Dr R Maroy (SHFJ – CEA Orsay, France) for his work on the development of the Anatomist/BrainVisa framework. Interleukin-1Ra was kindly provided by Amgen, USA.
NJR is a non-executive director of AstraZeneca, but there was no involvement of the company in these studies.
Footnotes
This work was funded by MRC and the European Union's 7th Framework Programme (FP7/2008-2013) under grant agreements no. 201024 and no. 202213 (European Stroke Network).
References
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- Anthony D, Dempster R, Fearn S, Clements J, Wells G, Perry VH, Walker K. CXC chemokines generate age-related increases in neutrophil-mediated brain inflammation and blood-brain barrier breakdown. Curr Biol. 1998;8:923–926. doi: 10.1016/s0960-9822(07)00373-9. [DOI] [PubMed] [Google Scholar]
- Banwell V, Sena ES, Macleod MR. Systematic review and stratified meta-analysis of the efficacy of interleukin-1 receptor antagonist in animal models of stroke. J Stroke Cerebrovasc Dis. 2009;18:269–276. doi: 10.1016/j.jstrokecerebrovasdis.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Calamante F, Lythgoe MF, Pell GS, Thomas DL, King MD, Busza AL, Sotak CH, Williams SR, Ordidge RJ, Gadian DG. Early changes in water diffusion, perfusion, T1, and T2 during focal cerebral ischemia in the rat studied at 8.5 T. Magn Reson Med. 1999;41:479–485. doi: 10.1002/(sici)1522-2594(199903)41:3<479::aid-mrm9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Castellanos M, Leira R, Serena J, Pumar JM, Lizasoain I, Castillo J, Davalos A. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke. 2003;34:40–46. [PubMed] [Google Scholar]
- Chapman KZ, Dale VQ, Denes A, Bennett G, Rothwell NJ, Allan SM, McColl BW. A rapid and transient peripheral inflammatory response precedes brain inflammation after experimental stroke. J Cereb Blood Flow Metab. 2009;29:1764–1768. doi: 10.1038/jcbfm.2009.113. [DOI] [PubMed] [Google Scholar]
- Chen ST, Hsu CY, Hogan EL, Maricq H, Balentine JD. A model of focal ischemic stroke in the rat: reproducible extensive cortical infarction. Stroke. 1986;17:738–743. doi: 10.1161/01.str.17.4.738. [DOI] [PubMed] [Google Scholar]
- Denes A, Ferenczi S, Halasz J, Kornyei Z, Kovacs KJ. Role of CX3CR1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. J Cereb Blood Flow Metab. 2008;28:1707–1721. doi: 10.1038/jcbfm.2008.64. [DOI] [PubMed] [Google Scholar]
- Denes A, Humphreys N, Lane TE, Grencis R, Rothwell N. Chronic systemic infection exacerbates ischemic brain damage via a CCL5 (regulated on activation, normal T-cell expressed and secreted)-mediated proinflammatory response in mice. J Neurosci. 2010a;30:10086–10095. doi: 10.1523/JNEUROSCI.1227-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denes A, Thornton P, Rothwell NJ, Allan SM. Inflammation and brain injury: acute cerebral ischaemia, peripheral and central inflammation. Brain Behav Immun. 2010b;24:708–723. doi: 10.1016/j.bbi.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Drake C, Boutin H, Jones MS, Denes A, McColl BW, Selvarajah JR, Hulme S, Georgiou RF, Hinz R, Gerhard A, Vail A, Prenant C, Julyan P, Maroy R, Brown G, Smigova A, Herholz K, Kassiou M, Crossman D, Francis S, Proctor SD, Russell JC, Hopkins SJ, Tyrrell PJ, Rothwell NJ, Allan SM. Brain inflammation is induced by co-morbidities and risk factors for stroke. Brain Behav Immun. 2011;25:1113–1122. doi: 10.1016/j.bbi.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley HC, Smith CJ, Georgiou RF, Vail A, Hopkins SJ, Rothwell NJ, Tyrrell PJ. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry. 2005;76:1366–1372. doi: 10.1136/jnnp.2004.054882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JH, Liu KF, Relton JK. Interleukin-1 receptor antagonist decreases the number of necrotic neurons in rats with middle cerebral artery occlusion. Am J Pathol. 1995;147:1477–1486. [PMC free article] [PubMed] [Google Scholar]
- Gasche Y, Fujimura M, Morita-Fujimura Y, Copin JC, Kawase M, Massengale J, Chan PH. Early appearance of activated matrix metalloproteinase-9 after focal cerebral ischemia in mice: a possible role in blood-brain barrier dysfunction. J Cereb Blood Flow Metab. 1999;19:1020–1028. doi: 10.1097/00004647-199909000-00010. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD, Busto R. Rodent models of cerebral ischemia. Stroke. 1989;20:1627–1642. doi: 10.1161/01.str.20.12.1627. [DOI] [PubMed] [Google Scholar]
- Greenhalgh AD, Galea J, Denes A, Tyrrell PJ, Rothwell NJ. Rapid brain penetration of interleukin-1 receptor antagonist in rat cerebral ischaemia: pharmacokinetics, distribution, protection. Br J Pharmacol. 2010;160:153–159. doi: 10.1111/j.1476-5381.2010.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum CH, Wilcox CJ, Arend WP, Joslin FG, Dripps DJ, Heimdal PL, Armes LG, Sommer A, Eisenberg SP, Thompson RC. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990;343:336–340. doi: 10.1038/343336a0. [DOI] [PubMed] [Google Scholar]
- Hennig J, Nauerth A, Friedburg H. RARE imaging: a fast imaging method for clinical MR. Magn Reson Med. 1986;3:823–833. doi: 10.1002/mrm.1910030602. [DOI] [PubMed] [Google Scholar]
- Hunter AJ, Hatcher J, Virley D, Nelson P, Irving E, Hadingham SJ, Parsons AA. Functional assessments in mice and rats after focal stroke. Neuropharmacology. 2000;39:806–816. doi: 10.1016/s0028-3908(99)00262-2. [DOI] [PubMed] [Google Scholar]
- Liu TH, Beckman JS, Freeman BA, Hogan EL, Hsu CY. Polyethylene glycol-conjugated superoxide dismutase and catalase reduce ischemic brain injury. Am J Physiol. 1989;256:H589–H593. doi: 10.1152/ajpheart.1989.256.2.H589. [DOI] [PubMed] [Google Scholar]
- Loddick SA, Rothwell NJ. Neuroprotective effects of human recombinant interleukin-1 receptor antagonist in focal cerebral ischaemia in the rat. J Cereb Blood Flow Metab. 1996;16:932–940. doi: 10.1097/00004647-199609000-00017. [DOI] [PubMed] [Google Scholar]
- Madrigal JL, Caso JR, de Cristobal J, Cardenas A, Leza JC, Lizasoain I, Lorenzo P, Moro MA. Effect of subacute and chronic immobilisation stress on the outcome of permanent focal cerebral ischaemia in rats. Brain Res. 2003;979:137–145. doi: 10.1016/s0006-8993(03)02892-0. [DOI] [PubMed] [Google Scholar]
- Mangat R, Su J, Scott PG, Russell JC, Vine DF, Proctor SD. Chylomicron and apoB48 metabolism in the JCR:LA corpulent rat, a model for the metabolic syndrome. Biochem Soc Trans. 2007;35:477–481. doi: 10.1042/BST0350477. [DOI] [PubMed] [Google Scholar]
- McColl BW, Rose N, Robson FH, Rothwell NJ, Lawrence CB. Increased brain microvascular MMP-9 and incidence of haemorrhagic transformation in obese mice after experimental stroke. J Cereb Blood Flow Metab. 2010;30:267–272. doi: 10.1038/jcbfm.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27:4403–4412. doi: 10.1523/JNEUROSCI.5376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl BW, Rothwell NJ, Allan SM. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci. 2008;28:9451–9462. doi: 10.1523/JNEUROSCI.2674-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl BW, Allan SM, Rothwell NJ. Systemic infection, inflammation and acute ischemic stroke. Neuroscience. 2009;158:1049–1061. doi: 10.1016/j.neuroscience.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Mohamed AA, Gotoh O, Graham DI, Osborne KA, McCulloch J, Mendelow AD, Teasdale GM, Harper AM. Effect of pretreatment with the calcium antagonist nimodipine on local cerebral blood flow and histopathology after middle cerebral artery occlusion. Ann Neurol. 1985;18:705–711. doi: 10.1002/ana.410180613. [DOI] [PubMed] [Google Scholar]
- Mulcahy NJ, Ross J, Rothwell NJ, Loddick SA. Delayed administration of interleukin-1 receptor antagonist protects against transient cerebral ischaemia in the rat. Br J Pharmacol. 2003;140:471–476. doi: 10.1038/sj.bjp.0705462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul SL, Srikanth VK, Thrift AG. The large and growing burden of stroke. Curr Drug Targets. 2007;8:786–793. doi: 10.2174/138945007781077418. [DOI] [PubMed] [Google Scholar]
- Pinteaux E, Rothwell NJ, Boutin H. Neuroprotective actions of endogenous interleukin-1 receptor antagonist (IL-1ra) are mediated by glia. Glia. 2006;53:551–556. doi: 10.1002/glia.20308. [DOI] [PubMed] [Google Scholar]
- STAIR Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- Relton JK, Martin D, Thompson RC, Russell DA. Peripheral administration of Interleukin-1 Receptor antagonist inhibits brain damage after focal cerebral ischemia in the rat. Exp Neurol. 1996;138:206–213. doi: 10.1006/exnr.1996.0059. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Yanez M, Castillo J. Role of inflammatory markers in brain ischemia. Curr Opin Neurol. 2008;21:353–357. doi: 10.1097/WCO.0b013e3282ffafbf. [DOI] [PubMed] [Google Scholar]
- Rothwell N. Interleukin-1 and neuronal injury: mechanisms, modification, and therapeutic potential. Brain Behav Immun. 2003;17:152–157. doi: 10.1016/s0889-1591(02)00098-3. [DOI] [PubMed] [Google Scholar]
- Schuett H, Luchtefeld M, Grothusen C, Grote K, Schieffer B. How much is too much? Interleukin-6 and its signalling in atherosclerosis. Thromb Haemost. 2009;102:215–222. doi: 10.1160/TH09-05-0297. [DOI] [PubMed] [Google Scholar]
- Simi A, Tsakiri N, Wang P, Rothwell NJ. Interleukin-1 and inflammatory neurodegeneration. Biochem Soc Trans. 2007;35:1122–1126. doi: 10.1042/BST0351122. [DOI] [PubMed] [Google Scholar]
- Stroemer RP, Rothwell NJ. Cortical protection by localized striatal injection of IL-1ra following cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1997;17:597–604. doi: 10.1097/00004647-199706000-00001. [DOI] [PubMed] [Google Scholar]
- Wegener S, Weber R, Ramos-Cabrer P, Uhlenkueken U, Sprenger C, Wiedermann D, Villringer A, Hoehn M. Temporal profile of T2-weighted MRI distinguishes between pannecrosis and selective neuronal death after transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 2006;26:38–47. doi: 10.1038/sj.jcbfm.9600166. [DOI] [PubMed] [Google Scholar]