Abstract
Single photon emission computed tomography (SPECT) is used widely in clinical studies. However, the technique requires image reconstruction and the methods for correcting scattered radiation and absorption are not standardized among SPECT procedures. Therefore, quantitation of cerebral blood flow (CBF) may not be constant across SPECT models. The quantitative SPECT (QSPECT) software package has been developed for standardization of CBF. Using the QSPECT/dual-table autoradiographic (DTARG) method, CBF and cerebral vascular reactivity (CVR) at rest and after acetazolamide challenge can be evaluated using 123I-iodoamphetamine in a single SPECT session. In this study, we examined the reproducibility of quantitative regional CBF and CVR in QSPECT/DTARG using different SPECT models at two facilities. The subjects were nine patients with chronic cerebral ischemic disease who underwent QSPECT/DTARG at both facilities with use of different γ-cameras and collimators. There were significant correlations for CBF at rest and after acetazolamide challenge measured at the two facilities. The consistency of the CBFs of the patients measured at the two facilities were good in all cases. Our results show that CBF measured by QSPECT/DTARG in the same patients is reproducible in different SPECT models. This indicates that standardized evaluation of CBF can be performed in large multicenter studies.
Keywords: cerebral blood flow, 123I-iodoamphetamine, quantitation, SPECT, vascular reactivity
Introduction
Several imaging modalities for measurement of cerebral circulation have been developed, including positron emission tomography (PET), single photon emission computed tomography (SPECT), perfusion CT, Xe-CT, perfusion magnetic resonance imaging, ultrasonography, and near-infrared spectroscopy. Among these techniques, 15O-PET is excellent in terms of quantitation and space resolution (Frackowiak et al, 1980) and can detect the severity of hemodynamic cerebral ischemia as the rise of the oxygen extraction fraction (OEF) (Yamauchi et al, 1999). An elevated OEF is defined as misery perfusion (Yamauchi et al, 1999), which may be a risk for recurrent stroke and a cause of selective neuronal damage in patients with major cerebral arterial occlusive disease. Therefore, evaluation of the severity of cerebral ischemia is important in predicting prognosis and determining the therapeutic strategy (Yamauchi et al, 2007). Ideally, all patients with ischemic cerebrovascular disease should be assessed by PET, but this is impractical because PET facilities are limited. In contrast, SPECT is more commonly available and has broader versatility than PET. Therefore, the use of SPECT for evaluation of the severity of cerebral ischemia could have significant benefits for more patients with ischemic cerebrovascular disorders.
Single photon emission computed tomography cannot be used to observe OEF directly, but quantitation of cerebral blood flow (CBF) using the N-isopropy1-[123I]p-iodoamphetamine (IMP) autoradiographic (ARG) method (Hatazawa et al, 1997; Iida et al, 1994, 1996) can be used to measure thresholds for CBF at rest, determine the cerebrovascular reserve, and detect misery perfusion (Hirano et al, 1994). Moreover, the development of the Dual-Table ARG (DTARG) method has made it possible to evaluate CBF at rest and to determine cerebral vascular reactivity (CVR) after acetazolamide challenge using 123I-IMP in a single day session (Kim et al, 2006). However, the reproducibility of these methods and standardization among different SPECT models have not been established. In particular, SPECT has a perceived problem that different models of γ-cameras and collimators may give different quantitative values. Therefore, standardization among models and ensuring reproducibility of measured values would be big steps towards utilizing the versatility of SPECT.
To address these issues, QSPECT (quantitative SPECT image reconstruction) has been developed as a program that automatically and accurately corrects attenuated absorption and scattered radiation (Iida et al, 1998). This program has been combined with DTARG to give the QSPECT/DTARG method (Iida et al, 2010; Kim et al, 2006). However, the reproducibility and errors of CBF and CVR measured in the same patients with different SPECT models using QSPECT/DTARG have not been evaluated. In this study, we retrospectively analyzed QSPECT/DTARG data for patients treated at Yamaguchi University Hospital (hereinafter referred to as institution Y) and its affiliated hospital, Ogori Daiichi General Hospital (institution O). These two institutions use different models of γ-cameras and collimators for SPECT, and this provided an opportunity for validation of the reproducibility and determination of the errors of the measured values.
Patients and methods
Patients
The study had a noninterventional, crosssectional design. The protocol was approved by the Yamaguchi University Hospital Institutional Review Board (No. H22-19) and followed the principles of the Declaration of Helsinki. All patient information was anonymized and protected. Among patients who received medical treatment at institution O from September 2005 to August 2009 and were diagnosed with chronic cerebral infarction with cerebrovascular stenosis and underwent QSPECT/DTARG, 25 patients required surgical revascularization and were referred to institution Y, at which QSPECT/DTARG was performed again. Among the 25 patients, 9 (five males and four females) had no differences in medications or clinical conditions at the times QSPECT/DTARG was performed at both facilities. The other 16 patients received additional administration of cilostazol, an antithrombotic agent, before the SPECT examination at institution Y. One of these patients was also treated with pioglitazone for diabetes. Cilostazol and pioglitazone may both increase CBF (Kai et al, 2011; Matsumoto et al, 2011; Sato et al, 2011). Therefore, the 16 patients who received these drugs before the second SPECT examination were excluded because the drugs might have affected the quantitative reproducibility in the study.
Values of CBF in the middle cerebral artery (MCA) territories at rest and after acetazolamide challenge measured at both facilities were examined in these nine patients.
Iodoamphetamine Single Photon Emission Computed Tomography Study Protocol
Procedures for QSPECT/DTARG (Iida et al, 2010) are shown in Figure 1. In institution Y, SPECT was performed with a three-headed ã-camera (GCA-9300A/PI; Toshiba Medical Systems, Tokyo, Japan) and a LESHR fan beam collimator (Toshiba Medical Systems). The energy range was centered at 160 keV with a width of 20%, and 2-minute rotation was performed 14 times in continuous mode. The matrix size was 64 × 64 pixels. Using the GMS-5500A/PI (Toshiba Medical Systems), fan beam data were converted to parallel data. In institution O, the SPECT machine has a two-headed ã-camera (E.CAM; Toshiba Medical Systems) and a LMEGP parallel collimator (Toshiba Medical Systems). The energy range was centered on 158 keV with a width of 20%, and 2-minute data collection per 180° was performed 14 times in continuous mode. The matrix size was 64 × 64 pixels.
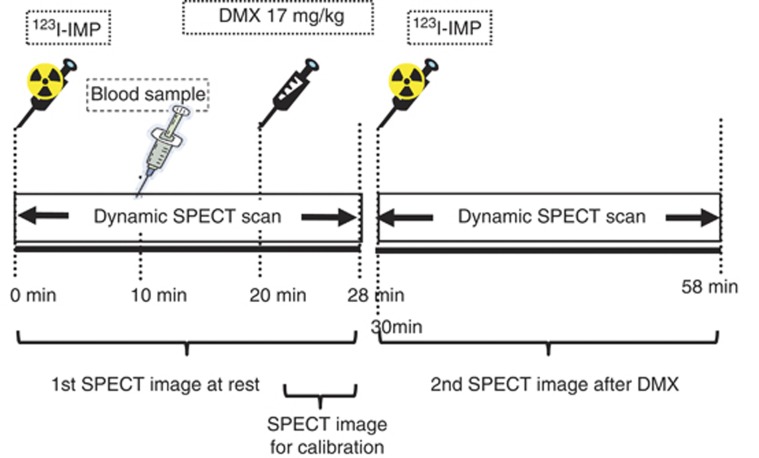
Figure 1.
Protocol of the quantitative single photon emission computed tomography/dual-table autoradiographic (QSPECT/DTARG) method. Iodoamphetamine (IMP) was initially intravenously administered for 1 minute and dynamic cerebral blood flow (CBF) SPECT was started simultaneously and continued for 58 minutes (2 minutes × 14 revolutions, two cycles). Ten minutes after IMP administration, arterial blood sampling was performed and an input function was obtained. After 20 minutes, acetazolamide (Diamox) (17 mg/kg) was intravenously administered for 1 minute. After 30 minutes, the same quantity of IMP as in the first administration was intravenously administered for 1 minute. CBF at rest was measured using data collected from 0 to 28 minutes. CBF after acetazolamide challenge was determined using data from 30 to 58 minutes.
In both facilities, an intravenous injection of two bottles of IMP (167 MBq each) was performed at an interval of 30 minutes, using a constant-rate infusion pump (TE-311; Termo, Tokyo, Japan) for 1 minute (Figure 1). Single photon emission computed tomography data collection was started at the same time as the intravenous injection. Dynamic SPECT scanning was performed for 28 minutes with one rotation of 2 minutes performed two times. The first scan lasted from 0 to 28 minutes and the second scan from 30 to 58 minutes, with each scan collecting data for seven frames of four minutes each. At 20 minutes after the first IMP intravenous injection, acetazolamide (Diamox) loading (17 mg/kg) was performed. At 10 minutes after the first IMP intravenous injection, 4 mL of arterial blood was obtained from the radial artery and the standard input function was calibrated. Whole blood radioactivity was measured with a well counter calibrated to the SPECT apparatus. The calibration between the well counter and the SPECT apparatus was performed using a pool phantom with a uniform diameter of 16 cm filled with ∼20 MBq of the 123I solution (height; 15 cm), with SPECT scanning using the same protocol as that for clinical testing. The radioactivity of the sampled solution was measured with the well counter used to measure arterial blood radioactivity in clinical testing. For the first and second scans, all frames were summed and images were reconstructed as described below. Gas measurements in the arterial blood were also performed.
Single Photon Emission Computed Tomography Image Reconstruction
Using data from both facilities, images were reconstructed and CBF quantitation was performed using the QSPECT image reconstruction package developed by Iida et al (2010) and Kim et al (2006). The objectives of QSPECT are to exclude variable factors that occur in SPECT imaging and image reconstruction, to minimize errors between facilities, and to improve quantitation of images. In this software, a series of programs for reconstruction and quantitation are consolidated. Quantitative SPECT extracts head outlines from projection data, makes μ maps, and performs correction of scatted radiation using transmission-dependent convolution subtraction. Transmission-dependent convolution subtraction uses the line spread function obtained from experiments to determine a relational expression among scattered radiation and absorption attenuation coefficient distribution, and the scattered radiation is corrected using this expression. By utilizing the μ map, attenuation correction with ordered subset expectation maximization is performed, the images are reconstructed, and quantitative images are made. These images show the distribution of radioactive concentrations as absolute values expressed as Bq per unit volume.
Single Photon Emission Computed Tomography Image Processing
Single photon emission computed tomography image processing was achieved with the SEE-JET (stereotactic extraction estimation based on the JET study) program (Mizumura et al, 2004), using quantitative image data obtained from the QSPECT/DTARG method at rest and after acetazolamide challenge with three-dimensional stereotactic surface projection (3D-SSP). In 3D-SSP, the image tilt of individual subjects is adjusted and linearly converted into 3D stereotaxic coordinates (Talairach standard brain) through the AC–PC line. After anatomic standardization, brain surface extraction is performed with the maximum pixel value shown in the vertical direction in the cerebral cortex from a prescribed brain surface on the stereotaxic coordinate system in the cortex of a standard brain (Minoshima et al, 1994). The SEE-JET program can also measure CBF values at rest and after acetazolamide challenge for the territories of the anterior cerebral artery, MCA, and posterior cerebral artery.
The SEE-JET can also automatically calculate the percentage vascular reserve (%VR) for all cerebral coordinate systems, and make ‘vascular reserve images'. %VR is defined as ([CBF after acetazolamide challenge−CBF at rest]/CBF at rest) × 100. The program can also classify the severity of hemodynamic brain ischemia for all cerebral coordinate systems into stage 0 to II based on %VR, as described by Nakagawara (1999) and Nakagawara et al (2000), and make cerebral surface ‘stage images' in 3D-SSP format. The stages are defined as follows: stage 0, resting CBF >15 mL per minute per 100 g and VR >30% stage I, 34 mL per minute per l00 g (80% of normal CBF) > resting CBF >15 mL per minute per l00 g and 30% > VR >10%, or CBF >34 mL per minute per l00 g and 30% > VR >−30% and stage II, 34 mL per minute per l00 g > resting CBF >15 mL per minute per l00 g and 10% > VR >−30% (Nakagawara, 1999; Nakagawara et al, 2000). Therefore, SEE-JET can perform objective, universal CBF assessment with automatic analysis that is independent of the operator. The SEE-JET was used on a Windows PC.
Data Analysis
In this study, we investigated CBF in the MCA territories by automatic calculation with SEE-JET. First, Wilcoxon signed-ranks tests were performed on three sets of data for the right and left MCA regions of each patient in institutions O and Y: (1) at rest, (2) after acetazolamide challenge, (3) %VR. Scatter diagrams and linear regression lines were calculated for each data set using Spearman correlation analysis to examine correlations between the two facilities. We also examined interobserver reliability for (1) to (3) using ICCs (intraclass correlation coefficients). Finally, the consistency of MCA CBF measured at the two facilities for each data set was evaluated using Bland–Altman plots (Bland and Altman, 1986). Differences in CBF and %VR were calculated as the value at institution O—that at institution Y. A difference with P<0.05 was considered significant.
Results
The subjects were five male and four female patients (59 to 78 years old, mean±s.d. 68.8±7.1 years old). Five of the patients had ischemic heart disease and one had chronic obstructive pulmonary disease. Four patients were current smokers. Cerebral blood flow in the MCA in each patient in the affected and left hemispheres extracted with SEE-JET, CBF after acetazolamide challenge, and %VR, %Stage II values in the right and left hemispheres are shown in Table 1. These data obtained at the two facilities were compared using Wilcoxon signed-ranks tests. This comparison gave values of P=0.34 at rest and P=0.48 after acetazolamide challenge for CBF in the right MCA territories (n=9); P=0.91 at rest and P=0.64 after acetazolamide challenge for CBF in the left MCA (n=9); P=0.93 at rest and P=0.93 after acetazolamide challenge for CBF in the right and left MCA (n=18); P=0.24 for %VR in the right MCA territories (n=9) and P=0.16 for %VR in the left MCA territories (n=9). Therefore, the absolute CBF values and %VR for each patient showed no significant differences between the two facilities.
Table 1. Patient characteristics, MCA CBF quantitation, and %stage II values using the SEE-JET program.
|
Institution O |
Institution Y |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of scanner: two-head, parallel-beam collimator |
Type of scanner: three-head, fan-beam collimator |
||||||||||||||||||||
|
Present disease |
CBF at rest |
CBF after ACZ |
%VRa |
%Stage IIb |
CBF at rest |
CBF after ACZ |
%VRa |
%Stage IIb |
|||||||||||||
| Case | Age | Gender | IHD | COPD | Smoking | Rt. MCA | Lt. MCA | Rt. MCA | Lt. MCA | Rt. MCA | Lt. MCA | Rt. MCA | Lt. MCA | Rt. MCA | Lt. MCA | Rt. MCA | Lt. MCA | Rt. MCA | Lt. MCA | Rt. MCA | Lt. MCA |
| 1 | 74 | F | Yes | Yes | — | 43.8 | 42.4 | 74.7 | 71.0 | 70.7 | 67.2 | 0.0 | 0.0 | 40.2 | 39.9 | 63.4 | 58.6 | 57.9 | 47.1 | 0.0 | 0.0 |
| 2 | 78 | M | Yes | — | Yes | 27.6 | 25.7 | 45.5 | 34.3 | 64.8 | 33.2 | 0.0 | 3.1 | 29.3 | 27.7 | 42.6 | 35.5 | 45.6 | 28.0 | 0.0 | 7.1 |
| 3 | 74 | F | — | — | — | 28.8 | 35.2 | 24.8 | 52.8 | −13.9 | 50.2 | 83.6 | 0.0 | 30.2 | 33.5 | 28.4 | 54.3 | −5.9 | 61.9 | 69.1 | 0.0 |
| 4 | 59 | F | — | — | Yes | 28.9 | 29.0 | 49.6 | 50.5 | 71.8 | 74.2 | 0.0 | 0.0 | 31.9 | 31.6 | 50.8 | 50.5 | 59.3 | 59.6 | 0.10 | 0.0 |
| 5 | 79 | M | — | — | — | 32.3 | 35.2 | 39.7 | 49.7 | 22.9 | 41.1 | 4.8 | 0.10 | 29.3 | 31.5 | 31.7 | 40.5 | 8.3 | 28.4 | 55.1 | 7.5 |
| 6 | 62 | M | Yes | — | Yes | 17.7 | 22.0 | 20.6 | 37.6 | 16.0 | 71.2 | 37.6 | 0.0 | 24.9 | 30.7 | 24.9 | 42.1 | 0.08 | 37.3 | 71.0 | 1.7 |
| 7 | 62 | F | — | — | Yes | 47.7 | 76.8 | 64.0 | 59.7 | 34.2 | −22.3 | 0.0 | 0.0 | 60.0 | 58.0 | 65.1 | 60.9 | 8.6 | 5.00 | 0.0 | 0.0 |
| 8 | 76 | M | Yes | — | — | 37.0 | 31.3 | 49.6 | 49.8 | 34.2 | 59.3 | 0.0 | 0.0 | 31.5 | 28.9 | 33.1 | 32.0 | 4.8 | 10.9 | 40.2 | 38.8 |
| 9 | 74 | M | Yes | — | — | 25.4 | 23.5 | 29.8 | 24.6 | 17.5 | 4.7 | 22.4 | 67.0 | 27.7 | 26.1 | 32.9 | 24.0 | 19.0 | −8.2 | 25.6 | 69.9 |
ACZ, acetazolamide; CBF, cerebral blood flow (mL per 100 g per minute); COPD, chronic obstructive pulmonary disease; IHD, ischemic heart disease; MCA, middle cerebral artery; SEE-JET, stereotactic extraction estimation based on the JET study; VR, vascular reserve.
%VR is defined as ([CBF after ACZ−CBF at rest]/CBF at rest) × 100.
Stage II is classified as severe hemodynamic brain ischemia. The %stage II value is the proportion of the Stage II area in the MCA territory.
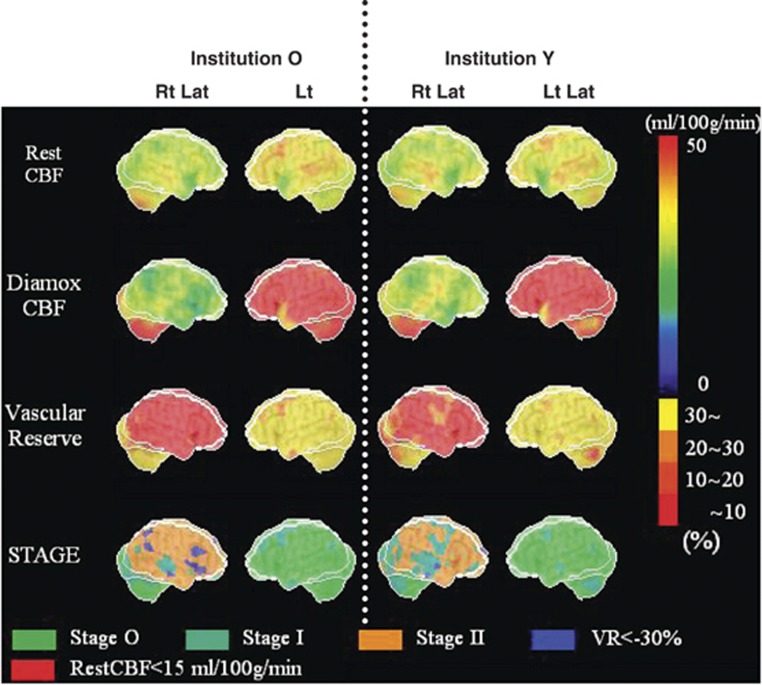
In Figure 2, three-dimensional cerebral surface CBF extracted images automatically displayed by SEE-JET are shown for data collected in both facilities for patient 3. This patient had symptoms of right internal carotid artery occlusion and the least CVR in the hemisphere among the nine patients. Images from this patient are shown as a representative case. Right and left hemisphere cortical images from both institutions are shown for CBF at rest, CBF after acetazolamide challenge, cerebrovascular reserve, and severity of hemodynamic cerebral ischemia. None of these images showed a major difference between the two facilities, even in a case in which almost all of the right hemisphere was without cerebrovascular reserve; that is, almost all of the hemisphere had hemodynamic brain ischemia.
Figure 2.
Stereotactic extraction estimation based on the JET study (SEE-JET) images of patient 3 obtained in institutions O and Y. Rt Lat and Lt Lat indicate right hemisphere and left hemisphere outer lateral images, respectively. Cerebral blood flow at rest (Rest CBF), CBF after acetazolamide challenge (Diamox CBF), cerebrovascular reserve (vascular reserve), and severity of hemodynamic cerebral ischemia (STAGE) are shown as three-dimensional cerebral surface images. Images in institutions O and Y are visually almost identical.
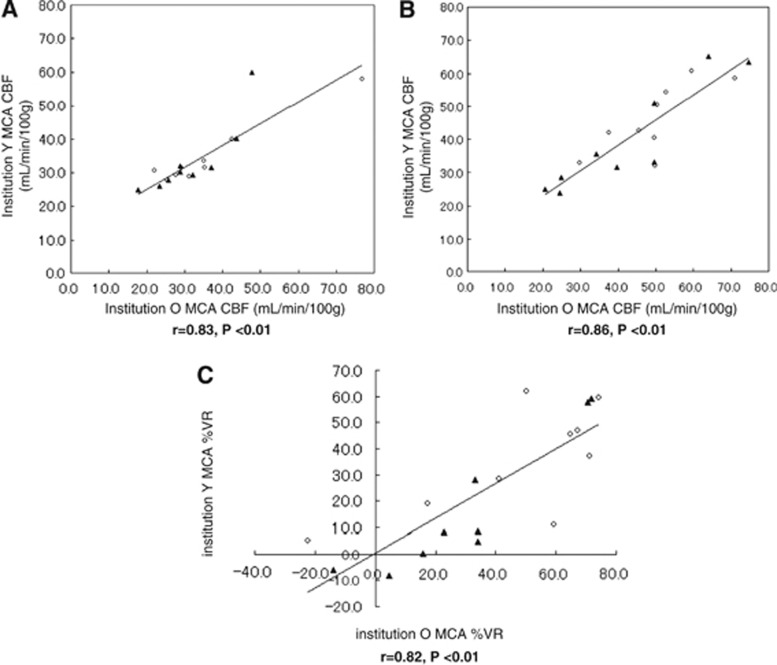
In Figure 3, scatter diagrams and linear regression lines comparing data between institutions are shown for MCA CBF in the affected and unaffected hemispheres at rest (Figure 3A), after acetazolamide challenge (Figure 3B), and for %VR (Figure 3C). In Spearman correlation analysis, all four comparisons showed significant correlations between the two facilities (at rest, r=0.83, P<0.01, n=18; after acetazolamide challenge, r=0.86, P<0.01, n=18; %VR, r=0.82, P<0.01, n=18). Regarding interobserver reliability, the ICCs were 0.847 (95% confidence interval (CI): 0.634 to 0.940) for CBF at rest, 0.860 (95% CI: 0.656 to 0.946) after acetazolamide, and 0.727 (95% CI: 0.276 to 0.899) at %VR.
Figure 3.
Scatter diagrams and regression lines of middle cerebral artery (MCA) cerebral blood flow (CBF) quantitation and percentage vascular reserve (%VR) in the two facilities. CBF quantitation and %VR in institutions O and Y are plotted on the x-axis and y-axis, respectively. Data are shown for the affected hemisphere (closed triangles) and the unaffected hemisphere (open circles). There were significant correlations between the two facilities for (A) CBF at rest (9 patients, n=18 data points, r=0.83, P<0.01) (B) CBF after acetazolamide challenge (9 patients, n=18, r=0.86, P<0.01), and (C) %VR for the affected and unaffected hemispheres (9 patients, n=18, r=0.82, P<0.01).
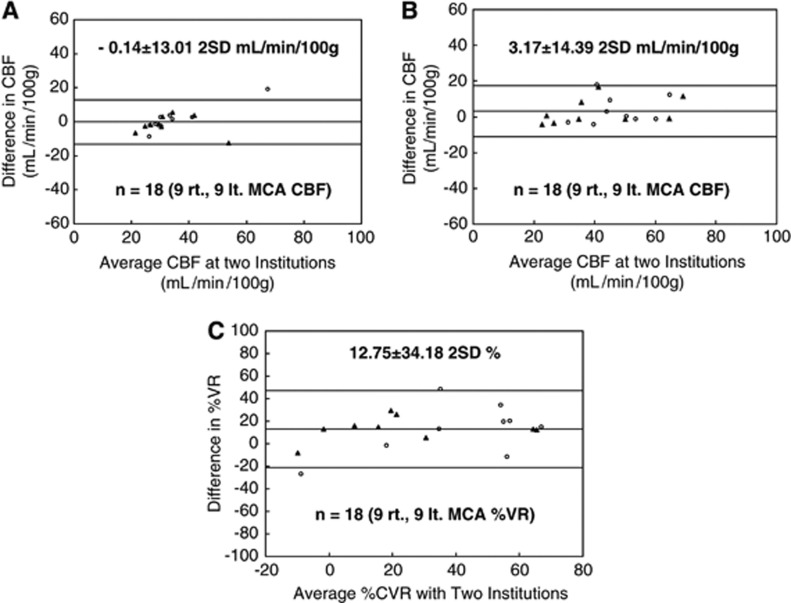
Bland–Altman plots showing the consistency among MCA CBF data measured in the two facilities are shown for MCA CBF at rest (Figure 4A), after acetazolamide challenge (Figure 4B), and for %VR (Figure 4C). For CBF at rest, the mean difference was −0.14 mL per minute per 100 g, the 2 s.d. value was 13.01 mL per minute per 100 g, and 1 of 18 data points was out of the 2 s.d. range. These respective numbers were 3.17 mL per minute per 100 g, 14.39 mL per minute per 100 g, and 1 of 18 data points out of the 2 s.d. range for CBF after acetazolamide challenge; and 12.75%, 34.18% and 2 of 18 data points out of the 2 s.d. range for %VR.
Figure 4.
Bland–Altman plots of the consistency of middle cerebral artery (MCA) cerebral blood flow (CBF) measured in the two facilities. Data are shown for the affected hemisphere (closed triangles) and the unaffected hemisphere (open circles). Differences in CBF and percentage vascular reserve (%VR) were calculated as the value at institution O—that at institution Y. (A) CBF at rest (9 patients, n=18 data points). A small bias was detected (mean difference, −0.14 mL per 100 g per minute) and the 2 s.d. was moderate (13.01 mL per 100 g per minute). (B) CBF after acetazolamide challenge (9 patients, n=18). A small bias was detected (mean difference, 3.17 mL per 100 g per minute) and the 2 s.d. was moderate (14.39 mL per 100 g per minute). (C) %VR for the right and left hemispheres (9 patients, n=18). A moderate bias was detected (mean difference, 12.75%) and the 2 s.d. was moderate (34.18%).
Discussion
Surgical treatment policies for ischemic cerebral diseases have been determined based on the degree of vascular narrowing, as shown in the North American Symptomatic Carotid Endarterectomy Trial study (North American Symptomatic Carotid Endarterectomy Trial Collaborators, 1991). Evaluation of cerebral perfusion has remained at the qualitative level based on relative comparison of the affected and unaffected hemispheres. In recent years, imaging methods such as IMP-ARG (Hatazawa et al, 1997; Iida et al, 1994, 1996) have been developed and quantitation of CBF has become possible in daily diagnosis. Findings for the relationship between misery perfusion and recurrent symptomatic cerebral infarction suggest that some patients with hemodynamic cerebral ischemia have an increased risk of recurrent ischemic stroke, and that these patients could be detected based on an increase in OEF (Yamauchi et al, 1999). Therefore, it is important to evaluate misery perfusion quantitatively to confirm the presence of hemodynamic cerebral ischemia, since this may contribute to prevention of recurrent ischemic stroke. To make further progress in this direction, techniques with broad versatility and standardized quantitation are required for large-scale studies.
Positron emission tomography can be used to assess the circulatory and metabolic states in the brain, but only a few facilities have installed PET systems and use of 15O is not common; thus, the versatility of PET is low. In contrast, SPECT is used in most facilities and has broad versatility, but differences in models of γ-cameras and collimators may cause large interinstitutional differences. The QSPECT/DTARG method was developed to resolve errors caused by differences in SPECT models, and we have used this method in our group since December 2004. Such an image reconstruction method with high accuracy and improved quantitation may be helpful for determination of the indication and judgment of the effects of treatment in ischemic cerebral diseases and other diseases. In a multicenter trial, Iida et al (2010) found good reproducibility of QSPECT/DTARG. Correction of errors between facilities is also theoretically possible using this method, but this has not been verified by comparison of data from different clinical sites.
In this study, we verified that CBF values measured in nine patients with a cerebral artery stenotic lesion showed reproducibility between facilities. That is, these data showed significant correlations between facilities (institutions Y and O) for CBF at rest (r=0.83, P<0.01), CBF after acetazolamide challenge (r=0.86, P<0.01), CBF at rest and after acetazolamide challenge (r=0.91, P<0.01), and %VR (r=0.82, P<0.01). Good interobserver reliability was obtained, based on respective ICCs of 0.847 (95% CI: 0.634 to 0.940), 0.860 (0.656 to 0.946), 0.872 (0.764 to 0.932), and 0.727 (0.276 to 0.899). A GCA-9300A/PI ã-camera with three detectors was used in institution Y, whereas an E.CAM ã-camera with two detectors was used in institution O, and the collimators also differed between the institutions. Despite these differences, strong correlations were found between data collected at the two facilities. This finding is important for performance of multicenter studies. It is also important that the test protocol is strictly defined, as shown in Figure 1, and that the timings of agent administration and blood collection are sufficiently standardized. However, a good correlation was observed in data between the two institutions, both of which followed the test protocol, but there was a tendency for CBF in the higher flow region to be lower at institution Y compared with institution O (Figures 3A and 3B). This might have happened because there was a minimum error when CBF of the same patient was determined with different γ-cameras and collimators using the QSPECT/DTARG method. A further limitation in the study may have been caused by the small sample size. In this study, we focused on data from MCA territories, but the results for the anterior cerebral artery and posterior cerebral artery also showed significant correlations (data not shown).
Good consistency of CBF was also obtained at the two facilities (Figure 4), but some measured values did fall outside the 2 s.d. range. These included one data point for CBF at rest (patient 7, Figure 4A), one after acetazolamide challenge (patient 8, Figure 4B), and two for %VR (patients 7 and 8, Figure 4C). In addition, as seen in Table 1, %VR for the right MCA in cases 7 and 8 at institution Y was significantly lower than that at institution O. In this study, there were no changes in the progress of symptoms and drug administration during the study period for all patients in the two facilities. Furthermore, there were no technical errors in performance of SPECT, time of drug administration, dosage, and leakage in injection. The differences in %VR for patients 7 and 8 suggest a progressive disease phase from the standpoint of cerebral circulation, despite no apparent clinical aggravation. As described above, there were two factors that might have caused the large 2 s.d. range in Figure 4C: the data for Cases 7 and 8 differed significantly between institutions O and Y (Table 1); and the results at institution Y seemed to be slightly lower than those at institution O (Figures 3A to 3C). It was difficult to eliminate intrinsic limitations such as aggravation of cerebral circulation and the reserve of the cerebral circulation because of the retrospective nature of the study. A prospective study with more subjects and a defined observation period is required to confirm the findings of this study.
The QSPECT/DTARG results suggest that this method can be used for objective evaluation as an indication for treatment of ischemic cerebral diseases. In addition, since the reproducibility is high, the method can be applied for observation of time-dependent changes in the same patient. In current medicine, ‘standardization' has become important. Standardization of SPECT diagnosis of CBF is important to establish standard therapeutic policies for stroke prevention. This will be facilitated by improved accuracy of quantitative measurements using techniques such as QSPECT/DTARG and of diagnosis of severity using stereotaxic and quantitative image analysis such as SEE analysis. In the current study, CBF assessment with QSPECT/DTARG was significantly correlated between facilities and showed good reproducibility. This method may enable accurate determination of CBF and cerebrovascular reserve capacity at any institution, with standardization of the therapeutic index of patients with ischemic cerebral disease in terms of cerebral circulation.
Acknowledgments
The authors are grateful for excellent technical assistance to Hideyuki Iwanaga and Yona Oishi.
The authors declare no conflict of interest.
References
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Frackowiak RS, Lenzi GL, Jones T, Heather JD. Quantitative measurement of regional cerebral blood flow and oxygen metabolism in man using 15O and positron emission tomography: theory, procedure, and normal values. J Comput Assist Tomogr. 1980;4:727–736. doi: 10.1097/00004728-198012000-00001. [DOI] [PubMed] [Google Scholar]
- Hatazawa J, Iida H, Shimosegawa E, Sato T, Murakami M, Miura Y. Regional cerebral blood flow measurement with iodine-123-IMP autoradiography: normal values, reproducibility and sensitivity to hypoperfusion. J Nucl Med. 1997;38:1102–1108. [PubMed] [Google Scholar]
- Hirano T, Minematsu K, Hasegawa Y, Tanaka Y, Hayashida K, Yamaguchi T. Acetazolamide reactivity on 123I-IMP single photon emission computed tomography in patients with major cerebral artery occlusive disease: correlation with positron emission tomography parameters. J Cereb Blood Flow Metab. 1994;14:763–770. doi: 10.1038/jcbfm.1994.97. [DOI] [PubMed] [Google Scholar]
- Iida H, Akutsu T, Endo K, Fukuda H, Inoue T, Ito H, Koga S, Komatani A, Kuwabara Y, Momose T, Nishizawa S, Odano I, Ohkubo M, Sasaki Y, Suzuki H, Tanada S, Toyama H, Yonekura Y, Yoshida T, Uemura K. A multicenter validation of regional cerebral blood flow quantitation using [123I]iodoamphetamine and single photon emission computed tomography. J Cereb Blood Flow Metab. 1996;16:781–793. doi: 10.1097/00004647-199609000-00003. [DOI] [PubMed] [Google Scholar]
- Iida H, Itoh H, Nakazawa M, Hatazawa J, Nishimura H, Onishi Y, Uemura K. Quantitative mapping of regional cerebral blood flow using iodine-123-IMP and SPECT. J Nucl Med. 1994;35:2019–2030. [PubMed] [Google Scholar]
- Iida H, Nakagawara J, Hayashida K, Fukushima K, Watabe H, Koshino K, Zeniya T, Eberl S. Multicenter evaluation of a standardized protocol for rest and acetazolamide CBF assessment using quantitative SPECT reconstruction program and split-dose 123I-IMP. J Nucl Med. 2010;51:1624–1631. doi: 10.2967/jnumed.110.078352. [DOI] [PubMed] [Google Scholar]
- Iida H, Narita Y, Kado H, Kashikura A, Sugawara S, Shoji Y, Kinoshita T, Ogawa T, Eberl S. Effects of scatter and attenuation correction on quantitative assessment of regional cerebral blood flow with SPECT. J Nucl Med. 1998;39:181–189. [PubMed] [Google Scholar]
- Kai Y, Watanabe M, Morioka M, Hirano T, Yano S, Ohmori Y, Kawano T, Hamada J, Kuratsu J. Cilostazol improves symptomatic intracranial artery stenosis -- evaluation of cerebral blood flow with single photon emission computed tomography. Surg Neurol Int. 2011;2:8. doi: 10.4103/2152-7806.76145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KM, Watabe H, Hayashi T, Hayashida K, Katafuchi T, Enomoto N, Ogura T, Shidahara M, Takikawa S, Eberl S, Nakazawa M, Iida H. Quantitative mapping of basal and vasoreactive cerebral blood flow using split-dose 123I-iodoamphetamine and single photon emission computed tomography. Neuroimage. 2006;33:1126–1135. doi: 10.1016/j.neuroimage.2006.06.064. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Shimodozono M, Miyata R, Kawahira K. Effect of cilostazol administration on cerebral hemodynamics and rehabilitation outcomes in poststroke patients. Int J Neurosci. 2011;121:271–278. doi: 10.3109/00207454.2010.551431. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Koeppe RA, Frey KA, Kuhl DE. Anatomic standardization: linear scaling and nonlinear warping of functional brain images. J Nucl Med. 1994;35:1528–1537. [PubMed] [Google Scholar]
- Mizumura S, Nakagawara J, Takahashi M, Kumita S, Cho K, Nakajo H, Toba M, Kumazaki T. Three-dimensional display in staging hemodynamic brain ischemia for JET study: objective evaluation using SEE analysis and 3D-SSP display. Ann Nucl Med. 2004;18:13–21. doi: 10.1007/BF02985609. [DOI] [PubMed] [Google Scholar]
- Nakagawara J. Clinical neuroimaging of cerebral ischemia. No To Shinkei. 1999;51:502–513. [PubMed] [Google Scholar]
- Nakagawara J, Hyogo T, Kataoka T, Hayase K, Kasuya J, Kamiyama K. Role of neuroimaging (SPECT/PET, CT/MRI) in thrombolytic therapy. No To Shinkei. 2000;52:873–882. [PubMed] [Google Scholar]
- North American Symptomatic Carotid Endarterectomy Trial Collaborators Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T. Efficacy of PPAR-γ agonist pioglitazone in mild Alzheimer disease. Neurobiol Aging. 2011;32:1626–1633. doi: 10.1016/j.neurobiolaging.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Yamauchi H, Fukuyama H, Nagahama Y, Nabatame H, Ueno M, Nishizawa S, Konishi J, Shio H. Significance of increased oxygen extraction fraction in five-year prognosis of major cerebral arterial occlusive disease. J Nucl Med. 1999;40:1992–1998. [PubMed] [Google Scholar]
- Yamauchi H, Kudoh T, Kishibe Y, Iwasaki J, Kagawa S. Selective neuronal damage and chronic hemodynamic cerebral ischemia. Ann Neurol. 2007;61:454–465. doi: 10.1002/ana.21104. [DOI] [PubMed] [Google Scholar]