Dear Editor,
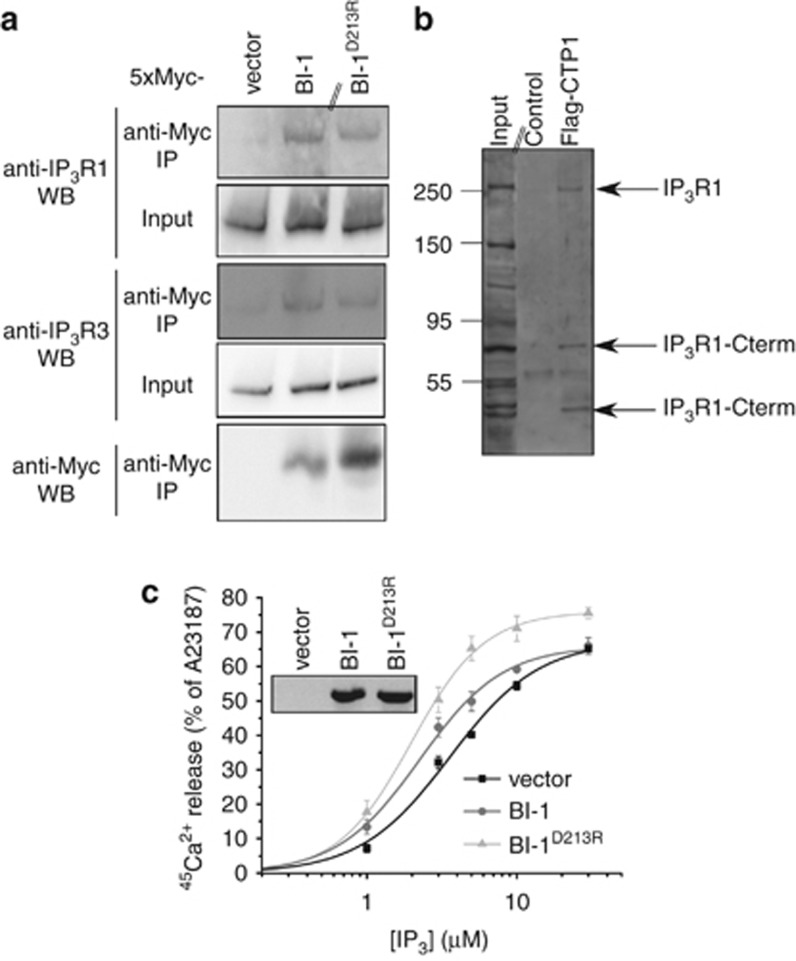
Bax Inhibitor-1 (BI-1) is an evolutionary conserved endoplasmic reticulum (ER)-located protein that protects against ER stress-induced apoptosis.1 This function has been closely related to its ability to permeate Ca2+ from the ER2 and to lower the steady-state [Ca2+]ER.3 BI-1 may function as an H+/Ca2+-antiporter2 or Ca2+ channel.4 Recently, BI-1 was proposed as a negative regulator of autophagy through IRE1α.5 However, recent findings indicate that BI-1 may promote autophagy.6 The latter required the presence of the inositol 1,4,5-trisphosphate (IP3) receptor (IP3R). The observations were explained through BI-1-enhanced IP3R activity, which lowered steady-state [Ca2+]ER, reducing ER-mitochondrial Ca2+ transfer and decreasing mitochondrial bio-energetics.7 However, direct evidence that BI-1 binds to IP3Rs and sensitizes IP3-induced Ca2+ release (IICR) is lacking. Therefore, we studied the regulation of IP3R function by BI-1 (see Supplementary Information for Methods). We constructed a 5xMyc-BI-1-expression plasmid, allowing the detection and purification of ectopically expressed BI-1 from transfected HeLa cells using anti-Myc-agarose beads (Figure 1a). Using isoform-specific IP3R antibodies, we demonstrated the co-immunoprecipitation of IP3R1 and IP3R3 with 5xMyc-BI-1 from HeLa cell lysates. Next, we screened for the subdomain of BI-1 responsible for IP3R interaction. We found that a synthetic Flag-tagged peptide containing BI-1's Ca2+-channel pore domain (CTP1; amino acids 198–217 of human BI-1) interacted with IP3R1 (Figure 1b). Lysates not exposed to Flag-CTP1 served as negative control. Moreover, proteolytic fragments of the IP3R containing its C terminus (indicated as IP3R1-Cterm in Figure 1b) were immunoprecipitated with Flag-CTP1. These C-terminal fragments were recognized by our antibody (Rbt03) that has its epitope in the last 15 C-terminal amino acids of the IP3R1.8 These fragments include the Ca2+-channel pore of the IP3R1, indicating that the Ca2+-channel pore domain of BI-1 interacted with the Ca2+-channel pore domain of IP3R1. Next, we examined the effect of BI-1 on IP3R function. Therefore, we used BI-1−/− mouse embryonic fibroblasts (MEF) and stably and ectopically overexpressed either empty vector (RFP-only), wild-type BI-1 or BI-1D213R with a bi-cistronic C-terminal IRES-RFP reporter. BI-1D213R is a mutant, in which the Asp213 critical for BI-1-mediated Ca2+ flux is altered into an Arg and which fails to lower [Ca2+]ER.4 BI-1-mRNA expression was detected using specific primers, and similar expression levels were found for wild-type BI-1 and BI-1D213R, while no signal was observed in vector-expressing BI-1−/− MEF cells (inset Figure 1c). Wild-type BI-1, but not BI-1D213R, overexpression significantly improved cell survival after thapsigargin exposure, an irreversible SERCA inhibitor, which kills cells through ER stress (empty vector: 33.65±4.48% wild-type BI-1: 44.39±5.31%* BI-1D213R: 34.14±4.19% surviving cells after 48 h, 20 nM thapsigargin normalized to vehicle-treated cells expressing empty vector. Mean±S.E.M. of four pooled experiments done in triplicates is shown, *P<0.05 Student's t-test). These data indicate that BI-1's Ca2+-flux properties are essential for BI-1's anti-apoptotic function. Next, we analyzed the direct effect of ectopically expressed BI-1 on IP3R function in the absence of endogenous BI-1 (Figure 1c). We used a unidirectional 45Ca2+-flux assay in saponin-permeabilized BI-1−/− MEF cells, allowing direct ER access and an accurate analysis of IP3R function in the absence of plasmalemmal Ca2+ fluxes, SERCA activity or mitochondrial Ca2+ uptake.8 Cells ectopically overexpressing BI-1 displayed a sensitized IICR and concomitant decrease in EC50 from 3.57 μM to 2.25 μM IP3. To exclude that Ca2+ flux mediated by BI-1 indirectly sensitized IP3Rs through Ca2+-induced Ca2+ release, we examined the effect of BI-1D213R overexpression on IP3R function. BI-1D213R also sensitized IICR and concomitantly decreased the EC50 from 3.57 μM to 1.98 μM IP3. This correlates with the ability of BI-1D213R to co-immunoprecipitate with IP3Rs (Figure 1a). Collectively, these data indicate a direct sensitizing effect of BI-1 on IP3Rs, which may contribute to a decrease in steady-state [Ca2+]ER and mitochondrial bioenergetics and subsequent induction of basal autophagy.
Figure 1.
(a) Interaction of 5xMyc-BI-1 and 5xMyc-BI-1D213R with IP3R channels. BI-1 and BI-1D213R were expressed as 5xMyc-tagged fusion proteins. The empty 5xMyc vector was used as negative control. The vectors were transfected into HeLa cells for 2 days allowing the expression of the 5xMyc-tagged proteins. Cell lysates were prepared using 1% CHAPS buffer and the overexpressed 5xMyc-tagged proteins were purified using anti-Myc-agarose beads (Sigma, Saint Louis, MO, USA). After washing the beads three times with CHAPS buffer, proteins were eluted using urea sample buffer and the immunoprecipitated samples were analyzed via SDS-PAGE and western blotting analysis (antibodies used for immunoblotting are indicated above WB). The double line on the western blot indicates that lanes from another part of the same gel and exposure time were merged. Using this immunoprecipitation approach, we found that both IP3R1 and IP3R3 co-immunoprecipitated with 5xMyc-BI-1 and 5xMyc-BI-1D213R, but not with 5xMyc vector. (b) Residues 198–217 of human BI-1 were synthesized as a Flag-tagged peptide (Flag-CTP1), which was applied in co-immunoprecipitation experiments using anti-Flag-M2-agarose beads (Sigma) and cell lysates from DT40 triple-IP3R knockout cells ectopically expressing IP3R1. The double line on the western blot indicates that lanes from another part of the same gel and same exposure time were merged. From the western blot analysis using the Rbt03 anti-IP3R1 C-terminal antibody, it is clear that full-length IP3R1 as well as C-terminal fragments interacted with Flag-CTP1 (indicated as IP3R1-Cterm). The numbers indicate Mw markers in kilodalton. (c) Inset is an RT-PCR showing similar mRNA-expression levels of ectopically expressed BI-1 and BI-1D213R in BI-1−/− MEF cells using a bi-cistronic C-terminal IRES-RFP reporter as vector. The main panel shows the results obtained from unidirectional 45Ca2+-flux assays in saponin-permeabilized BI-1−/− MEF cells comparing IP3-induced Ca2+ release between vector-expressing, BI-1-expressing and BI-1D213R-expressing cells, indicating IP3R sensitization by BI-1 independent of BI-1's Ca2+-flux properties. For analysis, cells were grown to the same density to perform an accurate comparison of the IP3-induced Ca2+-release responses between the different cell lines. Data represent mean±S.E.M. from three to five independent experiments using two replicates
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft Grant ME1922/9-1 and the Forschungskommission of the Heinrich-Heine University Dusseldorf (to AM), by Research Foundation-Flanders (FWO) grants G.0604.07N (to HDS), G.0788.11N (to GB) and G.0724.09 (to LM), by the Research Council of the KU Leuven via the Concerted Actions program (GOA/09/012) and an OT START (STRT/10/044) and by Interuniversity Attraction Poles Program Belgian Science Policy P6/28 (to HDS, JBP and LM). We thank Dr. JC Reed (Sanford-Burnham Medical Research Institute, La Jolla, CA) for providing the BI-1−/− mice.
The authors declare no conflict of interest
Footnotes
Supplementary Information accompanies the paper on Cell Death and Disease website (http://www.nature.com/cddis)
Supplementary Material
References
- Xu Q, Reed JC. Mol Cell. 1998. pp. 337–346. [DOI] [PubMed]
- Kim HR, et al. J Biol Chem. 2008. pp. 15946–15955. [DOI] [PMC free article] [PubMed]
- Westphalen BC, et al. Cell Death Differ. 2005. pp. 304–306. [DOI] [PubMed]
- Bultynck G, et al. J Biol Chem. 2012. pp. 2544–2557. [DOI] [PMC free article] [PubMed]
- Castillo K, et al. EMBO J. 2011. pp. 4465–4478. [DOI] [PMC free article] [PubMed]
- Sano R, et al. Genes Dev. 2012. pp. 1041–1054. [DOI] [PMC free article] [PubMed]
- Cardenas C, et al. Cell. 2010. pp. 270–283. [DOI] [PMC free article] [PubMed]
- Bultynck G, et al. Biochem J. 2004. pp. 87–96. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.