Abstract
The novel anticancer drug ABT-737 is a Bcl-2 Homology 3 (BH3)-mimetic that induces apoptosis by inhibiting pro-survival Bcl-2 proteins. ABT-737 binds with equal affinity to Bcl-2, Bcl-xL and Bcl-w in vitro and is expected to overrule apoptosis resistance mediated by these Bcl-2 proteins in equal measure. We have profiled ABT-737 specificity for all six pro-survival Bcl-2 proteins, in p53 wild-type or p53-mutant human T-leukemic cells. Bcl-B was untargeted, like Bfl-1 and Mcl-1, in accord with their low affinity for ABT-737 in vitro. However, Bcl-2 proved a better ABT-737 target than Bcl-xL and Bcl-w. This was reflected in differential apoptosis-sensitivity to ABT-737 alone, or combined with etoposide. ABT-737 was not equally effective in displacing BH3-only proteins or Bax from Bcl-2, as compared with Bcl-xL or Bcl-w, offering an explanation for the differential ABT-737 sensitivity of tumor cells overexpressing these proteins. Inducible expression demonstrated that BH3-only proteins Noxa, but not Bim, Puma or truncated Bid could overrule ABT-737 resistance conferred by Bcl-B, Bfl-1 or Mcl-1. These data identify Bcl-B, Bfl-1 and Mcl-1, but also Bcl-xL and Bcl-w as potential mediators of ABT-737 resistance and indicate that target proteins can be differentially sensitive to BH3-mimetics, depending on the pro-apoptotic Bcl-2 proteins they are complexed with.
Keywords: ABT-737, Bcl-2, Leukemia, resistance, apoptosis
In many tumors, overexpression of pro-survival Bcl-2 proteins contributes to apoptosis resistance.1, 2 The Bcl-2 protein family controls mitochondrial outer membrane permeabilization (MOMP) and the ensuing caspase activation. Bcl-2 proteins are therefore critical regulators of the apoptotic response. The family comprises the pro-apoptotic Bcl-2 Homology 3 (BH3) domain-only proteins, Bax, Bak, and the pro-survival proteins Bcl-2, Bcl-xL, Bcl-w, Bfl-1, Mcl-1 and Bcl-B.1, 2 Bax and Bak are the effector proteins that, upon their activation, bring about MOMP by forming large homomultimeric pores. The activity of Bax and Bak is constrained by the pro-survival Bcl-2 proteins that prevent their homomultimerization. In response to apoptotic stimuli, BH3-only proteins enable the effector function of Bax and Bak. It is thought that the BH3-only proteins Bid, Bim and possibly also Puma can activate Bax and Bak by direct interaction. Other BH3-only proteins, like Noxa and Bad, do this by liberating activated Bax and Bak from their pro-survival counterparts. They can also act more indirectly, by liberating other BH3-only proteins from pro-survival Bcl-2 proteins, allowing these to activate Bax and Bak.1, 2, 3, 4, 5, 6 The type of BH3-only protein that is mobilized depends on the stimulus and the cell type, but multiple BH3-only proteins can respond to a single stimulus in one cell.7, 8
BH3-mimetics represent a new class of anticancer drugs that mimic the function of BH3-only proteins.9 These compounds act in a tumor-selective way, most likely because tumor cells are ‘primed for death'.10 Their rapid proliferation under stressful conditions is thought to activate BH3-only proteins and Bax/Bak, whose function is counteracted by elevated expression of pro-survival Bcl-2 proteins. BH3-mimetics release the BH3-only proteins and Bax/Bak from their pro-survival counterparts, which kills the tumor cell. Bax/Bak and BH3 domain-only proteins bind to the pro-survival Bcl-2 family proteins with their α-helical BH3 domain that fits into a groove formed by the BH1–3 domains of the partner protein.2 The BH3-mimetic ABT-737 was identified by NMR-based screening of a chemical library for high-affinity binding to the hydrophobic BH3-binding groove of recombinant Bcl-xL.11 In vitro, ABT-737 binds Bcl-xL, Bcl-2 and Bcl-w with comparable high affinity, while it has over 1000-fold lower affinities for Bcl-B, Bfl-1 and Mcl-1.11 ABT-263 is a closely related compound that is orally bioavailable.12 It displays a similar binding selectivity for pro-survival Bcl-2 proteins in vitro as ABT-737 and is currently in clinical trials under the name navitoclax.13
ABT-737 showed impressive single-agent activity, in particular against leukemias, lymphomas and small-cell lung cancer (SCLC), but resistance was often encountered as well.11, 14, 15, 16, 17, 18 It was found at an early stage that Mcl-1 is untargeted and mediates ABT-737 resistance.14, 15, 16 However, the contribution of all six pro-survival Bcl-2 family members to ABT-737 resistance has never been compared side-by-side in a cellular context. Combination of ABT-737 with various anticancer drugs often leads to increased cell death,9 but these compounds often induce multiple BH3-only proteins.8 How individual BH3-only proteins contribute to synergy with ABT-737 is therefore unclear.
We present here the selectivity of ABT-737 for all human full-length pro-survival Bcl-2 family members in human p53 wild-type and -mutant T-leukemic cells. Bcl-B was identified as mediator of ABT-737 resistance, like Mcl-1 and Bfl-1 were previously,14, 15, 16 in accordance with the low affinity of ABT-737 for these pro-survival proteins in vitro. Contrary to in vitro affinity studies, however, we find that ABT-737 targets Bcl-2 with preference over Bcl-xL and Bcl-w. BH3-only protein and Bax displacement revealed that ABT-737 differs in its ability to disrupt complexes between these proteins and Bcl-2, Bcl-xL or Bcl-w. This explains the differential targeting of these proteins in the cellular context. Using cell lines with inducible expression of Noxa, Bim, Puma or truncated Bid, we found that only Noxa could synergize with ABT-737 in cells expressing the untargeted proteins Bcl-B, Bfl-1 or Mcl-1. Accordingly, Noxa-inducing anticancer drug bortezomib synergized with ABT-737 in case of Bcl-B, Bfl-1 or Mcl-1 overexpression. These data provide additional guidelines for design and selection of novel BH3-mimetic drugs.
Results
In a cellular context, ABT-737 targets Bcl-2 with greater efficiency than all other pro-survival proteins
For this study, we used the two well-characterized human T-acute lymphoblastic leukemia (T-ALL) cell lines MOLT-4 and J16 that have a wild-type and mutant p53 status, respectively. We stably expressed each of the six pro-survival Bcl-2 proteins in these cell lines by retroviral transduction. Overexpression of the Bcl-2 proteins was confirmed by western blotting (Supplementary Figure 1). To determine their sensitivity to ABT-737, the cell lines were cultured for 48 h with a dose range of the drug or its negative enantiomer, and cell death was read out by propidium iodide (PI) uptake. Cell death was classified as apoptosis by nuclear fragmentation and complete inhibition by the pan-caspase inhibitor z-VAD-fmk (Supplementary Figure 2). Cell death induced by the enantiomer was differentially inhibited by the various Bcl-2 family members, and therefore this compound was disqualified as a control (Supplementary Figure 3).
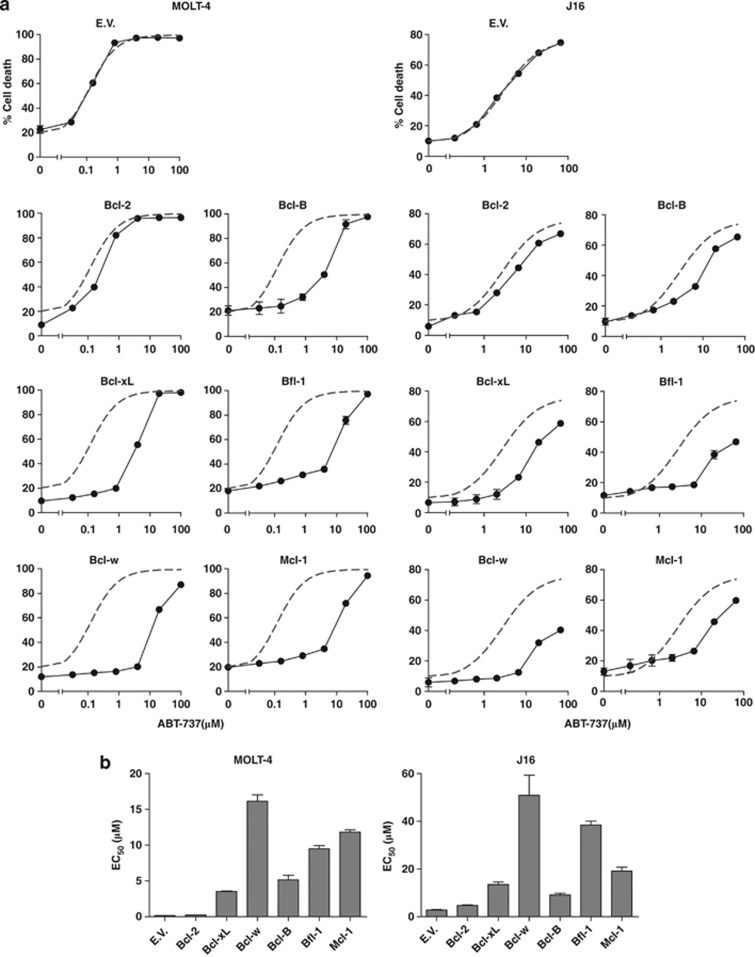
MOLT-4 and J16 empty-vector control cell lines died in a dose-dependent manner in response to ABT-737 treatment (Figure 1a), with EC50 values of about 0.1 μM and 8 μM, respectively (Figure 1b). For the cell lines overexpressing the different Bcl-2 family members, we have depicted the fitted response curve of the empty-vector control cells in each plot, as a reference for ABT-737 sensitivity (Figure 1a). Both MOLT-4 and J16 cells that overexpressed Bcl-2 died as effectively as empty-vector control cells in response to ABT-737 (Figures 1a and b), confirming that ABT-737 effectively targets Bcl-2. Bcl-B conferred resistance, as did Mcl-1 and Bfl-1, both in MOLT-4 and J16 cells. Surprisingly, despite their similar in vitro affinity for ABT-737, Bcl-xL and Bcl-w were not equivalent to Bcl-2 in their sensitivity to ABT-737. In both cell types, Bcl-xL and in particular Bcl-w conferred resistance as revealed by a right shift of the curves and increased EC50 values (Figures 1a and b).
Figure 1.
Of all six pro-survival Bcl-2 proteins, Bcl-2 appears the optimal target for ABT-737. (a) MOLT-4 and J16 (Jurkat) T-ALL cell lines that had been transduced to stably express the indicated pro-survival Bcl-2 family members or empty control vector (EV) were treated with a dose range of ABT-737 (μM). Cell death was assessed by PI uptake, 48 h after ABT-737 addition. Gray dashed line indicates the curve fit for the EV dose–response data that is included in every graph for easy reference. Data shown are mean values+S.D. derived from one experiment with triplicate samples and are representative of two to three independent experiments. (b) EC50 values of the dose–response curves in (a) as determined by curve fitting
Correction for protein turnover shows that Bcl-2 is the optimal target of ABT-737, preferred over Bcl-xL and Bcl-w, while Mcl-1 and Bfl-1 are untargeted
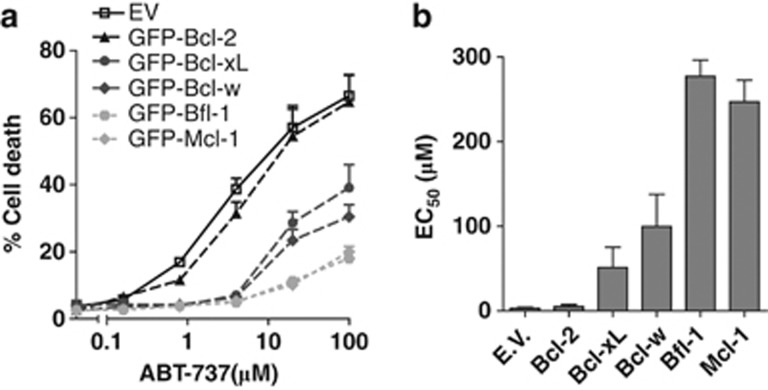
As ABT-737 seemed more selective for Bcl-2 than previously anticipated, we aimed to substantiate this finding by excluding differences in expression levels of the pro-survival Bcl-2 family members. Bfl-1 and Mcl-1 in particular are subject to constitutive ubiquitination and proteasomal turnover,19, 20 which may affect their antiapoptotic capability. For this purpose, we stably expressed all pro-survival Bcl-2 family members in J16 cells as amino-terminal fusions with green fluorescent protein (GFP). This enabled us to flow cytometrically sort the cell lines on equal expression levels of the pro-survival Bcl-2 proteins using GFP fluorescence (Supplementary Figure 4). We thus corrected for differences in protein turnover that played a role in the experiments depicted in Figure 1, where untagged Bcl-2 proteins were expressed. Bcl-B expressing cells were excluded from this analysis, as they also expressed GFP as non-fusion protein, apparently due to proteolytic cleavage (Supplementary Figure 4). The cells were exposed to a dose range of ABT-737 and cell death was assayed after 48 h, as before. Clearly, Bcl-2 was the optimal target, as Bcl-2 overexpressing cells were the only ones that displayed equal sensitivity to ABT-737 as empty-vector control cells (Figures 2a and b). Bfl-1 and Mcl-1 were highly insensitive to ABT-737, with EC50 values that were over 50-fold higher than those for Bcl-2. Importantly, the assay demonstrated conclusively that at equal expression levels, Bcl-xL and Bcl-w are targeted by ABT-737 with lower efficiency than Bcl-2. This is apparent from the shift in the dose–response curves (Figure 2a) and the 10 to 20-fold higher EC50 values (Figure 2b). We conclude that in the cellular context, ABT-737 targets Bcl-xL and Bcl-w to a lesser extent than Bcl-2 and does not target Bfl-1 or Mcl-1.
Figure 2.
Correction for protein turnover shows that Bcl-2 is a better target for ABT-737 than Bcl-xL and Bcl-w, while Bfl-1 and Mcl-1 are untargeted. (a) J16 cell line was transduced to stably express one of the indicated GFP-Bcl-2 fusion proteins or GFP control vector (EV) and cells were sorted on equal expression of GFP. Resulting stable cell lines with comparable expression of the GFP-tagged Bcl-2 proteins (Supplementary Figure 4) were subsequently treated with a dose range of ABT-737 (μM). Cell death was assessed by PI uptake, 48 h after ABT-737 addition. Data shown are mean values+S.D. derived from three independent experiments. (b) EC50 values of the dose–response curves in (a) as determined by curve fitting
ABT-737 and etoposide synergize more strongly in tumor cells that overexpress Bcl-2, as compared with Bcl-xL or Bcl-w
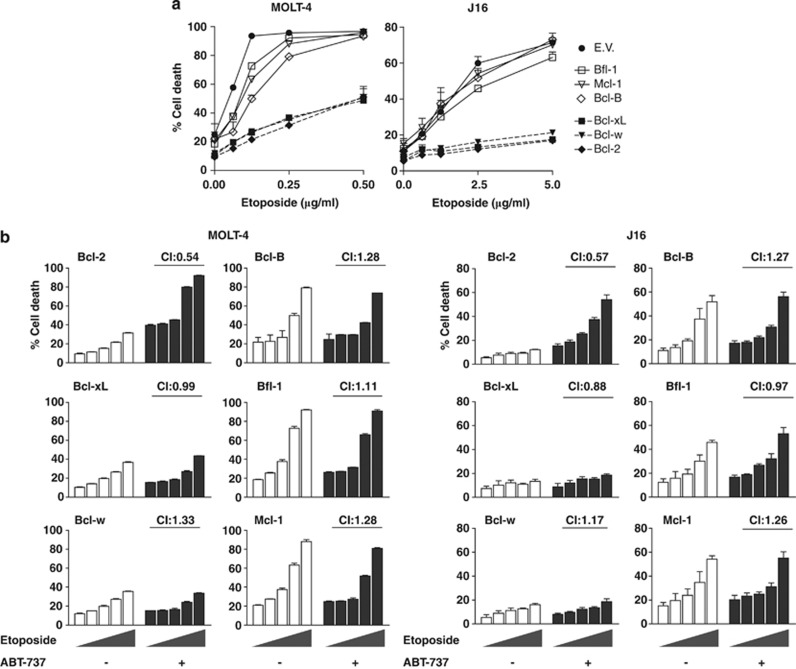
Next, we investigated in which cases ABT-737 could alleviate resistance to a conventional therapeutic regimen. To this end, we tested the cell line panel expressing the untagged pro-survival proteins for responsiveness to etoposide, a topoisomerase II inhibitor and an inducer of DNA double-strand breaks. MOLT-4 was much more sensitive to etoposide than J16 (Figure 3a), in agreement with its wild-type p53 status. Overexpression of Bcl-2, Bcl-xL and Bcl-w strongly inhibited etoposide-induced cell death in MOLT-4 and conferred almost complete resistance against etoposide in J16, at all tested concentrations (Figure 3a). In contrast, Bcl-B, Bfl-1 or Mcl-1 overexpression had little effect on etoposide-induced death in MOLT-4 and none in J16 (Figure 3a). It was expected that ABT-737 would sensitize the etoposide-resistant cells for cell death induction, in case they overexpressed Bcl-2 or other ABT-737 target proteins. To test this, all cell lines were exposed to a dose range of etoposide, in the absence or presence of a low dose of ABT-737. Strikingly, the combined effect of ABT-737 and etoposide was only synergistic in case of Bcl-2 overexpression, both in MOLT-4 and J16 cells (Figure 3b). This was defined by the calculated combination index (C.I.) of 0.54, which is below the cutoff for synergy of 0.9. In case of Bcl-B, Bfl-1 and Mcl-1, the CI was higher than 1, confirming that these proteins are not targeted by ABT-737. More importantly, in case of Bcl-xL and Bcl-w, the combined effect with ABT-737 was hardly or not at all synergistic (Figure 3b). At a higher dose of ABT-737, synergy was observed in Bcl-xL- and Bcl-w-overexpressing cells (results not shown), confirming that these proteins were targeted, but with lower efficacy than Bcl-2. Experiments in J16 cells, combining ABT-737 with the death receptor ligand TRAIL or ER stressor thapsigargin, confirmed the selectivity of ABT-737 for Bcl-2 as compared with the other pro-survival Bcl-2 proteins (results not shown). We conclude that ABT-737 can act synergistically with a conventional DNA-damaging anticancer regimen to alleviate the apoptotic blockade imposed by Bcl-2, but to a much lesser extent that imposed by Bcl-xL or Bcl-w. This is the case in both p53 wild-type and mutant leukemic cells.
Figure 3.
In J16 and MOLT-4 T-ALL, Bcl-2, Bcl-xL and Bcl-w mediate etoposide resistance, which can be alleviated by ABT-737 only in case of Bcl-2. (a) Single-agent treatment. J16 and MOLT-4 cell lines expressing empty vector (EV), or overexpressing one of the indicated pro-survival Bcl-2 proteins, were treated with the indicated doses of etoposide (μg/ml). Cell death was measured as PI uptake, 48 h after addition of the drug. Data shown are mean values+S.D. derived from one experiment with triplicate samples that is representative of three experiments. (b) Combined modality treatment. Cells were treated with a dose range of etoposide (0, 0.3, 0.6, 1.25, or 2.5 μg/ml for J16 and 0, 0.031, 0.063, 0.125, or 0.25 μg/ml for MOLT-4) in the absence of presence of ABT-737. ABT-737 was used at a concentration of 0.6 μM for J16 and at 0.16 μM for MOLT-4. Cell death was measured as PI uptake, 48 h after addition of the drugs. Data shown are mean values+S.D. derived from one experiment with triplicate samples that is representative of two experiments. CI were calculated as described in Materials and Methods and indicate synergy when <0.9
ABT-737 selectivity for Bcl-2 over Bcl-xL and Bcl-w is explained by differential displacement of BH3-only proteins and Bax
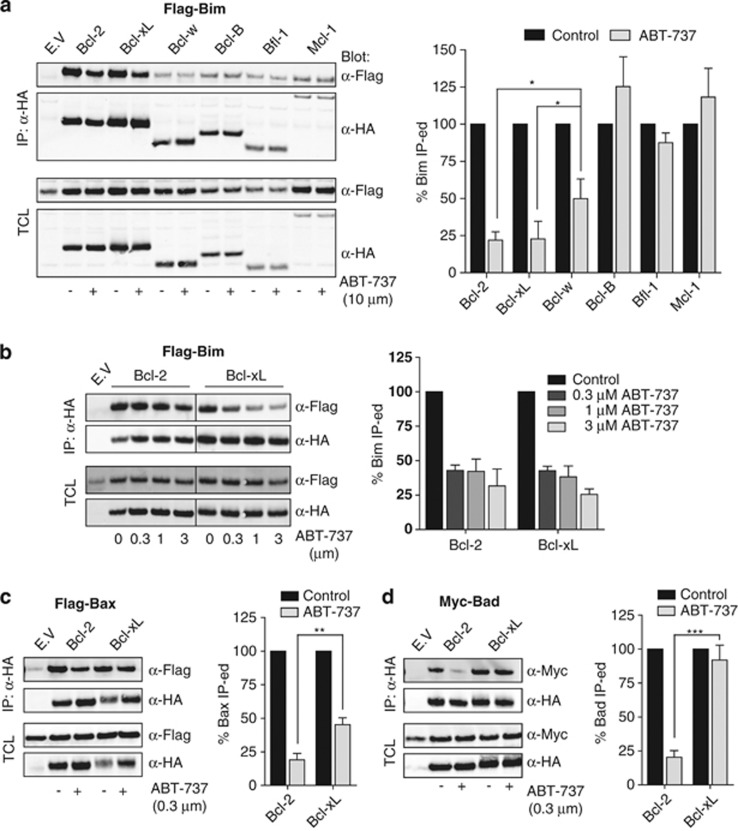
BH3-mimetics are thought to induce cell death by releasing BH3-only proteins like Bim, or an active pool of Bak/Bax from sequestration by pro-survival Bcl-2 family members.10, 14, 17 To gain further insight into the selectivity of ABT-737 in cells, we tested its potential to displace BH3-only proteins or Bax from the different pro-survival Bcl-2 family members. We first focused on Bim that has been implicated as a major factor in sensitivity to ABT-737.17 HEK 293T cells expressing Flag-tagged Bim, in combination with each of the HA-tagged pro-survival Bcl-2 proteins were treated for 8 h with a high dose (10 μM) of ABT-737 or solvent control. Pro-survival Bcl-2 proteins were immunoprecipitated and the amount of Bim that was bound to them was determined by western blotting (Figure 4a, left panel). Western blotting of total cell lysates indicated that overall expression levels of Bim and the inhibitory Bcl-2 proteins were comparable before and after ABT-737 treatment. Western blot signals in the immunoprecipitates were quantified and the ratio between Bim and the inhibitory Bcl-2 proteins was calculated (Figure 4a, right panel). The amount of Bim recovered in the absence of ABT-737 was set at 100% in each case. This analysis revealed that ABT-737 could not displace Bim from Bcl-B, Bfl-1 and Mcl-1, even at this high dose, in line with the data we obtained in the cell death assays. At 10 μM, ABT-737 displaced Bim from Bcl-2 and Bcl-xL in equal measure, by about 75% (Figure 4a, right panel). Also at a lower dose range from 3 to 0.3 μℳ, ABT-737 displaced Bim from Bcl-2 and Bcl-xL with equal efficiency (Figure 4b). However, at 10 μℳ, ABT-737 displaced Bim with significantly lower efficacy from Bcl-w than from Bcl-2 or Bcl-xL (Figure 4a, right panel). This is in agreement with the fact that Bcl-w conferred ABT-737 resistance in our cell lines.
Figure 4.
ABT-737 displaces Bad and Bax with greater efficacy from Bcl-2 than from Bcl-xL or Bcl-w. (a–d) HEK 293T cells were transfected to express each of the indicated HA-tagged pro-survival Bcl-2 proteins together with Flag-tagged Bim (a and b), Bax (c), or Myc-tagged Bad (d). At 24 h after transfection, ABT-737 or solvent control were added to the indicated final concentrations and cells were cultured for another 8 h in the presence of z-VAD-fmk (25 μM). Next, cells were harvested, lysed and subjected to immunoprecipitation (IP) with antibody directed at the HA tag. Before IP, samples were taken for control of expression of HA-tagged pro-survival and pro-apoptotic Bcl-2 proteins in total cell lysates (TCL). Samples of TCL and anti (α)-HA IP were separated by SDS-PAGE and subjected to western blotting with fluorochrome-conjugated α-HA, α-Flag and α-Myc antibodies. Imaging and quantification was done using the Odyssey Imager and associated software. Bar diagrams represent the ratio between the amounts of the pro-apoptotic and the pro-survival Bcl-2 family protein in the IP. The signal in the IP sample from mock-treated cells (control) was set at 100% for each of the pro-survival Bcl-2 proteins. Results shown are the mean+S.D. of three independent experiments. Asterisks denote statistical significant differences as determined by Student′s t-test (*P<0.05, **P<0.01, ***P<0.001)
We considered that looking solely at disruption of Bim complexes is not fully informative of ABT-737 action, as it did not explain the differential sensitivity of Bcl-2 and Bcl-xL overexpressing cells. We therefore tested displacement of other pro-apoptotic proteins from Bcl-2 and Bcl-xL. Flag-tagged Bax (Figure 4c) or Myc-tagged Bad (Figure 4d) were expressed in conjunction with HA-tagged Bcl-2 or Bcl-xL, cells were treated for 8 h with 0.3 μM of ABT-737 or solvent control and samples were analyzed as stated before. These assays revealed that ABT-737 displaced Bax from Bcl-2 with a significantly higher efficacy than from Bcl-xL (Figure 4c). Moreover, Bad displacement from Bcl-2 was very efficient at this 0.3 μM dose of ABT-737, while Bad displacement from Bcl-xL was completely ineffective (Figure 4d). Also at a high ABT-737 dose of 10 μM, Bad was still more efficiently displaced from Bcl-2 than from Bcl-xL (results not shown). These assays indicate that ABT-737 discriminates in cells between Bcl-2, Bcl-xL and Bcl-w complexes, despite similar in vitro affinity for the pro-survival proteins themselves.
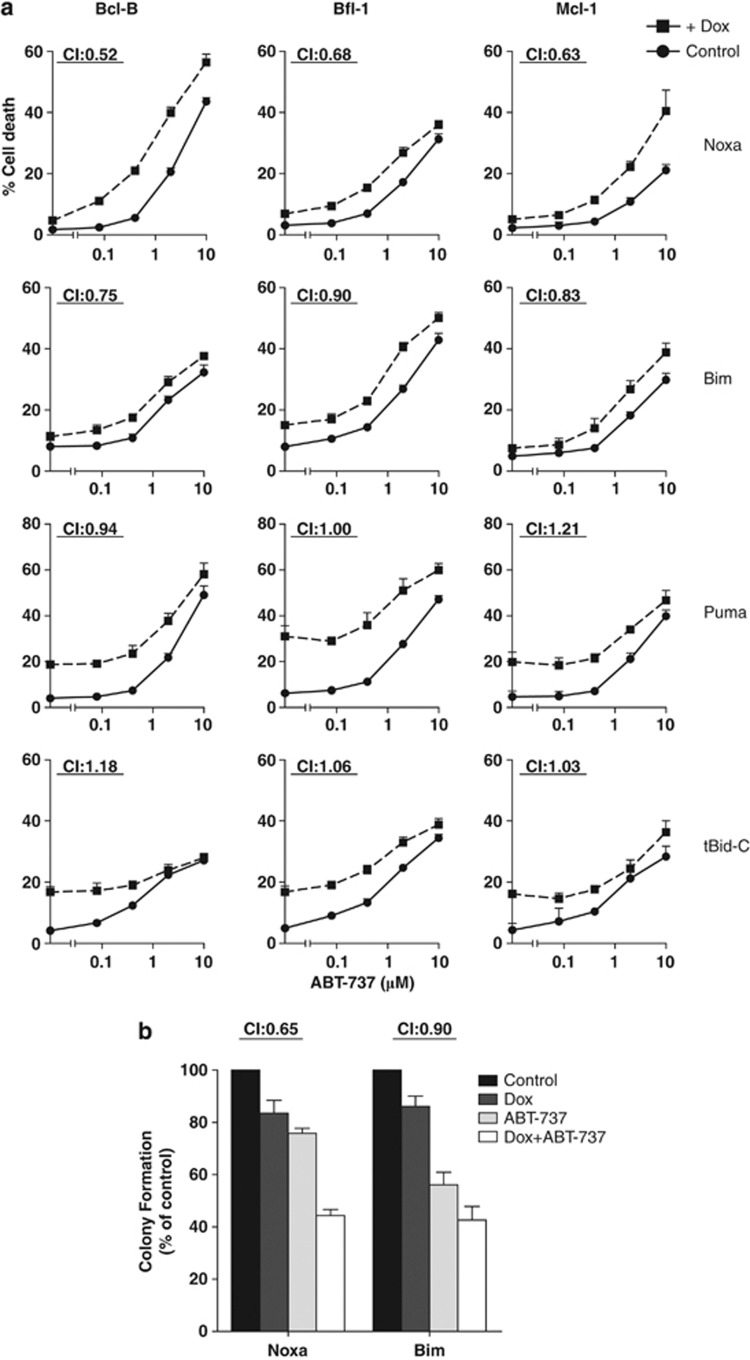
Noxa, but not Bim, Puma or tBid-C synergizes with ABT-737 in cells that overexpress the non-targeted pro-survival proteins Bcl-B, Bfl-1 or Mcl-1
Both apoptosis assays and Bim displacement studies indicated that Bcl-B, Bfl-1 and Mcl-1 are not targeted by ABT-737. Various drugs and stimuli have been tested for their potential to overcome ABT-737 resistance by Mcl-1 and various BH3-only proteins have been suggested to have a role.21, 22, 23 To test individual BH3-only proteins for their capacity to alleviate ABT-737 resistance as mediated by all three untargeted proteins, we enabled J16 cells to inducibly express BH3-only proteins in response to doxycycline (Dox). This allowed us to specifically study the contribution of one BH3-only protein at a time, which is not possible when broadly acting stimuli are used that may activate multiple (unknown) BH3-only proteins at once.7, 8 We tested Bim, Puma and the active carboxy-terminal fragment of Bid (tBid-C), as they reportedly bind to all pro-survival Bcl-2 family members and can directly activate Bax/Bak.3, 4, 24 We also tested Noxa, as it has a Bcl-2 family binding profile that is exactly complementary to that of ABT-737.3, 24 The Bim-, Puma-, tBid-C- and Noxa-inducible cell lines that were created died in a dose-dependent manner upon treatment with Dox and induction of the BH3-only proteins was confirmed by western blotting (Supplementary Figure 5). After this validation, the cell lines were retrovirally transduced to express Bcl-B, Bfl-1 or Mcl-1, which made them resistant to ABT-737.
These cell lines were treated for 48 h with a dose range of ABT-737, in the absence or presence of Dox, after which cell death was monitored. Noxa showed a clear synergistic interaction with ABT-737 in cells that expressed either Bcl-B, Bfl-1 or Mcl-1 (Figure 5a). A weak synergy was observed for Bim, but not for Puma or tBid-C, even at higher Dox concentrations (results not shown), or when cells were pre-treated with Dox (Supplementary Figure 6). To test combined effects on clonogenic survival, the Mcl-1 overexpressing cells with inducible Noxa- or Bim expression were cultured as single cells in the presence of ABT-737, Dox or the combination. BH3 protein induction or ABT-737 treatment alone reduced clonal outgrowth in both cases (Figure 5b). However, only in case of Noxa, the combination of ABT-737 treatment and BH3-only protein induction was synergistic as indicated by a CI of 0.65. Collectively, the data show that Noxa acts synergistically with ABT-737 to induce cell death in cells that express the non-targeted proteins Bcl-B, Bfl-1 or Mcl-1, while Bim, Puma and tBid-C do not.
Figure 5.
Only Noxa synergizes with ABT-737 in Bcl-B, Bfl-1 and Mcl-1 overexpressing cells. J16 clones with Dox-inducible expression of the indicated BH3-only proteins and overexpression of Bcl-B, Bfl-1 or Mcl-1, were tested for interaction of the BH3-only protein with ABT-737. (a) Cell death assay: Cells were treated with ABT-737 at 80, 400, 2000, or 10000 nℳ, in the absence or presence of Dox (Bim and Noxa, 1 μg/ml; Puma, 0.25 μg/ml; tBid-C, 0.03 μg/ml). Cell death was assessed by PI uptake after 48 h. Data shown are mean values+S.D. derived from three independent experiments. CI indicate synergy. (b) Clonogenic assay: Cells were sorted at one cell per well into round-bottom 96-well plates, with medium alone (control), or added ABT-737 (1 μM), Dox (2 μg/ml), or both. Cells were allowed to proliferate for 3 weeks, and wells with evident colonies were scored positive. Colony formation in medium alone was set at 100%. Data shown are mean values+S.D. derived from three independent experiments
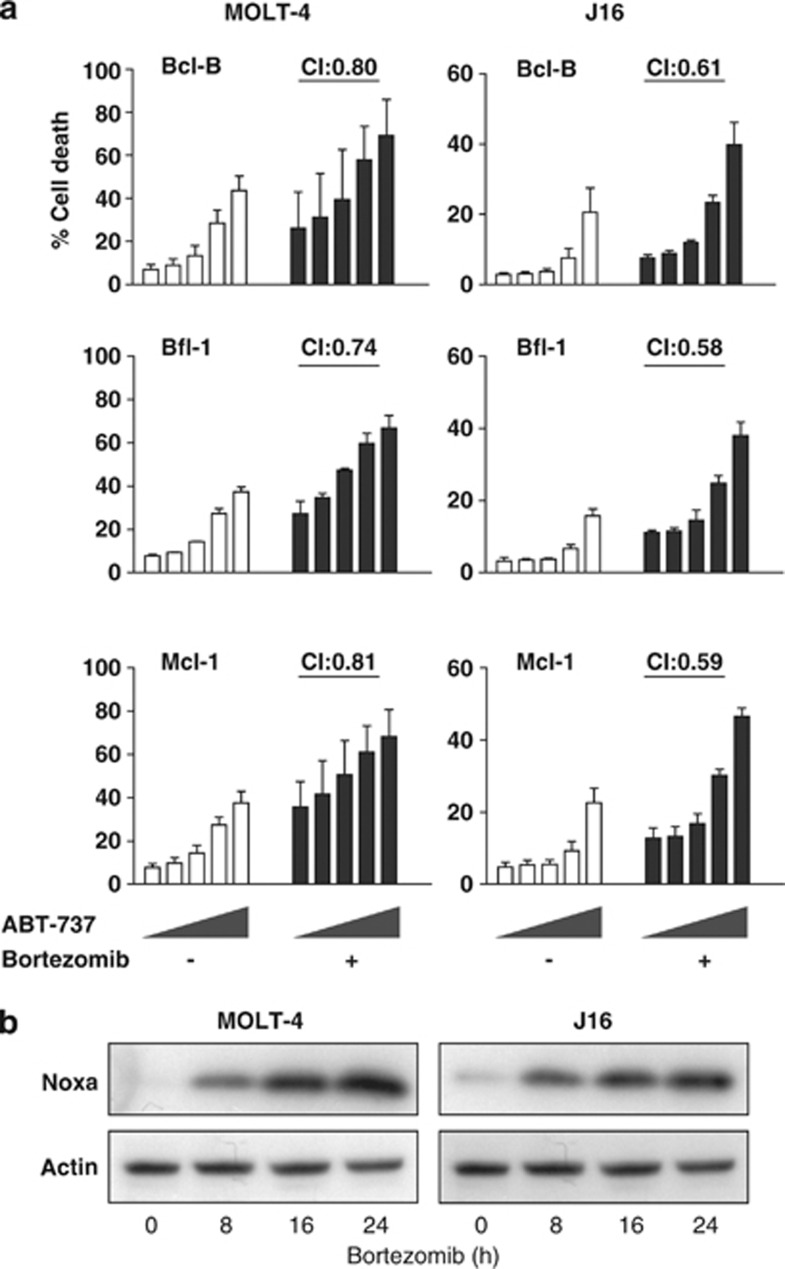
Noxa-inducing anticancer drug bortezomib synergizes with ABT-737 in Bcl-B-, Bfl-1- or Mcl-1- overexpressing leukemic cells
To extrapolate our findings to a clinically relevant setting, we tested whether the proteasome inhibitor bortezomib could act synergistically with ABT-737 in cells that overexpressed any of the non-targeted pro-survival Bcl-2 proteins. Bortezomib is clinically applied and induces Noxa in a p53-independent manner.25, 26 J16 and MOLT-4 cell lines overexpressing Bcl-B, Bfl-1 or Mcl-1 were treated for 48 h with different doses of ABT-737, in the absence or presence of bortezomib. The cell lines showed a response to single-agent treatment, but the combination synergistically induced cell death in all cases (Figure 6a).
Figure 6.
Proteasome inhibitor bortezomib increases Noxa expression, and works synergistically with ABT-737. (a) J16 and MOLT-4 cells, stably overexpressing Bcl-B, Bfl-1 or Mcl-1 that are not targeted by ABT-737, were incubated with ABT-737 (0,16, 80, 400, 2000, nM) in the absence or presence of bortezomib (15 nM and 5 nM for J16 and MOLT-4, respectively). After 24 h, cell death induction was assessed by PI uptake. Data shown are mean values+S.D. derived from three independent experiments. (b) J16 and MOLT-4 cells were treated for indicated periods of time with bortezomib supplemented with pan-caspase inhibitor to block cell death. Lysates were prepared and analyzed by western blotting for Noxa protein induction, using a Noxa-specific antibody. The blot was re-probed with an Actin-specific antibody to confirm equal loading
Accordingly, bortezomib dramatically increased Noxa protein expression, both in J16 and MOLT-4 cells (Figure 6b). Among all pro- and antiapoptotic Bcl-2 family proteins both in J16 and MOLT-4 only Noxa was consistently upregulated at the mRNA level in both cell lines (Supplementary Figure 7). Noxa downregulation by RNA interference was unsuccessful (results not shown), but bortezomib kills Jurkat cells via Noxa induction.27 These results show that bortezomib upregulates Noxa expression in p53 wild-type and mutant leukemias, which is accompanied by increased sensitivity to ABT-737, despite overexpression of the non-targeted proteins Bcl-B, Bfl-1 and Mcl-1.
Discussion
Our study revealed that Bcl-2, Bcl-xL and Bcl-w are not targeted with equal efficiency by ABT-737 in the cellular context. It thereby identified Bcl-w and Bcl-xL as potential mediators of ABT-737 resistance. Although this is surprising in the light of their similar in vitro targeting,11 other data also point in this direction.15, 28, 29, 30 Whitecross et al.29 reported that Bcl-w conferred resistance to ABT-737 in Eμ-Myc transformed mouse lymphoma cells, while Van Delft et al.15 found that mouse embryonic fibroblasts were less sensitive to ABT-737 upon overexpression of Bcl-xL as compared with Bcl-2. Furthermore, high levels of Bcl-2, but not Bcl-xL or Bcl-w were found to correlate with ABT-737 or ABT-263 sensitivity in CLL.17, 30 Also, Tahir et al.31 found a positive correlation between ABT-263 resistance and high Bcl-w mRNA levels in SCLC cell lines and with high Bcl-xL and low Bcl-2 protein levels in leukemia cell lines. Nevertheless, a major dose-limiting toxicity of ABT-263 in patients is apoptosis of platelets that depend on Bcl-xL for their survival.32, 33 It appears therefore that Bcl-xL can mediate resistance in some cell types, but that it is efficiently targeted in others.
Our findings offer an explanation for this paradox. ABT-737 induces apoptosis by disrupting pre-existing complexes between pro- and antiapoptotic Bcl-2 family proteins. We demonstrate that its ability to do so is different for different complexes, which explains the differential targeting of Bcl-xL and Bcl-w observed in cells. ABT-737 displaced Bim with lower efficiency from Bcl-w than from Bcl-xL or Bcl-2 (Figure 4). ABT-737 displaced Bim with similar efficiency from Bcl-2 and Bcl-xL, but differential targeting of Bcl-2 and Bcl-xL became apparent when we examined displacement of Bax and Bad. Our data indicate that not the binding affinity for the pro-survival Bcl-2 proteins as such, but rather the ability to disrupt complexes with their pro-apoptotic relatives determines the potency of ABT-737 and ABT-263. The fact that complexes of pro-survival Bcl-2 proteins are targeted, rather than the ‘empty' molecules, is important for the therapeutic window of BH3-mimetics. ABT-263 has dose-limiting normal tissue toxicity in patients, due to thrombocytopenia and T-cell lymphopenia.33 Platelets rely on Bcl-xL for survival, which seems to be dependent on sequestration of Bak32 and not Bid or Bim.34 Compounds that would specifically disrupt Bcl-xL complexed with Bim but not with Bak may therefore be more tumor-selective and less toxic on normal tissue.
In our comprehensive specificity profiling, we consistently found that Bfl-1- and Mcl-1-mediated ABT-737 resistance, confirming data by others.14, 15, 16, 28, 29 We found likewise that Bcl-B caused ABT-737 resistance in both cell lines. This is a novel finding, consistent with the low in vitro affinity of ABT-737 for Bcl-B.11 Bcl-B was recently found to be upregulated in primary CLL upon ABT-737 treatment and suggested to contribute to ABT-737 resistance.30 Bcl-B is also expressed in diffuse large B-cell lymphoma and SCLC, which are tumor types currently treated with ABT-263 in clinical trials13, 33 and it may therefore be of value as a predictive marker.
ABT-737 is presently under clinical evaluation as a single-agent therapy, but preclinical studies emphasize its improved potential in combined modality therapies.9 Many studies have focused on Mcl-1 neutralization to achieve synergy between ABT-737 and other drugs,9, 35 but we aimed to identify the BH3-only protein(s) that overrule ABT-737 resistance, as conferred by all untargeted pro-survival Bcl-2 proteins. We tested Puma, Bim and tBid-C, as they reportedly interact with all pro-survival Bcl-2 proteins and Noxa because it is known to neutralize Mcl-1.24 Although Puma and Bim have been previously suggested to synergize with ABT-737,21, 22 our data show that only Noxa provided synergy, in case of Mcl-1, Bfl-1, as well as Bcl-B overexpression. This is most likely because of its complementarity, as Noxa cannot only interact with Mcl-1, but also with Bfl-120 and with Bcl-B, as we have recently found (data not shown). These findings are corroborated by a vast amount of literature that implies Noxa as a crucial determinant of ABT-737 sensitivity in various tumor cell lines and in synergy with diverse agents.9, 23, 31 However, we are the first to show that selective induction of Noxa, but not Bim, Puma or tBid-C, alleviates ABT-737 resistance not only as imposed by Mcl-1, but by all three untargeted pro-survival proteins. In accordance, loss of Noxa, and not any other BH3-only protein, was found in a genome-wide screen in chronic myeloid leukemia cells as a key determinant for ABT-737 resistance.36 Thus, Noxa induction is the optimal remedy to overcome ABT-737 resistance as conveyed by Mcl-1, Bfl-1, as well as Bcl-B.
Given the occurrence of tumor cell resistance and normal tissue toxicity, the search is on for new BH3-mimetics that more selectively target certain pro-survival Bcl-2 family proteins, including those that are untargeted by ABT-263. Our work implies that such drugs must be selected for the ability to compete with BH3 domains from different pro-apoptotic proteins in binding to pro-survival Bcl-2 family members. This will be necessary to find the compounds that most effectively kill tumor cells, but also provide a therapeutic window in regards to normal tissue toxicity.
Materials and Methods
Constructs
Vectors encoding Bcl-2, Bfl-1, or tBid-C have been described previously.37 Vectors encoding Bcl-xL, Mcl-1, Noxa, Puma or Bax were kindly provided by various investigators. cDNA clones for Bcl-w, Bcl-B, Bad, Bim were obtained from the IMAGE library (ImaGenes, Berlin, Germany). For stable expression in MOLT-4 or J16 cells, cDNAs encoding all six pro-survival Bcl-2 proteins were cloned into the retroviral vector pMX-IRES-Blasticidin for expression as untagged protein, or into LZRS-GFP-IRES-Zeo for expression as N-terminally GFP-tagged fusion protein. For Dox-inducible, stable expression in J16 cells, HA-tagged Bid, Bim, Noxa and Puma cDNA′s were cloned into the lentiviral vector pLVX-Tight (Clontech, Mountain View, CA, USA). For transfection into HEK 293T cells, they were cloned in frame into the pHA-C2 vector, a modified version of pEGFP-C2 (Clontech), in which EGFP has been replaced by a double HA-tag (kindly provided by L Janssen, The Netherlands Cancer Institute, Amsterdam, The Netherlands). The cDNA encoding Bim or Bax was cloned into the vector p3xFlag (Sigma-Aldrich, St. Louis, MO, USA) and cDNA of Bad was cloned into pCDNA3 (Invitrogen, Carlsbad, CA, USA) that encodes a double Myc-tag. All cDNA clones used encode the full-length human proteins. All cloning was done using standard cloning and PCR techniques. All constructs were verified by dideoxynucleotide sequencing.
Cell lines
Two T-ALL cell lines were used as model systems in this study: The p53-mutant Jurkat derived clone J1637 and the p53 wild-type cell line MOLT-4.38 The p53 status of the cell lines was confirmed by sequencing of all exons of the p53 gene (data not shown). J16 and MOLT-4 cell lines stably overexpressing unmodified pro-survival Bcl-2 family members were obtained by retroviral transduction with pMX-IRES-Blasticidin. Cell lines expressing the pro-survival Bcl-2 proteins as amino-terminally GFP-tagged fusions were obtained using the LZRS-GFP-IRES-Zeo vector. To get equal GFP-fusion Bcl-2 protein expression after transductions, the resulting cell lines were selected on Zeocin for 2 weeks, and subsequently sorted on equal GFP signal using a FACSAria (BD Biosciences, San Jose, CA, USA). J16 cell lines with stable, Dox-inducible expression of BH3-only proteins were created by sequential lentiviral transduction with pLVX-Tet-On Advanced (Clontech) and pLVX-tight encoding the BH3-only proteins. Confirmed clones were retrovirally transduced to stably express the untagged versions of Bcl-B, Bfl-1 or Mcl-1, to make them resistant to ABT-737. Methodology used for virus production, gene transduction and cell selection is detailed in the Supplementary Information. Cell lines were cultured in either IMDM (J16 and MOLT-4) or DMEM (Phoenix-Ampho and HEK 293T), supplemented with 8% heat-inactivated fetal bovine serum and antibiotics in a humidified incubator at 37 °C and 5% CO2.
Reagents
To make stock solutions, etoposide (Sigma, St. Louis, MO, USA), z-VAD-FMK (Bachem, Bubendorf, Switzerland) and Q-VD-OPH (SM Biochemicals, Anaheim, CA, USA) were dissolved in DMSO to a concentration of 10 mg/ml, 50 mM and 20 mM, respectively. Dox (Sigma) was dissolved in culture medium at 2 mg/ml. ABT-737 and its negative enantiomer were kindly provided by Abbott Laboratories (Abbott Park, IL, USA) and dissolved in DMSO to 20 mM. Bortezomib was kindly provided by Dr. Huib Ovaa (The Netherlands Cancer Institute, Amsterdam, The Netherlands), as a 10 μM stock solution in DMSO.
Cell death and clonogenic assays
For cell death assays, cells were plated in round-bottom 96-well plates, at 25 000 cells/well in 100 μl IMDM. Stimuli were given in 100 μl IMDM, keeping solvent constant and cells were subsequently placed in the incubator. At indicated time points, cells were washed in PBS, and subsequently stained with PI (1 μg/ml) in PBS with BSA for 5 min to monitor dead cells, or lysed in 0.1% Triton X-100, 0.1% sodium citrate and 50 μg/ml PI as described to monitor cells with subdiploid DNA content, as a hallmark of apoptosis.39 Cells were analyzed on either a FACSArray or a FACSCalibur (BD Biosciences), equipped with a plate loader. Clonogenic assays were performed by single-cell sorting of cells on a FACSAria (BD Biosciences) into 96-well plates, containing medium with or without a stimulus. Cells were allowed to grow for 3 weeks, after which positive wells were scored by eye.
Synergy calculations and curve fitting
Synergy calculations were based on the principle of Bliss independence.40 Combination indices (CI) were calculated using the formula CI=(r1+r2−r1*r2)/ r12, where r1, r2 and r12 denote the normalized response between 0 and 1 of a given dose of compound 1, 2 or the combination. Mean CI′s were calculated from at least three averaged data points on the curve. Interactions were considered synergistic at CI<0.9, additive at 0.9<CI<1.1 and antagonistic at CI>1.1. EC50 determinations and curve fitting was performed with Prism software (Graphpad, La Jolla, CA, USA) on normalized data using a constant Hill-factor.
Western blotting
Cell lysates were prepared in NP-40 buffer (1% Nonidet-P40, 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 5 mM EDTA), supplemented with PMSF and Complete Protease Inhibitor cocktail (Roche, Mannheim, Germany) and protein content was determined by the Bio-Rad Protein Assay (Hercules, CA, USA). Total cell lysate at 30 μg per lane was loaded on 4–12% Nu-Page Bis-Tris gradient gels (Invitrogen) and subsequent blotting was performed using the Trans-Blot Turbo (Bio-Rad). Antibodies used for detection are described in the Supplementary Information. Western Blot signals were visualized and quantified on the Odyssey Imaging System (LI-COR, Lincoln, NE, USA).
Immunoprecipitation
HEK 293 T cells were transfected to express Myc- or Flag-tagged pro-apoptotic proteins and HA-tagged pro-survival Bcl-2 family proteins or empty vector. At 24 h after transfection, ABT-737 or DMSO control was added to the indicated final concentrations and cells were incubated for another 8 h in the presence of 25 μM z-VAD-fmk to inhibit cell death. Cells were lysed in CHAPS buffer (1% CHAPS, 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1.5 mM MgCl2, 0.5 mM EGTA), supplemented with PMSF and complete protease inhibitor cocktail (Roche). Lysates were cleared at 4 °C by centrifugation at 17 000 × g for 10 min. Equal amounts of protein were incubated with anti-HA antibody (12CA5 clone) overnight at 4 °C, while tumbling. Next, 15 μl of Protein G Sepharose beads (GE Healthcare Life Sciences, Diegem, Belgium) was added and incubation continued for another 1 h. Beads were washed three times in CHAPS buffer, after which the beads were boiled in SDS sample buffer (Invitrogen) containing DTT and subjected to western blotting.
Note Added in Proof
Merino D et al. have recently also reported that ABT-737 targets Bcl-2 with higher efficacy than Bcl-xL or Bcl-w (Blood 119: 5807-5816, 2012).
Acknowledgments
We thank Abbott Laboratories for providing ABT-737 and its negative enantiomer, Evert de Vries for helpful advice and Madelon Paauwe, Roelof Pruntel, Frank van Diepen and Anita Pfauth for experimental assistance. This work was supported by grants from the Dutch Cancer Society to M Verheij and J Borst and by grant ECHO 700.57.008 from the Netherlands Organization for Scientific Research.
Glossary
- BH3
Bcl-2 Homology 3
- DOX
doxycycline
- MOMP
mitochondrial outer membrane permeabilization
- PI
propidium iodide
- SCLC
small-cell lung cancer
- T-ALL
T-acute lymphoblastic leukemia
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Disease website (http://www.nature.com/cddis)
Author Contributions
RWR designed and performed experiments, analyzed data and wrote the paper, BvdK performed experiments, JB and MV analyzed data and wrote the paper.
Edited by M Piacentini
Supplementary Material
References
- Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- Ren D, Tu HC, Kim H, Wang GX, Bean GR, Takeuchi O, et al. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science. 2010;330:1390–1393. doi: 10.1126/science.1190217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell JF, Billen LP, Bindner S, Shamas-Din A, Fradin C, Leber B, et al. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135:1074–1084. doi: 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happo L, Cragg MS, Phipson B, Haga JM, Jansen ES, Herold MJ, et al. Maximal killing of lymphoma cells by DNA damage-inducing therapy requires not only the p53 targets Puma and Noxa, but also Bim. Blood. 2010;116:5256–5267. doi: 10.1182/blood-2010-04-280818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labi V, Erlacher M, Kiessling S, Villunger A. BH3-only proteins in cell death initiation, malignant disease and anticancer therapy. Cell Death Differ. 2006;13:1325–1338. doi: 10.1038/sj.cdd.4401940. [DOI] [PubMed] [Google Scholar]
- Cragg MS, Harris C, Strasser A, Scott CL. Unleashing the power of inhibitors of oncogenic kinases through BH3 mimetics. Nature reviews. 2009;9:321–326. doi: 10.1038/nrc2615. [DOI] [PubMed] [Google Scholar]
- Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- Gandhi L, Camidge DR, Ribeiro de Oliveira M, Bonomi P, Gandara D, Khaira D, et al. Phase I study of navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol. 2011;29:909–916. doi: 10.1200/JCO.2010.31.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yecies D, Carlson NE, Deng J, Letai A. Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood. 2010;115:3304–3313. doi: 10.1182/blood-2009-07-233304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117:112–121. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell. 2007;12:171–185. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Zhong Q, Gao W, Du F, Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121:1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Fan G, Simmons MJ, Ge S, Dutta-Simmons J, Kucharczak J, Ron Y, et al. Defective ubiquitin-mediated degradation of antiapoptotic Bfl-1 predisposes to lymphoma. Blood. 2010;115:3559–3569. doi: 10.1182/blood-2009-08-236760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Dai Y, Pei XY, Grant S. Bim upregulation by histone deacetylase inhibitors mediates interactions with the Bcl-2 antagonist ABT-737: evidence for distinct roles for Bcl-2, Bcl-xL, and Mcl-1. Mol Cell Biol. 2009;29:6149–6169. doi: 10.1128/MCB.01481-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuling AM, Andrew SE, Tron VA. Inhibition of p38 MAPK enhances ABT-737-induced cell death in melanoma cell lines: novel regulation of PUMA. Pigment Cell Melanoma Res. 2010;23:430–440. doi: 10.1111/j.1755-148X.2010.00698.x. [DOI] [PubMed] [Google Scholar]
- Weber A, Kirejczyk Z, Potthoff S, Ploner C, Hacker G. Endogenous Noxa Determines the Strong Proapoptotic Synergism of the BH3-Mimetic ABT-737 with Chemotherapeutic Agents in Human Melanoma Cells. Transl Oncol. 2009;2:73–83. doi: 10.1593/tlo.08223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Nikiforov MA, Riblett M, Tang WH, Gratchouck V, Zhuang D, Fernandez Y, et al. Tumor cell-selective regulation of NOXA by c-MYC in response to proteasome inhibition. Proc Natl Acad Sci U S A. 2007;104:19488–19493. doi: 10.1073/pnas.0708380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Galan P, Roue G, Villamor N, Montserrat E, Campo E, Colomer D. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood. 2006;107:257–264. doi: 10.1182/blood-2005-05-2091. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Dai H, Correia C, Takahashi R, Lee SH, Schmitz I, et al. Noxa/Bcl-2 protein interactions contribute to bortezomib resistance in human lymphoid cells. J Biol Chem. 2011;286:17682–17692. doi: 10.1074/jbc.M110.189092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler M, Butterworth M, Majid A, Walewska RJ, Sun XM, Dyer MJ, et al. Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood. 2009;113:4403–4413. doi: 10.1182/blood-2008-08-173310. [DOI] [PubMed] [Google Scholar]
- Whitecross KF, Alsop AE, Cluse LA, Wiegmans A, Banks KM, Coomans C, et al. Defining the target specificity of ABT-737 and synergistic antitumor activities in combination with histone deacetylase inhibitors. Blood. 2009;113:1982–1991. doi: 10.1182/blood-2008-05-156851. [DOI] [PubMed] [Google Scholar]
- Al-Harbi S, BT Hill, Mazumder S, Singh K, Devecchio J, Choudhary G, et al. An antiapoptotic BCL-2 family expression index predicts the response of chronic lymphocytic leukemia to ABT-737. Blood. 2011;118:3579–3590. doi: 10.1182/blood-2011-03-340364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir SK, Wass J, Joseph MK, Devanarayan V, Hessler P, Zhang H, et al. Identification of expression signatures predictive of sensitivity to the Bcl-2 family member inhibitor ABT-263 in small cell lung carcinoma and leukemia/lymphoma cell lines. Mol Cancer Ther. 2010;9:545–557. doi: 10.1158/1535-7163.MCT-09-0651. [DOI] [PubMed] [Google Scholar]
- Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- Wilson WH, O′Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JP, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11:1149–1159. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T, Takehara T, Hikita H, Shimizu S, Shigekawa M, Li W, et al. BH3-only activator proteins Bid and Bim are dispensable for Bak/Bax-dependent thrombocyte apoptosis induced by Bcl-xL deficiency: molecular requisites for the mitochondrial pathway to apoptosis in platelets. J Biol Chem. 2011;286:13905–13913. doi: 10.1074/jbc.M110.195370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67:782–791. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- Carette JE, Guimaraes CP, Wuethrich I, Blomen VA, Varadarajan M, Sun C, et al. Global gene disruption in human cells to assign genes to phenotypes by deep sequencing. Nat Biotechnol. 2011;29:542–546. doi: 10.1038/nbt.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner AB, Tait SW, de Vries E, Eldering E, Borst J. Requirement for aspartate-cleaved bid in apoptosis signaling by DNA-damaging anti-cancer regimens. J Biol Chem. 2004;279:28771–28780. doi: 10.1074/jbc.M400268200. [DOI] [PubMed] [Google Scholar]
- Nakano H, Kohara M, Shinohara K. Evaluation of the relative contribution of p53-mediated pathway in X-ray-induced apoptosis in human leukemic MOLT-4 cells by transfection with a mutant p53 gene at different expression levels. Cell and tissue research. 2001;306:101–106. doi: 10.1007/s004410100438. [DOI] [PubMed] [Google Scholar]
- Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc. 2006;1:1458–1461. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
- Bliss CI. The Toxicity of Poisons Applied Jointly. Ann Appl Biol. 1939;26:585–615. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.