Abstract
How split genomes arise and evolve in bacteria is poorly understood. Since each replicon of such genomes encodes a specific partition (Par) system, the evolution of Par systems could shed light on their evolution. The cystic fibrosis pathogen Burkholderia cenocepacia has three chromosomes (c1, c2, and c3) and one plasmid (pBC), whose compatibility depends on strictly specific interactions of the centromere sequences (parS) with their cognate binding proteins (ParB). However, the Par systems of B. cenocepacia c2, c3, and pBC share many features, suggesting that they arose within an extended family. Database searching revealed seven subfamilies of Par systems like those of B. cenocepacia. All are from plasmids and secondary chromosomes of the Burkholderiales, which reinforces the proposal of an extended family. The subfamily of the Par system of B. cenocepacia c3 includes plasmid variants with parS sequences divergent from that of c3. Using electrophoretic mobility shift assay (EMSA), we found that ParB-c3 binds specifically to centromeres of these variants, despite high DNA sequence divergence. We suggest that the Par system of B. cenocepacia c3 has preserved the features of an ancestral system. In contrast, these features have diverged variably in the plasmid descendants. One such descendant is found both in Ralstonia pickettii 12D, on a free plasmid, and in Ralstonia pickettii 12J, on a plasmid integrated into the main chromosome. These observations suggest that we are witnessing a plasmid-chromosome interaction from which a third chromosome will emerge in a two-chromosome species.
INTRODUCTION
Low-copy-number bacterial plasmids encode partition (Par) systems that ensure active segregation of plasmid copies prior to cell division. Most Par systems consist of three elements: an NTPase (generally ATPase), a DNA-binding protein, and a centromere. The DNA-binding protein, generically termed ParB, interacts with the centromere, parS, to create a partition complex. The NTPase, ParA, is recruited by the partition complexes to move sister plasmids toward opposite cell poles. There are two main types of Par system, defined according to the nature of the ATPase (21, 22). Type II, currently the best understood, has an actin-like ATPase which by forming filaments pushes sister plasmids to the poles (7, 41). Type I, the most common, has a P-loop ATPase (45) which oscillates over the nucleoid (9, 26) and, at least in vitro, polymerizes (3, 6). How these ParA proteins segregate plasmids remains speculative.
Type I ParA and ParB proteins are diverse in sequence (5), but their genetic organization falls mostly into two subtypes: Ia (e.g., plasmids F and P1) and Ib (e.g., plasmids pTAR, TP228, and pB171-Par2) (21). Ia ParA proteins are large (Table 1), with an N-terminal extension that binds the promoter to regulate the parAB operon, while Ib ParA proteins are small and have no promoter-binding domain. Ia ParB proteins bind the centromere via a helix-turn-helix (HTH) domain. Ib ParB proteins are small and bind via a ribbon-helix-helix domain to a centromere which harbors the parAB promoter (48). More recently, type III and IV partition systems have been identified. The type III systems drive partition with tubulin-like GTPases, whereas the type IV systems use a single, non-NTPase coiled-coil protein (33, 49–51).
Table 1.
Features of par loci of B. cenocepacia J2315 replicons and other model systemsa
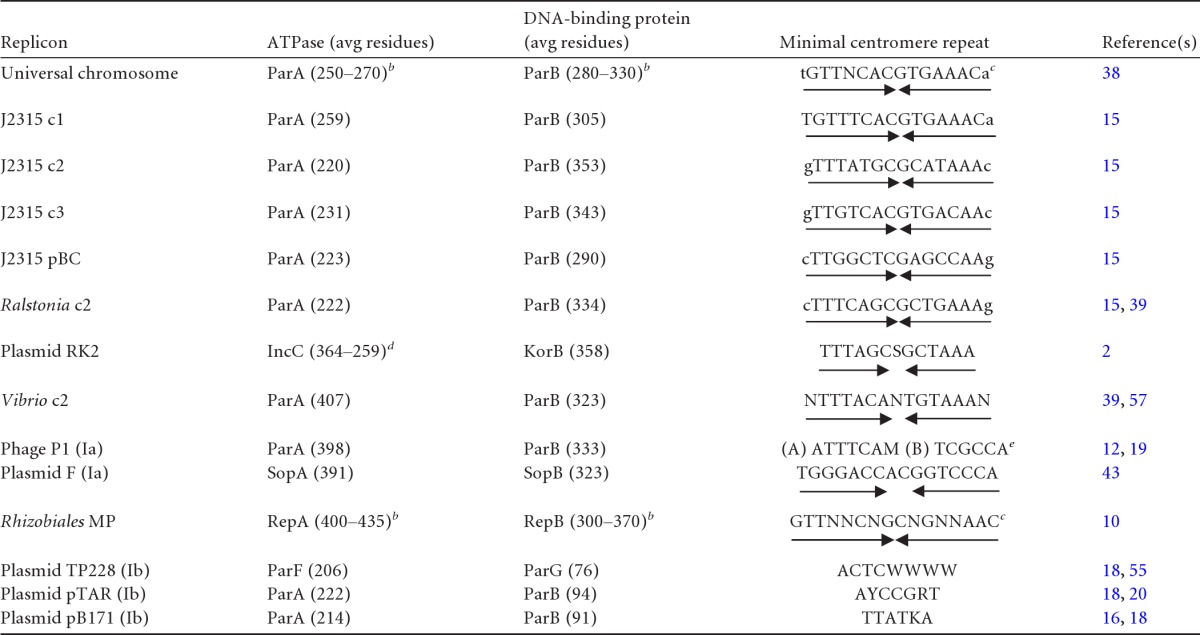
c1, c2, c3, and MP refer to chromosomes 1, 2, and 3 and to megaplasmids, respectively; Ia and Ib are Par system subtypes. Convergent arrows indicate symmetry. The single letter code is as follows: N = A, C, G, or T; S = C or G; M = A or C; W = A or T; Y = C or T; R = A or G; and K = G or T. Lowercase letters indicate bases that vary in the few natural repeats detected.
Size range of proteins in this category.
Consensus based on different replicons of the category.
Full and N-terminus-lacking forms of IncC.
(A) and (B) refer to the A and B boxes of P1 centromere.
Most bacterial chromosomes also encode Par systems, but the role of these in chromosome partitioning is often unclear. Their contribution may be evident only for cells in certain physiological states, such as growth deceleration in Pseudomonas putida and sporulation in Streptomyces coelicolor (23, 32); it may be restricted in scope, e.g., to positioning of new oriC replicas (34, 47, 52), and it may be indirect, as in regulation of the replication initiator DnaA (30, 44) and of the DNA condensin SMC (24). Nevertheless, chromosomal Par systems are true partitioning machines, since they can stabilize plasmids (15, 23, 56). Chromosomal Par systems constitute a specific subtype of type I (21, 56). Their ParA proteins have no N-terminal extension, like Ib ParA proteins, while their ParBs are large, with a central HTH domain (37), like Ia ParB proteins. Unlike plasmid centromeres, which are highly variable (Table 1) and located next to their parAB genes, chromosomal parS units are 16-bp palindromes of remarkably uniform (“universal”) sequence (38), dispersed within the region surrounding the replication origin (4, 39).
Although bacteria typically possess a single chromosome, some have more than one, notably proteobacteria in the orders Vibrionales, Rhodobacterales, Rhizobiales, and Burkholderiales. Split genomes are organized in a characteristic pattern: the largest chromosome has most of the housekeeping genes, an oriC, and a Par system similar to those of monochromosome species; secondary chromosomes have fewer housekeeping genes, a plasmid-like oriC, and a specific Par system (15, 17). The last feature suggests a crucial role for Par systems in the maintenance of multichromosome genomes.
One such genome is that of Burkholderia cenocepacia ET12, a bacterium of soils and roots also identified as an aggravating agent in cystic fibrosis (28). The first ET12 isolate to have its genome sequenced, J2315 (27), has three chromosomes (c1 to c3, defined as carrying essential genes), of 3.9, 3.2, and 0.9 Mb, and a plasmid of 93 kb (pBC). Although the par loci of all four replicons are strictly specific with regard to partition (15), those of the secondary chromosomes, c2 and c3, and the plasmid pBC are very similar with respect to protein size, clustering of parS sites upstream of parAB, and, more strikingly, the structure of parS (Table 1) (15, 39). These similarities suggest evolution from a common ancestor, within either B. cenocepacia or its predecessors. The in silico analysis of bacterial genomes we report here strengthens this hypothesis by revealing a distinct set of B. cenocepacia-like partition systems, all on secondary chromosomes and plasmids of the wider Burkholderiales group. To further characterize a link between these Par systems, we explored the possibility of so-far-undetected cross-reactions between ParB proteins and noncognate parS sites of related species. The cross-reactions we found suggest that extant Par systems indeed retain the capacity to bind centromeres of their family despite high DNA sequence divergence.
MATERIALS AND METHODS
In vivo determination of parS-induced growth inhibition.
parS sites of J2315 and mutated derivatives were obtained by annealing two oligonucleotides (Eurogentec) complementary at the 3′ end but carrying at the 5′ end either an EcoRI or an MluI overhang. The duplex was cloned between the EcoRI and MluI sites of the broad-host-range, multicopy, Cmr vector pMMB206 (42). parS+ c2 and parS+ pBC clusters were obtained by PCR amplification of J2315 genomic DNA. PCR fragments were cut with ApaI and HindIII or ApaI and HpaI and cloned in pMMB206 similarly cut. Recombinant plasmids were introduced into the J2315 derivative strain Mex1 by electrotransformation as described previously (14). Cells were spread on LB agar with chloramphenicol (40 μg/ml) and incubated at 37°C. From day 2 to day 7, colonies were scored twice a day.
Sources of Burkholderiales ParB proteins.
The sources of the parB genes were Burkholderia cenocepacia J2315 (DSMZ), Burkholderia vietnamiensis G4 (J. Tiedje, Michigan State University), Polaromonas naphthalenivorans CJ2 (DSMZ), Polaromonas sp. strain JS666 (ATCC), Ralstonia pickettii 12D and 12J (T. Marsh, Michigan State University), Ralstonia solanacearum GMI1000 (M. Arlat, INRA, Toulouse, France), and Rhodoferax ferrireducens DSM 15236 (D. Lovley, University of Massachusetts).
ParB cloning and extract preparation.
Wild-type parB genes of J2315 and other Burkholderiales were amplified by PCR from start to stop codon on genomic DNA. Primers were designed with a consensus Shine-Dalgarno sequence inserted 7 bp upstream of the start codon. parB genes were inserted under the control of the pBad promoter into the pDAG127 vector (35) with a deletion of the sopA gene by NheI/HindIII or EcoRI/HindIII digestion. Directed mutagenesis of the putative HTH of B. cenocepacia J2315 c3 and Ralstonia pickettii 12D plasmid 1parB genes in pDAG127 was ordered from GenScript. Recombinant plasmids, verified by sequencing, were electroporated into the Escherichia coli strain DLT812 (36). Cells grown in LB medium at 37°C to an optical density at 600 nm (OD600) of 0.25 were induced with 0.1% arabinose (final concentration), incubated for 4 h at 37°C, and chilled on ice for 10 min. Subsequent steps were carried out at 4°C. Cells were centrifuged, washed with TNE (50 mM Tris-HCl [pH 7.5], 50 mM NaCl, 1 mM EDTA), and resuspended in TNES (TNE, 20% sucrose) at an OD600 of 300. Lysozyme was added at 400 μg/ml; cells were then incubated for 5 min, frozen with liquid nitrogen, and thawed on ice. Lysis buffer (TNES, 660 mM NaCl, 4.5 mM dithiothreitol) was added to bring the OD600 to 200, the mixture was incubated on ice for 10 min, and the cells were lysed by sonication. The lysate was centrifuged for 15 min at 10,600 × g and the supernatant centrifuged at 20,800 × g. The final supernatant was then frozen with liquid nitrogen and stored at −20°C. Total protein concentrations were measured using the Bradford method (Bradford protein assay; Bio-Rad). Samples containing 3.5 μg of protein were electrophoresed in 4 to 12% polyacrylamide bis-tris denaturing gels (NuPage; Invitrogen) at a constant voltage of 200 V for 55 min and then stained with a Coomassie blue derivative (InstantBlue Expedeon) for estimation of ParB concentration.
Electrophoretic mobility shift assays (EMSA).
parS DNA probes obtained by annealing 26-base oligonucleotides (PAGE purified by Sigma) were 32P labeled using T4 polynucleotide kinase. Total protein extracts of E. coli (0.2 to 25 μg for initial titration tests and 10 μg for subsequent tests) were added to the probes (1 nM in 50 mM Tris-HCl [pH 7.5], 100 mM NaCl, 10% glycerol) on ice and then incubated for 10 min at room temperature prior to electrophoresis on 5% polyacrylamide gels in TBE buffer (90 mM Tris-borate, 1 mM EDTA) at 160 V for 2 h at 4°C. The gels were dried on Whatman DE81 paper and exposed to a PhosphorImager screen (Fuji). Proportions of shifted and unshifted probes were measured using the MultiGauge program (Fujifilm).
Bioinformatic analysis.
For detection of parS motifs, defined 14-bp palindromic sequences with a central CG were first used as queries at NCBI/BLAST, with the program adapted for short, nearly exact matches of the blastn suite on all bacterial genome sequences. A second analysis was carried out using the proteins ParA and ParB encoded by chromosome 2 of Ralstonia eutropha as queries to perform similarity searches with the BLASTP2 program of the BLAST suite (1). The search was carried out on all protein sequences deduced from the genome annotations of completely sequenced Burkholderiales genomes (chromosomes and plasmids) downloaded from the EBI site (http://www.ebi.ac.uk/genomes/) and incomplete genomes from the NCBI (http://www.ncbi.nlm.nih.gov/). The vicinity of parAB loci was explored using PatScan (13) for the presence of palindrome motifs of at least 14 bp with a central CG, with one mismatch allowed.
Multiple alignment was generated with MAFFT (31) and edited with Jalview (54). A maximum likelihood tree was generated with PhyML (25), using the JTT amino acid substitution model of evolution (29), four categories of substitution rate, and 1,000 bootstrap replicates. Tree topology, branch length, and substitution rate parameters were optimized. The tree was rooted using NJplot (46), drawn using Treedyn (11), and colored according to ParB subfamilies. Comparisons between chromosome 1 from Raltonia pickettii 12J and plasmid 2 from Ralstonia pickettii 12D sequences were performed with the Artemis Comparison Tool (8) (http://www.sanger.ac.uk/Software/ACT).
RESULTS
ParB/centromere selectivity in B. cenocepacia J2315.
When expressed in E. coli, the parAB operons of Burkholderia cenocepacia—c1, c2, c3, and pBC—each stabilize only the mini-F vector that carries its cognate parS (15), implying strict ParB/parS specificity. Nevertheless, similarities among the parS sites of the four replicons (Table 1) suggest some potential for cross-reaction.
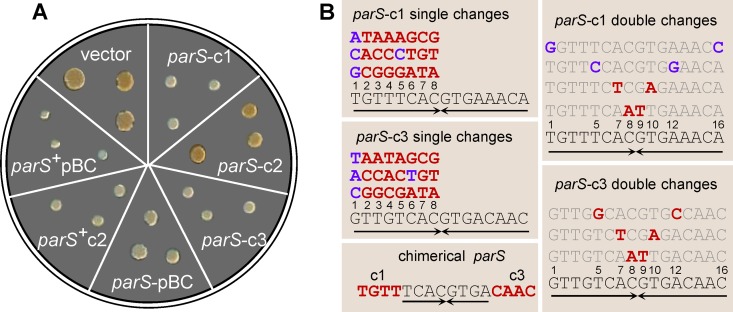
To investigate ParB-parS interactions in the natural host, we transformed B. cenocepacia with plasmids carrying each of the parS sites. Ability to bind ParB would provoke competition with native centromeres, which could well impair growth. Indeed, transformant colonies appeared later than those of cells transformed by the vector alone. The colony size 4 days after transformation is very reproducible and thus a sensitive indicator of growth impairment. In the case of c2 and pBC, the whole parS cluster (i.e., 5 and 3 parS sites, respectively; see operon structures in Fig. 3) was required for clear inhibition, whereas for c1 and c3 a single parS site was sufficient (Fig. 1A). We took advantage of the latter high sensitivity to assay the effects of all single base substitutions on one arm of the 16-bp palindromes parS-c1 and parS-c3 (Fig. 1B). In parS-c1 most single changes lead to loss of function, i.e., unperturbed growth of transformant colonies, whereas substitutions at position 1 and the transition T5C do not, i.e., maintenance of growth inhibition. Mutant parS-c1 sites with symmetric double changes at these positions remained partly (T1G-A16C) or fully (T5C-A12G) inhibitory for growth. Among parS-c3 mutants, those with changes at position 1 or the transition C6T imparted a degree of inhibition, the others none. As the 8-bp core sequences of parS-c1 and -c3 are identical, we tested a hybrid parS-c1/c3 (Fig. 1B). This sequence was not inhibitory, i.e., it did not compete in vivo with either parS-c1 or parS-c3.
Fig 3.
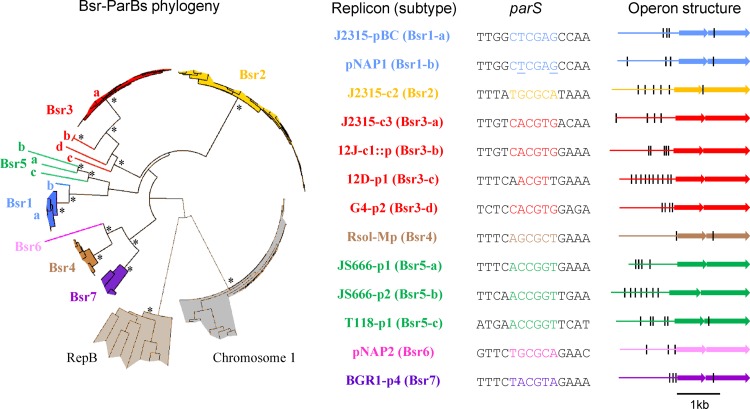
Bsr families and corresponding parS palindromes. Each color corresponds to one of the seven Bsr families. ParB proteins and loci are listed in Table S1 in the supplemental material. For clarity, only the bootstrap values superior to 800 concerning the deep branches are indicated (*). RepB proteins from Rhizobiales megaplasmids and chromosome 1 ParB proteins from Burkholderiales are indicated as outgroups. Protein and parS variations allow identification of 13 Bsr subtypes. Each subtype is exemplified (right) by one replicon: B. cenocepacia J2315 plasmid pBC (J2315-pBC), B. cenocepacia J2315 chromosome 2 (J2315-c2), B. cenocepacia J2315 chromosome 3 (J2315-c3), P. naphthalenivorans CJ2 plasmid 1 (pNAP1), P. naphthalenivorans CJ2 plasmid 2 (pNAP2), plasmid integrated in chromosome 1 of R. pickettii 12J (12J-c1::p), R. pickettii 12D plasmid 1 (12D-p1), B. vietnamiensis G4 plasmid 2 (G4-p2), R. solanacearum GMI 1000 megaplasmid (Rsol-Mp), Polaromonas sp. strain JS666 plasmid 1 (JS666-p1), Polaromonas sp. strain JS666 plasmid 2 (JS666-p2), R. ferrireducens T118 plasmid 1 (T118-p1), and Burkholderia glumae BGR1 plasmid 4 (BGR1-p4). The minimal 14-bp palindrome (parS) near the parAB operon is indicated. In the case of pNAP1 parS, underlined letters indicate positions of degeneracy. The structure of each parAB operon is shown (black line, minimal palindrome; first arrow, parA; second arrow, parB). ParA proteins vary from 217 residues (BGR1-p4) to 242 (JS666-p2), and ParB proteins vary from 290 (J2315-pBC) to 376 (JS666-p2).
Fig 1.
Extra parS sites inhibit growth in B. cenocepacia J2315. (A) Growth inhibition induced by wild-type parS sequences. Each sector shows three colonies of J2315-Mex1 4 days after transformation with the pMMB206 vector or recombinant derivatives carrying parS-c1, -c2, -c3, or -pBC in single copy or the parS-c2 and -pBC clusters (denoted parS+). (B) Mutated parS-c1 and parS-c3 sequences tested for growth inhibition. Each single-base change in parS-c1 and parS-c3 (left top and middle boxes) is shown above its corresponding wild-type base (numbered), in blue when silent (i.e., still growth inhibitory) or in red when leading to loss of function (i.e., allowing normal growth). Doubly mutated parS-c1 and parS-c3 (right boxes) are shown above the wild-type sequence, with changed bases in blue or red as described above. The chimeric parS-c1/c3 sequence, a loss-of-function mutant, is shown (bottom left), with the noncomplementary parS-c1 and parS-c3 ends indicated in red. Arrows, inverted repeated sequences.
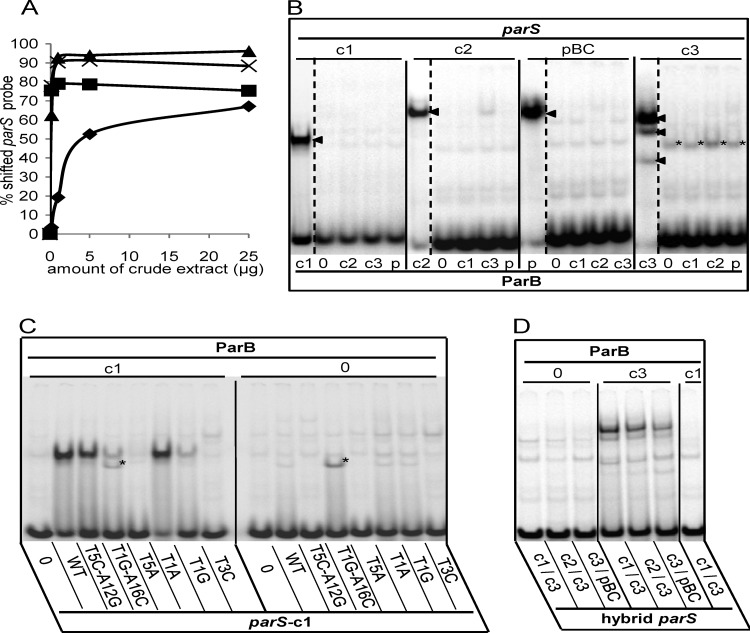
To complement these results, we carried out electrophoretic mobility shift assays (EMSA) using short duplex parS probes and extracts of ParB-overproducing Escherichia coli cells (see Materials and Methods). Extracts were used rather than purified ParB proteins, owing to the impracticability of purifying certain proteins which, despite strong transcription of their genes, could be obtained only at low concentrations. Because of variation of ParB concentrations between extracts (see Fig. S1 and Table S1 in the supplemental material), we determined the quantities of extract appropriate for EMSA by measuring the binding of each parS probe as a function of the quantity of extract protein containing the cognate ParB (Fig. 2A). For ParB-c2, -c3, and -pBC, 1 μg of extract gave maximal binding. For ParB-c1, >5 μg was required for a strong binding, even though this was the most concentrated of the ParB proteins. Variation in intrinsic affinity and in the active fraction of each ParB presumably accounts for the differences in binding efficiency. We carried out further EMSA using 10 μg for all ParB proteins, which ensures strong binding of the four ParB homologues to their cognate probes. Figure 2B shows that even at these higher-than-saturating ParB concentrations, each parS bound only its cognate ParB. For parS-c1, -c2, and -pBC, binding produced a single retarded species. In the case of parS-c3, three bands appeared. Although the two faster-migrating bands might be due to cleavage products of ParBc3, other explanations have not been ruled out (see the legend to Fig. 6).
Fig 2.
EMSA of the Par systems of B. cenocepacia. (A) Binding of each B. cenocepacia parS probe was tested with increasing amounts (0.2, 1, 5, and 25 μg) of crude extracts of E. coli cells overproducing its cognate ParB (▲, c3; ×, pBC; ■, c2; ◆, c1). (B) Radiolabeled parS probes, denoted above each group of lanes, were incubated with 10 mg of crude extract protein containing ParB-c1 (lanes c1), -c2 (lanes c2), -c3 (lanes c3), or -pBC (lanes p) or no ParB (lanes 0). The cognate ParB-parS interactions produce a single shifted complex in the case of c1, c2, and pBC and a three-band pattern in the case of c3 (arrowheads). The parS-c3 fragment is also retarded by an unknown E. coli protein (*). (C) Protein extracts containing ParB-c1 or no ParB, indicated by “c1” and “0” above the lane groups, were incubated with the radiolabeled 26-bp probes indicated below each lane as follows: negative-control sequence CTAGTCGTACGACTAG (lanes 0), wild-type parS-c1 (lanes WT), and parS-c1 mutated as indicated (other lanes). The mutated parS-c1 T1G-A16C fragment is shifted by an unknown E. coli protein (*). (D) Cell extracts containing no ParB, ParB-c3, or ParB-c1 (as indicated) were incubated with the hybrids indicated below each lane.
Fig 6.
Modifications in a putative HTH domain of ParB-Bsr3. (A) Comparison of the putative HTH domains of ParB-c3, -12J, -G4, and -12D. Amino acids identical to ParB-c3 are in black, others in red. Positions are numbered and presumed helices are shown. (B) EMSA of ParB proteins with the HTH domain exchanged (from −3 to 20 according to the numbering in panel A) or modified as shown above each panel, e.g., for c3A5K, ParB-c3 with Lys substituted for Ala at position 5. The parS probes are denoted below each lane as follows: 0, negative-control sequence CTAGTCGTACGACTAG; c3, parS-c3; D, parS Ralstonia pickettii 12D plasmid 1; J, main parS Ralstonia pickettii 12J. The parS-c3 fragment is also retarded by an unknown E. coli protein (*). Note that the three-band pattern typical of ParB-c3, possibly resulting from protein cleavage at sites distant from the HTH, is replaced by a single band after substitution with the 12D HTH. This exchange would not be expected to eliminate protease sensitivity of two sites, suggesting that the three-band pattern could have another explanation, such as formation by ParB-c3 of three types of complex with parS sites, each with a different gel electrophoretic mobility.
We then examined the binding of ParB-c1 and ParB-c3 to mutant parS-c1 sites. Mutant parS-c1 that no longer inhibited growth also failed to bind ParB-c1, as illustrated by T5A and T3C (Fig. 2C), while those that did inhibit growth, such as T1A and T1G, still bound (Fig. 2C). The noninhibitory hybrid parS-c1/c3 also failed to bind ParB-c1 (Fig. 2D). Binding affinities reflected relative in vivo inhibition in some cases (compare T5C-A12G with T1G-A16C in Fig. 2C) but not all (T1A and T1G mutants appeared equally inhibitory). In contrast, ParB-c3 exhibited distinct binding properties; e.g., the hybrid sequence parS-c1/c3, although noninhibitory, bound ParB-c3 with somewhat reduced affinity, forming the characteristic three-band pattern (Fig. 2D), as did two other chimerae not tested in vivo, c2/c3 and c3/pBC (Fig. 2D; see Table 1 and Fig. 3 for parS sequences). In vitro binding of ParB-c3 appears to tolerate changes in one arm of the cognate sequence. This result appears to be at odds with the in vivo tests, in which most changes in one arm of parS-c3 abolished inhibition. In vivo, however, parS sites entering on a plasmid compete for ParB with chromosomal parS sites. Moreover, an entering single parS-c3 site must compete with a natural cluster of parS sites that binds ParB more strongly (15). Correspondence between the in vivo test and EMSA with ParB-c3 nevertheless appears: e.g., C6T is the only internal mutant that maintains the inhibition, and accordingly, the double symmetric mutant C6T-G11A is the only one to show ParB binding comparable to that of the wild type (see Fig. 5).
Fig 5.
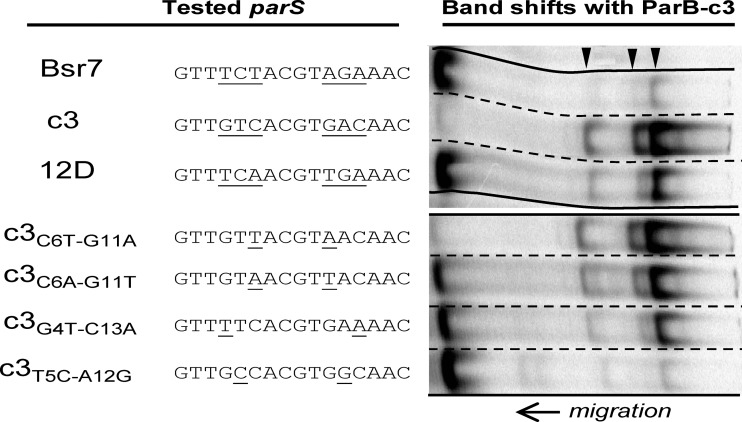
Binding of ParBc3 to parS-c3, parS-12D, parS-Bsr7, and related or mutated sequences was analyzed by EMSA. Positions 4, 5, and 6 and 11, 12, and 13 are underlined in parS-c3. Underlined bases in the other parS sequences differ from those in parS-c3. The characteristic three-band pattern of ParB-c3 is indicated by arrowheads.
These results confirm that ParB/parS systems of B. cenocepacia are highly specific and nonoverlapping. Some relaxation of specificity is nevertheless possible, suggesting that certain of these systems might cross-react with those of related bacteria.
Par families characteristic of secondary replicons of Burkholderiales.
We carried out a bioinformatic analysis of available eubacterial genomes to find centromeres similar to those of J2315. The criteria were (i) palindromes of at least 14 bp, (ii) a central CG dinucleotide, (iii) a T-rich 5′ end, and (iv) a location upstream of a parAB operon. We found such palindromes. In all cases, the adjacent parAB genes encode proteins with sizes similar to those of J2315 (Fig. 3). These loci were found only in species from the order Burkholderiales, most of them belonging to the family Burkholderiaceae (genera Ralstonia, Cupriavidus, and Burkholderia) and five to Comamonadaceae (genera Polaromonas and Rhodoferax). Essentially all these loci are in secondary chromosomes or large plasmids. The one apparent exception is a par locus on chromosome 1 (c1) of Ralstonia pickettii 12J; however, it appears to belong to a 380-kb plasmid integrated in the terminus region of c1. We identified 8 new palindromic parS motifs related to those of B. cenocepacia. On the basis of the phylogenies and the parS sequences, we could distinguish seven families of par loci, which we name Bsr (Burkholderiales secondary replicon) (Fig. 3). Bsr1 is the Par family of the plasmid pBC (15), and most Bsr1 ParB proteins are from Burkholderia plasmids, forming a narrow cluster (Bsr1-a [Fig. 3]). One Bsr1 ParB, more divergent, corresponds to the plasmid pNAP1 of Polaromonas naphthalenivorans CJ2 (Bsr1-b [Fig. 3]). Bsr1 members share the parS TTGGCTCGAGCCAA, but the pNAP1 parS set includes variants with a one-base transversion, T6G or G10T. Bsr2 is the family to which chromosome 2 parAB loci of Burkholderia species belong. Bsr3 consists mostly of a narrow cluster with a defined parS (Bsr3-a [Fig. 3]). This parS and the associated par genes appear characteristic of chromosome 3 of closely related species which together compose the Burkholderia cepacia complex (Bcc) (40, 53) rather than of Burkholderia in general. Three other Bsr3 ParB subtypes were found, each associated with a specific parS motif (Bsr3-b, -c, -d [Fig. 3]). Bsr4 is the family of Par systems of Ralstonia-Cupriavidus megaplasmid chromosome 2, previously identified (15, 39). Bsr5, Bsr6, and Bsr7 are novel. Bsr5 groups three Comamonadaceae plasmid Par systems whose parS sites share the core sequence ACCGGT. Bsr6 is defined by the Par system of plasmid 2 of Polaromonas naphthalenivorans CJ2. Bsr7 groups the par loci of Burkholderia glumae BGR1 plasmid 4 and some of the unassembled contigs of Burkholderia species (see Table S2 in the supplemental material).
ParB-parS cross-reactions within Bsr families.
The Bsr families with variant parS sites and ParB proteins appeared well suited to addressing the question of whether ParB proteins bind natural noncognate centromeres. The parB genes of representatives of Bsr families 1 to 6 and their subfamilies (Fig. 3) were amplified, cloned, and expressed in E. coli, and the ParB proteins in cell extracts were tested by EMSA for binding to various parS sites. All ParB/parS cognate shifts were detected (Fig. 2 and 4; see also Fig. S2 in the supplemental material) except for the three plasmids of the Bsr5 family whose poor ParB protein solubility precluded analysis.
Fig 4.
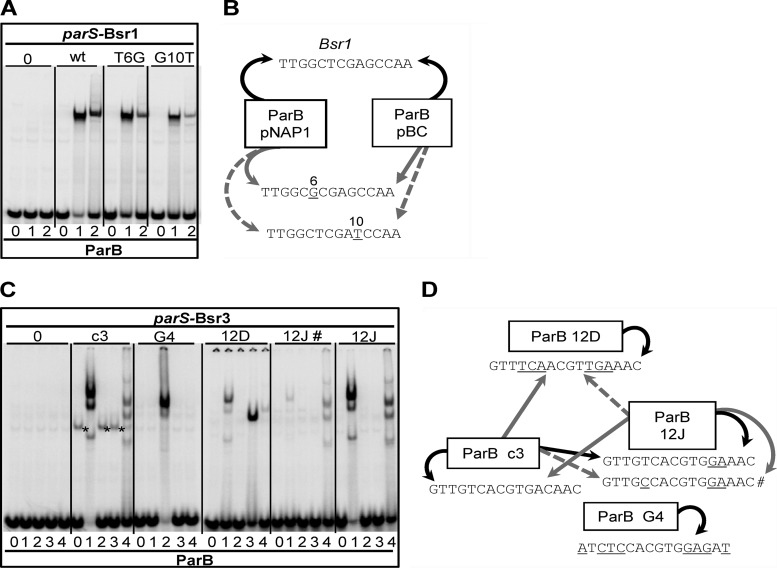
Cross-reactions in Bsr1 and Bsr3 families. (A) EMSA of Bsr1 ParB proteins and parS sites. Samples are grouped according to parS, indicated below: 0, negative-control sequence TAGTCGTACGACTA; wt, parS Bsr1, TTGGCTCGAGCCAA, common to pBC (Bsr1-a) and pNAP1 (Bsr1-b); T6G and G10T, parS derivatives exclusive to pNAP1. ParB proteins are denoted above parS as follows: 0, no ParB; 1, ParB-pBC; 2, ParB-pNAP1. (B) Bsr1 interaction summary. Arrows indicate that ParB in the box binds the parS pointed to. Curved and straight arrows represent cognate and noncognate binding, respectively. Black, gray, and dashed arrows correspond, respectively, to strong, medium, and weak binding relative to the specific binding. pNAP1-parS sequences are shown with bases changed relative to Bsr1 underlined and numbered. (C) EMSA of Bsr3 ParBs and parS sites. Samples are grouped according to parS: 0, negative-control sequence CTAGTCGTACGACTAG; c3, parS chromosome 3; G4, parS plasmid 2 of B. vietnamiensis G4; 12D, parS plasmid 1 of R. pickettii 12D; 12J #, secondary parS of R. pickettii 12J; 12J, main parS of R. pickettii 12J. ParB proteins are denoted as follows: 0, no-ParB extract; 1, ParB-c3; 2, ParB-G4; 3, ParB-12D; 4, ParB-12J. The parS-c3 fragment is also retarded by an unknown E. coli protein (*). (D) Bsr3 interaction summary. Arrows signify interactions as described for panel B. parS sequences are shown below each box, with bases changed relative to parS-c3 underlined.
We then sought cross-reactions between and within families whose parS elements shared sequence similarity. None were found among the Bsr2, Bsr4, and Bsr6 families (Fig. S2 in the supplemental material). On the other hand, the Bsr1 ParB of pBC bound not only to the parS characteristic of its family but also to the two variant parS sites specific to the Bsr1-b locus of pNAP1. Moreover, the pNAP1 and pBC ParB proteins showed the same order of affinity for the three parS sites: wild type > T6G > G10T (Fig. 4A and B). This result confirms the phylogenic link between the two ParBs and suggests that the changes in the parS sites of pNAP1 affect bases not essential for binding of these proteins.
Cross-reaction was also found in Bsr3. This result was more surprising because proteins and parS sites of this family exhibit considerable divergence. The main subtype (Bsr3-a [Fig. 3]) is that of B. cenocepacia J2315 chromosome 3 (c3). The other subtypes, i.e., Bsr3-b, -c, and -d (Fig. 3), are from, respectively, (i) a plasmid integrated into chromosome 1 of Ralstonia pickettii 12J (referred to as 12J; this system is also found on plasmid 2 of Ralstonia pickettii 12D), (ii) plasmid 1 of Ralstonia pickettii 12D (referred to as 12D), and (iii) plasmid 2 of Burkholderia vietnamiensis G4 (referred to as G4).
Cross-recognition between these subtypes was tested by EMSA (Fig. 4C and D). ParB-c3 bound the incomplete palindromic parS-12J almost as strongly as its own parS and more strongly than the nonpalindromic hybrid parS sites shown in Fig. 2D. Reciprocally, ParB-12J bound parS-c3 strongly. Also, both proteins formed a multiband pattern, further implying their relatedness. There are nevertheless obvious differences in the binding specificities of these proteins; e.g., (i) binding to the T5C variant of parS-12J (parS-12J# in Fig. 4C and D) was scarcely detected, whereas ParB-12J showed significant binding; (ii) ParB-c3 bound parS-12D better than ParB-12J did; and (iii) unlike parS-c3, parS-12J was not inhibitory when introduced into B. cenocepacia.
ParB-c3 bound to parS-12D, which was surprising given that parS-12D diverges from parS-c3 by three substitutions in each arm: G4T, T5C, and C6A and, symmetrically, C13A, A12G, and G11T. Bsr7-parS (Fig. 3) differs from parS-c3 by the same substitutions as parS-12D except with C6T-G11A instead of C6A-G11T. However, Bsr7-parS was bound by ParB-c3 very weakly (Fig. 5), which led us to examine the role of each of the symmetrical base change pairs in determining binding of ParB-c3 (Fig. 5). By itself, T5C-A12G, common to parS-12D and parS-Bsr7, drastically reduced binding. G4T-C13A, also common to both parS sites, allowed moderate binding. C6T-G11A, exclusive to parS-Bsr7, allowed binding comparable to that of the wild type, and C6A-G11T, exclusive to parS-12D, allowed strong binding. It appears that parS-12D is bound by ParB-c3 specifically despite its differences from parS-c3, which include even the detrimental T5C-A12G double change.
Identification of a domain involved in specific binding.
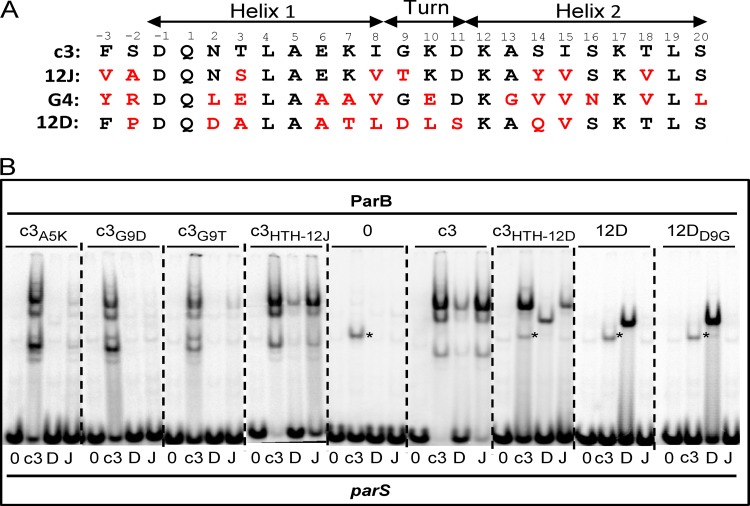
The foregoing results show that in vitro, ParB-c3 binds different parS motifs of the Bsr3 family, notably parS-12D, while ParB-12D fails to bind parS-c3. What are the determinants responsible for such differences? Typically, a helix-turn-helix (HTH) constitutes the core of the DNA-binding domain of ParB proteins. The prediction of an HTH motif for ParB-c3 was weak (15), and we have found this to be so for the other Bsr3-ParB subtypes. Nevertheless, protein alignments revealed amino acid conservation in the region where most ParB proteins have a putative or verified HTH domain. Moreover, certain observations are compatible with the assignment of DNA recognition and binding to this region of ParB: (i) mutation of the highly conserved Ala5 of helix 1 (Fig. 6A) reduced binding of ParB-c3 to both cognate and noncognate parS sites (Fig. 6B, c3A5K lanes); (ii) replacement of Gly 9 of ParB-c3 by Asp, as in ParB-12D, or by Thr, as in ParB-12J, reduces binding to parS (Fig. 6B, c3G9D and c3G9T lanes); (iii) in contrast, replacement of Asp 9 by Gly in ParB-12D results in binding at least as strong as that with the wild type (Fig. 6B). Points ii and iii corroborate the role of Gly 9 in ParB-c3 as the highly conserved interhelix hinge residue of HTH motifs. Hence, although designation of these regions as HTH domains is provisional, we refer to them as such hereafter.
To analyze its role in centromere binding, we exchanged the whole HTH of ParB-c3, as depicted in Fig. 6A, for those of ParB-G4, -12J, and -12D. The three hybrid ParB-c3 proteins were produced at comparable concentrations (see Fig. S1 and Table S1 in the supplemental material) and were functional in the sense that they all bound to parS-c3 and parS-12J. ParB-c3HTH-12J binding is little impaired compared with that of ParB-c3, even though the amino acid changes include G9T, which by itself reduces binding strongly (Fig. 6B; compare lanes c3G9T, c3HTH-12, and c3), implying that the exchanged region acts as a functional unit. Nevertheless, no clear shift in binding specificity was seen with the G4 (data not shown) and 12J hybrids, suggesting that some determinants of specificity lie outside the HTH.
On the other hand, the binding characteristics of ParB-c3HTH-12D plainly differed from those of ParB-c3. While ParB-c3 bound each of the three parS probes to form the same pattern of three retarded bands, ParB-c3HTH12D formed a single complex with parS-12D which migrated like that formed with its cognate ParB, indicating a clear alteration in binding behavior associated with the exchange of putative HTH motifs. The change in specificity is partial, however, since like ParB-c3 but unlike ParB-12D, the 12D hybrid binds to the c3 and 12J parS sites. These results imply that in the Bsr3 group of ParB proteins, elements both inside and outside the putative HTH motif determine parS binding specificity.
DISCUSSION
Although the strong similarities among the Par systems of B. cenocepacia could lead one to expect that they interact, the results of all tests applied in vivo (stabilization in E. coli and growth inhibition in B. cenocepacia) and in vitro (EMSA) attest to their nonoverlapping specificities. It is rather in the taxonomic neighborhood of B. cenocepacia that we have discovered cross-reactions between Par systems allowing us to propose evolutionary relationships.
The Bsr families of Burkholderiales and their centromeres.
To identify Par systems similar to those of B. cenocepacia J2315, we focused our analysis on the search for new centromeres. Livny et al. (39), using sequence matrices based on the chromosomal parS sites then known, revealed the wide distribution of such sites among bacterial species and replicons but identified no new centromere motifs. Our search was based on the centromere structure, i.e., palindromes of at least 14 bp with a central 5′-CG dinucleotide. It led to the identification of 8 new parS motifs, including one in Rhodoferax ferrireducens plasmid 1 (Bsr5-c [Fig. 3]) missed in the previous analysis. We brought to light seven families of such Par systems, all exclusive to secondary chromosomes and large plasmids of Burkholderiales, which by itself implies a phylogenetic linkage. Incidentally, in the case of Bsr3-a, i.e., the Par subtype characteristic of the chromosome 3 of Bcc members, this feature could aid in identification of Bcc pathogens. For example, the pathogen Burkholderia ubonensis, classified with difficulty as a Bcc member because of an atypical phenotype (53), has the Bsr3-a subtype. Conversely, Burkholderia xenovorans has a third chromosome, like Bcc members, but does not belong to the Bcc and, accordingly, does not carry a Bsr3-a subtype.
A notable parallel to the Bsr family of Par systems is found in the Rhizobiales, whose secondary replicons and megaplasmids are characterized by loci (repABC) that combine both partition (RepAB, analogous to ParAB) and replication (RepC) functions (10). As in the Bsr families, the parS sites of RepAB families are palindromes of 16 bp with a T-rich 5′ end. The only prominent difference is that whereas the central dinucleotide in Bsr-parS sites is CG, in Rep-parS sites it is GC. Rhizobiales and Burkholderiales belong to different classes, Alphaproteobacteria and Betaproteobacteria, respectively, but are both remarkable for the frequency of species that exhibit division of their genome into large replicons. Although the advantage conferred by split genomes is unclear, the diversification of an ancestral partition system (Rep or Bsr) in compatible descendants would have been central to the stability of large secondary replicons.
Further analysis of the newly identified parS families reveals some interesting aspects. The internal 14 bp appears to be the essential component of parS palindromes: although palindromes may extend to 16 bp, positions 1 and 16 are naturally the most variable, and our tests of parS-c1 and parS-c3 in vivo and in vitro show that these positions are less important. On the other hand, the 6-bp core of Bsr centromeres varies less than could be expected on the basis of the core sequences of B. cenocepacia c2, c3, and pBC and of Ralstonia solanacearum c2, i.e., the models for our search for new centromeres. These sequences, TGCGCA, CACGTG, CTCGAG, and AGCGCT, respectively, all consist of 2 C's, 2 G's, 1 A, and 1 T (Table 1). Keeping the central CG and the palindromic structure, this base composition rule would predict four additional sequences: ACCGGT, GACGTC, GTCGAC, and TCCGGA. We found the core ACCGGT in the Bsr5 family (Fig. 3) but were unable to find the three other motifs within putative centromeres. Unless genomes containing such parS sites have not yet been sequenced, this result suggests tighter constraint on parS base composition. However, some families, e.g., Bsr5 and Bsr3, display clear variation in parS (border or core sequences) associated with ParB variants (Fig. 3). This observation suggests that an ancestral ParB-parS cognate pair may relax its specificity, coevolve, and generate a diversity of subtypes.
Binding of ParB-c3 to family centromeres: is it ancestral?
The Bsr3 family includes four clearly distinct Par subtypes: C3 (chromosome 3 of J2315 and all Bcc members), G4 (B. vietnamiensis G4 plasmid 2), 12J (R. pickettii 12J plasmid integrated in chromosome 1), and 12D (R. pickettii 12D plasmid 1). Bsr3 appeared well-suited to testing ParB-parS cross-interaction within the family and to tracking a Par evolutionary pathway. In assessing which of these Par systems is closest to an ancestral form, we assume that an ancestor possesses the most general properties and that descendants acquire a more restricted binding specificity that allows compatibility with other Par systems. G4 is an example of the latter. B. vietnamiensis G4 is a Bcc member, and as such, it carries a C3 system. Accordingly, G4 and C3 do not cross-react. Indeed, despite their common core, CACGTG, parS-G4 and parS-c3 differ by 8 bases, and the ParB-G4 and ParB-c3 HTH domains differ at many residues (Fig. 6A), suggesting evolution for compatibility between G4 and C3.
In contrast, there is no compatibility constraint between subtypes C3 and 12J or between C3 and 12D, C3 being from Burkholderia, the latter two from Ralstonia. We found indeed that ParB-c3 binds to parS-12J and, more weakly, to parS-12D. 12J has one arm of parS identical to one parS-c3 arm, and its ParB HTH is similar to that of C3 (Fig. 6A). These similarities explain the cross-reaction between 12J and C3. In contrast, the cross-reaction between 12D and C3, even though weak, is unexpected. The HTH domain of ParB-12D strongly diverges from that of ParB-c3 (Fig. 6A), and parS-12D differs from parS-c3 by three double changes (positions 4 to 6 and symmetric 11 to 13). Besides, one of these changes introduced alone in parS-c3 (positions 5 and 12 [Fig. 5]) almost completely abolished the binding to ParB-c3 (Fig. 5). It appears that binding of ParB-c3 to parS-12D is based not on mere residual affinity for a degenerate site but on the recognition of this parS in its entirety.
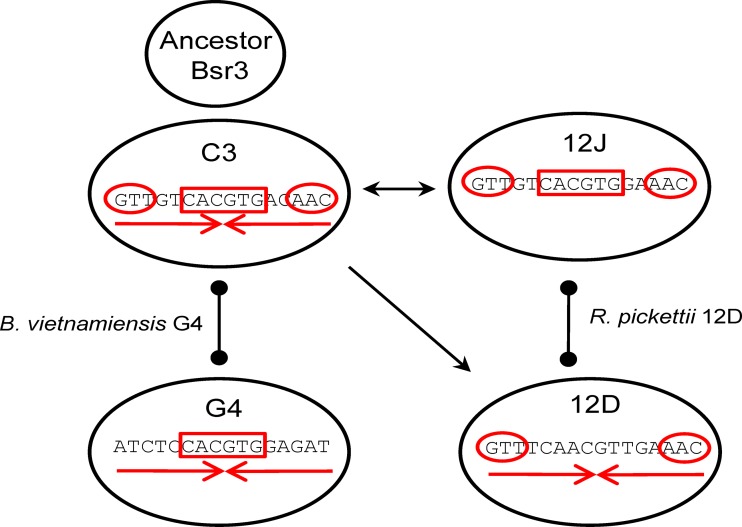
ParB-c3 is the least specialized of the Bsr3 proteins tested. Its HTH domain has an interhelix glycine, a feature of most HTHs which was adaptable here to the HTH of ParB-12D. In contrast, the HTH of ParB-12D has an atypical interhelix Asp, which prevents significant binding of the hybrid protein to noncognate parS sites, suggesting that the HTH domain of ParB-12D is more specialized. Besides, ParB-c3 binds noncognate parS, even the distantly related parS-12D. The frame of ParB-c3 is sufficiently adaptable to accommodate related DNA-binding domains in functional form. The swapped ParB-c3HTH12D even binds parS-12D, producing a single retarded species similar to that observed with ParB-12D (Fig. 6B). These observations argue for ParB-c3 as the ancestor of the Bsr3 group. Likewise, parS-c3, with its symmetrical form, core CACGTG, and terminal GTT, appears ancestral; each related parS has lost one of these features—12J the symmetry, 12D the core, and G4 the ends (see Fig. 7 for a summary).
Fig 7.
Evolution of an ancestral Bsr3-Par system. The Par system of Burkholderia cepacia complex chromosome 3 (C3) is proposed to be the most likely ancestor. Its ParB has a relatively wide binding specificity, and its parS comprises the features of the other systems: GTT ends (red circles), CACGTG core (red rectangle), and symmetry (red convergent arrows). An ancestral plasmid form would have been captured and frozen by C3 but would have spread on other plasmid replicons and diverged to generate the Par systems 12J, 12D, and G4, each with a parS having lost one feature. Where evolution has proceeded in different species, without the requirement for compatibility, ParB-parS cross-reaction can be detected (arrows). When managing two replicons in the same species (e.g., B. vietnamiensis G4 and R. pickettii 12D), Par systems must diverge for the replicons to be compatible and ParB-parS cross-reaction is detected barely or not at all (ball lines).
Is a third chromosome emerging in Ralstonia pickettii?
R. pickettii 12D carries not only a 12D Bsr3-Par subtype, on its plasmid 1, but also a 12J subtype, on its 270-kb plasmid 2. The latter is an exact copy—proteins, parS sequence, and repeat arrangement—of that found in R. pickettii 12J, on a 380-kb replicon integrated in c1. Remarkably, the 380-kb integrant of R. pickettii 12J corresponds to the 270-kb plasmid 2 of R. pickettii 12D (Fig. 8), sharing with it blocks of DNA 99% identical. The integrant, however, has been enlarged to 380 kb by the acquisition of blocks of heterologous DNA. Presumably this accretion can continue, up to and beyond the eventual excision of the replicon. It is frequently proposed that secondary chromosomes are derived from plasmids by horizontal gene transfer and genome shuffling. We seem to be witnessing this situation in R. pickettii 12J. This strain is a melting pot from which a chromosome 3 is emerging, under the necessary constraints of a Par system (subtype 12J) that is most similar to the Par system (subtype C3) controlling chromosome 3 of the Burkholderia cepacia complex.
Fig 8.
Ralstonia pickettii 12J chromosome 1 compared to R. pickettii 12D plasmid 2. Ralstonia pickettii 12J chromosome 1, nucleotides 1,560,000 to 1,950,000, is represented at the top, the whole Ralstonia pickettii 12D plasmid 2 at the bottom. They share extensive sequence homology and gene order. Conserved blocks with 99% identity are shown in red. Blue blocks correspond to inversions with sequence conservation above 80%. Duplications of 6 kb, presumably arising during plasmid integration, are highlighted as yellow blocks. (See also Materials and Methods.)
Supplementary Material
ACKNOWLEDGMENTS
We thank M. Arlat, D. Lovley, T. Marsh, and J. Tiedje for providing genomic DNA and strains of Ralstonia solanacearum, Rhodoferax ferrireducens, Ralstonia pickettii 12J and 12D, and Burkholderia vietnamiensis G4. We also thank the diligent reviewers whose remarks improved the manuscript. We are grateful to J.-Y. Bouet for advice on EMSA and to J. Rech for technical assistance and logistics.
This work was supported by ANR grant 06-BLAN-0280-01.
Footnotes
Published ahead of print 20 April 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Altschul SF, et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balzer D, Ziegelin G, Pansegrau W, Kruft V, Lanka E. 1992. KorB protein of promiscuous plasmid RP4 recognizes inverted sequence repetitions in regions essential for conjugative plasmid transfer. Nucleic Acids Res. 20:1851–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barillà D, Rosenberg MF, Nobbmann U, Hayes F. 2005. Bacterial DNA segregation dynamics mediated by the polymerizing protein ParF. EMBO J. 24:1453–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bartosik AA, Jagura-Burdzy G. 2005. Bacterial chromosome segregation. Acta Biochim. Pol. 52:1–34 [PubMed] [Google Scholar]
- 5. Bignell C, Thomas CM. 2001. The bacterial ParA-ParB partitioning proteins. J. Biotechnol. 91:1–34 [DOI] [PubMed] [Google Scholar]
- 6. Bouet J-Y, Ah-Seng Y, Benmeradi N, Lane D. 2007. Polymerization of SopA partition ATPase: regulation by DNA binding and SopB. Mol. Microbiol. 63:468–481 [DOI] [PubMed] [Google Scholar]
- 7. Campbell CS, Mullins RD. 2007. In vivo visualization of type II plasmid segregation: bacterial actin filaments pushing plasmids. J. Cell Biol. 179:1059–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carver TJ, et al. 2005. ACT: the Artemis Comparison Tool. Bioinformatics 21:3422–3423 [DOI] [PubMed] [Google Scholar]
- 9. Castaing J-P, Bouet J-Y, Lane D. 2008. F plasmid partition depends on interaction of SopA with non-specific DNA. Mol. Microbiol. 70:1000–1011 [DOI] [PubMed] [Google Scholar]
- 10. Cevallos MA, Cervantes-Rivera R, Gutiérrez-Ŕios RM. 2008. The repABC plasmid family. Plasmid 60:19–37 [DOI] [PubMed] [Google Scholar]
- 11. Chevenet F, Brun C, Bañuls A-L, Jacq B, Christen R. 2006. TreeDyn: towards dynamic graphics and annotations for analyses of trees. BMC Bioinformatics 7:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davis MA, Martin KA, Austin SJ. 1990. Specificity switching of the P1 plasmid centromere-like site. EMBO J. 9:991–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dsouza M, Larsen N, Overbeek R. 1997. Searching for patterns in genomic data. Trends Genet. 13:497–498 [DOI] [PubMed] [Google Scholar]
- 14. Dubarry N, Du W, Lane D, Pasta F. 2010. Improved electrotransformation and decreased antibiotic resistance of the cystic fibrosis pathogen Burkholderia cenocepacia strain J2315. Appl. Environ. Microbiol. 76:1095–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dubarry N, Pasta F, Lane D. 2006. ParABS systems of the four replicons of Burkholderia cenocepacia: new chromosome centromeres confer partition specificity. J. Bacteriol. 188:1489–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ebersbach G, Gerdes K. 2001. The double par locus of virulence factor pB171: DNA segregation is correlated with oscillation of ParA. Proc. Natl. Acad. Sci. U. S. A. 98:15078–15083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Egan ES, Fogel MA, Waldor MK. 2005. Divided genomes: negotiating the cell cycle in prokaryotes with multiple chromosomes. Mol. Microbiol. 56:1129–1138 [DOI] [PubMed] [Google Scholar]
- 18. Fothergill TJG, Barillà D, Hayes F. 2005. Protein diversity confers specificity in plasmid segregation. J. Bacteriol. 187:2651–2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Funnell BE, Gagnier L. 1993. The P1 plasmid partition complex at parS. II. Analysis of ParB protein binding activity and specificity. J. Biol. Chem. 268:3616–3624 [PubMed] [Google Scholar]
- 20. Gallie DR, Kado CI. 1987. Agrobacterium tumefaciens pTAR parA promoter region involved in autoregulation, incompatibility and plasmid partitioning. J. Mol. Biol. 193:465–478 [DOI] [PubMed] [Google Scholar]
- 21. Gerdes K, Møller-Jensen J, Bugge Jensen R. 2000. Plasmid and chromosome partitioning: surprises from phylogeny. Mol. Microbiol. 37:455–466 [DOI] [PubMed] [Google Scholar]
- 22. Gerdes K, Howard M, Szardenings F. 2010. Pushing and pulling in prokaryotic DNA segregation. Cell 141:927–942 [DOI] [PubMed] [Google Scholar]
- 23. Godfrin-Estevenon A-M, Pasta F, Lane D. 2002. The parAB gene products of Pseudomonas putida exhibit partition activity in both P. putida and Escherichia coli. Mol. Microbiol. 43:39–49 [DOI] [PubMed] [Google Scholar]
- 24. Gruber S, Errington J. 2009. Recruitment of condensin to replication origin regions by ParB/SpoOJ promotes chromosome segregation in B. subtilis. Cell 137:685–696 [DOI] [PubMed] [Google Scholar]
- 25. Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52:696–704 [DOI] [PubMed] [Google Scholar]
- 26. Hatano T, Yamaichi Y, Niki H. 2007. Oscillating focus of SopA associated with filamentous structure guides partitioning of F plasmid. Mol. Microbiol. 64:1198–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holden MTG, et al. 2009. The genome of Burkholderia cenocepacia J2315, an epidemic pathogen of cystic fibrosis patients. J. Bacteriol. 191:261–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Isles A, et al. 1984. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J. Pediatr. 104:206–210 [DOI] [PubMed] [Google Scholar]
- 29. Jones DT, Taylor WR, Thornton JM. 1992. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 8:275–282 [DOI] [PubMed] [Google Scholar]
- 30. Kadoya R, Baek JH, Sarker A, Chattoraj DK. 2011. Participation of chromosome segregation protein ParAI of Vibrio cholerae in chromosome replication. J. Bacteriol. 193:1504–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Katoh K, Kuma K, Toh H, Miyata T. 2005. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 33:511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim HJ, Calcutt MJ, Schmidt FJ, Chater KF. 2000. Partitioning of the linear chromosome during sporulation of Streptomyces coelicolor A3(2) involves an oriC-linked parAB locus. J. Bacteriol. 182:1313–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Larsen RA, et al. 2007. Treadmilling of a prokaryotic tubulin-like protein, TubZ, required for plasmid stability in Bacillus thuringiensis. Genes Dev. 21:1340–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee PS, Grossman AD. 2006. The chromosome partitioning proteins Soj (ParA) and Spo0J (ParB) contribute to accurate chromosome partitioning, separation of replicated sister origins, and regulation of replication initiation in Bacillus subtilis. Mol. Microbiol. 60:853–869 [DOI] [PubMed] [Google Scholar]
- 35. Lemonnier M, Bouet JY, Libante V, Lane D. 2000. Disruption of the F plasmid partition complex in vivo by partition protein SopA. Mol. Microbiol. 38:493–505 [DOI] [PubMed] [Google Scholar]
- 36. Lemonnier M, Lane D. 1998. Expression of the second lysine decarboxylase gene of Escherichia coli. Microbiology 144(Part 3):751–760 [DOI] [PubMed] [Google Scholar]
- 37. Leonard TA, Butler PJG, Löwe J. 2004. Structural analysis of the chromosome segregation protein Spo0J from Thermus thermophilus. Mol. Microbiol. 53:419–432 [DOI] [PubMed] [Google Scholar]
- 38. Lin DC, Grossman AD. 1998. Identification and characterization of a bacterial chromosome partitioning site. Cell 92:675–685 [DOI] [PubMed] [Google Scholar]
- 39. Livny J, Yamaichi Y, Waldor MK. 2007. Distribution of centromere-like parS sites in bacteria: insights from comparative genomics. J. Bacteriol. 189:8693–8703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mahenthiralingam E, Urban TA, Goldberg JB. 2005. The multifarious, multireplicon Burkholderia cepacia complex. Nat. Rev. Microbiol. 3:144–156 [DOI] [PubMed] [Google Scholar]
- 41. Møller-Jensen J, Jensen RB, Löwe J, Gerdes K. 2002. Prokaryotic DNA segregation by an actin-like filament. EMBO J. 21:3119–3127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Morales VM, Bäckman A, Bagdasarian M. 1991. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene 97:39–47 [DOI] [PubMed] [Google Scholar]
- 43. Mori H, Kondo A, Ohshima A, Ogura T, Hiraga S. 1986. Structure and function of the F plasmid genes essential for partitioning. J. Mol. Biol. 192:1–15 [DOI] [PubMed] [Google Scholar]
- 44. Murray H, Errington J. 2008. Dynamic control of the DNA replication initiation protein DnaA by Soj/ParA. Cell 135:74–84 [DOI] [PubMed] [Google Scholar]
- 45. Ogura T, Hiraga S. 1983. Partition mechanism of F plasmid: two plasmid gene-encoded products and a cis-acting region are involved in partition. Cell 32:351–360 [DOI] [PubMed] [Google Scholar]
- 46. Perrière G, Gouy M. 1996. WWW-query: an on-line retrieval system for biological sequence banks. Biochimie 78:364–369 [DOI] [PubMed] [Google Scholar]
- 47. Saint-Dic D, Frushour BP, Kehrl JH, Kahng LS. 2006. A parA homolog selectively influences positioning of the large chromosome origin in Vibrio cholerae. J. Bacteriol. 188:5626–5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schumacher MA. 2008. Structural biology of plasmid partition: uncovering the molecular mechanisms of DNA segregation. Biochem. J. 412:1. [DOI] [PubMed] [Google Scholar]
- 49. Simpson AE, Skurray RA, Firth N. 2003. A single gene on the staphylococcal multiresistance plasmid pSK1 encodes a novel partitioning system. J. Bacteriol. 185:2143–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tang M, Bideshi DK, Park H-W, Federici BA. 2007. Iteron-binding ORF157 and FtsZ-like ORF156 proteins encoded by pBtoxis play a role in its replication in Bacillus thuringiensis subsp. israelensis. J. Bacteriol. 189:8053–8058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tinsley E, Khan SA. 2006. A novel FtsZ-like protein is involved in replication of the anthrax toxin-encoding pXO1 plasmid in Bacillus anthracis. J. Bacteriol. 188:2829–2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Toro E, Hong S-H, McAdams HH, Shapiro L. 2008. Caulobacter requires a dedicated mechanism to initiate chromosome segregation. Proc. Natl. Acad. Sci. U. S. A. 105:15435–15440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vanlaere E, et al. 2008. Burkholderia latens sp. nov., Burkholderia diffusa sp. nov., Burkholderia arboris sp. nov., Burkholderia seminalis sp. nov. and Burkholderia metallica sp. nov., novel species within the Burkholderia cepacia complex. Int. J. Syst. Evol. Microbiol. 58:1580–1590 [DOI] [PubMed] [Google Scholar]
- 54. Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. 2009. Jalview Version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics 25:1189–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wu M, et al. 2011. Segrosome assembly at the pliable parH centromere. Nucleic Acids Res. 39:5082–5097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yamaichi Y, Niki H. 2000. Active segregation by the Bacillus subtilis partitioning system in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 97:14656–14661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yamaichi Y, Fogel MA, McLeod SM, Hui MP, Waldor MK. 2007. Distinct centromere-like parS sites on the two chromosomes of Vibrio spp. J. Bacteriol. 189:5314–5324 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.