Abstract
Strains of enterohemorragic Escherichia coli (EHEC) O157:H7 that are non-sorbitol fermenting (NSF) and β-glucuronidase negative (GUD−) carry a large virulence plasmid, pO157 (>90,000 bp), whereas closely related sorbitol-fermenting (SF) E. coli O157:H− strains carry plasmid pSFO157 (>120,000 bp). GUD+ NSF O157:H7 strains are presumed to be precursors of GUD− NSF O157:H7 strains that also carry pO157. In this study, we report the complete sequence of a novel virulence plasmid, pO157-2 (89,762 bp), isolated from GUD+ NSF O157:H7 strain G5101. PCR analysis confirmed the presence of pO157-2 in six other strains of GUD+ NSF O157:H7. pO157-2 carries genes associated with virulence (e.g., hemolysin genes) and conjugation (tra and trb genes) but lacks katP and espP present in pO157. Comparative analysis of the three EHEC plasmids shows that pO157-2 is highly related to pO157 and pSFO157 but not ancestral to pO157. These results indicated that GUD+ NSF O157:H7 strains might not be direct precursors to GUD− NSF O157:H7 as previously proposed but rather have evolved independently from a common ancestor.
INTRODUCTION
Non-sorbitol-fermenting (NSF) enterohemorrhagic Escherichia coli (EHEC) of serotype O157:H7 frequently causes large outbreaks of severe enteric infections including bloody diarrhea, hemorrhagic colitis, and hemolytic uremic syndrome worldwide (7, 10). It has been proposed that highly pathogenic E. coli O157:H7 arose from an ancestral enteropathogenic E. coli (EPEC) O55:H7 through sequential acquisition of virulence factors, phenotypic traits, and serotypic change (8, 9, 28). In this evolutionary model for O157:H7, six clonal complexes (A1 to A6) carrying characteristic traits were proposed. Ancestral O55:H7 EPEC strains belong to clonal complexes (CCs) A1 and A2. The somatic (O) antigen change from O55 to O157 gave rise to a hypothetical intermediary (CC A3) (29). This was followed by a divergence in two separate O157 clonal lineages of nonmotile sorbitol-fermenting (SF) O157:H− strains (A4) and non-sorbitol-fermenting (NSF) O157:H7 strains (CC A5). The most typical O157:H7 phenotype at present (CC A6) is believed to have emerged from CC A5 strains by the loss of β-glucuronidase activity (GUD−), caused by a mutational inactivation of the uidA (9). These CC A6 strains have spread widely into disparate locales and now account for most food-borne illness caused by EHEC (29).
The pathogenicity of O157:H7 is among other factors associated with the possession of a large EHEC virulence plasmid. CC A6 strains carry pO157 (∼90 kb), and CC A4 strains carry a larger plasmid (∼120 kb), namely, pSFO157 (2). Different genes and gene clusters including the EHEC hemolysin (hly) operon located on pO157 have been linked to virulence (21, 23). pO157 furthermore carries katP, encoding a periplasmic catalase-peroxidase (3); a type II secretion system (etp operon) (22), toxB, involved in adherence (26); and espP, encoding a secreted serine protease (4). Although NSF O157 and SF O157 strains are closely related and share virulence factors such as the attaching and effacing (eae) locus and Shiga toxin (stx) genes, pSFO157-carrying strains do not show hemolytic activity despite carrying the hyl operon and lacking katP and espP. Ancestral O55:H7 strains carry pO55, which does not possess most virulence genes present on pO157 or pSFO157; however, pO55 does contain the etp operon (31). All three plasmids contain incomplete tra gene clusters and thus are presumably incapable of conjugative transfer, as many of the missing genes are required for conjugation (16). Thereby, it appears that the plasmids were inherited from a common ancestor of O157:H7 and O55:H7 but have undergone major structural changes (31).
Brunder at al. (1) suggested that pO157 and pSFO157 evolved from a common ancestor plasmid probably present in CC A3 by the acquisition and loss of various genes due to insertion/transposition events and possibly plasmid fusion. Results by Rump et al. (20) showed that the virulence plasmid in CC A5 strains lack some of the pO157 regions containing insertion sequence 629 (IS629), suggesting the presence of a different plasmid. Due to these discrepancies with the proposed stepwise emergence of E. coli O157:H7, we determined the complete sequence of the plasmids present in CC A5 strain (G5101) and two CC A4 strains (493/89 and H2687) in order to gain an insight into the emergence of pO157 and pSFO157.
MATERIALS AND METHODS
Bacterial strains.
Strains used in this study are listed in Table 1. The SF O157 strains (493/89 and H2687) were originally isolated in Germany (12) and Scotland (27) and carry stx2, representing strains of CC A4 (9). GUD+ NSF O157:H7 strain G5101 harbors both stx1 and stx2 and belongs to CC A5. All strains examined were from the culture collection of the Division of Microbiology at the U.S. Food and Drug Administration (FDA), Center for Food Safety and Applied Nutrition (CFSAN). Strains were grown overnight in Luria-Bertani (LB) medium at 37°C with shaking (250 rpm).
Table 1.
Serotype, sequence type, characteristics, and isolation information of E. coli strains used in this study
| Strain | Serotype | stxa | Special characteristics |
STd | CCe | Sourcef | Countryf | Yearf | Accession no. or reference | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GUDb | SORc | Plasmid | |||||||||
| Sakai | O157:H7 | 1,2 | − | − | pO157 | 66 | A6 | Human | Japan | 1996 | NC_002695 |
| EDL933 | O157:H7 | 1,2 | − | − | pO157 | 66 | Food | USA | 1982 | AE005174 | |
| EC4115 | O157:H7 | 1,2 | − | − | pO157 | 66 | Food | USA | 2006 | NC_011353 | |
| TW14359 | O157:H7 | 1,2 | − | − | pO157 | 66 | ? | USA | 2006 | CP001368 | |
| EDL931 | O157:H7 | 1,2 | − | − | pO157 | 66 | Human | USA | 1983 | 24 | |
| MA6 | O157:H7 | 2 | − | − | pO157 | 66 | Food | Malaysia | 1998 | 11 | |
| 550654 | O157:H7 | 2 | − | − | pO157 | 66 | ? | USA | 2009 | ||
| FDA 413 | O157:H7 | 2 | − | − | pO157 | 66 | ? | ? | ? | 11 | |
| G5101 | O157:H7 | 1,2 | + | − | pO157_2 | 65 | A5 | Human | USA | 1995 | 12 |
| TW06289 | O157:H7 | 1,2 | + | − | pO157_2 | 65 | ? | USA | 1997 | 25 | |
| TW06290 | O157:H7 | 1,2 | + | − | pO157_2 | 65 | ? | USA | 1997 | 12 | |
| EC97144 | O157:H7 | 1,2 | + | + | pO157_2 | 65 | ? | Japan | 1997 | 8 | |
| EC96038 | O157:H7 | 1,2 | + | + | pO157_2 | 65 | ? | Japan | 1996 | 12 | |
| EC96012 | O157:H7 | 1,2 | + | + | pO157_2 | 65 | ? | Japan | 1996 | 12 | |
| 493-89 | O157:H− | 2 | + | + | pSFO157 | 75 | Human | Germany | 1989 | 12 | |
| 5412-89 | O157:H− | 2 | + | + | pSFO157 | 75 | A4 | Human | Germany | 1989 | 24 |
| H56929 | O157:H− | 2 | + | + | pSFO157 | 76 | ? | Finland | 1999 | 12 | |
| H56909 | O157:H− | 2 | + | + | pSFO157 | 76 | ? | Finland | 1999 | 12 | |
| H1085c | O157:H− | 2 | + | + | pSFO157 | 76 | Cat | Scotland | 2003 | 12 | |
| H2687 | O157:H− | 2 | + | + | pSFO157 | 76 | Human | Scotland | 2003 | 12 | |
stx, Shiga toxin gene.
GUD, β-glucuronidase activity.
SOR, sorbitol fermentation.
ST, sequence type as determined by a combination of seven genes (http://www.shigatox.net/ecmlst/cgi-bin/dst).
CC, clonal complex (12).
?, Unknown.
Plasmid extraction, sequencing, and contig assembly.
Plasmid DNAs were extracted from overnight cultures of strains G5101, 493/89, and H2687 using the PureYield plasmid maxiprep system kit (Promega, Madison, WI) following the manufacturer's instructions. The plasmid genomes were sequenced using 454 Titanium pyrosequencing (Roche, Branford, CT) according to the manufacturer's instructions at 20× to 100× coverage. Plasmid sequence contigs were assembled using CLC Genomics Workbench software version 4.7.1 (CLC bio, Germantown, MD) with the following reference sequences: pSFO157 of E. coli O157:H− strain 3072/96 (GenBank accession no. AF401292) (1) and pO157 of E. coli O157:H7 strain Sakai (GenBank accession no. NC_002128) (17). Contigs were manually arranged according to their contiguous sequence homologies using BioEdit version 7.0.9.0 (11). Contig organization for the novel G5101 plasmid (pO157-2) was confirmed by PCR bridging adjacent fragments. Sequences were annotated using the NCBI Prokaryotic Genomes Automatic Annotation Pipeline (http://www.ncbi.nlm.nih.gov/genomes/static/Pipeline.html) (14). Comparison of the sequenced plasmids with pO157 and pSFO157 was performed with Mauve version 2.3.1 (6) and visualized with CGView (http://wishart.biology.ualberta.ca/cgview/index.html) (25). The overall similarities of the considered plasmid core genes (excluding mobile elements) of pO157-2 with pO157 and pSFO157 were 99.8 and 99.4%, respectively.
Plasmid confirmation by PCR.
Specific PCRs were conducted to establish the presence of each individual plasmid in the strains analyzed. Primers employed are listed in Table S1 in the supplemental material. The following genes or regions were used as target indicators for the presence of each plasmid: espP (pO157), traC (pSFO157), and repA3-repE (pO157-2, region 4); yihA (each plasmid produced a different PCR product size); and letA-etpH (pO157/pSFO157; negative for pO157-2). PCR amplifications were performed using 5 ng of plasmid DNA in a final volume of 30 μl. The PCR mixture contained 1 U of Taq polymerase (Qiagen, Valencia, CA), 1× Taq polymerase buffer, 3.5 mM MgCl2, 125 μM each deoxynucleoside triphosphate (dNTP), 150 nM each primer pair. PCR conditions were as follows: 1 cycle of 95°C for 2 min, followed by 30 cycles of 95°C for 1 min, 58°C for 1 min, and 72°C for 1.5 min, and a final extension at 72°C for 10 min. Amplicons were visualized on a 1% agarose gel in Tris-borate-EDTA (TBE) buffer containing 0.3 μg/ml ethidium bromide.
Nucleotide sequence accession numbers.
The plasmid sequences for pO157-2 (G5101) and pSFO157 (493/89 and H2687) are available in GenBank under accession numbers AETX01000217, AETY01000205, and AETZ01000210, respectively.
RESULTS
Plasmid content in E. coli strains 493/89, H2687, and G5101.
The assembly of plasmid contigs confirmed the presence of a plasmid highly similar to pSFO157 from strain 3072/96 in two O157:H− strains (493/89 and H2687) belonging to CC A4 (1), showing only minor differences (single nucleotide polymorphisms [SNPs]). pSF493/89 had 3 SNPs (positions 32471, 87701, and 115470) and pSF2687 had 7 SNPs (positions 6860, 47558, 55997, 87698, 87701, 90799, and 115470) compared to pSFO157 of strain 3072/96. PCR analysis confirmed the presence of pSFO157 in four other O157:H− CC A4 strains. Strain G5101 contains a novel plasmid (pO157-2) resembling both pO157 and pSFO157 (Fig. 1A and B).
Fig 1.
EHEC plasmid pO157-2 map and comparison with two other EHEC plasmids. (A) Genetic map of the novel virulence EHEC plasmid pO157-2 generated with CGView (25). Blue block arrows in outer circle denote coding regions in plasmid indicating ORF transcription direction. G+C content is shown in the middle circle, and deviation from the average G+C content (47.71%) is shown in the innermost circle. BLAST comparisons with other two EHEC plasmids are shown in red (pO157) and green (pSFO157). (B) Comparison of whole sequences of plasmid pO157-2 and plasmids pO157 and pSFO157 with MAUVE. Each plasmid genome is laid out in a horizontal track, and homologous segments are indicated in the same color and connected across genomes. Respective scales show the sequence coordinates in base pairs. A colored similarity plot is shown for each genome, the height of which is proportional to the level of sequence identity in that region.
Assembly and characterization of pO157-2 from E. coli O157:H7 strain G5101.
Twelve contigs were obtained for pO157-2. The assembly of contigs, closing gaps using sequenced PCR products, revealed a circular plasmid of 89,762 bp (GC content of 47.71%) that contained 107 annotated open reading frames (ORFs), including multiple transposons and insertion elements (Fig. 1A; also see Table S2 in the supplemental material). pO157-2 carries virulence-associated regions like the hly operon, the etp operon, and toxB but lacks the espP and katP genes. Although pO157-2 shares more sequence similarities with pO157 (Fig. 1), its sequence analysis is based on pSFO157, since pSFO157 is thought to be evolutionarily older than pO157 (1). In accordance with the annotations in pSFO157 (5), pO157-2 was divided into five discrete regions with the origins of replication defining the regions (1) and the finO gene as the first gene of the analysis (Fig. 2).
Fig 2.
Schematic comparison of plasmids pO157-2, pO157, and pSFO157. Genes are shown in blocks; shaded blocks share less similarity with other genes. (A to E) Comparative analysis of gene contents for all three plasmids (pO157, pO157-2, and pSFO157) divided by regions according to pSFO157 (1). Block coloring indicates type of region: insertion sequences are represented by red blocks, transposons are dark orange, hly genes are dark green, etp genes are blue, tra genes are light green, and toxB is light orange. Areas not present in the plasmids are shown with dotted lines. tr, truncated; hp, genes coding for a hypothetical protein.
Region 1 (finO to repA1) (position 1 to 4353) carries eight potential ORFs of an IncFII minimal replication origin (1). Shared genes of pO157 and pO157-2 are 100% similar in sequence, compared to 93 to 97% similarity to genes in pSFO157 (see Table S2 in the supplemental material). Major differences between the three plasmids are in yihA and copB, with pSFO157 carrying a complete yihA (590 bp), which is truncated in both pO157 and pO157-2 (339 bp; internal deletion). A truncated IS629 interrupts yihA upstream (position 88 and 89) in pO157-2 (Fig. 2A). In addition, both pO157-2 and pO157 carry a copB gene that shared 65% sequence identity with its homologous gene in pSFO157, located at the end of this region (Fig. 2A).
Region 2 differs significantly between plasmids (Fig. 2B). pO157-2 shares none of the genes present in pSFO157 (sfp cluster encoding fimbriae and mannose-resistant hemagglutination genes) and pO157 (katP and espP) and containing solely complete and incomplete ISs and transposons (IS1294, truncated Tn2501, ISEc8). pO157-2 shares most of the ISs flanking the sfp cluster with pSFO157 with the addition of ISEc8, which is the second most prevalent IS found in O157:H7 strains (after IS629). ISEc8 is unique to pO157-2 but is also present in pO55 of E. coli O55:H7 and in the O157:H7 genome in multiple copies (19).
Region 3 in pSFO157 carries virulence genes (msbB, etp operon, hly operon) and the eae-positive conserved fragment (ecf). Both pO157 and pO157-2 lack the replication origin and ISs with the exception of iso-IS1 remnants. However, all of the following genes and operons in this region are present and ≥99% identical to those found in pSFO157 (see Table S2 in the supplemental material), showing that this region is more conserved in all three plasmids than other regions. One exception is an additional IS30a located between a truncated IS911 and the e-hlyC in pSFO157.
Region 4 in pSFO157 starts at the third origin of replication in the plasmid (repA3) and comprises seven ORFs (1). This region is present in pO157 with the exception of a hypothetical transposon (42 to 44 partial) but entirely absent from pO157-2. pO157 carries an additional complete IS629 at the end of this region truncating repE.
Region 5 in pSFO157 comprises genes related to F-like plasmids. It was shown previously that pO157 and pSFO157 differed notably in length in this particular region, which is mainly due to the lack of a significant amount of transfer genes (tra and trb) in pO157 (Fig. 2F; also see Table S2 in the supplemental material) (1), while repE is truncated in both pO157-2 and pO157 and the latter contains an IS629. sopA, sopB, and several other genes found on the F-plasmid (AP001918) until parB are >99% identical in sequence in all three plasmids analyzed. The exception is yccB, which is truncated at the 3′ end in pO157 by an unknown element (a possible RNA-directed DNA polymerase or transposon of ∼2,400 bp). Major differences between plasmids are present in the region containing transfer genes (Fig. 2E and F). The whole transfer gene region between parB and traH observed in pSFO157 is missing from pO157. Instead, this region in pO157 contains toxB flanked by several complete and incomplete ISs elements (IS3, IS21, IS629). pO157-2 also carries toxB including its surrounding ISs and shares the upstream transfer genes (traM to traB) present in pSFO157. pSFO157, contrary to both pO157 and pO157-2, lacks toxB and carries several more transfer genes. traB is either truncated in or absent from all three plasmids: truncated (41%) in both pSFO157 and pO157-2 and entirely absent from pO157.
Distribution of pO157-2 plasmids in E coli strains belonging to the stepwise emergence of E. coli O157:H7.
PCR results show that pO157, pO157-2, and pSFO157 in the strains analyzed are distributed according to CCs (see Table S3 in the supplemental material), whereas all CC A6 strains, CC A5 strains, and CC A4 strains carry pO157, pO157-2, and pSFO157, respectively.
DISCUSSION
In this study, we described a novel, potentially nonconjugative plasmid present in GUD+ E. coli O157:H7 strains. According to the stepwise evolutionary model for O157:H7 (8), strain G5101 was thought to be a precursor of SOR−, GUD− O157:H7 strains (9). It was previously assumed that clinical O157:H7 isolates carry a virulence plasmid resembling pO157 (24, 30). However, in this study we showed that O157:H7 strains could carry virulence plasmids other than pO157.
The three closely related plasmids pO157, pSFO157, and pO157-2 found in O157 strains shared several genes and regions including highly conserved virulence factors (etp and hly operons, mspB, sopA, sopB) and the F-plasmid-like leading region (yccB-parB). Sequences within the leading region are also conserved among other F-like plasmids belonging to the Inc9, B, I, K, and Y incompatibility groups (18). Since none of the plasmids appears to be conjugative, a recent introduction into E. coli O157 is unlikely. DNA sequence analysis indicated that they have undergone major structural changes. The plasmids lack a significant number of transfer genes: 38 for pO157 (traM-traI), 30 for pO157-2 (traP-traI), and 6 for pSFO157 (traB-traG, artA, trbJ, and trbF) in the F-plasmid-like region. However not all transfer genes are essential for plasmid conjugation. traB, however, which is either missing or truncated in all three plasmids, is essential for conjugative transfer (13), indicating that none of the plasmids is likely conjugative. Comparative analysis showed numerous evidences for a separate emergence of the three plasmids and most likely an early split of pSFO157. Besides a significantly greater number of transfer genes present in pSFO157, the plasmid also carries a complete yihA and a distinct copB in region 1. The hypothetical ancestral plasmid pO157A thereby would have likely carried more transfer genes, a functional yihA, and a different copB. Presumably after the split from pSFO157, transfer genes were lost and the two genes were truncated in hypothetical common ancestor plasmid pO157B (Fig. 3). Other evidences of this early split and the ongoing changes are also present; for example, pSFO157 carries the sfp gene cluster, a functional repE, several differences in IS content, and multiple nucleotide differences in shared genes (see Table S1 in the supplemental material).
Fig 3.
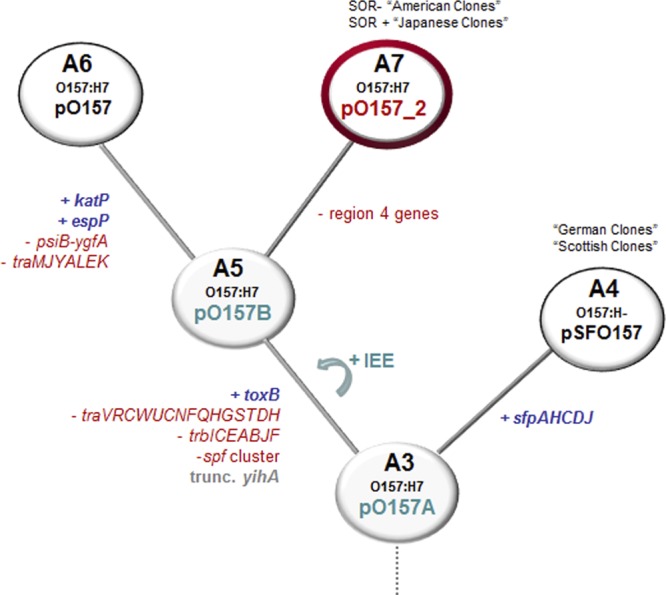
Proposed evolution of plasmid pO157-2 based on the stepwise evolution model for EHEC O157:H7. Possible acquisitions and/or losses of genes are indicated in branches. Clonal complexes (CCs) A3 to A7 are shown; changes predicted to have occurred: blue, gain of genes or regions; red, loss of genes or regions; gray, particular gene was truncated; green arrow, introduction of insertion sequence excision enhancer (IEE) in strains. Red circle, newly introduced CC A7 with plasmid pO157-2. Ancestral strain and plasmids A3 and A5 (shaded circles) have not been reported.
pO157 and pO157-2 are more closely related to each other than to pSFO157, which is confirmed by the presence of toxB and copB and the absence of the sfp gene cluster. However, there are several additional features indicating that pO157-2, although highly related to pO157, evolved independently from hypothetical pO157B and unlikely gave rise to plasmid pO157 (Fig. 3). pO157 shares genes with pSFO157 in region 4 (ydiA, etpH, kfrA, redD), but these are absent from pO157-2. Since pO157 and pSFO157 share genes of this region, genes should be present in pO157-2 in order to be a possible progenitor to pO157. The deletion of those genes in pO157-2 therefore indicates a divergence of pO157 and pO157-2 from pO157B and excludes the possibility of A5 CC strains being precursor strains to CC A6 strains. Furthermore, after this split, pO157 likely incorporated katP and espP, additional transfer genes (psiB-traK) were lost, and different ISs were also lost and/or acquired in diverse regions. It is thereby apparent that especially since it misses elements shared by pSFO157 and pO157, the newly sequenced plasmid pO157-2 is unlikely to be the precursor of pO157.
The three plasmids contain several ISs, with the majority belonging to the IS3 and IS21 family located in regions differing between plasmids (see Table S2 in the supplemental material). Additionally, pO157 carries IS91 and pO157-2 carries IS66 family members. pSFO157 is almost identical in CC A4 strains, but pO157 differs greatly between closely related strains. The recently described IS excision enhancer (IEE) protein, exclusively present in O157:H7 strains, significantly increases IS3 family transposition (15). It would therefore be interesting to speculate that the presence of IS3 family members and IEE lead to rearrangements, insertions, and deletions in pO157B from which pO157 and pO157-2 evolved (Fig. 3). This hypothesis suggests that virulence plasmids in O157:H7 strains are still changing rapidly, thereby contributing to pathogenic potential. They could be used for additional characterization of strains and also serve as markers for relatedness analysis among strains.
In the present study, we have shown that O157:H7 strains, although displaying characteristics for pO157, could carry distinct plasmids. Plasmid pO157-2 shares high similarity with pO157; nevertheless, structural evidences showed that it evolved independently from a common ancestor plasmid and pO157-2 carrying strains belong to a separate clonal lineage (designated CC A7). Zhou et al. (31) also suggested an early split of GUD+ NSF O157:H7 strains using a core genome alignment of several O55:H7, O157:H− and O157:H7 genomes. In this model, GUD+ strain G5101 (containing pO157-2) split from the O157:H7 evolutionary path and did not appear to be the ancestral strain for GUD− NSF CC A6, as also suggested by our results. Therefore, GUD+ O157:H7 strains which either are SOR+ (“American clones”) or SOR− (“Japanese clones”) belong to CC A7 and are not precursors to CC A6 strains as initially proposed (8). This analysis resulted in a novel evolutionary path that may offer insight into the evolutionary history of O157 populations.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ruth E. Timme for her assistance with the annotation of the plasmids.
This project was supported by an appointment to L.V.R. through the Research Fellowship Program for the Center for Food Safety and Applied Nutrition administered by the Oak Ridge Associated Universities through a contract with the FDA. This project was supported by the FDA Foods Program Intramural Funds.
Footnotes
Published ahead of print 20 April 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Brunder W, Karch H, Schmidt H. 2006. Complete sequence of the large virulence plasmid pSFO157 of the sorbitol-fermenting enterohemorrhagic Escherichia coli O157:H− strain 3072/96. Int. J. Med. Microbiol. 296:467–474 [DOI] [PubMed] [Google Scholar]
- 2. Brunder W, Schmidt H, Frosch M, Karch H. 1999. The large plasmids of Shiga-toxin-producing Escherichia coli (STEC) are highly variable genetic elements. Microbiology 145:1005–1014 [DOI] [PubMed] [Google Scholar]
- 3. Brunder W, Schmidt H, Karch H. 1996. KatP, a novel catalase-peroxidase encoded by the large plasmid of enterohaemorrhagic Escherichia coli O157:H7. Microbiology 142:3305–3315 [DOI] [PubMed] [Google Scholar]
- 4. Brunder W, Schmidt H, Karch H. 1997. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7 cleaves human coagulation factor V. Mol. Microbiol. 24:767–778 [DOI] [PubMed] [Google Scholar]
- 5. Burland V, et al. 1998. The complete DNA sequence and analysis of the large virulence plasmid of Escherichia coli O157:H7. Nucleic Acids Res. 26:4196–4204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Darling ACE, Mau B, Blattner FR, Perna NT. 2004. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 14:1394–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feng P. 1995. Escherichia coli serotype O157:H7: novel vehicles of infection and emergence of phenotypic variants. Emerg. Infect. Dis. 1:47–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feng P, Lampel KA, Karch H, Whittam TS. 1998. Genotypic and phenotypic changes in the emergence of Escherichia coli O157:H7. J. Infect. Dis. 177:1750–1753 [DOI] [PubMed] [Google Scholar]
- 9. Feng PC, et al. 2007. Genetic diversity among clonal lineages within Escherichia coli O157:H7 stepwise evolutionary model. Emerg. Infect. Dis. 13:1701–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Griffin PM, Tauxe RV. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 13:60–98 [DOI] [PubMed] [Google Scholar]
- 11. Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. 41:95–98 [Google Scholar]
- 12. Karch H, et al. 1993. Clonal structure and pathogenicity of Shiga-like toxin-producing, sorbitol-fermenting Escherichia coli O157:H−. J. Clin. Microbiol. 31:1200–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim SR, Funayama N, Komano T. 1993. Nucleotide sequence and characterization of the traABCD region of IncI1 plasmid R64. J. Bacteriol. 175:5035–5042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klimke W, et al. 2009. The National Center for Biotechnology Information's Protein Clusters Database. Nucleic Acids Res. 37:D216–D223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kusumoto M, et al. 2011. Insertion sequence-excision enhancer removes transposable elements from bacterial genomes and induces various genomic deletions. Nat. Commun. 2:152. [DOI] [PubMed] [Google Scholar]
- 16. Lawley TD, Klimke WA, Gubbins MJ, Frost LS. 2003. F factor conjugation is a true type IV secretion system. FEMS Microbiol. Lett. 224:1–15 [DOI] [PubMed] [Google Scholar]
- 17. Makino K, et al. 1998. Complete nucleotide sequences of 93-kb and 3.3-kb plasmids of an enterohemorrhagic Escherichia coli O157:H7 derived from Sakai outbreak. DNA Res. 5:1–9 [DOI] [PubMed] [Google Scholar]
- 18. Manwaring NP, Skurray RA, Firth N. 1999. Nucleotide sequence of the F plasmid leading region. Plasmid 41:219–225 [DOI] [PubMed] [Google Scholar]
- 19. Ooka T, et al. 2009. Inference of the impact of insertion sequence (IS) elements on bacterial genome diversification through analysis of small-size structural polymorphisms in Escherichia coli O157 genomes. Genome Res. 19:1809–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rump LV, Fischer M, Gonzalez-Escalona N. 2011. Prevalence, distribution and evolutionary significance of the IS629 insertion element in the stepwise emergence of Escherichia coli O157:H7. BMC Microbiol. 11:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmidt H, Beutin L, Karch H. 1995. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL 933. Infect. Immun. 63:1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schmidt H, Henkel B, Karch H. 1997. A gene cluster closely related to type II secretion pathway operons of Gram-negative bacteria is located on the large plasmid of enterohemorrhagic Escherichia coli O157 strains. FEMS Microbiol. Lett. 148:265–272 [DOI] [PubMed] [Google Scholar]
- 23. Schmidt H, Karch H, Beutin L. 1994. The large-sized plasmids of enterohemorrhagic Escherichia coli O157 strains encode hemolysins which are presumably members of the E. coli alpha-hemolysin family. FEMS Microbiol. Lett. 117:189–196 [DOI] [PubMed] [Google Scholar]
- 24. Schmidt H, Kernbach C, Karch H. 1996. Analysis of the EHEC hly operon and its location in the physical map of the large plasmid of enterohaemorrhagic Escherichia coli O157:H7. Microbiology 142:907–914 [DOI] [PubMed] [Google Scholar]
- 25. Stothard P, Wishart DS. 2005. Circular genome visualization and exploration using CGView. Bioinformatics 21:537–539 [DOI] [PubMed] [Google Scholar]
- 26. Tatsuno I, et al. 2001. toxB gene on pO157 of enterohemorrhagic Escherichia coli O157:H7 is required for full epithelial cell adherence phenotype. Infect. Immun. 69:6660–6669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taylor P, Allison L, Willshaw G, Cheasty T, Hanson M. 2003. Sorbitol-fermenting Escherichia coli O157 in Scotland, abstr. P280. 5th International Symposium on Shiga Toxin (Verotoxin)-Producing E. coli Infections Edinburgh, Scotland [Google Scholar]
- 28. Whittam TS, et al. 1993. Clonal relationships among Escherichia coli strains that cause hemorrhagic colitis and infantile diarrhea. Infect. Immun. 61:1619–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wick LM, Qi W, Lacher DW, Whittam TS. 2005. Evolution of genomic content in the stepwise emergence of Escherichia coli O157:H7. J. Bacteriol. 187:1783–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yoon JW, Hovde CJ. 2008. All blood, no stool: enterohemorrhagic Escherichia coli O157:H7 infection. J. Vet. Sci. 9:219–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou Z, et al. 2010. Derivation of Escherichia coli O157:H7 from its O55:H7 precursor. PLoS One 5:e8700 doi:10.1371/journal.pone.0008700 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





