Abstract
Our objective was to determine whether melatonin increases retinal ganglion cell (RGC) survival in ischemic mouse retina. Transient retinal ischemia was induced by an acute elevation of intraocular pressure in C57BL/6 mice. To evaluate the effect of melatonin on retinal ischemia, an equal amount of either melatonin or vehicle was intraperitoneally injected into the mice 1 hour before ischemia, at the time of ischemia, and 1 hour after ischemia. Hypoxia inducible factor 1α (HIF-1α) and glial fibrillary acidic protein (GFAP) expression were assessed 6, 12, and 24 hours after ischemia-reperfusion by Western blot. RGC survival was measured 2 weeks after ischemia-reperfusion. The expression of HIF-1α and GFAP peaked 24 hours after ischemia-reperfusion in ischemic retina. The treatment of ischemic retina with melatonin resulted in the inhibition of increased expression of HIF-1α and GFAP. RGC survival was greater in retinas treated with melatonin than in retinas treated with vehicle 2 weeks after ischemia-reperfusion. On the basis of our results, we suggest that melatonin treatment increased RGC survival in ischemic mouse retina. The neuroprotective effect of melatonin is mediated by the inhibition of HIF-1α stabilization and reduced activity of glial cells in ischemic mouse retina.
Keywords: Glaucoma, Ischemia, Melatonin, Neuroprotection
INTRODUCTION
Glaucoma is one of the leading causes of irreversible blindness.1 Glaucoma is an optic neuropathy characterized by retinal ganglion cell (RGC) death, axon loss, and an excavated appearance to the optic nerve (ON) head.2 In glaucoma, elevated intraocular pressure (IOP) and other factors, such as oxidative stress and interruption of axoplasmic transport, have been implicated in RGC death.3-8
Although elevated IOP is the most significant risk factor for glaucomatous ON damage and RGC loss, several studies have shown a relation between oxidative stress and RGC loss in glaucoma.8,9-11 In addition, there is much evidence of a dysregulated blood supply to the ON head and abnormal retinal blood flow in glaucoma patients and in a chronic ocular hypertensive animal model.12-16
Histopathologically, the loss of RGCs in glaucoma is accompanied by morphologic and functional changes in Müller cells the main type of glial cell in the retina.17 Müller cells are activated in the retina under stressful conditions, such as elevated IOP or ischemia.17-19 A key feature of activation of Müller cells is the upregulation of the intermediate filament glial fibrillary acidic protein (GFAP).20-23
Under normal conditions, Müller cells are responsible for the protection of RGCs by releasing neurotrophic factors and the secretion of glutathione, which has an antioxidant effect.24-26 However, activated Müller cells negatively affect RGC survival in ischemic retina.26-28
Melatonin was recently found to be an antioxidant and a free radical scavenger.29,30 It is a highly effective direct scavenger of reactive radicals and their intermediates, such as hydrogen peroxide, nitric oxide, and peroxynitrite. Because of its lipophilic characteristic, which allows it to cross the blood-brain barrier, melatonin has been proposed to endogenously protect against oxidative damage to the brain.31,32
In this study, we investigated the expression of hypoxia inducible factor 1α (HIF-1α) and GFAP in ischemic mouse retina. In addition, to determine the neuroprotective effect of melatonin, RCG counting by retrograde FluoroGold labeling was performed in ischemic retina treated with either vehicle or melatonin.
MATERIALS AND METHODS
1. Animals
All experiments were conducted in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. The protocol was approved by the Institutional Animal Care and Use Committee of Chonnam National University Hospital. C57BL/6 mice (3 months of age, 20-25 g in weight) were housed in individual cages under controlled lighting conditions (12 hours light/12 hours dark) and given tap water and food ad libitum.
2. Transient retinal ischemia
For transient retinal ischemia, C57BL/6 mice were anesthetized with a mixture of tiletamine/zolazepam (4 mg/kg; Zoletil 50, Virbac, France) and xylazine hydrochloride (1 mg/kg, Rompun; Bayer Korea, Seoul, Korea) by intramuscular injection. A cannula was inserted into the anterior chamber, which was connected by flexible tubing to the saline reservoir. IOP was elevated above systolic blood pressure (100-120 mmHg) for 60 minutes by raising the reservoir. Retinal ischemia was confirmed by the whitening of the anterior segment of the eye, blanching of the iris vessels, and loss of retinal red reflex. Previous studies have shown that neuronal degeneration occurs mainly in the thinner retinal layer, especially in the ganglion cell layer, after 60 minutes of transient retinal ischemia.18,33
3. Melatonin treatment
Two groups of mice were studied following unilateral transient retinal ischemia: one group was treated with vehicle (saline, n=10) and the other group was treated with melatonin (40 mg/kg, n=10) 1 hour before ischemia, at the time of ischemia, and 1 hour after ischemia. The dose and delivery route of melatonin were based on previous reports for melatonin treatment.34
4. Tissue preparations
The retinas were dissected from the choroid and fixed in 4% paraformaldehyde in phosphate buffered saline (pH 7.4) for 2 hours at 4℃ for retinal flat mounting. For Western blot analyses, whole retinas were immediately used or frozen in liquid nitrogen and stored at -70℃ until used.
5. Western blot analysis
Retinal tissues were homogenized in a glass-polytetrafluoroethylene Potter homogenizer in lysis buffer (PRO-PREP™; iNtRoN Biotechnology, Kyungki-Do, Korea). Each sample (10 µg) was separated in a 10% polyacrylamide mini-gel at 150 V for 1 hour. The transferred membranes were incubated for 1 hour at room temperature in TBS-T solution [10 mM Tris-HCl (pH 7.6), 150 mM NaCl, and 0.1% Tween-20] containing 5% non-fat dry milk. Blocking membranes were incubated overnight at 4℃ with mouse monoclonal anti-HIF-1α (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse monoclonal anti-GFAP antibody (1:3000; Cell Signaling Technology), or mouse polyclonal anti-actin antibody (1:4000; Santa Cruz Biotechnology) in TBS-T solution containing 5% non-fat dry milk. After being washed three times with TBS-T, the membranes were incubated for 1 hour at room temperature with peroxidase-conjugated goat anti-mouse IgG (1:2000; Santa Cruz Biotechnology) in TBS-T containing 5% non-fat dry milk. Blots were developed with enhanced chemiluminescence and quantified by using an LAS-3000 image-analyzer (Fujifilm, Tokyo, Japan).
6. Retrograde labeling and counting of RGCs
FluoroGold (1 µL/injection of 4%; Fluorochrome, Inc., Denver, CO, USA) diluted in saline was microinjected bilaterally into the superior colliculi of anesthetized mice in a stereotactic apparatus 1 week before enucleation. To evaluate the loss of RGCs, each retinal quadrant was divided into three zones: center, middle, and peripheral retina (one-sixth, three-sixths, and five-sixths of the retinal radius). The numbers of RGCs were counted in 32 distinct areas of 0.48 mm2 (two areas at the center and three areas at the middle and periphery per retinal quadrant) by two investigators in a masked fashion, and the scores were averaged. The images were analyzed by confocal microscopy with a 20× objective with a laser scanning microscope (LSM 510; Carl Zeiss, Jena, Germany).
7. Statistical analysis
The experiments were repeated at least three times. The data are presented as means±SDs. An unpaired Student's t-test was used for the comparison between two experimental conditions. A p value <0.05 was considered to be statistically significant.
RESULTS
1. Expression of HIF-1α and GFAP on ischemic mouse retina according to time interval
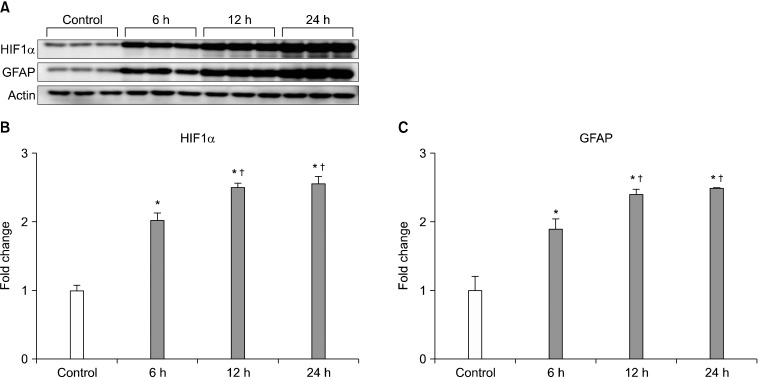
Western blot showed increased expression of HIF-1α and GFAP in ischemic mouse retina within 24 hours after ischemia-reperfusion. Expression of HIF-1α and GFAP peaked 24 hours after ischemia-reperfusion (p<0.05; Fig. 1).
FIG. 1.
Expression of HIF-1α and GFAP protein on ischemic mouse retina according to time interval. HIF-1α and GFAP expression was increased within 24 hours in ischemic mouse retina (A-C). Relative intensity of chemiluminescence for HIF-1α and GFAP protein bands was normalized using actin as a calibrator. *Significant at p<0.05 compared with control mouse retina. †Significant at p<0.05 compared with ischemic mouse retina-6 h, Error bars, SD. HIF-1α: hypoxia inducible factor 1α, GFAP: glial fibrillay acidic protein.
2. Effect of melatonin on HIF-1α and GFAP expression
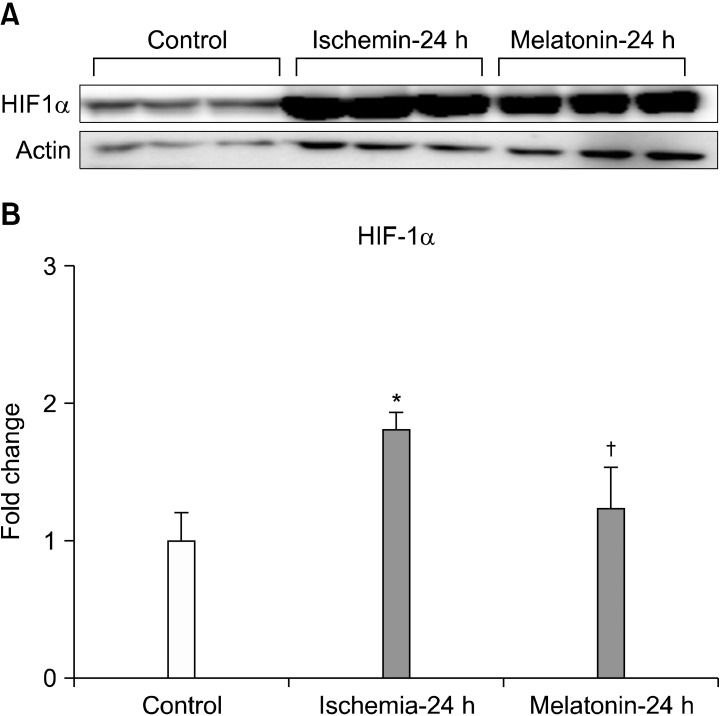
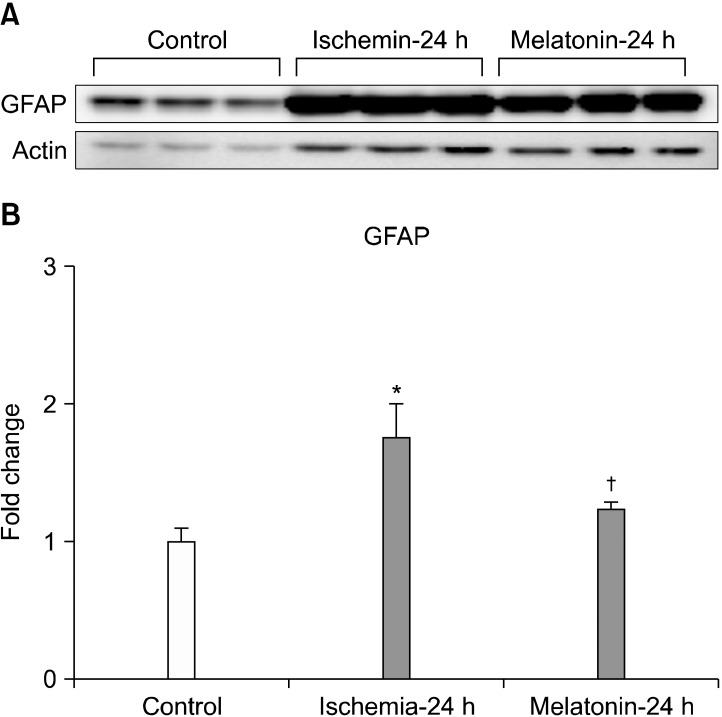
Increased expression of HIF-1α induced by ischemia-reperfusion, after acute IOP elevation, decreased significantly after treatment with melatonin (p<0.05; Fig. 2). In addition, melatonin treatment significantly inhibited the increased expression of GFAP in ischemic mouse retina (p<0.05; Fig. 3).
FIG. 2.
The effect of melatonin on HIF-1α expression in ischemic mouse retina. Melatonin treatment significantly decreased HIF-1α protein expression in ischemic retina at 24 hours (A, B). Relative intensity of chemiluminescence for HIF-1α protein band was normalized using actin as a calibrator. *Significant at p<0.05 compared with control mouse retina. †Significant at p<0.05 compared with ischemic mouse retina-24 h. Error bars, SD. HIF-1α: hypoxia inducible factor 1α.
FIG. 3.
The effect of melatonin on GFAP expression in ischemic mouse retina. Melatonin treatment significantly decreased GFAP protein expression in ischemic retina at 24 hours (A, B). Relative intensity of chemiluminescence for GFAP protein band was normalized using actin as a calibrator. Error bars, SD. *Significant at p<0.05 compared with control mouse retina. †Significant at p<0.05 compared with ischemic mouse retina-24 h. Error bars, SD. GFAP: glial fibrillay acidic protein.
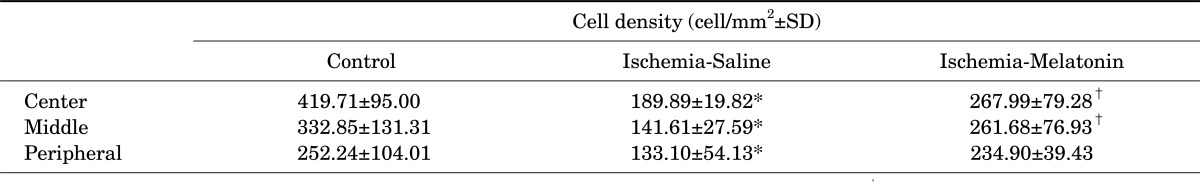
3. Effect of melatonin on RGC loss
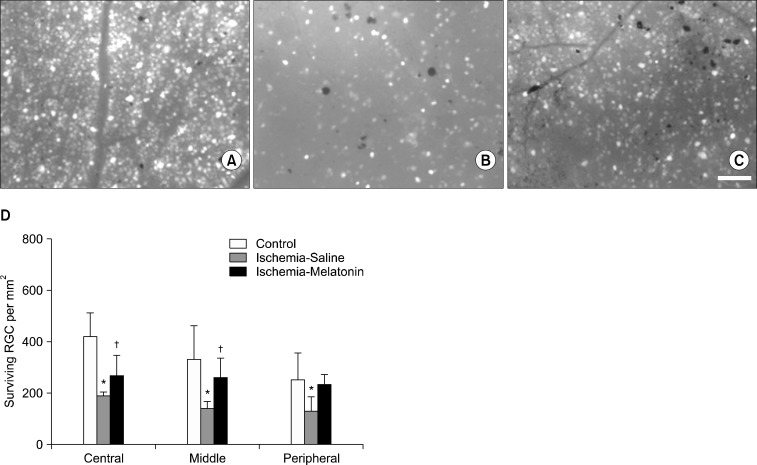
Compared with the control retina, acute IOP elevation induced RGC loss by approximately 45% in the central, 58% in the middle, and 48% in the peripheral areas of vehicle-treated ischemic retina 2 weeks after ischemia-reperfusion (Fig. 4, A, B, and D; Table 1). In contrast, melatonin treatment increased RGC survival in ischemic mouse retina, especially in the central and middle areas compared with vehicle-treated ischemic retina (p<0.05; Fig. 4, C and D; Table 1).
FIG. 4.
The effect of melatonin on RGC survival in ischemic mouse retina. The retinal flat mounts of control (A), vehicle-treated ischemic retina (B) and melatonin-treated ischemic retina (C). The quantitative analysis of RGC survival (D). *Significant at p<0.05 compared with control mouse retina. †Significant at p<0.05 compared with ischemic mouse retina treated with saline. Error bars, SD. Scale bars, 100 µm.
TABLE 1.
The effect of melatonin on retinal ganglion cell (RGC) survival in ischemic mouse retina
Data are expressed as the mean±SD. *p<0.05, comparison between control vs. ischemia-saline. †p<0.05, comparison between ischemia-saline vs. ischemia-melatonin. RGC: retinal ganglion cell.
DISCUSSION
This study showed that melatonin treatment inhibited the increased expression of HIF-1α and GFAP and prevented RGC death in ischemic mouse retina. When adequate oxygen is supplied to the cell, HIF-1α is usually degraded in the cytoplasm. However, under hypoxic conditions, stabilized HIF-1α forms heterodimer with HIF-1β in the nucleus.35,36 This heterodimer transcribes several genes associated with cellular adaptation to hypoxia, such as erythropoietin, vascular endothelial growth factor, and nitric oxide.37
Therefore, modulation of HIF-1α is tightly regulated by the level of tissue hypoxia. However, excessive expression of HIF-1α can lead to neuronal cell death.38,39
Our result showed that melatonin inhibits increased expression of HIF-1α in ischemic retina compared with ischemic retina treated with vehicle. We suggested that the possible mechanism of this inhibition might be mediated by the antioxidant effect of melatonin and that blockage of subsequent hypoxic responses via inhibition of HIF-1α expression results in increased RGC survival in ischemic retina. In addition, we investigated the effect of melatonin on GFAP expression in ischemic retina. Melatonin effectively reduced GFAP expression and increased RGC survival in ischemic retina. Müller cells, the principal glial cells of the retina, support neurons with blood-derived nutrients, by removing metabolic waste, and by maintaining the homeostasis of the retinal extracellular milieu (ions, water, neurotransmitter molecules, and pH).40 Müller cells become activated or reactive in response to pathological alteration of the retina, including ischemia-reperfusion or elevated IOP. Furthermore, activated Müller cells may participate in damaging neurons41,42 and are characterized by upregulation of the intermediate filament protein GFAP.43
However, the exact mechanism responsible for activation of Müller cells is still unknown. Activated Müller cells increase the expression of the inducible form of nitric oxide synthase and decrease glutamate uptake in ischemic retina.44,45 Tezel and Wax46 reported that increased production of tumor necrosis factor-α and nitric oxide by retinal glial cells exposed to elevated hydrostatic pressure and simulated ischemia induced apoptosis in cocultured RGCs.
Melatonin is an indoleamine produced in the pineal gland and the retina. Melatonin is known to be involved in diverse functions in the human body, such as temperature regulation, blood pressure regulation, and sleep-cycle control.47 In the retina, melatonin receptors are clustered on the photoreceptors, inner segments, and ganglion cells.46 Melatonin is known to inhibit the nitridergic pathway, which has a protective effect on the photoreceptor's outer membranes and reverses the effect of ocular hypertension on retinal function. In addition, in an experimental animal model, the concentration of melatonin in the retina of glaucomatous rats with high IOP was significantly reduced.48 Melatonin has diverse direct and indirect antioxidant effects and also acts as a free radical scavenger. Several reports have indicated the potent antioxidant and neuroprotective effects of melatonin. Treatment with melatonin after neonatal hypoxia-ischemia was shown to reduce neuronal cell death and white-matter demyelination in rats.49 In addition, injection of melatonin after induction of hypoxic-ischemic injury reduced oxidative stress and inflammatory cell recruitment in the cerebral cortex of rats.50 Moreover, melatonin counteracted ischemia-induced apoptosis in cultured human retinal pigment cells.51 The antioxidant functions of melatonin include direct free radical scavenging, stimulation of antioxidative enzymes, increased efficiency of mitochondrial oxidative phosphorylation, reduction in electron leakage, and augmentation of the efficiency of other antioxidants.52-56 Therefore, as a potent antioxidant, melatonin can induce functional changes in Müller cells during ischemia-reperfusion injury. We believe that the decreased activation of Müller cells resulting from melatonin treatment can reduce RGC death during ischemia-reperfusion injury. Thus, the use of melatonin may be an effective therapeutic strategy for preventing glaucomatous RGC death. In combination with the effects of traditional anti-glaucoma medication, the antioxidative effect of melatonin may benefit patients with glaucoma by protecting against RGC death resulting from ischemic injury. In conclusion, our findings provide evidence that the functional state of retinal glial cells may be important in determining the ultimate fate of RGCs and that melatonin has a neuroprotective effect on RGC in ischemic retina, and this effect is mainly mediated by a reduction in the stabilization of HIF-1α and activation of Müller cells. The specific molecular events that occur in Müller cells after melatonin treatment need to be determined.
ACKNOWLEDGEMENTS
This study was supported by grant 2010-CURIMS-DR002 from the Research Institute of Medical Sciences, Chonnam National University.
References
- 1.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley HA, Green WR. The histology of human glaucoma cupping and optic nerve damage: clinicopathologic correlation in 21 eyes. Ophthalmology. 1979;86:1803–1830. doi: 10.1016/s0161-6420(79)35338-6. [DOI] [PubMed] [Google Scholar]
- 3.Anderson DR. What happens to the optic disc and retina in glaucoma? Ophthalmology. 1983;90:766–770. doi: 10.1016/s0161-6420(83)34490-0. [DOI] [PubMed] [Google Scholar]
- 4.Kelly MEM, Barnes S. Physiology and pathophysiology of nitric oxide in the retina. Neuroscientist. 1997;3:357–360. [Google Scholar]
- 5.Nickells RW. Apoptosis of retinal ganglion cells in glaucoma: an update of the molecular pathways involved in cell death. Surv Ophthalmol. 1999;43(Suppl 1):S151–S161. doi: 10.1016/s0039-6257(99)00029-6. [DOI] [PubMed] [Google Scholar]
- 6.Sucher NJ, Lei SZ, Lipton SA. Calcium channel antagonists attenuate NMDA receptor-mediated neurotoxicity of retinal ganglion cells in culture. Brain Res. 1991;551:297–302. doi: 10.1016/0006-8993(91)90944-q. [DOI] [PubMed] [Google Scholar]
- 7.Osborne NN, Chidlow G, Nash MS, Wood JP. The potential of neuroprotection in glaucoma treatment. Curr Opin Ophthalmol. 1999;10:82–92. doi: 10.1097/00055735-199904000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Osborne NN, Ugarte M, Chao M, Chidlow G, Bae JH, Wood JP, et al. Neuroprotection in relation to retinal ischemia and relevance to glaucoma. Surv Ophthalmol. 1999;43(Suppl 1):S102–S128. doi: 10.1016/s0039-6257(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 9.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 10.Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999;18:39–57. doi: 10.1016/s1350-9462(98)00014-7. [DOI] [PubMed] [Google Scholar]
- 11.Chung HS, Harris A, Evans DW, Kagemann L, Garzozi HJ, Martin B. Vascular aspects in the pathophysiology of glaucomatous optic neuropathy. Surv Ophthalmol. 1999;43(Suppl 1):S43–S50. doi: 10.1016/s0039-6257(99)00050-8. [DOI] [PubMed] [Google Scholar]
- 12.Tezel G, Wax MB. Hypoxia-inducible factor 1alpha in the glaucomatous retina and optic nerve head. Arch Ophthalmol. 2004;122:1348–1356. doi: 10.1001/archopht.122.9.1348. [DOI] [PubMed] [Google Scholar]
- 13.Yang J, Tezel G, Patil RV, Romano C, Wax MB. Serum autoantibody against glutathione S-transferase in patients with glaucoma. Invest Ophthalmol Vis Sci. 2001;42:1273–1276. [PubMed] [Google Scholar]
- 14.Tezel G, Yang X, Cai J. Proteomic identification of oxidatively modified retinal proteins in a chronic pressure-induced rat model of glaucoma. Invest Ophthalmol Vis Sci. 2005;46:3177–3187. doi: 10.1167/iovs.05-0208. [DOI] [PubMed] [Google Scholar]
- 15.Moreno MC, Campanelli J, Sande P, Sánez DA, Keller Sarmiento MI, Rosenstein RE. Retinal oxidative stress induced by high intraocular pressure. Free Radic Biol Med. 2004;37:803–812. doi: 10.1016/j.freeradbiomed.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Tezel G. Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res. 2006;25:490–513. doi: 10.1016/j.preteyeres.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inman DM, Horner PJ. Reactive nonproliferative gliosis predominates in a chronic mouse model of glaucoma. Glia. 2007;55:942–953. doi: 10.1002/glia.20516. [DOI] [PubMed] [Google Scholar]
- 18.Kim IB, Kim KY, Joo CK, Lee MY, Oh SJ, Chung JW, et al. Reaction of Müller cells after increased intraocular pressure in the rat retina. Exp Brain Res. 1998;121:419–424. doi: 10.1007/s002210050476. [DOI] [PubMed] [Google Scholar]
- 19.Osborne NN, Block F, Sontag KH. Reduction of ocular blood flow results in glial fibrillary acidic protein (GFAP) expression in rat retinal Müller cells. Vis Neurosci. 1991;7:637–639. doi: 10.1017/s0952523800010427. [DOI] [PubMed] [Google Scholar]
- 20.Fletcher EL, Downie LE, Ly A, Ward MM, Batcha AH, Puthussery T, et al. A review of the role of glial cells in understanding retinal disease. Clin Exp Optom. 2008;91:67–77. doi: 10.1111/j.1444-0938.2007.00204.x. [DOI] [PubMed] [Google Scholar]
- 21.Francke M, Faude F, Pannicke T, Uckermann O, Weick M, Wolburg H, et al. Glial cell-mediated spread of retinal degeneration during detachment: a hypothesis based upon studies in rabbits. Vision Res. 2005;45:2256–2267. doi: 10.1016/j.visres.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 22.Kanamori A, Nakamura M, Nakanishi Y, Yamada Y, Negi A. Long-term glial reactivity in rat retinas ipsilateral and contralateral to experimental glaucoma. Exp Eye Res. 2005;81:48–56. doi: 10.1016/j.exer.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Kawasaki A, Otori Y, Barnstable CJ. Müller cell protection of rat retinal ganglion cells from glutamate and nitric oxide neurotoxicity. Invest Ophthalmol Vis Sci. 2000;41:3444–3450. [PubMed] [Google Scholar]
- 24.Harada T, Harada C, Watanabe M, Inoue Y, Sakagawa T, Nakayama N, et al. Functions of the two glutamate transporters GLAST and GLT-1 in the retina. Proc Natl Acad Sci USA. 1998;95:4663–4666. doi: 10.1073/pnas.95.8.4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newman E, Reichenbach A. The Müller cell: a functional element of the retina. Trends Neurosci. 1996;19:307–312. doi: 10.1016/0166-2236(96)10040-0. [DOI] [PubMed] [Google Scholar]
- 26.Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, et al. Müller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25:397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi M, Kuroiwa T, Shimokawa R, Okeda R, Tokoro T. Nitric oxide synthase expression in ischemic rat retinas. Jpn J Ophthalmol. 2000;44:235–244. doi: 10.1016/s0021-5155(99)00220-8. [DOI] [PubMed] [Google Scholar]
- 28.Bringmann A, Pannicke T, Biedermann B, Francke M, Iandiev I, Grosche J, et al. Role of retinal glial cells in neurotransmitter uptake and metabolism. Neurochem Int. 2009;54:143–160. doi: 10.1016/j.neuint.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Hardeland R. Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine. 2005;27:119–130. doi: 10.1385/endo:27:2:119. [DOI] [PubMed] [Google Scholar]
- 30.Marshall KA, Reiter RJ, Poeggeler B, Aruoma OI, Halliwell B. Evaluation of the antioxidant activity of melatonin in vitro. Free Radic Biol Med. 1996;21:307–315. doi: 10.1016/0891-5849(96)00046-9. [DOI] [PubMed] [Google Scholar]
- 31.Costa EJ, Lopes RH, Lamy-Freund MT. Permeability of pure lipid bilayers to melatonin. J Pineal Res. 1995;19:123–126. doi: 10.1111/j.1600-079x.1995.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 32.Siu AW, Ortiz GG, Benitez-King G, To CH, Reiter RJ. Effects of melatonin on the nitric oxide treated retina. Br J Ophthalmol. 2004;88:1078–1081. doi: 10.1136/bjo.2003.037879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adachi M, Takahashi K, Nishikawa M, Miki H, Uyama M. High intraocular pressure-induced ischemia and reperfusion injury in the optic nerve and retina in rats. Graefes Arch Clin Exp Ophthalmol. 1996;234:445–451. doi: 10.1007/BF02539411. [DOI] [PubMed] [Google Scholar]
- 34.Sinha K, Degaonkar MN, Jagannathan NR, Gupta YK. Effect of melatonin on ischemia reperfusion injury induced by middle cerebral artery occlusion in rats. Eur J Pharmacol. 2001;428:185–192. doi: 10.1016/s0014-2999(01)01253-5. [DOI] [PubMed] [Google Scholar]
- 35.Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 36.Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- 37.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 38.Halterman MW, Miller CC, Federoff HJ. Hypoxia-inducible factor-1alpha mediates hypoxia-induced delayed neuronal death that involves p53. J Neurosci. 1999;19:6818–6824. doi: 10.1523/JNEUROSCI.19-16-06818.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–10903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Akiyama H, Nakazawa T, Shimura M, Tomita H, Tamai M. Presence of mitogen-activated protein kinase in retinal Müller cells and its neuroprotective effect ischemia-reperfusion injury. Neuroreport. 2002;13:2103–2107. doi: 10.1097/00001756-200211150-00022. [DOI] [PubMed] [Google Scholar]
- 41.Pannicke T, Iandiev I, Uckermann O, Biedermann B, Kutzera F, Wiedemann P, et al. A potassium channel-linked mechanism of glial cell swelling in the postischemic retina. Mol Cell Neurosci. 2004;26:493–502. doi: 10.1016/j.mcn.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Tezel G, Chauhan BC, LeBlanc RP, Wax MB. Immunohistochemical assessment of the glial mitogen-activated protein kinase activation in glaucoma. Invest Ophthalmol Vis Sci. 2003;44:3025–3033. doi: 10.1167/iovs.02-1136. [DOI] [PubMed] [Google Scholar]
- 43.Bignami A, Dahl D. The radial glia of Müller in the rat retina and their response to injury. An immunofluorescence study with antibodies to the glial fibrillary acidic (GFA) protein. Exp Eye Res. 1979;28:63–69. doi: 10.1016/0014-4835(79)90106-4. [DOI] [PubMed] [Google Scholar]
- 44.Abu El-Asrar AM, Desmet S, Meersschaert A, Dralands L, Missotten L, Geboes K. Expression of the inducible isoform of nitric oxide synthase in the retinas of human subjects with diabetes mellitus. Am J Ophthalmol. 2001;132:551–556. doi: 10.1016/s0002-9394(01)01127-8. [DOI] [PubMed] [Google Scholar]
- 45.Napper GA, Pianta MJ, Kalloniatis M. Reduced glutamate uptake by retinal glial cells under ischemic/hypoxic conditions. Vis Neurosci. 1999;16:149–158. doi: 10.1017/s0952523899161108. [DOI] [PubMed] [Google Scholar]
- 46.Tezel G, Wax MB. Increased production of tumor necrosis factor-alpha by glial cells exposed to simulated ischemia or elevated hydrostatic pressure induces apoptosis in cocultured retinal ganglion cells. J Neurosci. 2000;20:8693–8700. doi: 10.1523/JNEUROSCI.20-23-08693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lundmark PO, Pandi-Perumal SR, Srinivasan V, Cardinali DP. Role of melatonin in the eye and ocular dysfunctions. Vis Neurosci. 2006;23:853–862. doi: 10.1017/S0952523806230189. [DOI] [PubMed] [Google Scholar]
- 48.Agorastos A, Huber CG. The role of melatonin in glaucoma: implications concerning pathophysiological relevance and therapeutic potential. J Pineal Res. 2011;50:1–7. doi: 10.1111/j.1600-079X.2010.00816.x. [DOI] [PubMed] [Google Scholar]
- 49.Alonso-Alconada D, Alvarez A, Lacalle J, Hilario E. Histological study of the protective effect of melatonin on neural cells after neonatal hypoxia-ischemia. Histol Histopathol. 2012;27:771–783. doi: 10.14670/HH-27.771. [DOI] [PubMed] [Google Scholar]
- 50.Balduini W, Carloni S, Perrone S, Bertrando S, Tataranno ML, Negro S, et al. The use of melatonin in hypoxic-ischemic brain damage: an experimental study. J Matern Fetal Neonatal Med. 2012;25(Suppl 1):119–124. doi: 10.3109/14767058.2012.663232. [DOI] [PubMed] [Google Scholar]
- 51.Osborne NN, Nash MS, Wood JP. Melatonin counteracts ischemia-induced apoptosis in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1998;39:2374–2383. [PubMed] [Google Scholar]
- 52.Meyer P, Pache M, Loeffler KU, Brydon L, Jockers R, Flammer J, et al. Melatonin MT-1-receptor immunoreactivity in the human eye. Br J Ophthalmol. 2002;86:1053–1057. doi: 10.1136/bjo.86.9.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sáenz DA, Goldin AP, Minces L, Chianelli M, Sarmiento MI, Rosenstein RE. Effect of melatonin on the retinal glutamate/glutamine cycle in the golden hamster retina. FASEB J. 2004;18:1912–1913. doi: 10.1096/fj.04-2062fje. [DOI] [PubMed] [Google Scholar]
- 54.Tan DX, Chen LD, Poeggeler B, Manchester LC, Reiter RJ. Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocrine J. 1993;1:57–60. [Google Scholar]
- 55.Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36:1–9. doi: 10.1046/j.1600-079x.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 56.León J, Acuña-Castroviejo D, Escames G, Tan DX, Reiter RJ. Melatonin mitigates mitochondrial malfunction. J Pineal Res. 2005;38:1–9. doi: 10.1111/j.1600-079X.2004.00181.x. [DOI] [PubMed] [Google Scholar]