Abstract
The anatomical and biophysical specializations of octopus cells allow them to detect the coincident firing of groups of auditory nerve fibers and to convey the precise timing of that coincidence to their targets. Octopus cells occupy a sharply defined region of the most caudal and dorsal part of the mammalian ventral cochlear nucleus. The dendrites of octopus cells cross the bundle of auditory nerve fibers just proximal to where the fibers leave the ventral and enter the dorsal cochlear nucleus, each octopus cell spanning about one-third of the tonotopic array. Octopus cells are excited by auditory nerve fibers through the activation of rapid, calcium-permeable, α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate receptors. Synaptic responses are shaped by the unusual biophysical characteristics of octopus cells. Octopus cells have very low input resistances (about 7 MΩ), and short time constants (about 200 μsec) as a consequence of the activation at rest of a hyperpolarization-activated mixed-cation conductance and a low-threshold, depolarization-activated potassium conductance. The low input resistance causes rapid synaptic currents to generate rapid and small synaptic potentials. Summation of small synaptic potentials from many fibers is required to bring an octopus cell to threshold. Not only does the low input resistance make individual excitatory postsynaptic potentials brief so that they must be generated within 1 msec to sum but also the voltage-sensitive conductances of octopus cells prevent firing if the activation of auditory nerve inputs is not sufficiently synchronous and depolarization is not sufficiently rapid. In vivo in cats, octopus cells can fire rapidly and respond with exceptionally well-timed action potentials to periodic, broadband sounds such as clicks. Thus both the anatomical specializations and the biophysical specializations make octopus cells detectors of the coincident firing of their auditory nerve fiber inputs.
Most acoustic information arrives at the brainstem of mammals through large, myelinated auditory nerve fibers that form a single, tonotopically organized pathway. In the synaptic connection of auditory nerve fibers with distinct groups of principal cells, the auditory pathway branches into multiple, parallel ascending pathways. The two groups of principal cells of the dorsal cochlear nucleus, fusiform and giant cells, project directly to the inferior colliculus. Pathways through the ventral cochlear nucleus (VCN) diverge through bushy, D stellate, T stellate, and octopus cells to take part in intermediate integrative circuits before converging again in the inferior colliculus. How these pathways contribute to the fundamental biological tasks of localizing and interpreting sounds is only partly understood. There is strong evidence that pathways through bushy cells and their targets in the medial and lateral superior olivary nuclei contribute to the localization of sound in the horizontal plane (1, 2). What integrative tasks are performed through other pathways is less well understood. The possibility has been raised that in mammals pathways through the dorsal cochlear nucleus might be involved in analyzing spectral cues for localization in the vertical plane (3). In birds, which lack a structure like the mammalian dorsal cochlear nucleus, localization in the vertical plane seems to be accomplished through homologues of T stellate cells of the mammalian VCN (4). Very little is known about how the pathways through the brainstem in vertebrates contribute to the recognition of acoustic patterns such as those in speech.
The possibility that octopus cells are involved in the recognition of natural sounds, including speech, is intriguing but untested. Octopus cells detect synchrony in the firing of groups of auditory nerve fibers, a pattern that is important for the understanding of speech. Studies from a variety of perspectives have concluded that the temporal structure in the firing of auditory nerve fibers is important in the representation of speech sounds (5, 6). Not only is phase locking important for the recognition of such fundamental features of sounds as pitch but broadband transients and gaps are critical features of consonants in speech. A second intriguing aspect of the role of octopus cells is that they are involved in largely monaural neuronal circuits. The observation that the loss of hearing in one ear does not significantly hinder speech recognition in quiet environments indicates that pattern recognition is a monaural function; octopus cells project to the contralateral ventral nucleus of the lateral lemniscus (VNLL), a nucleus that is largely monaural in most species and is likely to be involved in fundamental functions as it is present not only in mammals but also in birds and reptiles (7–10). Octopus cells also project to the superior paraolivary nucleus, a nucleus about which less is known but which is innervated mainly by the contralateral cochlear nucleus (11). The ventral nuclei of the lateral lemniscus receive input mainly from the contralateral VCN in most, but not all, species. A third intriguing observation is that there is considerable variation in the structure and in the relative proportions of inputs to the VNLL between species. It is possible that this variability reflects differences in the needs of different species in extracting biological meaning from sounds in their environment. In cats, bats, and guinea pigs the monaural, ventral lemniscal nuclei are divided into the ventral and intermediate nuclei of the lateral lemniscus on the basis of the clustering and innervation by the medial nucleus of the trapezoid body (9, 12, 13) whereas in rats and oppossums two subnuclei cannot be distinguished in this area (14, 15). Interestingly the specialized region of the VNLL that is innervated by octopus cells comprises 38% of the nucleus in humans whereas it occupies only 4% of the nucleus in cats (8). Lastly, patients with auditory neuropathy whose auditory brainstem responses revealed abnormally low synchrony in auditory nerve discharge have deficits in speech recognition that are disproportionate to their hearing losses (16).
Projection of the Auditory Nerve on Octopus Cells
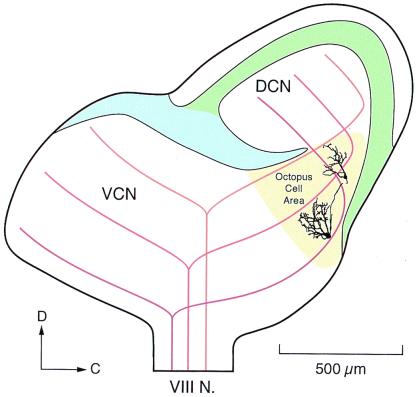
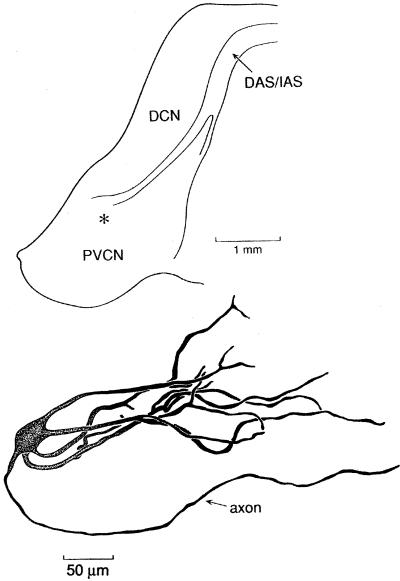
The tonotopic array of auditory nerve fibers is tapped systematically by octopus cell dendrites (Fig. 1). The octopus cell area occupies the most dorsal and caudal tail of the VCN where auditory nerve fibers bundle closely (17). In mice the octopus cell area has sharply defined borders and contains only octopus cells (18–20) but in other species it may be heterogeneous. Each auditory nerve fiber bifurcates at the nerve root sending one branch caudally through the posteroventral to the dorsal cochlear nucleus. Terminals of auditory nerve fibers are subtly different in the octopus cell area than in other regions of the VCN. Auditory nerve terminals that innervate bushy and stellate cells rostrally to the octopus cell area are variable in size and shape; large and small end bulbs lie intermingled with large and small boutons. In the octopus cell area, in contrast, terminals of auditory nerve fibers are uniformly small boutons. In the octopus cell area of mice, the fibers are tonotopically organized in the parasagittal plane, with fibers encoding the highest frequencies lying rostrally and those encoding the lowest frequencies caudally. The dendrites of octopus cells emanate from the rostral pole of the cell bodies so that octopus cells receive input from fibers that encode low frequencies near the cell body and from those that encode higher frequencies progressively more distally on the dendrites (17, 19–21). Octopus cell dendrites span only about one-third of the tonotopic array of auditory nerve fibers (19–21). If mice hear over a range of about 8 octaves (22), individual octopus cells would be expected to receive input from auditory nerve fibers that encode roughly between 2 and 3 octaves. In mice about 200 octopus cells (23) sample the array of about 12,000 auditory nerve fibers (24). As all auditory nerve fibers have been observed to terminate in the octopus cell area, octopus cells receive on average at least 60 inputs (25, 26). However, the number of auditory nerve inputs onto octopus cells may be several times 60 because many auditory nerve fibers probably innervate multiple octopus cells. Although most excitatory input to octopus cells is from auditory nerve fibers, in mice octopus cells also are excited through collaterals of octopus cells (19). In other species the arrangement of auditory nerve inputs on octopus cells appears to be similar but has not been investigated in as much detail. Fig. 2 shows an anatomical reconstruction of an octopus cell from a cat that was labeled by the intracellular injection of label. The dendrites of this cell also emanate from one pole. The relationship of the tonotopic arrangement of auditory nerve fibers with respect to the dendrites of octopus cells is less clear in cats where the tonotopic axis is not aligned in the conventional planes of section.
Figure 1.
Anatomical reconstructions of cell body and dendrites of two intracellularly labeled octopus cells from mice are shown on a schematic version of the cochlear nuclear complex in the parasagittal plane. The granule cell lamina (blue) separates the unlayered VCN from the layered dorsal cochlear nucleus (DCN). Octopus cells occupy an area (yellow) at the most caudal and dorsal extreme of the VCN where auditory nerve fibers are closely bundled as they cross from the VCN to the DCN. Auditory nerve fibers that encode high frequencies (light brown) terminate rostrally and those that encode low frequencies (dark brown) terminate caudally in the octopus cell area. The dendrites of octopus cells extend rostrally from the cell body. Adapted from the results of Golding et al. (19).
Figure 2.
Reconstruction with a camera lucida of an octopus cell in a cat that was labeled by an intraxonal injection. The location of the octopus cell body in the coronal section of the cochlear nuclear complex with respect to the posteroventral cochlear nucleus (PVCN), dorsal cochlear nucleus (DCN), dorsal acoustic stria (DAS), and intermediate acoustic stria (IAS) is indicated by * (Upper).
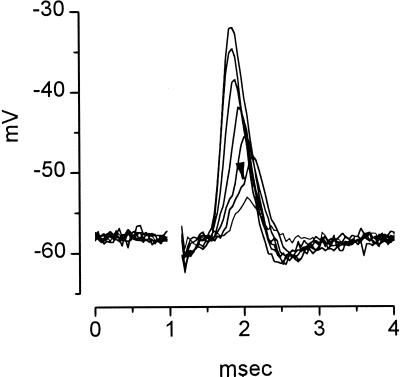
The convergent input from a relatively large number of auditory nerve fibers is reflected in the responses of octopus cells to the activation of the auditory nerve with shocks in slices. Synaptic responses grow incrementally as more and more auditory nerve fibers are simultaneously brought to threshold with brief (0.1 msec) shocks of increasing strength (Fig. 3). Several features of synaptic responses in octopus cells are noteworthy. First, the amplitude of excitatory postsynaptic potentials varied over a wide range, from just detectable responses to weak shocks to about 30-mV responses to strong shocks; maximum amplitudes ranged between about 15 and 50 mV in different cells (19). The responses are so finely graded with shock strength that incremental responses from individual auditory nerve fiber inputs could not be resolved. Second, one small jump in amplitude, which was accompanied by a small action potential, was consistently detected at intermediate stimulus strengths (19) (Fig. 3, arrowhead). Responses to shocks recorded at the cell body comprised small action potentials superimposed on large synaptic potentials. Such an arrangement allows the timing of the synaptic inputs to be reflected in the timing of the action potential with precision because the relatively small action potential distorts the timing of the peak of the synaptic response only minimally. Third, over the entire range of suprathreshold responses the timing of the peaks of responses varied by only about 300 μsec. The timing of peaks was not only consistent but also precise.
Figure 3.
Octopus cells fire in response to the coincident activation of many, but not necessarily all, of the auditory nerve fibers by which they are innervated. Seven superimposed responses are shown to shocks of the auditory nerve of 0.1-msec duration and of varying strength (1–10 V) delivered through a pair of tungsten electrodes. Responses were recorded with a sharp microelectrode filled with 4 M potassium acetate from an octopus cell in a parasagittal slice from the cochlear nucleus of a mouse. The extracellular saline was saturated with 95% oxygen/5% carbon dioxide and contained 130 mM NaCl, 3 mM KCl, 1.3 mM MgSO4, 2.4 mM CaCl2, 20 mM NaHCO3, 3 mM Hepes, 10 mM glucose, 1.2 mM KH2PO4, pH 7.4. Shocks produced artifacts that serve as markers of their occurrence and whose removal left a blank space in the traces. The amplitude of responses was a monotonic function of the shock strength with the weakest shocks producing the smallest responses and the strongest shocks producing the largest responses. The appearance of a small action potential, whose inflection point is marked with an arrowhead, shows where the response was just large and rapid enough to cause firing in the octopus cell. In larger responses the action potential and synaptic potential cannot be resolved. The recording was made by N. L. Golding (19).
Octopus cells detect coincidence of firing in the population of auditory nerve fibers by which they are innervated by requiring the summation of multiple synaptic inputs to reach threshold. In all recordings from octopus cells the amplitude of subthreshold synaptic responses was graded, indicating that inputs from multiple auditory nerve fibers had to sum to produce an action potential in octopus cells. The brevity of synaptic responses makes summation possible only when auditory nerve fibers are activated within about 1 msec. When auditory nerve fibers are activated with shocks in slices, activation is synchronous and summing is optimal. Under these conditions activation of roughly between one-tenth to one-third of the auditory nerve fiber inputs is required to bring octopus cells to threshold. In responses to sound in vivo, when auditory nerve fibers are not necessarily activated in such perfect synchrony, a larger proportion of inputs may be required to activate octopus cells.
The firing of octopus cells can follow the activation of auditory nerve fibers with temporal precision even at high rates. When shocks are delivered to octopus cells at 1/sec, the timing of the peak of the response had a standard deviation of between 20 and 40 μsec (19). Octopus cells can respond to repeated shocks to the auditory nerve to the maximum rate at which auditory nerve fibers can be driven, about 1,000/sec. The responses of octopus cells to activation of the auditory nerve with trains of shocks to the maximum firing rate that is observed in vivo, 300/sec, show no depression. The timing of the peaks of responses with respect to the shock are constant. Only at unphysiological stimulation rates do the responses to octopus cells show signs of depression. Responses to the last of a 10-msec train of shocks at 714 Hz were reduced in amplitude by 25% and had a latency about 200 μsec longer than responses to the first (19). The observed depression arises only in part from synaptic depression because the amplitude of action potentials in auditory nerve fibers are reduced at high firing rates. The ability to fire rapidly and with temporal precision also is observed in responses to sound in vivo. Octopus cells can respond to tones of 800 Hz at every cycle of the tone (27). In vivo, therefore, octopus cells have maximum firing rates that are more than double that of their auditory nerve inputs.
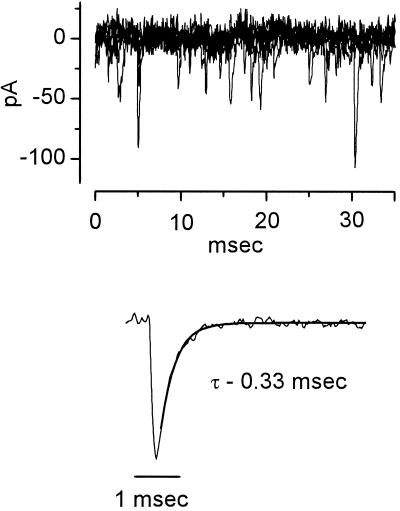
The finding that the terminals of auditory nerve fibers contain high levels of glutamate suggests that glutamate is the neurotransmitter that mediates excitation (28). The glutamate released by auditory nerve fibers acts on the AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate) subtype of glutamate receptors on their targets (19). Under voltage-clamp at the resting potential numerous miniature excitatory postsynaptic currents (mEPSCs) that are sensitive to 6,7-dinitroquinoxaline-2,3-dione and insensitive to tetrodotoxin are observed (Fig. 4). Like other AMPA receptors in brainstem auditory neurons of mice and rats and their avian homologues, the AMPA receptors of octopus cells are exceptionally rapid, rising from 10–90% in 0.20 ± 0.12 msec and decaying with time constants of 0.35 ± 0.16 msec at 33°C (29–31). The mEPSCs in octopus cells of mice show little sign of dendritic filtering, not only when they were recorded with patch pipettes that contained Cs+ (31) but also when they were recorded with potassium gluconate-containing pipettes (Fig. 4). The finding that the AMPA receptors of octopus cells are blocked by the polyamine-containing wasp toxin, philanthotoxin (31), indicates that the receptors in octopus cells lack GluR2 subunits and therefore would be expected to be permeable to calcium (30, 32). It has been shown that calcium-permeable AMPA receptors have single-channel conductances that are 2–3 times larger than calcium-impermeable AMPA receptors (33–35). The low input resistance of octopus cells requires that the robust, suprathreshold synaptic potentials that are observed in octopus cells be driven by large synaptic currents. Possibly the large number of receptors required for the activation of octopus cells and their calcium permeability account for the high levels of calretinin in octopus cells (8).
Figure 4.
Spontaneous miniature synaptic currents recorded from an octopus cell of a mouse under voltage clamp. Patch recording from an octopus cell was made in the whole-cell configuration by using a potassium gluconate-filled pipette. (Upper) Five traces are superimposed to illustrate the frequent spontaneous events when the cell was held near its resting potential at −65 mV. (Lower) Ensemble average of 113 events in the same cell. The decay of currents was well fit with a single exponential with a time constant (τ) equal to 0.33 msec. The pipette solution contained 108 mM potassium gluconate, 9 mM Hepes, 9 mM EGTA, 4.5 mM MgCl2, 14 mM phosphocreatinine (Tris salt), 4 mM ATP (Na salt), and 0.3 mM GTP (Tris salt); pH was adjusted to 7.4 with KOH. The composition of the extracellular saline is given in the legend to Fig. 3. The results have been corrected for a junction potential of −12 mV.
Biophysical Characteristics of Octopus Cells
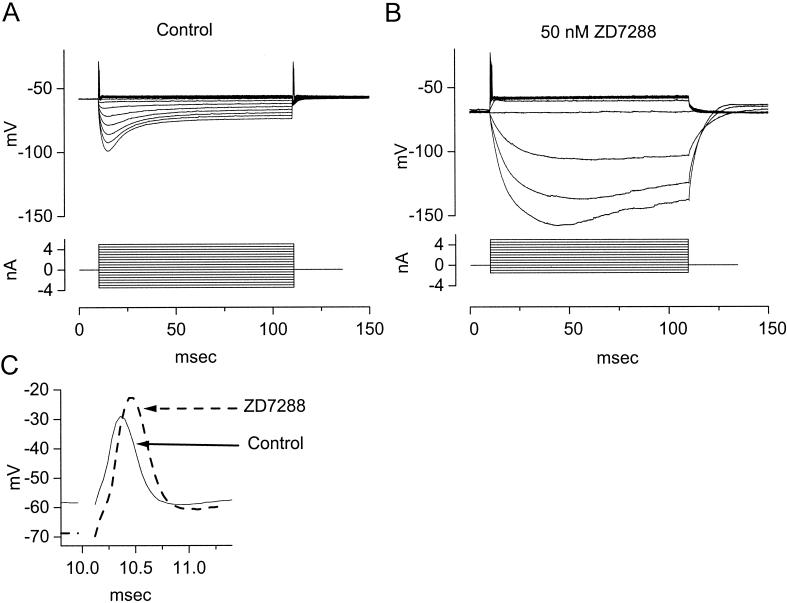
The intrinsic biophysical properties of octopus cells have been studied in slices from mice. Fig. 5A illustrates the responses of octopus cells to current pulses. The resting potential of octopus cells measured with patch-clamp electrodes is 62 ± 2 mV (n = 135) (36). The voltage changes produced by current pulses are small both in the hyperpolarizing and depolarizing directions. When they are depolarized with current pulses greater than about 1 nA, octopus cells fire only a single, small action potential. When they are hyperpolarized, the membrane potential of octopus cells sags back toward rest after the initial hyperpolarization. The voltage-current relationships plotted from peak or steady-state levels are nonlinear (20, 36). Estimates of the input resistance, made from the slope of voltage/current relationships in the voltage range just negative to the resting potential show that octopus cells have input resistances of about 2 and 7 MΩ when measured from steady-state and peak voltage changes, respectively (20, 36).
Figure 5.
(A) Polarization of an octopus cell with current pulses (−3.5 to 5 nA in 0.5-nA steps) reveals the biophysical characteristics of the cell. Depolarizing current pulses greater than 1 nA produced small action potentials at the onset of the depolarization. After the single action potential, the octopus cell remained depolarized by a few mV. Hyperpolarizing current pulses produced transient hyperpolarizations that sagged back toward rest. (B) In the presence of 50 nM ZD7288, a blocker of gh, the cell hyperpolarized and the input resistance in the hyperpolarizing voltage range increased. The increase in input resistance is reflected in that the current pulses produced larger and slower hyperpolarizations. The rectification in the depolarizing voltage range, reflected in the cluster of traces in responses to depolarizing currents, is not affected by ZD7288. (C) Blocking gh shapes responses in the physiological voltage range. Expansions of the onset of responses to the largest depolarizations shown in A and B show that action potentials are taller and broader in the absence of gh. The voltage drop across the resistance of the electrode was balanced off-line. Whole-cell patch recording from an octopus cell with solutions as in Fig. 4.
Octopus cells have conventional regenerative currents that underlie the firing of action potentials. They generate all-or-none action potentials that are sensitive to tetrodotoxin (20). Octopus cells have exceptionally large axons (9, 11, 19, 21, 37) from which trains of action potentials have been recorded in responses to sound (38). These action potentials presumably appear small in recordings from the cell body because they are generated at an electrically distant site near the axon hillock and are attenuated as they spread back to the cell body. In the presence of α-dendrotoxin action potentials are large, suggesting that a potassium conductance provides a pathway for the leakage of depolarizing current as the action potential spreads to the cell body (M. Ferragamo and D.O., unpublished results). Octopus cells also have a weak, voltage-sensitive calcium conductance whose existence was demonstrated by blocking voltage-sensitive Na+ and repolarizing K+ channels and evoking broad, regenerative, calcium-sensitive action potentials (20).
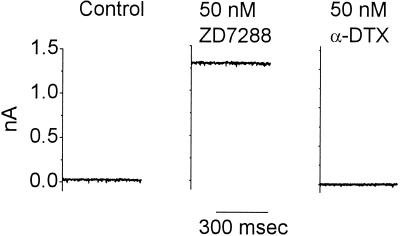
Two voltage-sensitive conductances that are activated at rest dominate the biophysical properties of octopus cells. One is a hyperpolarization-activated, ZD7288-sensitive, mixed-cation conductance, gh, and the other is a depolarization-activated, α-dendrotoxin-sensitive, low-threshold potassium conductance, gK(L). Although these conductances are activated by voltage changes in the opposite direction, the voltage range of activation of the conductances overlap at the resting potential. Together these conductances set the resting potential to a level near −62 mV at which the inward current, Ih, balances the outward current, IK(L) (36). The experiment illustrated in Fig. 6 illustrates the balance in one cell. On average the magnitude of the inward current blocked by ZD7288 was 1,280 ± 270 (mean ± SD) pA; the addition of 50 nM α-dendrotoxin in those same seven cells left an outward current of 33 ± 46 pA. The simultaneous activation of these two conductances not only makes the input resistance of octopus cells low but also endows octopus cells with biophysical characteristics that promote firing in response to synchronous inputs and prevent firing when inputs are not synchronous.
Figure 6.
At the resting potential Ih is roughly balanced by IK(L). The murine octopus cell was held at its resting potential, −63 mV, under voltage clamp with whole-cell patch clamp under conditions such as those described for Fig. 4. In the presence of ZD7288, a large, steady, outward current developed. Application of α-dendrotoxin blocked a current exactly equal to that which had developed in the presence of ZD7288.
The hyperpolarization-activated, mixed-cation conductance, gh, in octopus cells resembles such conductances in other cells but is unusually large and has a half-maximal activation that is more depolarized than in most neurons (19, 20, 36). This conductance is sensitive to extracellular Cs+ and ZD7288 (36). The reversal potential of the current through this conductance was −38 mV under normal physiological conditions and was sensitive to extracellular concentrations of both K+ and Na+ (20, 36). The permeability ratio PNa/PK of gh in octopus cells was about 0.2 (36). When fully activated at hyperpolarizing potentials, the maximum gh was 150 ± 30 nS (36). The half-maximal activation voltage, Vhalf, is unusually depolarized, lying at −65 mV. As a result of the large maximal conductance, of which a high proportion is activated at the resting potential, gh contributes substantially to the total input conductance. At rest gh contributed from 35 to 85 nS, with a mean of 62 nS, to the total input conductance, which was on average 149 nS (36). In the presence of ZD7288 the resting potential of octopus cells hyperpolarizes by about 10 mV (Fig. 5B). The characteristics of gh in octopus cells indicate that this conductance is mediated by a class of ion channels that has been termed HCN (for hyperpolarization-activated and cyclic nucleotide-gated channels) (39, 40). The activation and deactivation of gh are relatively slow with respect to the signaling of octopus cells. The fast and slow time constants of activation, τfast and τslow, were voltage-dependent with τfast = 44 ± 6 ms and τslow = 181 ± 39 ms at −77 mV and decreasing to τfast = 16 ± 3 ms and τslow = 84 ± 20 ms at −107 mV (36). Deactivation was fit with single exponentials 126 ± 15 ms at −62 mV and 178 ± 33 ms at −87 mV. Although gh is activated by hyperpolarization, this conductance nevertheless shapes responses in the physiological, depolarizing voltage range because the rates of activation and deactivation are slow relative to the duration of synaptic potentials and action potentials. In the presence of ZD7288 action potentials rose more slowly, reached higher peaks, and were broader than under control conditions (Fig. 5C).
A depolarization-activated, low-threshold K+ conductance also contributes to the unusual properties of octopus cells (19, 20, 36). Low-threshold K+ conductances are prominent in many neurons in the auditory brainstem nuclei of vertebrates, causing them to fire only at the onset of current pulses (41, 42). In octopus cells, as in other brainstem neurons, this conductance is sensitive to 4-aminopyridine and α-dendrotoxin (M. Ferragamo, R.B., and D.O., unpublished results) (20). The finding that 4-aminopyridine causes the resting potential of octopus cells to depolarize indicates that the threshold of activation of the K+ conductance is more hyperpolarized than the resting potential and identifies it as a low-threshold K+ conductance, gK(L) (20). Homomeric and heteromeric channels with Kv1.1, Kv1.2, and Kv1.3 subunits have low thresholds for activation (43, 44). Immunocytochemical labeling for α subunits of K+ channels of the Kv1 family suggests that potassium channels of this family may underlie gK(L). Potassium channel α subunits Kv1.1 and Kv1.2 (45) have been shown to be strongly expressed in the octopus cell area.
Less is known about other K+ conductances in octopus cells. Under conditions when gK(L) was blocked with 4-aminopyridine or α-dendrotoxin, action potentials repolarized slowly (20). Immunolabeling for high-threshold, Kv3.1 potassium channels has been detected in the cell bodies of octopus cells (46).
Despite the large conductances that are active at rest, three experimental observations suggest that dendritic filtering is surprisingly low in octopus cells. The first is that recordings of miniature synaptic currents in octopus cells showed no sign of dendritic filtering (31). Octopus cells receive input from auditory nerve fibers on dendrites (19). Dendritic filtering would be expected to produce a positive correlation between rise and fall times and a negative correlation between rise time and amplitude but none was observed (31). Miniature synaptic currents were uniformly rapid not only when intracellular Cs+ in the recording pipettes were used to block leakage in octopus cells but also when pipettes contained potassium gluconate. The second observation indicative of isopotentiality and lack of filtering in octopus cell dendrites is that Ih recorded under voltage clamp was well-behaved; chord conductances converged at a single point under a wide range of conditions. Third, in the study of gh the reversal potential of Ih, a mixed-cation current, was measured when the extracellular Na+ and K+ concentrations, and therefore the reversal potential, was varied. To test whether the reversal potentials measured under these conditions were consistent, the relative permeabilities to Na+ and K+ were calculated and compared and found not to be statistically different from one another (36). Whether the low dendritic filtering results primarily from the large size of dendrites or from a favorable spatial distribution of ion channels is not clear.
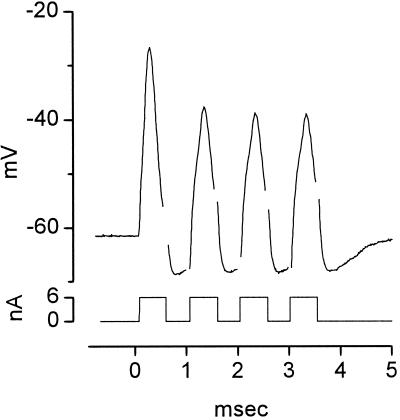
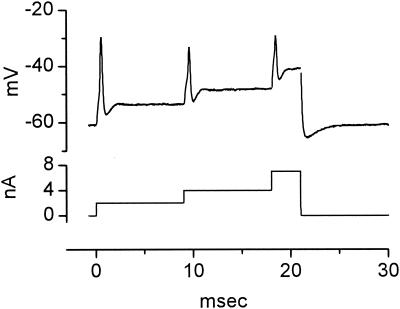
The interplay of conductances gives octopus cells unusual biophysical properties. When depolarized with a steady pulse of current, octopus cells fire only once at the onset (Fig. 5); in no octopus cell have multiple action potentials ever been observed in responses to depolarizing current pulses. The presence of two voltage-sensitive conductances at rest also makes the firing of octopus cells sensitive to the rate at which they are depolarized. Octopus cells fire when they are depolarized rapidly but fail to fire when they are depolarized slowly (M. Ferragamo and D.O., unpublished results) (47). The finding that octopus cells fire only once in response to long depolarizations does not preclude their being able to fire rapidly. A train of current pulses presented at 1,000/sec drives action potentials in an octopus cell with every pulse (Fig. 7). Not surprisingly, the first action potential is larger than the later ones, which rise from the undershoot of the preceding action potentials. These observations raise the question to what extent octopus cells are refractory after the first action potential. The experiment illustrated in Fig. 8 shows that octopus cells can be induced to fire even when they are steadily depolarized. This octopus cell was depolarized with a current pulse of 2 nA and then the current was increased in two steps. With each increase the octopus cell fired an action potential. The later action potentials were smaller, when measured from the inflection to the peak, than the first, presumably because the regenerative inward current had to counter the larger, steady outward current. The large after-hyperpolarization that followed the offset of the current reflects the deactivation of the potassium conductance that had been activated by the previous depolarization.
Figure 7.
Octopus cells can fire rapidly. A train of depolarizing current pulses presented at 1,000/sec evoked action potentials at every pulse. The voltage drop across the resistance of the electrode was balanced off-line; removal of transient artifacts left brief gaps in the trace. Whole-cell patch recording from an octopus cell of a mouse with solutions as in Fig. 4.
Figure 8.
When steadily depolarized, octopus cells are in a relative, but not absolute, refractory period. This octopus cell was depolarized with increasing current steps. Depolarization with a current pulse of 2 nA produced an action potential. Further step depolarizations from 2 to 4 nA and from 4 to 7 nA caused the octopus cell to fire again. Action potentials that were evoked by superimposed step depolarizations were smaller than the initial action potential. At the offset of the current pulse, the octopus cell undershot the resting potential. The voltage drop across the resistance of the electrode was balanced off-line and transient artifacts were made blank. Whole-cell patch recording from a murine octopus cell with solutions as in Fig. 4.
Responses to Sound
Few reports have been published of the responses to sound of neurons that are identified with some certainty of being octopus cells. Godfrey et al. (48) concluded that octopus cells in cats respond to tones >2 kHz with sharply timed action potentials at the onset. This conclusion was confirmed by later studies allowing octopus cells to be identified by their onset responses (27, 37, 38, 49, 50). Recordings in vivo indicate that the anatomical and biophysical features of octopus cells that have been revealed in vitro are correlated with the ability of neurons to encode temporal features of acoustic stimuli with greater precision than their auditory nerve inputs and with greater precision that other groups of neurons in the cochlear nuclei. Consistent with the observation that octopus cells are innervated by many auditory nerve fibers and require the synchronous activation of a substantial fraction of those inputs, octopus cells are broadly tuned and have high thresholds to pure tones (27, 48, 51). At high intensities a broad range of auditory nerve fibers can respond to tones of frequencies less than about 2 kHz, with discharges that are locked to a particular phase of each stimulus cycle. Octopus cells can respond to such tones with a single well-timed spike at every stimulus cycle for frequencies up to 800 Hz, firing at rates that are unprecedented in the central nervous system. They respond to tones above about 2 kHz with a single action potential at the onset of the tone, presumably because it is only at stimulus onset that the firing of auditory nerve inputs fire in sufficient synchrony to drive octopus cells. Octopus cells also respond to broadband transients such as clicks with exceptionally well-timed action potentials. Of all cells in the cochlear nucleus, the octopus cells show the strongest synchronization to amplitude-modulated stimuli (49) and to the fundamental frequency of simple speech-like sounds (50). Not only is the precision in the timing of firing remarkable but these cells also show sharp tuning to modulation frequency in terms of average firing rate.
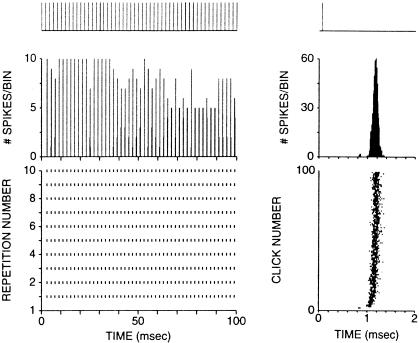
Responses to clicks in one octopus cell from a cat are illustrated in Fig. 9. The recording was made intracellularly from the axon with a dye-filled pipette, making it possible to reconstruct the cell from which the recording was made after the end of the experiment (Fig. 2). The cell responded to a train of clicks at 500 Hz with trains of action potentials whose timing followed the stimulus with precision (Fig. 9). The dot raster in the lower left panel, showing the spike response to 10 repetitions of a 100-ms train of clicks, is regular. The histogram in the left, middle panel shows that the spikes fall into either one or two 0.2-ms bins. The timing of the firing is illustrated in greater resolution on the right as a function of the 2-ms period of the stimulus. The timing of firing of individual action potentials is shown in the dot raster plot at the bottom and is shown as a histogram with 8-μsec bins in the middle. These records show that the jitter in the timing of firing of octopus cells is less than 200 μsec.
Figure 9.
Response of an octopus cell to clicks in a cat in vivo. (Left) Responses to 10 repetitions of a train of acoustic clicks (20-μsec duration) spaced at 2-msec (500 Hz) intervals. (Top) Trace shows the timing of the click stimuli that were presented 30 dB above threshold, (Middle) the poststimulus time histogram (0.2-msec binwidth), and (Bottom) dot rasters to 10 click trains with each symbol representing one action potential. (Right) Responses on an expanded time scale as a function of the 2-msec period of the stimulus. (Top) Trace shows position of the click in the period, (Middle) the period histogram using 8-μsec binwidths, and (Bottom) dot rasters ordered by click number (response to first click on bottom, response to 100th click on top). The threshold of responses to tones at the characteristic frequency, 9 kHz was 52 dB sound pressure level.
Despite the large size of octopus cells, in vivo recordings have proven to be surprisingly difficult to make. The biophysical properties of octopus cells perhaps can account for that difficulty. The finding that action potentials recorded at the cell body are small suggests that extracellular currents associated with those action potentials are also small and difficult to record. Although the action potentials associated with the axons are easier to record, the axons themselves are not easy to reach (38).
Acknowledgments
The ideas and conclusions summarized here reflect the thoughts and efforts of many people whose substantial contributions are a pleasure to acknowledge. When Shu Hui Wu first revealed the morphology of octopus cells, we had not yet appreciated how fascinating they were. It was the findings of Robert Wickesberg and Donna Whitlon that drew our attention to the octopus cell area. Nace Golding, Don Robertson, and Michael Ferragamo then made critical observations that serve as the basis of our recent conclusions. This work depended on the support by National Institutes of Health Grants DC00176 and DC00116.
Abbreviations
- VCN
ventral cochlear nucleus
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate
Footnotes
This paper was presented at the National Academy of Sciences colloquium “Auditory Neuroscience: Development, Transduction, and Integration,” held May 19–21, 2000, at the Arnold and Mabel Beckman Center in Irvine, CA.
References
- 1.Joris P X, Smith P H, Yin T C. Neuron. 1998;21:1235–1238. doi: 10.1016/s0896-6273(00)80643-1. [DOI] [PubMed] [Google Scholar]
- 2.Oertel D. Annu Rev Physiol. 1999;61:497–519. doi: 10.1146/annurev.physiol.61.1.497. [DOI] [PubMed] [Google Scholar]
- 3.Spirou G A, Davis K A, Nelken I, Young E D. J Neurophysiol. 1999;82:648–663. doi: 10.1152/jn.1999.82.2.648. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi T, Moiseff A, Konishi M. J Neurosci. 1984;4:1781–1786. doi: 10.1523/JNEUROSCI.04-07-01781.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young E D, Sachs M B. J Acoust Soc Am. 1979;66:1381–1403. doi: 10.1121/1.383532. [DOI] [PubMed] [Google Scholar]
- 6.Shannon R V, Zeng F G, Kamath V, Wygonski J, Ekelid M. Science. 1995;270:303–304. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- 7.Vater M, Feng A S. J Comp Neurol. 1990;292:373–395. doi: 10.1002/cne.902920305. [DOI] [PubMed] [Google Scholar]
- 8.Adams J C. Aud Neurosci. 1997;3:335–350. [Google Scholar]
- 9.Schofield B R, Cant N B. J Comp Neurol. 1997;379:363–385. doi: 10.1002/(sici)1096-9861(19970317)379:3<363::aid-cne4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 10.Carr C E, Code R A Springer Handbook of Auditory Research 13, editors. In: The Central Auditory System of Reptiles and Birds. Dooling R, Popper A N, Fay R R, editors. New York: Springer; 2000. pp. 197–248. [Google Scholar]
- 11.Schofield B R. J Comp Neurol. 1995;360:135–149. doi: 10.1002/cne.903600110. [DOI] [PubMed] [Google Scholar]
- 12.Glendenning K K, Brunso-Bechtold J K, Thompson G C, Masterton R B. J Comp Neurol. 1981;197:673–703. doi: 10.1002/cne.901970409. [DOI] [PubMed] [Google Scholar]
- 13.Covey E, Casseday J H. J Neurosci. 1986;6:2926–2940. doi: 10.1523/JNEUROSCI.06-10-02926.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willard F H, Martin G F. Neuroscience. 1983;10:1203–1232. doi: 10.1016/0306-4522(83)90109-4. [DOI] [PubMed] [Google Scholar]
- 15.Merchan M A, Berbel P. J Comp Neurol. 1996;372:245–263. doi: 10.1002/(SICI)1096-9861(19960819)372:2<245::AID-CNE7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Zeng F G, Oba S, Garde S, Sininger Y, Starr A. NeuroReport. 1999;10:3429–3435. doi: 10.1097/00001756-199911080-00031. [DOI] [PubMed] [Google Scholar]
- 17.Osen K K. Acta Otolaryngol (Stockh) 1969;67:352–359. doi: 10.3109/00016486909125462. [DOI] [PubMed] [Google Scholar]
- 18.Wickesberg R E, Whitlon D S, Oertel D. J Comp Neurol. 1994;339:311–327. doi: 10.1002/cne.903390302. [DOI] [PubMed] [Google Scholar]
- 19.Golding N L, Robertson D, Oertel D. J Neurosci. 1995;15:3138–3153. doi: 10.1523/JNEUROSCI.15-04-03138.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golding N L, Ferragamo M, Oertel D. J Neurosci. 1999;19:2897–2905. doi: 10.1523/JNEUROSCI.19-08-02897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oertel D, Wu S H, Garb M W, Dizack C. J Comp Neurol. 1990;295:136–154. doi: 10.1002/cne.902950112. [DOI] [PubMed] [Google Scholar]
- 22.Ehret G. In: Psychoacoustics. Willott J F, editor. Springfield: Thomas; 1983. pp. 13–56. [Google Scholar]
- 23.Willott J F, Bross L S. J Comp Neurol. 1990;300:61–81. doi: 10.1002/cne.903000106. [DOI] [PubMed] [Google Scholar]
- 24.Ehret G. J Comp Neurol. 1979;183:73–88. doi: 10.1002/cne.901830107. [DOI] [PubMed] [Google Scholar]
- 25.Lorente de No R. Laryngoscope. 1933;43:327–350. [Google Scholar]
- 26.Brown M C, Ledwith J V. Hear Res. 1990;49:105–118. doi: 10.1016/0378-5955(90)90098-a. [DOI] [PubMed] [Google Scholar]
- 27.Rhode W S, Smith P H. J Neurophysiol. 1986;56:261–286. doi: 10.1152/jn.1986.56.2.261. [DOI] [PubMed] [Google Scholar]
- 28.Hackney C M, Osen K K, Ottersen O P, Storm-Mathisen J, Manjaly G. Eur J Neurosci. 1996;8:79–91. doi: 10.1111/j.1460-9568.1996.tb01169.x. [DOI] [PubMed] [Google Scholar]
- 29.Raman I M, Trussell L O. Neuron. 1992;9:173–186. doi: 10.1016/0896-6273(92)90232-3. [DOI] [PubMed] [Google Scholar]
- 30.Geiger J R, Melcher T, Koh D S, Sakmann B, Seeburg P H, Jonas P, Monyer H. Neuron. 1995;15:193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 31.Gardner S M, Trussell L O, Oertel D. J Neurosci. 1999;19:8721–8729. doi: 10.1523/JNEUROSCI.19-20-08721.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jonas P, Burnashev N. Neuron. 1995;15:987–990. doi: 10.1016/0896-6273(95)90087-x. [DOI] [PubMed] [Google Scholar]
- 33.Koh D S, Geiger J R, Jonas P, Sakmann B. J Physiol (London) 1995;485:383–402. doi: 10.1113/jphysiol.1995.sp020737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raman I M, Trussell L O. Biophys J. 1995;68:137–146. doi: 10.1016/S0006-3495(95)80168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swanson G T, Kamboj S K, Cull-Candy S G. J Neurosci. 1997;17:58–69. doi: 10.1523/JNEUROSCI.17-01-00058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bal R, Oertel D. J Neurophysiol. 2000;84:806–817. doi: 10.1152/jn.2000.84.2.806. [DOI] [PubMed] [Google Scholar]
- 37.Rhode W S, Oertel D, Smith P H. J Comp Neurol. 1983;213:448–463. doi: 10.1002/cne.902130408. [DOI] [PubMed] [Google Scholar]
- 38.Smith P H, Joris P X, Banks M I, Yin T C T. In: The Mammalian Cochlear Nuclei: Organization and Function. Merchan M A, Juiz J M, Godfrey D A, Mugnaini E, editors. New York: Plenum; 1993. pp. 349–360. [Google Scholar]
- 39.Clapham D E. Neuron. 1998;21:5–7. doi: 10.1016/s0896-6273(00)80508-5. [DOI] [PubMed] [Google Scholar]
- 40.Seifert R, Scholten A, Gauss R, Mincheva A, Lichter P, Kaupp U B. Proc Natl Acad Sci USA. 1999;96:9391–9396. doi: 10.1073/pnas.96.16.9391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oertel D. Neuron. 1997;19:959–962. doi: 10.1016/s0896-6273(00)80388-8. [DOI] [PubMed] [Google Scholar]
- 42.Trussell L O. Annu Rev Physiol. 1999;61:477–496. doi: 10.1146/annurev.physiol.61.1.477. [DOI] [PubMed] [Google Scholar]
- 43.Stuhmer W, Ruppersberg J P, Schroter K H, Sakmann B, Stocker M, Giese K P, Perschke A, Baumann A, Pongs O. EMBO J. 1989;8:3235–3244. doi: 10.1002/j.1460-2075.1989.tb08483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hopkins W F, Allen M L, Houamed K M, Tempel B L. Pflügers Arch Eur J Physiol. 1994;428:382–390. doi: 10.1007/BF00724522. [DOI] [PubMed] [Google Scholar]
- 45.Wang H, Kunkel D D, Schwartzkroin P A, Tempel B L. J Neurosci. 1994;14:4588–4599. doi: 10.1523/JNEUROSCI.14-08-04588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perney T M, Kaczmarek L K. J Comp Neurol. 1997;386:178–202. [PubMed] [Google Scholar]
- 47.Cai Y, McGee J, Walsh E J. J Neurophysiol. 2000;83:301–314. doi: 10.1152/jn.2000.83.1.301. [DOI] [PubMed] [Google Scholar]
- 48.Godfrey D A, Kiang N Y S, Norris B E. J Comp Neurol. 1975;162:247–268. doi: 10.1002/cne.901620206. [DOI] [PubMed] [Google Scholar]
- 49.Rhode W S. Hear Res. 1994;77:43–68. doi: 10.1016/0378-5955(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 50.Rhode W S. Hear Res. 1998;117:39–56. doi: 10.1016/s0378-5955(98)00002-1. [DOI] [PubMed] [Google Scholar]
- 51.Cai Y, Walsh E J, McGee J. J Neurophysiol. 1997;78:872–883. doi: 10.1152/jn.1997.78.2.872. [DOI] [PubMed] [Google Scholar]