Summary
Cells perceive force through a variety of molecular sensors, of which the mechanosensitive ion channels are the most efficient and act the fastest. These channels apparently evolved to prevent osmotic lysis of the cell as a result of metabolite accumulation and/or external changes in osmolarity. From this simple beginning, nature developed specific mechanosensitive enzymes that allow us to hear, maintain balance, feel touch and regulate many systemic variables, such as blood pressure. For a channel to be mechanosensitive it needs to respond to mechanical stresses by changing its shape between the closed and open states. In that way, forces within the lipid bilayer or within a protein link can do work on the channel and stabilize its state. Ion channels have the highest turnover rates of all enzymes, and they can act as both sensors and effectors, providing the necessary fluxes to relieve osmotic pressure, shift the membrane potential or initiate chemical signaling. In this Commentary, we focus on the common mechanisms by which mechanical forces and the local environment can regulate membrane protein structure, and more specifically, mechanosensitive ion channels.
Key words: Activin, Cancer, Tumor microenvironment, Wound healing
Introduction
Cells and tissues respond to a variety of mechanical stresses that can be roughly classified as follows: (1) Osmotic swelling or shrinkage, which occurs in all cells but is particularly significant in epithelial tissues, such as the intestines, nephrons and the skin, where osmolarity can change drastically; (2) cell deformation as a result of actomyosin activity, which is exemplified in muscle tissues and migrating fibroblasts; (3) compressive or tensile stresses, such as those occurring in cartilage, bone and arteries; (4) Fluid shear stress, which acts on cell surfaces and protruding cilia; and (5) distortion of specialized structures, such as the stereocilia of auditory hair cells.
Although this Commentary will focus on mechanosensitive ion channels, it is important to emphasize that all proteins are deformable and hence subject to mechanical modulation. The primary structural components of a cell that bear stress and reorganize under forces are the cytoskeleton, the extracellular matrix (ECM) and adhesion complexes (Vogel, 2006; Hoffman et al., 2011), as well as membranes. Many enzymes, such as kinases, phosphatases, GTPases, cyclases and G-protein-coupled receptors (GPCRs) (Moore et al., 2010), change conformation in response to force, thereby creating transduction pathways that report mechanical stress. Force transduction can involve changes in the kinetic rate constant of a mechanosensitive enzyme or, more qualitatively, expose cryptic binding sites on a molecule (Sawada and Sheetz, 2002; Vogel, 2006; Discher et al., 2006).
Mechanosensitive channels (MSCs) form a special group of mechanosensors that can serve as both sensors and effectors as they modify the electrical potential of the cell and mediate a flux of specific ions, such as Ca2+, across the plasma membrane. MSCs are the fastest transducers of mechanical stress in cells. Signals initiated by conformational changes in MSCs are amplified by the electrochemical gradients that exist across the membrane. Because MSCs are embedded in a lipid bilayer they are sensitive to local, as well as global, stress in the bilayer, which leads to the question of why cells evolved these tools.
When the first membrane-delineated protocells developed metabolism, they also developed the need to deal with the osmotic gradients that can make cells swell and lyse. Cells evolved several mechanisms to deal with osmotic pressure. Bacteria and plants developed a rigid external cell wall to withstand the hydrostatic pressure from osmotic gradients. A second method, which is used by motile animal cells lacking a cell wall, is to develop a dynamic internal cytoskeleton that absorbs osmotic stress much like sponge swells in water. However, all cells, without exception, avoid excessive swelling by allowing excess solutes to leave the cell by means of channels or transporters, in a process called regulatory volume decrease. Particularly in bacteria, the fast exit of solutes takes place through multiple large, stretch-activated channels.
In multicellular organisms, mechanosensation evolved to perfection in the sensory receptors of the cochlea, the skin, muscles and joints (Gillespie and Müller, 2009; Lumpkin et al., 2010; Delmas et al., 2011), but it is important to recall that all cells respond to forces. The exquisite sensitivity of the sensory receptors does not originate from MSCs themselves, but from an efficient coupling between the channel gating machinery and the cellular structures that transmit the force. Mechanical sensitivity is a property of all deformable structures and in the case of ion channels, the deformations take the form of transitions between the closed and open states. In general, the open structures are larger than the closed structures, so channels are stretch activated. However, it is usually too simplistic to describe channels as simply being closed or open. The more general finding is that the channels have multiple states allowing them to adapt or become inactive. This adaptation means the system is most sensitive to changes in force rather than the force itself.
MSCs differ widely in their ion selectivity, ranging from being nonselective between cations and anions (Sukharev et al., 1993) to being cation selective (Zhou et al., 2003; Coste et al., 2012) and highly selective for K+ (Maingret et al., 2000). The apparent physiological functions that these channels serve undoubtedly vary as well, and they can be modulated by multiple other inputs. For example TREK1, a K+-selective MSC, seems to be involved in setting the resting potential of neurons, and hence their excitability, but TREK1 is also opened by general anesthetics (Patel and Honoré, 2003), and activation of TREK1 might underlie anesthesia. The large, nonselective channels found in bacteria serve as osmoprotectors, because osmotic pressure can be changed by the flux of a neutral species. We have chosen to define MSCs as channels that can be modulated over most of their dynamic range (i.e. the probability of being open) by mechanical forces alone. However, because all channels must change shape when they open, all channels (and for that matter all membrane-bound enzymes) are sensitive to mechanical stress to some degree. Channels known by their familiar gating variables, such as voltage-gated channels (VGCs), can also be substantially modulated by mechanical stress (Morris, 2011). The reverse interaction of channels with the mechanical environment has been explicitly demonstrated by (Lauritzen et al., 2005) who showed that expression of two-pore domain (2P) channels, even if they are non-conducting, has a large effect on actin cytoskeleton reorganization.
The first genes encoding MSCs that were identified are mscL and mscS, which were cloned from prokaryotes (Sukharev et al., 1994; Levina et al., 1999; Perozo and Rees, 2003). Crystallographic studies on these channels (Steinbacher et al., 2007) have provided the first three-dimensional view of this family of channels. Following that, eukaryotic MSCs belonging to several distinct families have been cloned and functionally characterized (Honoré et al., 2006; Su et al., 2011; Coste et al., 2010; Haswell et al., 2011). The recently reported crystal structure of a mammalian two-pore domain mechanosensitive channel, TRAAK (also known as KCNK4), now provides a foundation for mechanistic studies of these channels (Brohawn et al., 2012). In this Commentary, we will provide an overview of the physical principles involved in mechanosensation with a focus on ion channels.
Biophysical aspects of mechanosensation
The forces
On the scale of organisms, applied forces can vary over orders of magnitude: from the pressures in bones and joints of 106 Pa and blood pressures of 104 Pa to faint sounds of 10−4 Pa (105 Pa = 1 Atm = 15 psi). How can cells discriminate such a wide range of stimuli? First, these pressures and the forces they create are not applied to single cells but to cell populations that share the stress. Second, at the level of a single cell, especially in stiff tissues like bone, the pressures are shared by many elastic elements, such as the cytoskeleton and the ECM. Finally, measurements of MSC sensitivity in different cells made by using patch clamp assays have shown that the channels become activated in response to similar forces (Box 1) (Sachs, 1986; Duncan and Misler, 1989; Wu et al., 2011). We would like to emphasize that although hydrostatic pressure is commonly used to apply a mechanical stimulus to cells, channels do not respond to pressure, they respond to the mechanical stresses produced by the pressure. For example, the aortic endothelium is pushed outwards by the blood pressure, but according to Newton's third law, the cells push back equally strongly using the elasticity of the vessel. An increase in blood pressure will swell the vessel and stretch the layers of cells that form the wall of the blood vessel, but the hydrostatic pressure of the blood itself exerts minimal effect on cell physiology. The physiologically important stresses are those in the wall of the blood vessel. Those wall stresses are shared across many cells and finally filter down to the mechanical sensor molecules in the cells. When discussing MSCs and the location of channels in the membrane, we are referring to the lipid bilayer. However, the bilayer is only one component of the cell cortex, and mechanical stresses are distributed to all components of the cortex including the bilayer, the cytoskeleton and the ECM, and this balance is dynamic, varying in time and space. We would also like to draw the readers' attention to the fact that in the scientific literature almost no papers are able to address the forces that are actually applied to the MSCs, except when purified channel proteins are reconstituted in a pure bilayer (Häse et al., 1995; Sukharev et al., 1999; Moe and Blount, 2005).
Box 1. Applying forces to cells – methods to study mechanosensitivity in ion channels.
The way a stimulus is applied to a mechanosensor depends on the system under study: tension in a linear filament (del Rio et al., 2009; Meng and Sachs, 2011b; Wang et al., 2011; Brenner et al., 2011), tension in a bilayer or flattening of a molecule (Fig. 1). In an ideal experiment, one would like to apply a uniform mechanical stimulus and measure the response. The plasma membrane, however, is not well defined in mechanical terms, and stresses vary with the local curvature and stiffness (Sachs, 2010). By contrast to studies of voltage- or ligand-gated channels, it is difficult to apply a uniform mechanical stimulus to membrane-embedded channels unless they are in spherical vesicles.
In principle, an ion channel can respond to increases in membrane tension as a result of cell swelling (Hua et al., 2010), but the literature contains many examples in which osmotic stress is referred to as a mechanical stress, which might not always be correct (Spagnoli et al., 2008). Cells are not spherical shells and large forces are exerted by the cytoskeleton, which lies normal to the membrane. Much of the osmotic gradient energy is contained within the cytoskeleton and osmotic pressure is not generally converted into membrane tension.
If we had a planar bilayer and could uniformly stretch its perimeter, the tension would be evenly distributed between the two monolayers. Stretching membranes in a glass patch pipette (as in a patch clamp), however, does not provide such uniformity, and this approach comes with several caveats. One caveat is that adhesion to the glass causes the membrane to be under near-lytic tension. Therefore, even in the absence of transmembrane pressure (i.e. when the patch is flat) the resting stress is substantial (Suchyna et al., 2009; Ursell et al., 2011). A second caveat is that, although the transmembrane pressure gradient Δp causes the patch to bulge and the tension increases according to the law of Young–Laplace (γ = Δp•r/2), the radius of curvature (r) of the patch is rarely known (Ursell et al., 2011). A third caveat is, that only the outer leaflet contacts the pipette and will bear all the tension, whereas the inner monolayer can slide over the outer and relax (Evans and Yeung, 1994). This tension gradient, which is normal to the membrane, also leads to more efficient lipid exchange between monolayers. For example, MscL and MscS are more sensitive to tension in the inner leaflet (Belyy et al., 2010b). This uneven distribution of stress becomes more complicated when considering the properties of the cell cortex (Suchyna et al., 2009). In most cells, stresses are distributed in three dimensions: parallel to the bilayer and normal to it (Akinlaja and Sachs, 1998; Xiong et al., 2010). The biochemical dissection of stresses in the proteins of the cell cortex that are most effective in modulating MSCs has only just begun (Meng et al., 2008; Meng and Sachs, 2011b).
In yet another experimental approach, cells can be grown on an elastic substrate that is subsequently stretched. In this approach, however, one is pulling on focal cell adhesions and this stress is being passed onto the cytoskeleton through the adhesion complexes and an unknown fraction spreads into the bilayer (Alford et al., 2011). The bilayer is a liquid and it will flow under a tension gradient so its tension tends to be uniform.
It is possible to stimulate a cell locally by pressing on it with a blunt pipette (Coste et al., 2010), by applying a local water flow (S�nchez et al., 2007), by pushing or pulling with an atomic force microscopic cantilever (Beyder and Sachs, 2009), or by pulling on it with a magnetic bead (Lele et al., 2007). These stimuli do not produce uniform stress but spatial gradients in two and three dimensions that decrease with distance from the probe (Radmacher, 2007). The resulting stress is not distributed equally among the different biochemical components of the cortex and cell interior. In addition, these stresses are time dependent, and remodeling tends to reduce local stress (Niggel et al., 2000; Suchyna et al., 2009).
The energy involved in mechanical transduction
When force is applied to a deformable object, such as an MSC, it will move, and the energy expended for this process is equal to force × distance moved. This energy, which is relative to the surrounding noisy energy of thermal fluctuations, determines the probability that an MSC or other sensor will be in an active or inactive state (Markin and Sachs, 2004; Suchyna and Sachs, 2007). Stretching elastic objects such as proteins makes them bigger in the direction of stress so that large conformations are favored over smaller ones.
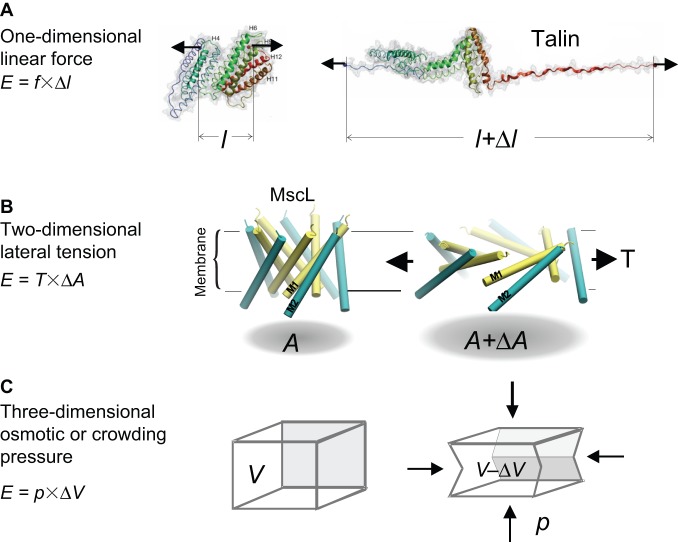
Applied forces do not move a molecular sensor in a clockwork manner, but rather they alter the probability that the sensor, which is constantly subjected to thermal forces, will reside in the active or inactive conformation. But if every molecule in a cell deforms in response to random thermal forces, how can a cell differentiate between random noise and a specific signal? The average thermal energy that is contained within all particles is kBT/2 (per degree of freedom) where kB is Boltzmann's constant and T is the Kelvin temperature. This means that, for a signal to emerge from the thermal noise, work has to be done by the environment on the sensor, and the energy expended must be larger than kBT for it to have a substantial effect. As energy is equal to force × distance, substantial energy can arise from a stiff object moving a small distance or a soft object moving a large distance (Markin and Sachs, 2004). In terms of molecular mechanics, 1 kBT is the work done by a force of 4.1 picoNewtons (pN) acting over a distance of 1 nm (kBT = 4.1 pN•nm). In two dimensions, the equivalent calculation states that kBT corresponds to the expansion of a channel protein by 4.1 nm2 under membrane tension of 1 mN/m (1 dyn/cm), or in three dimensions a shrinkage of a 1 nm3 structure under an osmotic pressure of 4.1 Atm (Fig. 1). This principle applies to all mechanical transducers, not just MSCs.
Fig. 1.
Mechanosensing in different dimensions. (A) A one-dimensional sensor, such as talin, can unfold and elongate with tension. Such a conformational change could expose cryptic binding sites within the protein [modified with permission from del Rio et al., 2009. Reprinted with permission from AAAS. (del Rio et al., 2009)]. (B) A membrane channel that opens in response to membrane tension is an example of a two-dimensional sensor that increases its in-plane area (A). The panel shows the predicted tilting motion of pairs of transmembrane helices of the bacterial mechanosensitive channel MscL, which are associated with a ∼20 nm2 change of in-plane area expansion [modified with permission from Sukharev et al., 2005, ASM Press, Washington, DC (Sukharev et al., 2005)]. (C) A hypothetical elastic structure that decreases its volume under osmotic pressure is an example of a three-dimensional mechanosensor.
Tuning mechanoreceptor sensitivity – pre-stress and adaptation
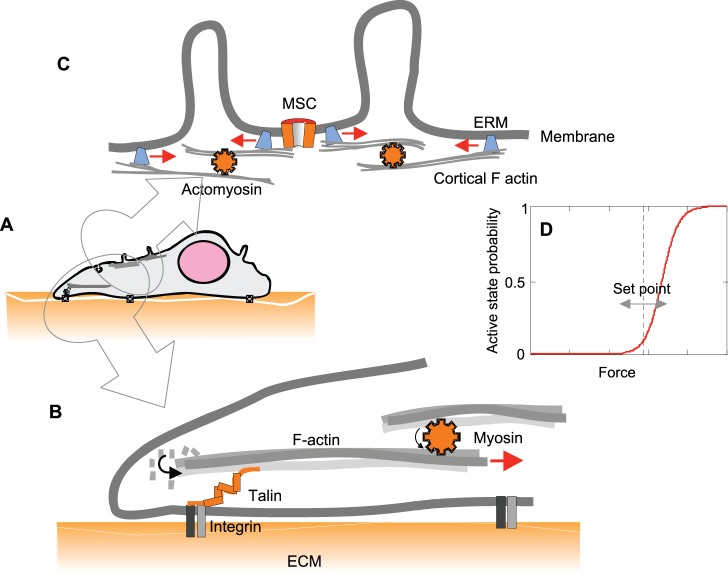
The dose–response curve of a mechanoreceptor is a sigmoidal function of stress. The receptor is most sensitive in the middle of the curve (i.e. where it is steepest) (Fig. 2D). The curve in Fig. 2D is a Boltzmann distribution that describes the occupancy of different states as a function of their energy. For simplicity, consider the one-dimensional case where the free energy is defined by ΔG = force × distance = Fx. For a two-state system (inactive and active), the probability of being active is given by Pact = 1/(1+exp((E0–Fx)/kBT)), where E0 is the energy difference between inactive and active states with no force. Pact is a nonlinear function of F, so resting forces will modulate the sensitivity of the sensor. This resting force (or pre-stress) can tune the system set-point such that the receptor is more or less sensitive to changes in force, ΔF. Fig. 2A–C shows some examples of how pre-stress might be applied to a sensor by a molecular motor. Talin is a component of the protein complex that links the actin cytoskeleton to integrins, and it becomes pre-stressed by myosin II motors (Moore et al., 2010) (Fig. 2B). Those picoNewton forces are strong enough to begin unfolding talin, which leads to the exposure of cryptic binding sites for vinculin, a protein that reinforces the link (del Rio et al., 2009). Similarly, actomyosin complexes anchored to the membrane through proteins of the ezrin–radixin–moesin (ERM) family might result in folding (or ‘wrinkling’) of a part of the membrane (Thery and Bornens, 2008), thereby creating pre-stress tension in adjacent areas that contain MSCs (Fig. 2C).
Fig. 2.
Pre-stressing sensors affects their sensitivity through mechanical adaptation. (A) An adherent cell spreads lamellipodia that probe the stiffness of the substrate by applying force to focal adhesions. (B) Diagrammatic representation of the actin–talin–integrin linkage, in which partial unfolding of talin that was pre-stressed through actomyosin leads to reinforcement of focal adhesions (reviewed by Moore et al., 2010). (C) Blebbing of the plasma membrane mediated by actomyosin, which is connected to the membrane through anchoring to ERM family proteins, at the same time, might pre-stress adjacent membranes that contain a mechanosensitive channel (MSC). The diagram is based on the ideas discussed by Thery and Bornens (Thery and Bornens, 2008). (D) Dose–response curve of an MSC. The curve has a sigmoid shape (Boltzmann distribution), in which the slope sensitivity (the change in active state probability for a given change in force) depends on the resting force. This defines the set-point and the dynamic range of response.
Whereas tuning the receptor midpoint can adjust sensitivity, it compromises the dynamic range: small forces can move the channel to saturation and make it insensitive to external stimuli. To preserve the ability of receptors to respond to changes in average force over a wide range, the process of adaptation evolved, which adjusts the resting force to keep the channels in a responsive range. This is a property similar to automatic volume controls in an audio recorder. This way, the system can preserve sensitivity over a wide dynamic range at the expense of making the system unresponsive to low frequencies of stimuli. In cochlear hair cells, adjacent stereocilia are connected by fibers called tip-links that pull on sensory MSCs, and tension within these links is set by slowly moving motors in the hair cell so that ∼15% of the channels are active, operating near the steep part of the gating curve (Howard and Hudspeth, 1988). This adaptation is familiar to airplane passengers; the change in airline cabin pressure is much larger than sound pressure, but our ears are not in saturation and we can hear. The adaptation process is characteristic of nearly all biological sensors (Gauthier et al., 2011; Rangamani et al., 2011; Xiong et al., 2010).
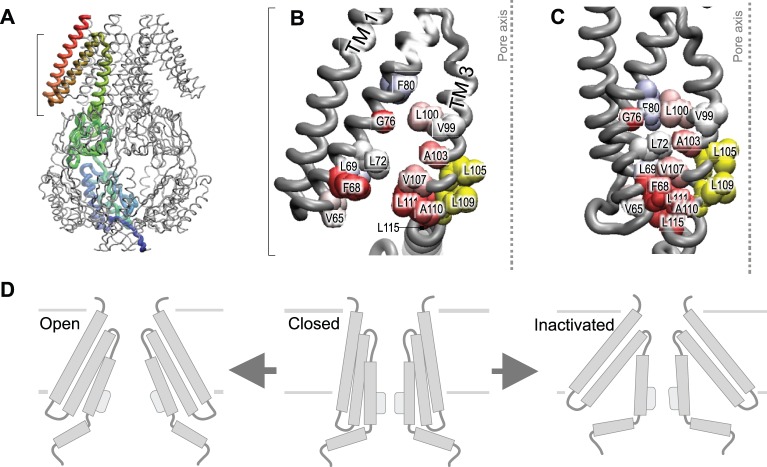
Adaptation can be accomplished passively by making the coupling between the applied force and the gate viscoelastic (Janmey, 1998). When rapidly changing external stresses are applied, the viscous component appears stiff and high frequencies of stimuli are transmitted to the receptor. If the applied force is sustained, the viscous element flows and the average stress decreases with time (Evans, 2001; Na et al., 2008). Viscosity can arise from the untangling of filamentous structures or the reversible breaking of non-covalent bonds (slip bonds) between structures or domains (Bustamante et al., 2004; Vogel, 2006; Moore et al., 2010). These slip bonds can also exist within the channel itself, as in the bacterial channel MscS (Akitake et al., 2005; Belyy et al., 2010a) (Fig. 3). It is noteworthy that adaptation can be confused with desensitization or inactivation. All these processes lead to a time-dependent decrease in current, but adaptation of the stimulus simply reduces the force applied to the gate, whereas desensitization implies that a channel transitions to a state of lower sensitivity (Honoré et al., 2006). Inactivation, by contrast, represents the channel reaching a non-conducting and tension-insensitive state (Akitake et al., 2005; Kamaraju et al., 2011a). Time-dependent stresses in single molecules have been measured with atomic-force microscopy (AFM), laser traps and force-sensitive fluorescent Förster resonance energy transfer (FRET) probes (Bustamante et al., 2004; Cornish and Ha, 2007; Meng et al., 2011; Meng and Sachs, 2011a; Meng and Sachs, 2011b). AFM and laser traps can measure the mechanics of single molecules in vitro, but FRET probes function in vivo and in vitro and experiments using these probes have revealed that all filamentous proteins in a cell seem to be under resting tension (Grashoff et al., 2010; Brenner et al., 2011; Meng and Sachs, 2011b; Rahimzadeh et al., 2011; Wang et al., 2011; Meng and Sachs, 2012). If proteinaceous fibers are to be used for signaling, it is important that resting tension is maintained (Ingber, 2008) because fibers under tension can transmit signals close to the speed of sound (Na et al., 2008).
Fig. 3.
Adaptive gating mechanism of the bacterial small-conductance MSC (MscS). (A) The crystal structure of the MscS (PDB ID 2OAU) heptamer with one subunit highlighted in color. The TM2–TM3 helices show an open crevice (B) and a closed crevice that has been generated by computational rearrangement (C). The TM2–TM3 hydrophobic link might serve as a ‘clutch’ that connects the peripheral helices to the gate (yellow). The Lys111Ser mutant shows a strong propensity toward inactivation as a result of compromised TM2–TM3 interactions, which transmit force from the membrane to the gate. Figures reproduced with permission from (Akitake et al., 2005; Belyy et al., 2010a).
Modulation of mechanosensors by the local environment
The local mechanics of cellular membranes are complicated by the presence of the non-uniformly distributed cytoskeleton and focal adhesions to the ECM. Both components are expected to bear most of the stress applied to the cell (or the patch membrane), which was recognized in studies in which MSCs in patches from blebs that contained much less cytoskeleton were sensitized (Zhang et al., 2000; Maksaev et al., 2011). Membranes also contain spatial domains that can be based on lipids [e.g. lipid rafts (Eisenberg et al., 2011; Thakur et al., 2011)] or heterogeneous [e.g. protein corrals (Corbett-Nelson et al., 2006; Leitner et al., 2000; Poudel et al., 2011; Sachs, 2010)]. The mechanical stress inside any domain will be different from that in the surrounding membrane, so that groups of mechanosensors can be collectively modulated by inclusion or exclusion from a domain. The effects of clustering and crowding of MSCs in the plane of the membrane have been analyzed theoretically, and the models predict that there would be changes in tension sensitivity under such conditions (Ursell et al., 2007; Lindén et al., 2012). If a domain such as a caveolus breaks open, a group of channels will suddenly be exposed to the exterior forces and this collective activation effect is often observed in patch recordings. The effect has also been demonstrated in intact heart cells where the mechanical sensitivity of a group of channels changes simultaneously (Bett and Sachs, 2000).
The set-point of MSCs can be changed by amphipaths that bind within a membrane domain. When hydrophobic agents dissolve in the bilayer they push on adjacent structures, including MSCs. These forces add to the pressure profile of the lipids that exists throughout the membrane: the lateral pressure is positive at the headgroups and in the center of the lipids (compression), and negative at the hydrophilic–hydrophobic boundary (tension) (Cantor, 1999; Lindahl and Edholm, 2000; Gullingsrud and Schulten, 2004). The influence of the pressure profile is seen in MscL, which is more sensitive to applied tension when reconstituted in phosphatidylcholine than in phosphatidylethanolamine (i.e. two lipids with different pressure profiles) (Moe and Blount, 2005). In another example, MscL and MscS have been shown to be activated by asymmetric lysolipids (Perozo et al., 2002b; Vásquez et al., 2008). The exceptional sensitivity of MscS to asymmetrical perturbations of lateral pressure by amphipaths has made it possible to use this channel as a sensor for the partitioning of biologically active molecules and synthetic analogs to the native bacterial membrane (Kamaraju et al., 2011b).
A notable group of amphipaths that affect MSCs are the general anesthetics. These agents activate mechanosensitive 2P K+ channels in neurons, thereby leading to hyperpolarization and decreased excitability, i.e. anesthesia (Patel et al., 1999). The action of the amphipaths is generally non-chiral given that they are just dissolving in the lipids. These long-range interactions emphasize the need for caution when interpreting the pharmacology of MSCs or other membrane proteins (Suchyna et al., 2004). For example, genistein is commonly used as a tyrosine kinase inhibitor. But it dissolves in membranes and changes the pressure profile and modulates MSCs such as gramicidin (Hwang et al., 2003; Suchyna et al., 2004), and this effect has nothing to do with kinase inhibition because gramicidin is not a substrate for kinases and no kinases are present in the bilayer studies.
Mechanosensitive channel structure
Mechanosensitive channels in bacteria
The Escherichia coli osmoregulatory system includes at least four types of MSCs: large-conductance MscL (Sukharev et al., 1994), small-conductance MscS (Levina et al., 1999), K+-dependent MscK (Li et al., 2002) and mini MscM (Schumann et al., 2010). The activities of these channels have been functionally characterized in patches made from spheroplasts of the inner membrane. MscL and MscS have also been studied in reconstituted liposomes (Sukharev et al., 1999; Sukharev, 2002; Moe and Blount, 2005), and these studies have shown that lipid tension provides all of the activating force. Functional reconstitution of eukaryotic MSCs has not yet been accomplished, but the lipid-based source of stress is a convenient working model.
The crystal structure of E. coli MscS (Bass et al., 2002; Steinbacher et al., 2007) and the mycobacterial MscL homolog (Chang et al., 1998) have been solved, and those structures have served as guides for modeling the opening transitions. The most-studied MSC, MscL, is a pentamer with two transmembrane (TM) domains per monomer. The change in external dimensions between the closed and open states corresponds to a lateral expansion of 20 nm2, and this is associated with the opening of a 3-nm-diameter pore through which anions and cations can pass (Betanzos et al., 2002; Perozo et al., 2002a; Sukharev et al., 2005) (Fig. 1B). The pore is so large that activation of a single channel [of the ∼1000 that are present on a single cell (Bialecka-Fornal et al., 2012)] could cause a disruption of vital metabolic gradients. To keep it from opening accidentally, nature arranged a large energy gap (∼125 kJ/mol) between the closed and open states in the resting cell. This energy barrier arises from unfavorable pore hydration in the open state (Anishkin et al., 2010), lateral interactions with lipids (Ollila et al., 2009) and hydrophobic mismatch (Ursell et al., 2007).
In contrast with MscL, which is only found in prokaryotes, MscS has multiple homologs in other organisms that possess cell walls (Balleza and Gómez-Lagunas, 2009). MscS-like channels regulate volume and division of plastids in algae (Nakayama et al., 2007) and higher plants, including Arabidopsis (Haswell and Meyerowitz, 2006; Peyronnet et al., 2008; Wilson et al., 2011). Both MscL and MscS are found in the same bacterial cell and show functional redundancy, but in contrast with MscL, MscS is a heptamer with three TM domains (Bass et al., 2002). It is noteworthy that the original crystal structure solved (Bass et al., 2002) was probably that of a non-conducting state (Anishkin and Sukharev, 2004), whereas a more recent structure of a mutant MscS (Wang et al., 2008) might be representative of a partially open state. The gating sensitivity of MscS is slightly lower than that recorded for MscL, and it corresponds to an area expansion of ∼15 nm2 and a 16-Å-diameter pore. Not only are these two MSCs different in terms of their molecular structure, they also have different functional cycles. MscL is essentially a two-state (closed–open) channel, whereas MscS exists in three or more states. Under sustained moderate tension, MscS enters the inactivated state where the gate is effectively uncoupled from stress (Fig. 3) (Belyy et al., 2010a; Kamaraju et al., 2011a). This inactivation helps bacterial survival during exposure to prolonged osmotic shock (Boer et al., 2011). These examples emphasize the need for caution in generalizing the properties of MSCs.
Eukaryotic MSCs
MSCs appear to be present in all types of animal cells, including red blood cells (Vandorpe et al., 2010), but only a few of the genes encoding these MSCs have been cloned. Eukaryotic MSCs are grouped into two classes on the basis of their ion selectivity. One class comprises the cationic channels, such as the Piezo family (Coste et al., 2010; Bae et al., 2011b; Coste et al., 2012), the transient receptor potential (TRP) family (Su et al., 2009; Patel et al., 2010; Arnadóttir and Chalfie, 2010; Gottlieb et al., 2008; Inoue et al., 2009) and the degenerin (DEG) family (Arnadóttir et al., 2011). The second type of eukaryotic MSCs are the K+-selective 2P channels (Chemin et al., 2005; Patel and Honoré, 2003; Honoré et al., 2006). Members of the first group of channels act as excitatory channels, as they tend to depolarize cells from their resting potential by activating VGCs. By contrast, the second group comprises inhibitory channels, because hyperpolarization turns off most VGCs. The structure of two members of the 2P channel family have been solved recently (Miller and Long, 2012; Brohawn et al., 2012), which has provided a framework for functional and modeling studies. The physiological role of MSCs has been difficult to establish, because there are almost no specific drugs that inhibit these channels, and all cells appear to express endogenous MSCs (Gottlieb et al., 2008).
Cellular roles of MSCs and potential clinical applications
To establish the cellular functions of MSCs, specific inhibitors or activators of these channels would be ideal. Gadolinium (Gd3+) was initially described as an inhibitor of cationic MSCs (Yang and Sachs, 1989), but it has since proved to be non-specific and probably acts by binding to anionic lipids and changing membrane stiffness (Ermakov et al., 2001; Ermakov et al., 2010). The only known specific inhibitor of MSCs is the amphipathic peptide GsMTx4, which was originally isolated from a tarantula venom (Bowman et al., 2007; Gottlieb et al., 2010; Hua et al., 2010; Bae et al., 2011a). Acting extracellularly, GsMTx4 inhibits cation-selective MSCs, probably by increasing the local pressure in the outer monolayer and thereby pushing the channel towards its closed state. The fact that this compound acts extracellularly implies that the gate apparatus of these channels is located in the outer monolayer (Suchyna et al., 2004). GsMTx4 affects many cationic MSCs, including gramicidin (Suchyna et al., 2004) and the bacterial MSCs (Hurst et al., 2009; Kamaraju et al., 2010), but in the bacterial channels the actions are not always inhibitory in the way they are for the eukaryotic cation-selective MSCs such as Piezo1. GsMTx4 has no effect on the 2P MSCs (Honoré et al., 2006; Bae et al., 2011b), possibly because the gating structure of K+ channels is located in the inner monolayer, which is inaccessible to the peptide.
To establish a cellular function for MSCs requires an answer to the question ‘what do the channels do in situ?’. Whereas we would like to extrapolate from patch clamp data (Box 1), the resting tension of a patch is far too high (Suchyna et al., 2009) to offer a representative cellular environment for MSCs, where the resting tension is small. However, we can assess the activity of MSCs in resting cells in culture, where most of the cell is free of stresses from the glass pipette, using whole cell recordings. Following the inhibition of MSCs with GsMTx4, there is no change in the holding current, that is the channels are not open at rest (Suchyna and Sachs, 2007). If the channels are not open at rest, what will cause them to open? Sufficiently large stresses will cause them to open and that can happen as a result of the application of external or internal forces or the loss of a reinforcement (Coste et al., 2010). In the case of muscular dystrophy, the reinforcing network made of dystrophin is broken and the stresses that should have been maintained within dystrophin are transferred, in part, to the bilayer, where they activate MSCs (Franco and Lansman, 1990). This transfer of stress is visible in dystrophic muscle in which the spontaneous activity of MSCs has been inhibited by GsMTx4, thereby reducing the stretch-induced Ca2+ uptake (Yeung et al., 2005; Suchyna and Sachs, 2007; Fanchaouy et al., 2009). Thus, one could view the function of cationic MSCs as ‘pain sensors’ that inform the cytoskeleton where the bilayer needs reinforcing. If MSCs are normally closed, applying GsMTx4 should have no effect on the host organism. Indeed, we have found that GsMTx4 administration to mice over long periods of time has no toxic effects. The inhibition by GsMTx4 is negligible in terms of the cationic channels in differentiated sensory organs, such as the cochlea, probably because in those systems force is transmitted directly to the channels through protein filaments and not through the bilayer. The in situ action of GsMTx4 has been tested in intact hearts swollen by changes in blood pressure. Atrial fibrillation is inhibited by 170 nM GsMTx4 (Bode et al., 2001). Other arrhythmias are also associated with mechanical defects (Kohl et al., 2011) and could possibly be treated with GsMTx4 (Sachs et al., 2004).
In the case of glia, cationic MSCs might contribute to cell volume regulation. Here, the mechanical stimulus is the swelling that is produced by the ion influx following nerve activity in the surrounding tissue (Niggel et al., 2000; Suchyna et al., 2000; Ostrow et al., 2011). As mentioned above, K+-selective 2P MSCs set the cellular resting potential and they might be involved in mediating the action of general anesthetics (Patel and Honore, 2003; Honoré, 2007). The Piezo family has recently been implicated in pain sensing and those channels might provide a useful target for future analgesic development (Kim et al., 2012; Gottlieb and Sachs, 2012). In individuals affected by sickle cell anemia, low oxygen pressures can cause hemoglobin to form crystals that push on the membrane causing MSCs to open, disturbing the regulation of cell volume, and those changes are inhibited by GsMTx4 (Vandorpe et al., 2010).
Perspectives – fundamental questions and applications
In this Commentary, we have outlined some general principles of mechanosensitivity and the energetics of the primary transduction events. We have highlighted the role of active and passive adaptation that allows the system to mechanically tune its sensitivity and dynamic range. In addition, we have pointed out how MSCs can amplify their conformational changes by expending the stored energy of the electrochemical gradient that exists across the cell membrane. We have pointed out a number of the experimental challenges that can arise with experiments on MSCs and how non-specific drug effects caused by bilayer partitioning can alter channel kinetics. We have discussed the rich diversity of structural designs of mechanosensitive channels, which suggests that mechanosensor evolution must have occurred many times.
There are still many fundamental and practical problems to be solved in the field. A primary biophysical question is whether the ability to sense force is distributed uniformly in the cell, or whether there are specific subdomains or clusters of channels. If so, do such domains contain multiple channel types? In order to gain a better understanding of how mechanosensitive channels affect cellular processes, we have to study the interactions between proteins and lipids that account for the sensitivity of MSCs to different lipids and amphipaths. We also need to understand the distribution of stresses among various cellular structures, such as membrane proteins, the lipid bilayer, the components of the ECM and the cytoskeleton. We need to understand the role of lipids and amphipaths in desensitization and adaptation of channels to different stimuli. In prokaryotes, we need to understand how global stresses are locally distributed to MSCs and why there is such a large excess of the number of channels than appear necessary for osmotic regulation.
In the long run, we hope to transfer basic knowledge of MSCs to develop clinical tools. As we discussed, there are already promising links to muscular dystrophy, sickle cell anemia and cardiac arrhythmias. There are also indications that bacterial MSCs are involved in ecological adaptation of many pathogenic microorganisms and in host–parasite interactions. Given the ubiquitous nature of MSCs, they are likely to be involved in a great deal of pathology.
Supplementary Material
Acknowledgments
The authors thank Phillip Gottlieb, Andriy Anishkin and Catherine Morris for helpful discussions.
Footnotes
Funding
The work of the authors' laboratories is supported by grants from the National Institutes of Health [grant numbers NS039314, GM075225 to S.S.]; and the United States Department of Defense, National Institutes of Health and Children's Guild of Buffalo to F.S. Deposited in PMC for release after 12 months.
This article is part of a Minifocus on Mechanotransduction. For further reading, please see related articles: ‘Deconstructing the third dimension – how 3D culture microenvironments alter cellular cues’ by Brendon M. Baker and Christopher S. Chen (J. Cell Sci. 125, 3015-3024). ‘Finding the weakest link – exploring integrin-mediated mechanical molecular pathways’ by Pere Roca-Cusachs et al. (J. Cell Sci. 125, 3025-3038). ‘Signalling through mechanical inputs – a coordinated process’ by Huimin Zhang and Michel Labouesse (J. Cell Sci. 125, 3039-3049). ‘United we stand – integrating the actin cytoskeleton and cell–matrix adhesions in cellular mechanotransduction’ by Ulrich S. Schwarz and Margaret L. Gardel (J. Cell Sci. 125, 3051-3060). ‘Mechanosensitive mechanisms in transcriptional regulation’ by Akiko Mammoto et al. (J. Cell Sci. 125, 3061-3073).
References
- Akinlaja J., Sachs F. (1998). The breakdown of cell membranes by electrical and mechanical stress. Biophys. J. 75, 247–254 10.1016/S0006-3495(98)77511-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akitake B., Anishkin A., Sukharev S. (2005). The “dashpot” mechanism of stretch-dependent gating in MscS. J. Gen. Physiol. 125, 143–154 10.1085/jgp.200409198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford P. W., Dabiri B. E., Goss J. A., Hemphill M. A., Brigham M. D., Parker K. K. (2011). Blast-induced phenotypic switching in cerebral vasospasm. Proc. Natl. Acad. Sci. USA 108, 12705–12710 10.1073/pnas.1105860108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anishkin A., Sukharev S. (2004). Water dynamics and dewetting transitions in the small mechanosensitive channel MscS. Biophys. J. 86, 2883–2895 10.1016/S0006-3495(04)74340-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anishkin A., Akitake B., Kamaraju K., Chiang C. S., Sukharev S. (2010). Hydration properties of mechanosensitive channel pores define the energetics of gating. J. Phys. Condens. Matter 22, 454120 10.1088/0953-8984/22/45/454120 [DOI] [PubMed] [Google Scholar]
- Arnadóttir J., Chalfie M. (2010). Eukaryotic mechanosensitive channels. Annu. Rev. Biophys. 39, 111–137 10.1146/annurev.biophys.37.032807.125836 [DOI] [PubMed] [Google Scholar]
- Arnadóttir J., O'Hagan R., Chen Y., Goodman M. B., Chalfie M. (2011). The DEG/ENaC protein MEC-10 regulates the transduction channel complex in caenorhabditis elegans touch receptor neurons. J. Neurosci. 31, 12695–12704 10.1523/JNEUROSCI.4580-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae C., Markin V., Suchyna T., Sachs F. (2011a). Modeling ion channels in the gigaseal. Biophys. J. 101, 2645–2651 10.1016/j.bpj.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae C., Sachs F., Gottlieb P. A. (2011b). The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry 50, 6295–6300 10.1021/bi200770q [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleza D., Gómez–Lagunas F. (2009). Conserved motifs in mechanosensitive channels MscL and MscS. Eur. Biophys. J. 38, 1013–1027 10.1007/s00249-009-0460-y [DOI] [PubMed] [Google Scholar]
- Bass R. B., Strop P., Barclay M., Rees D. C. (2002). Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science 298, 1582–1587 10.1126/science.1077945 [DOI] [PubMed] [Google Scholar]
- Belyy V., Anishkin A., Kamaraju K., Liu N., Sukharev S. (2010a). The tension-transmitting ‘clutch’ in the mechanosensitive channel MscS. Nat. Struct. Mol. Biol. 17, 451–458 10.1038/nsmb.1775 [DOI] [PubMed] [Google Scholar]
- Belyy V., Kamaraju K., Akitake B., Anishkin A., Sukharev S. (2010b). Adaptive behavior of bacterial mechanosensitive channels is coupled to membrane mechanics. J. Gen. Physiol. 135, 641–652 10.1085/jgp.200910371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betanzos M., Chiang C. S., Guy H. R., Sukharev S. (2002). A large iris-like expansion of a mechanosensitive channel protein induced by membrane tension. Nat. Struct. Biol. 9, 704–710 10.1038/nsb828 [DOI] [PubMed] [Google Scholar]
- Bett G C L., Sachs F. (2000). Whole-cell mechanosensitive currents in rat ventricular myocytes activated by direct stimulation. J. Membr. Biol. 173, 255–263 [DOI] [PubMed] [Google Scholar]
- Beyder A., Sachs F. (2009). Electromechanical coupling in the membranes of Shaker-transfected HEK cells. Proc. Natl. Acad. Sci. USA 106, 6626–6631 10.1073/pnas.0808045106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialecka–Fornal M., Lee H. J., DeBerg H. A., Gandhi C. S., Phillips R. (2012). Single-cell census of mechanosensitive channels in living bacteria. PLoS ONE 7, e33077 10.1371/journal.pone.0033077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode F., Sachs F., Franz M. R. (2001). Tarantula peptide inhibits atrial fibrillation. Nature 409, 35–36 10.1038/35051165 [DOI] [PubMed] [Google Scholar]
- Boer M., Anishkin A., Sukharev S. (2011). Adaptive MscS gating in the osmotic permeability response in E. coli: the question of time. Biochemistry 50, 4087–4096 10.1021/bi1019435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman C. L., Gottlieb P. A., Suchyna T. M., Murphy Y. K., Sachs F. (2007). Mechanosensitive ion channels and the peptide inhibitor GsMTx-4: history, properties, mechanisms and pharmacology. Toxicon 49, 249–270 10.1016/j.toxicon.2006.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M. D., Zhou R., Ha T. (2011). Forcing a connection: impacts of single-molecule force spectroscopy on in vivo tension sensing. Biopolymers 95, 332–344 10.1002/bip.21587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohawn S. G., del Mármol J., MacKinnon R. (2012). Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science 335, 436–441 10.1126/science.1213808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante C., Chemla Y. R., Forde N. R., Izhaky D. (2004). Mechanical processes in biochemistry. Annu. Rev. Biochem. 73, 705–748 10.1146/annurev.biochem.72.121801.161542 [DOI] [PubMed] [Google Scholar]
- Cantor R. S. (1999). Lipid composition and the lateral pressure profile in bilayers. Biophys. J. 76, 2625–2639 10.1016/S0006-3495(99)77415-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang G., Spencer R. H., Lee A. T., Barclay M. T., Rees D. C. (1998). Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science 282, 2220–2226 10.1126/science.282.5397.2220 [DOI] [PubMed] [Google Scholar]
- Chemin J., Patel A., Duprat F., Zanzouri M., Lazdunski M., Honoré E. (2005). Lysophosphatidic acid-operated K+ channels. J. Biol. Chem. 280, 4415–4421 10.1074/jbc.M408246200 [DOI] [PubMed] [Google Scholar]
- Corbett–Nelson E. F., Mason D., Marshall J. G., Collette Y., Grinstein S. (2006). Signaling-dependent immobilization of acylated proteins in the inner monolayer of the plasma membrane. J. Cell Biol. 174, 255–265 10.1083/jcb.200605044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish P. V., Ha T. (2007). A survey of single-molecule techniques in chemical biology. ACS Chem. Biol. 2, 53–61 10.1021/cb600342a [DOI] [PubMed] [Google Scholar]
- Coste B., Mathur J., Schmidt M., Earley T. J., Ranade S., Petrus M. J., Dubin A. E., Patapoutian A. (2010). Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60 10.1126/science.1193270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B., Xiao B., Santos J. S., Syeda R., Grandl J., Spencer K. S., Kim S. E., Schmidt M., Mathur J., Dubin A. E.et al. (2012). Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483, 176–181 10.1038/nature10812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio A., Perez–Jimenez R., Liu R., Roca–Cusachs P., Fernandez J. M., Sheetz M. P. (2009). Stretching single talin rod molecules activates vinculin binding. Science 323, 638–641 10.1126/science.1162912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas P., Hao J., Rodat–Despoix L. (2011). Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nat. Rev. Neurosci. 12, 139–153 10.1038/nrn2993 [DOI] [PubMed] [Google Scholar]
- Discher D. E., Bhasin N., Johnson C. P. (2006). Covalent chemistry on distended proteins. Proc. Natl. Acad. Sci. USA 103, 7533–7534 10.1073/pnas.0602388103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R., Misler S. (1989). Voltage-activated and stretch-activated Ba2+ conducting channels in an osteoblast-like cell line (UMR 106). FEBS Lett. 251, 17–21 10.1016/0014-5793(89)81420-6 [DOI] [PubMed] [Google Scholar]
- Eisenberg S., Beckett A. J., Prior I. A., Dekker F. J., Hedberg C., Waldmann H., Ehrlich M., Henis Y. I. (2011). Raft protein clustering alters N-Ras membrane interactions and activation pattern. Mol. Cell. Biol. 31, 3938–3952 10.1128/MCB.05570-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermakov Y. A., Averbakh A. Z., Yusipovich A. I., Sukharev S. (2001). Dipole potentials indicate restructuring of the membrane interface induced by gadolinium and beryllium ions. Biophys. J. 80, 1851–1862 10.1016/S0006-3495(01)76155-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermakov Y. A., Kamaraju K., Sengupta K., Sukharev S. (2010). Gadolinium ions block mechanosensitive channels by altering the packing and lateral pressure of anionic lipids. Biophys. J. 98, 1018–1027 10.1016/j.bpj.2009.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. (2001). Probing the relation between force–lifetime–and chemistry in single molecular bonds. Annu. Rev. Biophys. Biomol. Struct. 30, 105–128 10.1146/annurev.biophys.30.1.105 [DOI] [PubMed] [Google Scholar]
- Evans E., Yeung A. (1994). Hidden dynamics in rapid changes of bilayer shape. Chem. Phys. Lipids 73, 39–56 10.1016/0009-3084(94)90173-2 [DOI] [Google Scholar]
- Fanchaouy M., Polakova E., Jung C., Ogrodnik J., Shirokova N., Niggli E. (2009). Pathways of abnormal stress-induced Ca2+ influx into dystrophic mdx cardiomyocytes. Cell Calcium 46, 114–121 10.1016/j.ceca.2009.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier N. C., Fardin M. A., Roca–Cusachs P., Sheetz M. P. (2011). Temporary increase in plasma membrane tension coordinates the activation of exocytosis and contraction during cell spreading. Proc. Natl. Acad. Sci. USA 108, 14467–14472 10.1073/pnas.1105845108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie P. G., Müller U. (2009). Mechanotransduction by hair cells: models, molecules, and mechanisms. Cell 139, 33–44 10.1016/j.cell.2009.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb P., Folgering J., Maroto R., Raso A., Wood T. G., Kurosky A., Bowman C., Bichet D., Patel A., Sachs F.et al. (2008). Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflugers Arch. 455, 1097–1103 10.1007/s00424-007-0359-3 [DOI] [PubMed] [Google Scholar]
- Gottlieb P. A., Sachs F. (2012). Cell biology: The sensation of stretch. Nature 483, 163–164 10.1038/483163a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb P. A., Barone T., Sachs F., Plunkett R. (2010). Neurite outgrowth from PC12 cells is enhanced by an inhibitor of mechanical channels. Neurosci. Lett. 481, 115–119 10.1016/j.neulet.2010.06.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashoff C., Hoffman B. D., Brenner M. D., Zhou R., Parsons M., Yang M. T., McLean M. A., Sligar S. G., Chen C. S., Ha T.et al. (2010). Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, 263–266 10.1038/nature09198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullingsrud J., Schulten K. (2004). Lipid bilayer pressure profiles and mechanosensitive channel gating. Biophys. J. 86, 3496–3509 10.1529/biophysj.103.034322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haswell E. S., Meyerowitz E. M. (2006). MscS-like proteins control plastid size and shape in Arabidopsis thaliana. Curr. Biol. 16, 1–11 10.1016/j.cub.2005.11.044 [DOI] [PubMed] [Google Scholar]
- Haswell E. S., Phillips R., Rees D. C. (2011). Mechanosensitive channels: what can they do and how do they do it? Structure 19, 1356–1369 10.1016/j.str.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman B. D., Grashoff C., Schwartz M. A. (2011). Dynamic molecular processes mediate cellular mechanotransduction. Nature 475, 316–323 10.1038/nature10316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré E. (2007). The neuronal background K2P channels: focus on TREK1. Nat. Rev. Neurosci. 8, 251–261 10.1038/nrn2117 [DOI] [PubMed] [Google Scholar]
- Honoré E., Patel A. J., Chemin J., Suchyna T., Sachs F. (2006). Desensitization of mechano-gated K2P channels. Proc. Natl. Acad. Sci. USA 103, 6859–6864 10.1073/pnas.0600463103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J., Hudspeth A. J. (1988). Compliance of the hair bundle associated with gating of mechanoelectrical transduction channels in the bullfrog's saccular hair cell. Neuron 1, 189–199 10.1016/0896-6273(88)90139-0 [DOI] [PubMed] [Google Scholar]
- Hua S. Z., Gottlieb P. A., Heo J., Sachs F. (2010). A mechanosensitive ion channel regulating cell volume. Am. J. Physiol. Cell Physiol. 298, C1424–C1430 10.1152/ajpcell.00503.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst A. C., Gottlieb P. A., Martinac B. (2009). Concentration dependent effect of GsMTx4 on mechanosensitive channels of small conductance in E. coli spheroplasts. Eur. Biophys. J. 38, 415–425 10.1007/s00249-008-0386-9 [DOI] [PubMed] [Google Scholar]
- Hwang T. C., Koeppe R. E., 2nd, Andersen O. S. (2003). Genistein can modulate channel function by a phosphorylation-independent mechanism: importance of hydrophobic mismatch and bilayer mechanics. Biochemistry 42, 13646–13658 10.1021/bi034887y [DOI] [PubMed] [Google Scholar]
- Ingber D. E. (2008). Tensegrity-based mechanosensing from macro to micro. Prog. Biophys. Mol. Biol. 97, 163–179 10.1016/j.pbiomolbio.2008.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Jian Z., Kawarabayashi Y. (2009). Mechanosensitive TRP channels in cardiovascular pathophysiology. Pharmacol. Ther. 123, 371–385 10.1016/j.pharmthera.2009.05.009 [DOI] [PubMed] [Google Scholar]
- Janmey P. A. (1998). The cytoskeleton and cell signaling: component localization and mechanical coupling. Physiol. Rev. 78, 763–781 [DOI] [PubMed] [Google Scholar]
- Kamaraju K., Gottlieb P. A., Sachs F., Sukharev S. (2010). Effects of GsMTx4 on bacterial mechanosensitive channels in inside-out patches from giant spheroplasts. Biophys. J. 99, 2870–2878 10.1016/j.bpj.2010.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamaraju K., Belyy V., Rowe I., Anishkin A., Sukharev S. (2011a). The pathway and spatial scale for MscS inactivation. J. Gen. Physiol. 138, 49–57 10.1085/jgp.201110606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamaraju K., Smith J., Wang J., Roy V., Sintim H. O., Bentley W. E., Sukharev S. (2011b). Effects on membrane lateral pressure suggest permeation mechanisms for bacterial quorum signaling molecules. Biochemistry 50, 6983–6993 10.1021/bi200684z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. E., Coste B., Chadha A., Cook B., Patapoutian A. (2012). The role of Drosophila Piezo in mechanical nociception. Nature 483, 209–212 10.1038/nature10801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl P., Sachs F., Franz M. R. (2011). Cardiac mechano-electric coupling and arrhythmias. Oxford: Oxford University Press. [Google Scholar]
- Lauritzen I., Chemin J., Honoré E., Jodar M., Guy N., Lazdunski M., Jane Patel A. (2005). Cross-talk between the mechano-gated K2P channel TREK-1 and the actin cytoskeleton. EMBO Rep. 6, 642–648 10.1038/sj.embor.7400449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner D. M., Brown F. L., Wilson K. R. (2000). Regulation of protein mobility in cell membranes: a dynamic corral model. Biophys. J. 78, 125–135 10.1016/S0006-3495(00)76579-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lele T. P., Sero J. E., Matthews B. D., Kumar S., Xia S., Montoya–Zavala M., Polte T., Overby D., Wang N., Ingber D. E. (2007). Tools to study cell mechanics and mechanotransduction. Methods Cell Biol. 83, 443–472 [DOI] [PubMed] [Google Scholar]
- Levina N., Tötemeyer S., Stokes N. R., Louis P., Jones M. A., Booth I. R. (1999). Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 18, 1730–1737 10.1093/emboj/18.7.1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Moe P. C., Chandrasekaran S., Booth I. R., Blount P. (2002). Ionic regulation of MscK, a mechanosensitive channel from Escherichia coli. EMBO J. 21, 5323–5330 10.1093/emboj/cdf537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl E., Edholm O. (2000). Spatial and energetic-entropic decomposition of surface tension in lipid bilayers from molecular dynamics simulations. J. Chem. Phys. 113, 3882–3893 10.1063/1.1287423 [DOI] [Google Scholar]
- Lindén M., Sens P., Phillips R. (2012). Entropic tension in crowded membranes. PLOS Comput. Biol. 8, e1002431 10.1371/journal.pcbi.1002431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin E. A., Marshall K. L., Nelson A. M. (2010). The cell biology of touch. J. Cell Biol. 191, 237–248 10.1083/jcb.201006074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F., Lauritzen I., Patel A. J., Heurteaux C., Reyes R., Lesage F., Lazdunski M., Honoré E. (2000). TREK-1 is a heat-activated background K(+) channel. EMBO J. 19, 2483–2491 10.1093/emboj/19.11.2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksaev G., Milac A., Anishkin A., Guy H. R., Sukharev S. (2011). Analyses of gating thermodynamics and effects of deletions in the mechanosensitive channel TREK-1: comparisons with structural models. Channels 5, 34–42 10.4161/chan.5.1.13906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markin V. S., Sachs F. (2004). Thermodynamics of mechanosensitivity. Phys. Biol. 1, 110–124 10.1088/1478-3967/1/2/007 [DOI] [PubMed] [Google Scholar]
- Meng F., Sachs F. (2012). Orientation-based FRET sensor for real-time imaging of cellular forces. J. Cell Sci. 125, 743–750 10.1242/jcs.093104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F., Sachs F. (2011a). Visualizing dynamic cytoplasmic forces with a compliance-matched FRET sensor. J. Cell Sci. 124, 261–269 10.1242/jcs.071928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F., Sachs F. (2011b). Measuring strain of structural proteins in vivo in real time. Cardiac Mechano-Electric Coupling and Arrhythmia: From Pipette to Patient. (ed. Kohl P., Sachs F., Franz M R.), pp. 431–434 Oxford, UK: Oxford University Press; [Google Scholar]
- Meng F., Suchyna T. M., Sachs F. (2008). A fluorescence energy transfer-based mechanical stress sensor for specific proteins in situ. FEBS J. 275, 3072–3087 10.1111/j.1742-4658.2008.06461.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F., Suchyna T. M., Lazakovitch E., Gronostajski R. M., Sachs F. (2011). Real time FRET based detection of mechanical stress in cytoskeletal and extracellular matrix proteins. Cell. Mol. Bioeng. 4, 148–159 10.1007/s12195-010-0140-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. N., Long S. B. (2012). Crystal structure of the human two-pore domain potassium channel K2P1. Science 335, 432–436 10.1126/science.1213274 [DOI] [PubMed] [Google Scholar]
- Moe P., Blount P. (2005). Assessment of potential stimuli for mechano-dependent gating of MscL: effects of pressure, tension, and lipid headgroups. Biochemistry 44, 12239–12244 10.1021/bi0509649 [DOI] [PubMed] [Google Scholar]
- Moore S. W., Roca–Cusachs P., Sheetz M. P. (2010). Stretchy proteins on stretchy substrates: the important elements of integrin-mediated rigidity sensing. Dev. Cell 19, 194–206 10.1016/j.devcel.2010.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. E. (2011). Voltage-gated channel mechanosensitivity: fact or friction? Front Physiol. 2, 25 10.3389/fphys.2011.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na S., Collin O., Chowdhury F., Tay B., Ouyang M., Wang Y., Wang N. (2008). Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc. Natl. Acad. Sci. USA 105, 6626–6631 10.1073/pnas.0711704105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama Y., Fujiu K., Sokabe M., Yoshimura K. (2007). Molecular and electrophysiological characterization of a mechanosensitive channel expressed in the chloroplasts of Chlamydomonas. Proc. Natl. Acad. Sci. USA 104, 5883–5888 10.1073/pnas.0609996104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niggel J., Sigurdson W., Sachs F. (2000). Mechanically induced calcium movements in astrocytes, bovine aortic endothelial cells and C6 glioma cells. J. Membr. Biol. 174, 121–134 10.1007/s002320001037 [DOI] [PubMed] [Google Scholar]
- Ollila O. H., Risselada H. J., Louhivuori M., Lindahl E., Vattulainen I., Marrink S. J. (2009). 3D pressure field in lipid membranes and membrane-protein complexes. Phys. Rev. Lett. 102, 078101 10.1103/PhysRevLett.102.078101 [DOI] [PubMed] [Google Scholar]
- Ostrow L. W., Suchyna T. M., Sachs F. (2011). Stretch induced endothelin-1 secretion by adult rat astrocytes involves calcium influx via stretch-activated ion channels (SACs). Biochem. Biophys. Res. Commun. 410, 81–86 10.1016/j.bbrc.2011.05.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A., Sharif–Naeini R., Folgering J. R., Bichet D., Duprat F., Honoré E. (2010). Canonical TRP channels and mechanotransduction: from physiology to disease states. Pflugers Arch. 460, 571–581 10.1007/s00424-010-0847-8 [DOI] [PubMed] [Google Scholar]
- Patel A. J., Honore E. (2003). 2P domain K+ channels: novel pharmacological targets for volatile general anesthetics. Adv. Exp. Med. Biol. 536, 9–23 10.1007/978-1-4419-9280-2_2 [DOI] [PubMed] [Google Scholar]
- Patel A. J., Honoré E., Lesage F., Fink M., Romey G., Lazdunski M. (1999). Inhalational anesthetics activate two-pore-domain background K+ channels. Nat. Neurosci. 2, 422–426 10.1038/8084 [DOI] [PubMed] [Google Scholar]
- Perozo E., Rees D. C. (2003). Structure and mechanism in prokaryotic mechanosensitive channels. Curr. Opin. Struct. Biol. 13, 432–442 10.1016/S0959-440X(03)00106-4 [DOI] [PubMed] [Google Scholar]
- Perozo E., Cortes D. M., Sompornpisut P., Kloda A., Martinac B. (2002a). Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature 418, 942–948 10.1038/nature00992 [DOI] [PubMed] [Google Scholar]
- Perozo E., Kloda A., Cortes D. M., Martinac B. (2002b). Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat. Struct. Biol. 9, 696–703 10.1038/nsb827 [DOI] [PubMed] [Google Scholar]
- Peyronnet R., Haswell E. S., Barbier–Brygoo H., Frachisse J. M. (2008). AtMSL9 and AtMSL10: Sensors of plasma membrane tension in Arabidopsis roots. Plant Signal. Behav. 3, 726–729 10.4161/psb.3.9.6487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poudel K. R., Keller D. J., Brozik J. A. (2011). Single particle tracking reveals corralling of a transmembrane protein in a double-cushioned lipid bilayer assembly. Langmuir 27, 320–327 10.1021/la104133m [DOI] [PubMed] [Google Scholar]
- Radmacher M. (2007). Studying the mechanics of cellular processes by atomic force microscopy. Methods Cell Biol. 83, 347–372 10.1016/S0091-679X(07)83015-9 [DOI] [PubMed] [Google Scholar]
- Rahimzadeh J., Meng F., Sachs F., Wang J., Verma D., Hua S. Z. (2011). Real-time observation of flow-induced cytoskeletal stress in living cells. Am. J. Physiol. Cell Physiol. 301, C646–C652 10.1152/ajpcell.00099.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangamani P., Fardin M. A., Xiong Y., Lipshtat A., Rossier O., Sheetz M. P., Iyengar R. (2011). Signaling network triggers and membrane physical properties control the actin cytoskeleton-driven isotropic phase of cell spreading. Biophys. J. 100, 845–857 10.1016/j.bpj.2010.12.3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs F. (1986). Biophysics of mechanoreception. Membr. Biochem. 6, 173–195 10.3109/09687688609065448 [DOI] [PubMed] [Google Scholar]
- Sachs F. (2010). Stretch-activated ion channels: what are they? Physiology (Bethesda) 25, 50–56 10.1152/physiol.00042.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs F., Jalife J., Zipes D. (2004). Heart mechanoelectric transduction. Cardiac Electrophysiology: From Cell to Bedside (ed. Andjelkovic N., Gaillard J.), pp. 96–102 Philadelphia, PA: Saunders, Elsevier [Google Scholar]
- Sánchez D., Anand U., Gorelik J., Benham C. D., Bountra C., Lab M., Klenerman D., Birch R., Anand P., Korchev Y. (2007). Localized and non-contact mechanical stimulation of dorsal root ganglion sensory neurons using scanning ion conductance microscopy. J. Neurosci. Methods 159, 26–34 10.1016/j.jneumeth.2006.06.018 [DOI] [PubMed] [Google Scholar]
- Sawada Y., Sheetz M. P. (2002). Force transduction by Triton cytoskeletons. J. Cell Biol. 156, 609–615 10.1083/jcb.200110068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann U., Edwards M. D., Rasmussen T., Bartlett W., van West P., Booth I. R. (2010). YbdG in Escherichia coli is a threshold-setting mechanosensitive channel with MscM activity. Proc. Natl. Acad. Sci. USA 107, 12664–12669 10.1073/pnas.1001405107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnoli C., Beyder A., Besch S., Sachs F. (2008). Atomic force microscopy analysis of cell volume regulation. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 78, 031916 10.1103/PhysRevE.78.031916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbacher S., Bass R., Strop P., Rees D. C. (2007). Structures of the prokaryotic mechanosensitive channels MscL and MscS. Mechanosensitive Ion Channels. Part A 58, 1–24 10.1016/S1063-5823(06)58001-9 [DOI] [Google Scholar]
- Su Z., Anishkin A., Kung C., Saimi Y. (2011). The core domain as the force sensor of the yeast mechanosensitive TRP channel. J. Gen. Physiol. 138, 627–640 10.1085/jgp.201110693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchyna T. M., Sachs F. (2007). Mechanosensitive channel properties and membrane mechanics in mouse dystrophic myotubes. J. Physiol. 581, 369–387 10.1113/jphysiol.2006.125021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchyna T. M., Johnson J. H., Hamer K., Leykam J. F., Gage D. A., Clemo H. F., Baumgarten C. M., Sachs F. (2000). Identification of a peptide toxin from Grammostola spatulata spider venom that blocks cation-selective stretch-activated channels. J. Gen. Physiol. 115, 583–598 10.1085/jgp.115.5.583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchyna T. M., Tape S. E., Koeppe R. E., 2nd, Andersen O. S., Sachs F., Gottlieb P. A. (2004). Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature 430, 235–240 10.1038/nature02743 [DOI] [PubMed] [Google Scholar]
- Suchyna T. M., Markin V. S., Sachs F. (2009). Biophysics and structure of the patch and the gigaseal. Biophys. J. 97, 738–747 10.1016/j.bpj.2009.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev S. (2002). Purification of the small mechanosensitive channel of Escherichia coli (MscS): the subunit structure, conduction, and gating characteristics in liposomes. Biophys. J. 83, 290–298 10.1016/S0006-3495(02)75169-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev S., Anishkin A., Chiang C. S., Betanzos M., Guy H. R. (2005). MscL, a Bacterial Mechanosensitive Channel. Bacterial Ion Channels and Their Eukaryotic Homologs (ed. Kubalski A, Martinac B.), pp. 259–290 Washington, DC: ASM Press [Google Scholar]
- Sukharev S. I., Martinac B., Arshavsky V. Y., Kung C. (1993). Two types of mechanosensitive channels in the Escherichia coli cell envelope: solubilization and functional reconstitution. Biophys. J. 65, 177–183 10.1016/S0006-3495(93)81044-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev S. I., Blount P., Martinac B., Blattner F. R., Kung C. (1994). A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature 368, 265–268 10.1038/368265a0 [DOI] [PubMed] [Google Scholar]
- Sukharev S. I., Sigurdson W. J., Kung C., Sachs F. (1999). Energetic and spatial parameters for gating of the bacterial large conductance mechanosensitive channel, MscL. J. Gen. Physiol. 113, 525–540 10.1085/jgp.113.4.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur G., Pao C., Micic M., Johnson S., Leblanc R. M. (2011). Surface chemistry of lipid raft and amyloid Aβ (1-40) Langmuir monolayer. Colloids Surf. B Biointerfaces 87, 369–377 10.1016/j.colsurfb.2011.05.047 [DOI] [PubMed] [Google Scholar]
- Thery M., Bornens M. (2008). Get round and stiff for mitosis. HFSP J. 2, 65–71 10.2976/1.2895661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursell T., Huang K. C., Peterson E., Phillips R. (2007). Cooperative gating and spatial organization of membrane proteins through elastic interactions. PLOS Comput. Biol. 3, e81 10.1371/journal.pcbi.0030081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursell T., Agrawal A., Phillips R. (2011). Lipid bilayer mechanics in a pipette with glass-bilayer adhesion. Biophys. J. 101, 1913–1920 10.1016/j.bpj.2011.08.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandorpe D. H., Xu C., Shmukler B. E., Otterbein L. E., Trudel M., Sachs F., Gottlieb P. A., Brugnara C., Alper S. L. (2010). Hypoxia activates a Ca2+-permeable cation conductance sensitive to carbon monoxide and to GsMTx-4 in human and mouse sickle erythrocytes. PLoS ONE 5, e8732 10.1371/journal.pone.0008732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vásquez V., Sotomayor M., Cordero–Morales J., Schulten K., Perozo E. (2008). A structural mechanism for MscS gating in lipid bilayers. Science 321, 1210–1214 10.1126/science.1159674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel V. (2006). Mechanotransduction involving multimodular proteins: converting force into biochemical signals. Annu. Rev. Biophys. Biomol. Struct. 35, 459–488 10.1146/annurev.biophys.35.040405.102013 [DOI] [PubMed] [Google Scholar]
- Wang W., Black S. S., Edwards M. D., Miller S., Morrison E. L., Bartlett W., Dong C., Naismith J. H., Booth I. R. (2008). The structure of an open form of an E. coli mechanosensitive channel at 3.45 A resolution. Science 321, 1179–1183 10.1126/science.1159262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Meng F., Sachs F. (2011). Genetically encoded force sensors for measuring mechanical forces in proteins. Commun. Integr. Biol. 4, 385–390 10.4161/cib.4.2.14257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. E., Jensen G. S., Haswell E. S. (2011). Two mechanosensitive channel homologs influence division ring placement in Arabidopsis chloroplasts. Plant Cell 23, 2939–2949 10.1105/tpc.111.088112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Ganatos P., Spray D. C., Weinbaum S. (2011). On the electrophysiological response of bone cells using a Stokesian fluid stimulus probe for delivery of quantifiable localized picoNewton level forces. J. Biomech. 44, 1702–1708 10.1016/j.jbiomech.2011.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Rangamani P., Fardin M. A., Lipshtat A., Dubin–Thaler B., Rossier O., Sheetz M. P., Iyengar R. (2010). Mechanisms controlling cell size and shape during isotropic cell spreading. Biophys. J. 98, 2136–2146 10.1016/j.bpj.2010.01.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. C., Sachs F. (1989). Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science 243, 1068–1071 10.1126/science.2466333 [DOI] [PubMed] [Google Scholar]
- Yeung E. W., Whitehead N. P., Suchyna T. M., Gottlieb P. A., Sachs F., Allen D. G. (2005). Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J. Physiol. 562, 367–380 10.1113/jphysiol.2004.075275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Gao F., Popov V. L., Wen J. W., Hamill O. P. (2000). Mechanically gated channel activity in cytoskeleton-deficient plasma membrane blebs and vesicles from Xenopus oocytes. J. Physiol. 523, 117–130 10.1111/j.1469-7793.2000.t01-1-00117.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X. L., Batiza A. F., Loukin S. H., Palmer C. P., Kung C., Saimi Y. (2003). The transient receptor potential channel on the yeast vacuole is mechanosensitive. Proc. Natl. Acad. Sci. USA 100, 7105–7110 10.1073/pnas.1230540100 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.