Summary
Transcriptional regulation contributes to the maintenance of pluripotency, self-renewal and differentiation in embryonic cells and in stem cells. Therefore, control of gene expression at the level of transcription is crucial for embryonic development, as well as for organogenesis, functional adaptation, and regeneration in adult tissues and organs. In the past, most work has focused on how transcriptional regulation results from the complex interplay between chemical cues, adhesion signals, transcription factors and their co-regulators during development. However, chemical signaling alone is not sufficient to explain how three-dimensional (3D) tissues and organs are constructed and maintained through the spatiotemporal control of transcriptional activities. Accumulated evidence indicates that mechanical cues, which include physical forces (e.g. tension, compression or shear stress), alterations in extracellular matrix (ECM) mechanics and changes in cell shape, are transmitted to the nucleus directly or indirectly to orchestrate transcriptional activities that are crucial for embryogenesis and organogenesis. In this Commentary, we review how the mechanical control of gene transcription contributes to the maintenance of pluripotency, determination of cell fate, pattern formation and organogenesis, as well as how it is involved in the control of cell and tissue function throughout embryogenesis and adult life. A deeper understanding of these mechanosensitive transcriptional control mechanisms should lead to new approaches to tissue engineering and regenerative medicine.
Key words: Mechanical force, Transcription, Stem cells, Embryos, Pattern formation, Growth, Cytoskeleton, Nucleus, Prestress, Cell shape
Introduction
Tissue and organ architecture are constructed as a result of the complex orchestration of various cell types that exhibit specific behaviors (e.g. growth, differentiation, migration and apoptosis) in specific locations at precise times during embryogenesis. Transcriptional programs confer and maintain cellular identity and functions that are necessary for the maturation of embryonic cells into terminally differentiated tissues or organs, as well as for stem cell fate switching throughout adult life. These behaviors are regulated by turning genes on and off in a highly spatiotemporally controlled manner, and this process is mediated at the level of gene transcription through the interplay between RNA polymerase II, various transcription factors and their co-regulators (Box 1). Whereas most studies on transcriptional regulation have focused on genetic or chemical control mechanisms of transcription, recent work suggests that mechanical forces (Box 2) are equally important regulators of transcriptional control in embryonic and adult tissues. In this Commentary, we review these new insights into cellular mechanotransduction, and discuss how mechanical cues influence transcriptional regulation to govern organogenesis and to ensure tissue maintenance throughout adult life.
Box 1. Regulation of transcription.
Transcription: the synthesis of an RNA molecule from a complementary DNA sequence by RNA polymerase II that binds to the promoter region of a gene.
Transcription factor: regulatory protein that regulates transcription by binding with high affinity to its target promoter sequence on the DNA. Alone or in combination with other associated proteins, transcription factors promote or block recruitment of RNA polymerase II to the promoter regions of a gene.
Transcriptional regulation: controls the rate of transcription by regulating the binding of RNA polymerase II to the DNA strand.
Co-activator or co-repressor: associate with transcription factors to, respectively, up- or downregulate the rate of transcription.
Box 2. Mechanical forces involved in regulation of transcription.
Hydrostatic pressure: mechanical force applied by fluids or gases (e.g. blood or air) that perfuse or infuse living organs (e.g. blood vessels or lung).
Shear stress: frictional force of fluid flow on the surface of cells. The shear stress generated by the heart pumping blood through the systemic circulation has a key role in the determination of the cell fate of cardiomyocytes, endothelial cells and hematopoietic cells.
Compressive force: pushing force that shortens the material in the direction of the applied force.
Tensional force: pulling force that lengthens materials in the direction of the applied force.
Cell traction force: is exerted on the adhesion to the ECM and other cells as a result of the shortening of the contractile cytoskeletal actomyosin filaments, which transmit tensional forces across cell surface adhesion receptors (e.g. integrins, cadherins).
Cell prestress: stabilizing isometric tension in the cell that is generated by the establishment of a mechanical force balance within the cytoskeleton through a tensegrity mechanism. Pulling forces generated within contractile microfilaments are resisted by external tethers of the cell (e.g. to the ECM or neighboring cells) and by internal load-bearing structures that resist compression (e.g. microtubules, filipodia). Prestress controls signal transduction and regulates cell fate.
Mechanical determinants of transcriptional control during early embryogenesis
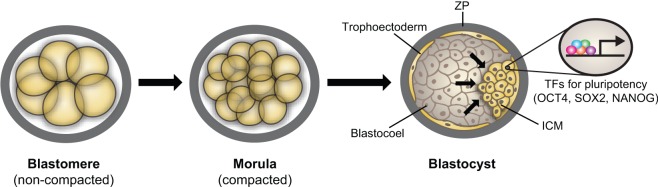
During development, individual cells sense changes in mechanical forces and transduce them into intracellular signals that drive alterations in cell growth, differentiation, migration and apoptosis that are required for tissue and organ development (reviewed in Farge, 2011; Mammoto and Ingber, 2010; Wozniak and Chen, 2009). Early in mammalian embryogenesis, the totipotent inner cell mass (ICM) of the blastocyst gives rise to embryonic stem cells (ESCs) as a result of the expansion of the blastocoel and secretion of liquid into the cavity. The accumulation of liquid pushes the ICM against the surrounding zona pellucida, and the ICM establishes a stable transcriptional regulatory circuit, whereby specific sets of transcription factors, including OCT4 (also known as POU5F1), SOX2 and NANOG, promote pluripotency (Rossant and Tam, 2009) (Fig. 1). Importantly, dense cultures of compact, rounded mouse and human ESCs exhibit higher expression of these pluripotent transcription factors than flattened (sparse) ESCs cultured in vitro (Hemsley et al., 2011; Peerani et al., 2007). Moreover, cell stretching induced by cyclic mechanical strain leads to downregulation of OCT4 gene expression in mouse ESCs, and this is dependent on the level of cytoskeletal tension inside the cell (Chowdhury et al., 2010). These findings suggest that the transcriptional regulation that is necessary for pluripotency can be maintained and modified by the local micromechanical environment in the embryo.
Fig. 1.
Mechanical transcriptional control and pluripotency. The expanding blastocoel pushes the inner cell mass (ICM) against the outer zona pellucida (ZP), which induces specific transcription factors (TFs) that are crucial for pluripotency.
Mechanical cues determine tissue pattern and size in the embryo
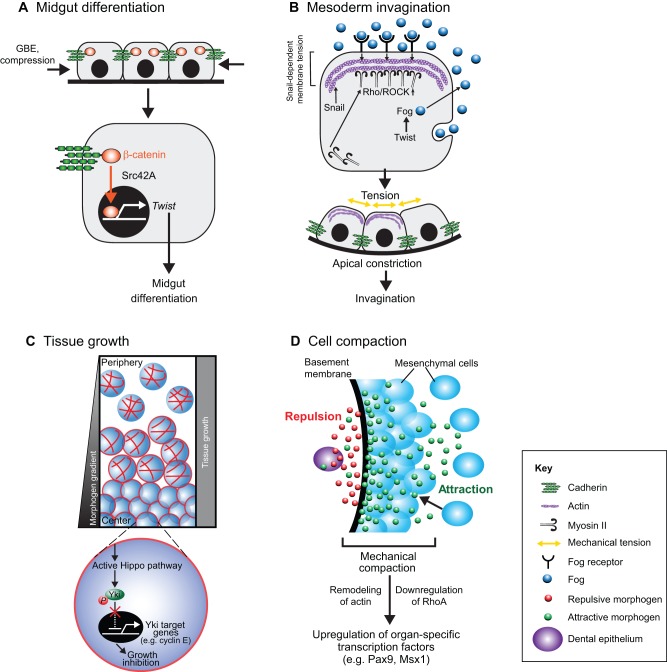
Studies of later developmental stages in the Drosophila wing have revealed that morphogen gradients determine tissue size and pattern, and that this process is mediated at the transcriptional level (Schwank and Basler, 2010). However, internal genetic programs and chemical cues do not appear to be sufficient to explain how these complex tissues form. Importantly, changes in the physical shape of the embryo can feed back to individual cells to mechanically control patterns of gene expression, and thereby govern tissue patterning during Drosophila morphogenesis (Farge, 2003). For instance, physiological deformations in embryonic Drosophila tissues caused by germband extension (GBE) triggers Src42A-dependent nuclear translocation of β-catenin from cell–cell junctions in the anterior pole of stomodeal cells, and activates the transcription of the basic helix-loop-helix transcription factor Twist, which is involved in pattern formation and is crucial for subsequent midgut differentiation (Desprat et al., 2008) (Fig. 2A).
Fig. 2.
Mechanical control of transcription during embryonic pattern formation. (A) Midgut differentiation. In Drosophila anterior stomodeal cells, mechanical compression as a result of germ band extension (GBE) induces expression of the gene encoding the transcription factor Twist, which is crucial for midgut differentiation through a Src42A-dependent mechanotransduction process that leads to the translocation of β-catenin into the nucleus. Modified with permission from Mammoto and Ingber, 2010 (Mammoto and Ingber, 2010). (B) Mesoderm invagination. In the Drosophila mesoderm, the Snail-dependent unstable pulses of apical constriction lead to a mechanically induced inhibition of Fog endocytosis through an increase in membrane tension. This activates the Fog–Rho–ROCK–Myo II signaling pathway, which leads to stable apical constriction and invagination. Fog is expressed under the control of Twist. Modified with permission from Mammoto and Ingber, 2010 (Mammoto and Ingber, 2010). (C) Tissue growth. During wing growth in Drosophila, cells in the central regions are compressed. This results in remodeling of actin in these cells, which activates the Hippo pathway and leads to phosphorylation of the transcription factor Yki, which prevents its translocation into the nucleus. Consequently, transcriptional activity of Yki target genes, such as cyclin E, is decreased and cell growth is inhibited. At the same time, cells at the periphery slow their proliferation because they have grown beyond the edges of the morphogen gradient. By employing these two mechanisms, the whole tissue grows uniformly. (D) Cell compaction. During teeth development in mouse, the early dental epithelium (DE) produces the antagonistic morphogens FGF8 (green) and semaphorin 3f (red) that attract and repulse mesenchymal cells, respectively. This causes mesenchymal cells to pack tightly during mesenchymal condensation in tooth development. The resultant mechanical compaction-induced changes in cell shape induce sets of transcription factors that are crucial for organ-specific morphogenesis.
Gastrulation in Drosophila is controlled by Twist in concert with the zinc-finger transcription factor Snail through Twist-dependent transcription of the gene encoding Folded gastrulation (Fog), and this process begins with mesoderm invagination (Seher et al., 2007) (Pouille et al., 2009) (Fig. 2B). First, Snail induces stochastic apical constrictions that are pulsed, and are subsequently stabilized by the secreted Fog protein (Martin et al., 2009). Mechanical tension that is generated in the apical membrane of the mesodermal cells by Snail-dependent constrictions inhibits Fog endocytosis, thereby enhancing the extracellular concentration and activating signaling pathways downstream of Fog (Pouille et al., 2009). These signals lead to local activation of the Rho and Rho-associated, coiled-coil containing protein kinase 1 (Rho–ROCK) pathway and myosin II (Myo-II) at the apical membrane, which subsequently drives mesoderm invagination (Dawes-Hoang et al., 2005). Twist is known to also activate another downstream signaling molecule, transmembrane protein T48, which reinforces apical constriction and subsequent invagination (Kolsch et al., 2007). Importantly, Myo-II, which is recruited locally in response to mechanical distortion, disappears from the membrane when mechanical tension is relieved during axis elongation in Drosophila (Fernandez-Gonzalez et al., 2009). These observations suggest that mechanical forces act in concert with chemical cues to orchestrate transcriptional and biochemical controls that are crucial for the formation of spatiotemporal patterns during early embryogenesis.
Mechanical cues also contribute to the control of cell proliferation, tissue growth and organ size during Drosophila development. For instance, computational modeling studies suggest that cells in the central parenchyma of the growing Drosophila wing become compressed, a cue that mechanically feeds back to suppress cell growth in the central area (Chen et al., 1997). Meanwhile, cell growth at the periphery of the wing will slow when the cells extend beyond the edges of the morphogen gradient. As a result, the model predicts that the tissue will grow uniformly (Hufnagel et al., 2007; Shraiman, 2005).
The Hippo pathway, a conserved signalling pathway that is crucial for the correct regulation of organ growth in Drosophila and vertebrates, appears to be involved in this feedback loop. The pathway is composed of the Hippo (Hpo) and Warts (Wts) kinases, together with their co-factors scaffold protein salvador (Sav) and MOB kinase activator-like 1 (Mats), and the transcriptional co-activator Yorkie (Yki; Yap1 in mammals) (Dick and Mymryk, 2011; Zhao et al., 2010). In both Drosophila and mice, active Hippo signaling results in phosphorylated Yki (and Yap1, respectively) being retained in the cytoplasm, which leads to transcriptional downregulation of target genes, such as the cell cycle regulator cyclin E (Fig. 2C) (Dong et al., 2007; Oh and Irvine, 2008; Oh and Irvine, 2009; Ren et al., 2010). This pathway, which enables cells to alter their proliferative rate in response to external mechanical cues, is sensitive to mechanical inputs from the extracellular matrix (ECM) and cell–cell contacts, and it requires an intact actin cytoskeleton (Assoian and Klein, 2008; Klein et al., 2007). Consistent with this observation, changes in the physical organization of the actin cytoskeleton can also alter cell growth by modulating Hippo signaling through Yap1. This further highlights a role for this cytoskeleton-dependent mechanical signaling pathway in transcription regulation, which leads to the inhibition of cell growth in response to environmental stimuli (Sansores-Garcia et al., 2011).
Mechanical control of organ-specific determination of the cell fate during development
Morphogen gradients in the embryo are converted into patterns of gene expression by concentration-specific responses of target genes (Wolpert, 1969). These gradients spatially limit the expression of target-differentiation genes and produce distinct organ-specific cell fates within precise physical boundaries of tissues (Small et al., 1992). Recent evidence suggests that external mechanical cues also contribute to the determination of tissue boundaries and organ-specific cell fates. This is important because maintaining the boundaries that are defined by these expression patterns is crucial for the maturation of these early patterns into stable tissue structures during embryogenesis (Dahmann et al., 2011). Studies in Drosophila have revealed that cytoskeletal tension – which is controlled by Rho, ROCK and Myo-II, and exerts tension on cell–cell adhesions – contributes to the formation of these discrete compartmental boundaries (Landsberg et al., 2009; Monier et al., 2010). Furthermore, during tooth formation in mouse, the dental epithelium produces morphogen gradients of opposing attractive and repulsive motility factors (FGF8 and semaphorin 3F, respectively) that generate defined areas of cell compaction in a process known as the ‘formation of the condensed mesenchyme’. This, in turn, triggers the protein expression of sets of transcription factors that orchestrate organ-specific morphogenesis as a result of cell distortion and subsequent inhibition of Rho signaling (Mammoto et al., 2011) (Fig. 2D). Thus, developmental patterning and organ-specific determination of the cell fate seem to be governed through a complex mechanochemical mechanism in which chemical cues manifest their actions largely through changes in the physical microenvironment that feed back into chemical signals – which subsequently alter transcriptional control. This theory is supported by observations during mouse mammary gland development: reorganization of mouse mammary epithelial cells into three-dimensional (3D) polarized acinar structures, which are induced by the ECM protein laminin, exposes prolactin receptors to the local prolactin hormone. This, and following the nuclear translocation of the transcription factor STAT5, activates the receptors and leads to β-casein expression, which induces differentiation of cells into the mammary-specific cell lineage (Xu et al., 2009).
Mechanosensitive transcriptional control during organ development and homeostasis
Mechanical forces not only have a role during developmental processes, but are also crucial for maintaining the function of most adult organs and organ systems, including heart (Groenendijk et al., 2007; McCain and Parker, 2011), blood vessels (Culver and Dickinson, 2010; Shiu et al., 2005), lungs (Hooper and Wallace, 2006), hematopoietic system (Adamo et al., 2009; North et al., 2009), gastrointestinal tract (Gayer and Basson, 2009) and musculoskeletal system (Huang and Ogawa, 2010; Tidball, 2005). Below, we provide examples of this form of mechanoregulation in relevant organ systems.
Heart
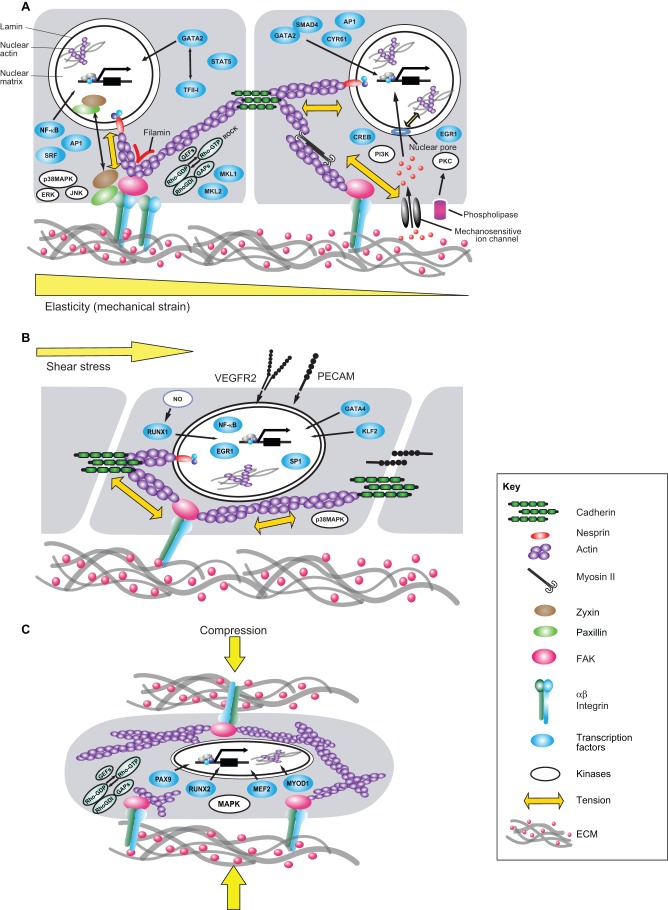
GATA-binding protein 4 (GATA4) is a transcription factor that regulates heart development (Bruneau, 2002), and is activated by vasopressin-induced increases in cardiac afterload and through direct stretching of the ventricles in rats (Fig. 3) (Hautala et al., 2001; Hautala et al., 2002). GATA4 has also been implicated as a mechanical load-responsive mediator of transcription that regulates the expression of genes that contribute to cardiac remodeling and ventricular hypertrophy, such as B-type natriuretic peptide (BNP) (Marttila et al., 2001).
Fig. 3.
Mechanotransduction pathways and transcriptional control in response to mechanical strain, fluid shear stress and compression. Cells and tissues are stabilized through a tensegrity mechanism in which a stabilizing tensional prestress or state of isometric tension is generated inside the cells by mechanical forces. The latter are generated by interactions of actin and myosin within the cytoskeleton that are worked against by integrin adhering to the ECM, cadherin adhering to neighboring cells and by internal cytoskeletal structures (Ingber, 2006). This mechanical equilibrium governs cell mechanics and hence, modulates the response of cells to external forces. (A) When cells and tissues experience mechanical strain, forces transmitted through integrin receptors and cadherins alter intracellular signaling pathways involving proteins such as kinases (e.g. PKC, MAPK14, ERKs and JNK), focal adhesion proteins (e.g. FAK, zyxin, paxillin) and Rho small GTPases and its guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs). Mechanical tension exerted on integrins also modulates mechanosensitive ion channels and phospholipases, which activate PI3K and PKC, respectively, through second messengers such as intracellular Ca2+, inositol lipids and arachidonic acid. All of these mechanochemical signals lead to the activation of transcription factors (e.g. GATA2, TFII-I, NF-κB, AP1, SRF, STAT5, MKL1, MKL2, SMAD4, CYR61, CREB and EGR1). Physical forces that are exerted on surface adhesion receptors and are transmitted directly to the nucleus along cytoskeletal filaments and molecules that connect the cytoskeleton to the nucleus, such as nesprin, can influence transcription activity more directly. (B) When adherent cells, such as endothelial cells, are exposed to apical fluid shear stress, the cells sense these forces through mechanosensors such as VEGFR2 or PECAM. Various transcriptional activities (e.g. RUNX1, GATA4, KLF2, NF-κB, EGR1 and SP1) are modulated through downstream changes in biochemical signaling (e.g. NO, p38MAPK) and cytoskeletal prestress. (C) Compressive forces generated by gravity or physical movements that are transmitted across the ECM can also alter activities of intracellular biochemical signaling molecules (e.g. Rho GTPases, their GEFs and GAPs and MAPK) and cytoskeletal tension. Again, these changes can modulate various transcriptional activities (involving for example PAX9, RUNX2, MEF2 and MYOD1) that are crucial for homeostasis of tissues that normally bear compressive forces, such as bone, cartilage and tooth. Parts of this figure were modified with permission from Mammoto and Ingber, 2010 (Mammoto and Ingber, 2010).
Formation of the heart valve is also flow-dependent (Bartman et al., 2004; Hove et al., 2003; Wirrig and Yutzey, 2011) and GATA4, which is also activated by the changes in blood flow in the heart (Huang et al., 2010), appears to partly mediate this response. For example, GATA4 interacts with SMAD4, another transcription factor that is part of the bone morphogenetic protein (BMP) and transforming growth factor β (TGF-β) TGF-β signaling cascade. In mouse, they co-regulate valve formation by controlling endocardial cushion development (Moskowitz et al., 2011). In zebrafish, Krüppel-like factor 2 (KLF2) – another flow-sensitive transcription factor that orchestrates the expression of angiogenic, antithrombotic and atheroprotective genes in endothelial cells (Lee et al., 2006) – has been shown to have crucial roles in valve morphogenesis (Vermot et al., 2009).
Hematopoietic system
Interestingly, in the mouse embryo, the initiation of heart pumping and blood flow also switches on the formation of blood cells, activating hematopoietic stem cells in the aorta–gonad–mesenephron (AGM) region, the origin of the dorsal aorta (Medvinsky and Dzierzak, 1996). This effect is mediated by the transcription factor RUNX1 that is induced by flow-sensitive production of nitric oxide (NO) in mice and zebrafish (Fig. 3) (Adamo et al., 2009; Lichtinger et al., 2010; North et al., 2009). Thus, mechanical forces generated by blood flow are central to the control of transcriptional activities that govern development of the circulatory system.
Blood vessels
Endothelial cells within blood vessels are constantly exposed to fluid shear stresses, pulsatile pressures and cyclic mechanical strain. The effects of fluid shear on the transcription of genes that encode proteins that are crucial for vascular homeostasis, such as platelet-derived growth factor B (PDGFB) (Resnick et al., 1993), nitric oxide synthase 3 (eNos, also known as NOS3) (Davis et al., 2004) and platelet endothelial cell adhesion molecule 1 (PECAM1) (Almendro et al., 1996), are regulated by shear-stress-responsive elements (SSREs) within their promoter regions. Fluid shear stress regulates binding of mechanosensitive transcription factors, such as nuclear factor kappa B (NF-κB) and early growth response 1 (EGR1), to SSREs in endothelial cells, where they maintain blood vessel homeostasis (Gimbrone et al., 2000). Endothelial cells also sense shear stress through a mechanosensory cell–cell adhesion complex containing PECAM-1, vascular endothelial (VE)-cadherin and vascular endothelial growth factor 2 (VEGFR2); flow-mediated mechanical distortion of the endothelial cells activates NF-κB through the integrin–p38 MAPK (also known as MAPK14) signaling cascade (Orr et al., 2005; Tzima et al., 2005). Activated NF-κB upregulates the expression of genes that encode proteins such as eNOS (Balligand et al., 2009; Davis et al., 2004; Ozawa et al., 2004), several cytokines (Allport et al., 2000; Martin et al., 1997) and adhesion molecules that are crucial for maintaining vascular homeostasis (Ahmad et al., 1998). In addition, fluid shear stress activates the transcription of the gene encoding VEGFR2 through binding of the transcription factor SP1 to its promoter, and this transcriptional regulation is crucial for endothelial cell survival (Urbich et al., 2003).
During angiogenesis, VEGFR2 protein synthesis and the formation of new microvessels are regulated by changes in ECM mechanics (e.g. stiffness or compliance), which alter the balance of activities between two transcription factors GATA2 and TFII-I (also known as GTF2I) in endothelial cells (Mammoto et al., 2009). In vascular smooth muscle cells, remodeling of the arteries in response to mechanical loads – such as changes in blood pressure – is also regulated at transcriptional level by the mechanosensitive transcription factor EGR1 (Morawietz et al., 1999). Mechanical strain induces transcription of the cysteine-rich angiogenic inducer 61 (CYR61), a crucial gene for vascular development and repair in smooth muscle cells (Chaqour and Goppelt-Struebe, 2006). In addition, cyclic stretching of rat aortic smooth muscle cells has been shown to increase the protein expression of endothelin B receptor, which is involved in blood-pressure-dependent arterial remodeling. At the transcriptional level this is mediated by activator protein 1 (AP1) and CCAAT/enhancer-binding protein (CEBP) following stimulation of the ROCK and the p38MAPK pathways (Fig. 3) (Cattaruzza et al., 2001).
Lung
Embryonic lung development is highly sensitive to mechanical cues (Costa et al., 2001; Mendelson, 2000; Minoo, 2000). Before birth, intraluminal pressures are generated by secretion of fluid by the pulmonary epithelium and the elastic recoil properties of the lung tissues (Harding et al., 1986; Vilos and Liggins, 1982). Physical stretching of lung epithelial cells activates NF-κB (Harding and Hooper, 1996), which enhances maturation of lung epithelial cells during embryogenesis in mouse (Londhe et al., 2008). In chicken lung, NF-κB activity in the mesenchyme also has a role during morphogenesis of lung branching (Muraoka et al., 2000). Similarly, the level of tensile prestress (isometric tension) generated by Rho-dependent changes in cytoskeletal contractility affects lung branching morphogenesis in mice (Moore et al., 2002; Moore et al., 2005). Mechanical stretching of the lung also drives myogenesis in airway smooth muscle, which is crucial for bronchial development. This process is mediated by the activation of the serum response factor (SRF) transcription factor (Badri et al., 2008; Yang et al., 2000). Overdistention of the fetal lung (as seen, for example, in congenital laryngeal atresia) results in the formation of larger lungs with an increased number of alveoli and a more mature architecture than expected for gestational age in humans (Wigglesworth et al., 1987). During the transition to breathing air at birth, the lung also undergoes dynamic physical changes that appear to influence formation and maturation of alveolar structures (Inanlou et al., 2005; Nelson et al., 2005; Wigglesworth et al., 1987). For example, in vitro studies have demonstrated that differentiation of rat embryonic lung cells into either type I or II alveolar cells, is modulated by mechanically distending cells in a way that mimics inflation and deflation of the alveolus during respiration (Gutierrez et al., 1999; Wang et al., 2006).
The gastrointestinal and musculoskeletal systems
Tissues within many other organs have been shown to exhibit mechanosensitive transcriptional regulation. For instance, mechanical pressure in the gut stimulates gene transcription and proliferation in rat gastrointestinal epithelial cells through MAPK-mediated activation of the transcription factor AP1 (Hirokawa et al., 2001). Exposure of these cells to pressure also modulates the synthesis of interleukin 6 (IL6) through the NF-κB pathway, which is crucial for gut homeostasis (Kishikawa et al., 2002). Mechanical loading, generated by gravity or physical movement, similarly stimulates the formation of bones, joints, tendons, ligaments, cartilage and muscle within the musculoskeletal system in humans and rats (Kahn et al., 2009; Lanyon, 1992; Raab-Cullen et al., 1994; Robling et al., 2006; Rubin and Lanyon, 1985). Many of these effects are mediated through the activation of specific transcription factors. For example, mechanical deformation of osteoblasts activates MAPK, which binds to and phosphorylates the transcription factor RUNX2, which subsequently controls expression of osteoblast-specific differentiation genes, including osteocalcin, type I collagen, bone sialoprotein, osteopontin and alkaline phosphatase in humans and rodents (Kanno et al., 2007; Ziros et al., 2008).
Similarly, during differentiation of skeletal muscle, the expression of specific sets of genes leads to the production of myofibril proteins and their assembly into contractile sarcomere units that are constantly remodeled in response to changes in mechanical load. Calcineurin, a molecular target of intracellular Ca2+–calmodulin signaling activates the transcription factors myocyte enhancer factor 2 (MEF2) and myogenic differentiation 1 (MYOD1), which mediate mechanical-load-induced myogenesis by inducing the expression of myogenin in mice (Friday et al., 2000; Friday et al., 2003; Parsons et al., 2004). Mechanosensitive endocytosis of BMP2 also has a crucial role in the transcriptional control of myogenic and osteoblastic differentiation in mouse mesenchymal stem cells (Rauch et al., 2002).
Deregulation of mechanosensitive transcriptional control and disease development
It is important to note that deregulation of normal mechanosensitive mechanisms of transcriptional control can lead to pathological changes (Table 1). In rodents, for example, excessive hemodynamic loading results in cardiac hypertrophy through exaggerated transcriptional activation of smooth muscle α-actin (ACTA2) (Mack and Owens, 1999; Zhao et al., 2007). The exposure of tissues to excessive mechanical forces increases the expression of pro-inflammatory proteins, such as interleukin-1β (IL1B), tumor necrosis factor α (TNFA), inducible oxide synthase (iNOS, also known as NOS2) and matrix metalloproteinases. Continued activation of these responses can lead to inflammatory diseases, such as arthritis (Agarwal et al., 2004; Ray et al., 2005; Yang et al., 2005), asthma (Kumar et al., 2003) and ventilator-induced lung injury (Ning and Wang, 2007).
Table 1.
Deregulated mechanotranscription in different disease-states
| Organ system | Mechanical deregulation | Pathological conditions | Transcriptional regulation | References |
| Heart | High pressure and/or volume | Cardiac hypertrophy | MKL1a | (Zhao et al., 2007) |
| Blood vessel | Turbulent flow | Atherosclerosis | NF-κBb, EGR1b | (Gimbrone et al., 2000) |
| Lung | High pressure | Asthma | NF-κBb | (Kumar et al., 2003) |
| Lung | High pressure | Ventilator-induced lung injury | NF-κBb | (Ning and Wang, 2007) |
| Gastrointestinal tract | High pressure | Irritable bowel syndrome | AP1b | (Hirokawa et al., 2001) |
| Bone | Low pressure | Osteoporosis | FOSb | (Bergmann et al., 2010) |
| Joint | High pressure | Arthritis | NF-κBb | (Agarwal et al., 2004) |
| Muscle | Low pressure | Muscle atrophy | FBXO32b, MURF1b | (Sandri, 2008) |
, co-activator;
, transcription factor.
At the same time, sustained application of mechanical force can sometimes repair diseased tissues. For instance, in a process referred to as distraction osteogenesis, gradual traction on bones is used to enhance osteogenesis for the treatment of skeletal deformities in dogs and humans (Ilizarov, 1989a; Ilizarov, 1989b; Aarnes et al., 2002). Similarly, constant stretching of the intestines leads to an increase in intestinal length in rats (Park et al., 2004) and stretching induces regeneration and lengthening of the esophagus in human infants with esophageal atresia (Foker et al., 1997). However, little is known about the transcriptional processes that mediate these mechanoresponses.
Mechanotransduction and transcriptional control
The multiple examples described above clearly demonstrate that mechanical forces can regulate the activity of transcription factors and the expression of genes that are crucial for developmental control and tissue maintenance. By contrast, the varied mechanisms through which mechanical forces applied at the cell surface or generated within the contractile cytoskeleton can produce these transcriptional alterations have only come to light recently.
Mechanosensitive transmembrane receptors
Mechanical forces can alter transcription by activating transmembrane receptors and harnessing intracellular signaling cascades that are used by soluble hormones and cytokines to alter the transcription factor activity. Work over the past two decades has revealed that mechanical signals are preferentially sensed by transmembrane surface adhesion receptors, such as integrins and cadherins, which can transfer physical forces across the plasma membrane (Fig. 3). Such mechanical signals are converted into cytoplasmic biochemical signals within cell surface focal adhesions and cell–cell adhesion complexes (reviewed in Mammoto and Ingber, 2010). These adhesion complexes can trigger transcriptional changes in the nucleus through modulating downstream signaling cascades (Ingber, 2006; Mammoto and Ingber, 2009; Mammoto et al., 2008; Orr et al., 2006). Mechanical signals can, thereby, elicit changes in the concentration of second messengers, such as Ca2+ flux, inositol trisphosphate (InsP3), diacylglycerol (DAG), cAMP and nitric oxide, or activate protein signaling molecules, such as MAPKs and Rho-family small GTPases, which are involved in controlling virtually all cellular processes, including migration, growth, differentiation and matrix remodeling (Alenghat et al., 2009; Balligand et al., 2009; Huang and Ingber, 2002; Mammoto and Ingber, 2009; Mammoto et al., 2008; Martinac, 2004; Orr et al., 2006; Papachristou et al., 2009; Sadoshima and Izumo, 1993).
One of the most rapid mechanical signaling pathways upstream of transcription involves the activation of mechanosensitive ion channels on the cell surface. In endothelial cells, cyclic mechanical strain applied through the ECM and ECM-bound integrin receptors induces Ca2+ influx through mechanosensitive transient receptor potential cation channel subfamily V member 4 (TRPV4) channels within 5 mseconds after the application of the mechanical force (Matthews et al., 2010). The resultant change in membrane potential stimulates phosphatidyl inositol-3-kinase (PI3K), which activates additional β1 integrin receptors and promotes cytoskeletal remodeling that is crucial for angiogenesis (Fig. 3) (Thodeti et al., 2009). In neurons, Ca2+ flux through mechanosensitive ion channels activates the ERK1 and ERK2 (ERK1/2, also known as MAPK3 and MAPK1, respectively) pathway and stimulates the expression of genes that are essential for neuronal survival, plasticity and memory formation through activation of the transcription factor cAMP responsive element binding protein (CREB) (Dolmetsch et al., 2001; Wheeler et al., 2008).
Mechanical forces activate MAPK pathways including the ERK1/2, p38 MAPK (also known as MAPK14) and Jun N-terminal kinase (JNK, also known as MAPK8) pathways in endothelial cells, osteoblasts and fibroblasts by stimulating focal adhesion kinase (FAK) in focal adhesions (Boutahar et al., 2004; D'Addario et al., 2002; Huang and Ingber, 2002; Ishida et al., 1996). Mechanically activated ERK1/2 and/or JNK signals are transmitted into the nucleus where they activate the transcription factor AP1 and, thereby, upregulate expression of molecules that are crucial for tissue formation and remodeling, such as type I collagen and osteopontin in bone (Hong et al., 2010; Jeon et al., 2009; Kook et al., 2009). Mechanical strain and distortion of the cell shape also activate membrane-associated phospholipases and, thereby, increase the metabolism of inositol lipids and arachidonic acid in the cytoplasm, which results in the release of Ca2+ from intracellular stores, activation of protein kinase C (PKC) and the remodeling of cardiomyocytes through activation of mechanosensitive transcription factors, such as EGR1 and AP1 (Fig. 3) (Bishop and Lindahl, 1999; Komuro et al., 1991; Sadoshima and Izumo, 1993; Tseng et al., 1994). These findings suggest that mechanical cues are transmitted to the nucleus through an interplay between mechanosensitive transmembrane receptors and a variety of second messengers that modulate transcriptional activities that are crucial for cell differentiation and tissue remodeling.
The cytoskeleton as a regulator of transcription
Cells and tissues that are tensionally prestressed are held in a state of isometric tension as a result of micromechanical contractile forces, which are generated by ECM adhesions as well as adhesive contacts to neighboring cells that balance the cytoskeletal actomyosin filaments. These forces, in turn, stabilize cell shape and mechanics through a tensional integrity (‘tensegrity’) mechanism (Ingber, 1993). Mechanotransduction is sensitive to this micromechanical level of prestress in the cells, which is stored and released to control mechanotransduction in a way that resembles the use of a bow and bow string (Ingber, 2006).
Focal adhesion proteins that physically couple actin filaments to integrins and mediate transmembrane mechanical force transmission, including talin, paxillin, filamin A and vinculin, also act as mechanotransducers, in part by regulating cytoskeletal tension (Chan et al., 2009; Riveline et al., 2001). Part of this response is mediated by regulating the physical strength of the focal adhesion that resists cell traction forces to sustain cytoskeletal prestress (Fig. 3) (Ezzell et al., 1997). These focal adhesion proteins also facilitate translation of mechanical cues into chemical signals that modulate the Rho signaling pathway, which feeds back to control phosphorylation of myosin II and the generation of cytoskeletal tension (Mammoto et al., 2007; Zhao et al., 2007). Moreover, some focal adhesion proteins, such as paxillin and zyxin, alter their binding kinetics in a force-dependent manner (Lele et al., 2006). This enables them to shuttle between the cytoplasm and nucleus where they can act directly as co-activators of transcription to regulate gene expression (Wang and Gilmore, 2003).
In addition to the involvement of various signaling and adhesion proteins, the actin cytoskeleton itself seems to have a central role in coupling mechanical forces to the regulation of tissue growth at a transcriptional level (Wang and Riechmann, 2007). For example, actin cytoskeleton-dependent control of Rho GTPase activity through p190RhoGAP (also known as ARHGAP35) mediates cell-shape-dependent changes in cell-cycle progression by altering the balance between ROCK and mammalian Diaphanous-related formins (mDia) (Mammoto et al., 2007; Mammoto et al., 2004). When the actin cytoskeleton is disrupted and cells are round, filamin A binds to p190RhoGAP and its activity as a GTPase activating protein (GAP) is inhibited, leading to the activation of Rho (Mammoto et al., 2007). By contrast, in spreading cells, filamin A, an actin-binding protein that crosslinks F-actin, is cleaved by calpain and p190RhoGAP dissociates from filamin A. Subsequently, RhoGAP moves to the lipid-raft fraction where it inactivates Rho (Mammoto et al., 2007). This distortion-dependent signaling mechanism mediates cell-shape-dependent changes in cell cycle progression (Mammoto et al., 2004; Mammoto et al., 2007). Myocardin-like proteins 1 and 2 (MKL1 and MKL2, respectively), which are co-activators of SRF, also have a key role in controlling Rho-dependent gene expression and cell growth (Descot et al., 2008). RhoA regulates the nuclear translocation of MKL1 and/or MKL2 and mediates the formation of stress fibers and focal adhesions by activating the transcription of cytoskeletal and/or focal adhesion genes (Morita et al., 2007).
Human mesenchymal stem cells (MSCs) express organ-specific transcription factors and undergo tissue-specific cell fate switches (e.g. neuron vs myocyte vs osteocyte) when cultured on ECMs with mechanical stiffnesses that mirror the physical properties (i.e. the Young's moduli) of their respective tissues (e.g. brain vs muscle vs bone) (Engler et al., 2006). Changes in ECM compliance alter cytoskeletal tension, actin structure and cell shape (Ingber, 2003; Polte et al., 2004), and the effects of ECM compliance and cell shape on MSC fate switching is mediated by Rho and Myo-II, which generate cytoskeletal tension (Fig. 3) (Engler et al., 2004; Ren et al., 2009). These findings further suggest that the actin cytoskeleton and tensional prestress are central mechanosensitive control elements, which are involved in a complex feedback loop: they both mediate the effects of mechanical cues on transcription regulation and alter their functional state in response to changes in gene transcription.
The nucleus as a mechanical point of control of transcription
The cytoskeleton is coupled to the nuclear membrane, intranuclear scaffolds and chromatin, and molecular connections between integrins, cytoskeletal filaments as well as nuclear scaffolds provide a discrete path for the transmission of mechanical signals in response to changes in adhesion to the ECM or in the cytoskeletal prestress (Maniotis et al., 1997b) (Fig. 3). In fact, changes in nuclear mechanics and architecture that are modulated by the ECM substrate, focal adhesions and the cytoskeleton, have crucial roles in the transcriptional control of cell fate determination (Buxboim et al., 2010). Importantly, changes in cell fate between growth, differentiation and apoptosis can be controlled by altering cell shape (Chen et al., 1997; Dike et al., 1999), ECM elasticity (Engler et al., 2006), cytoskeletal contractility through Rho–ROCK signaling (McBeath et al., 2004) and tissue patterns (Ruiz and Chen, 2008). Given that nuclear mechanics are regulated by these same parameters (Khatau et al., 2010; Kihara et al., 2011; Sims et al., 1992), the nucleus itself might act as a mechanosensor that directly modulates regulation of transcription during cell fate determination in these cells. In support of this possibility, inhibition of Myo-II with blebbistatin or disruption of actin microfilaments with cytochalasin D, results in a dramatic decrease in the nuclear size of adherent cells, whereas inhibition of microtubule polymerization with nocodazole increases the nuclear size (Mazumder and Shivashankar, 2010). Thus, the area of the cell surface and the size of nuclei are directly coupled to integrin through cytoskeletal linkages; this physical connection results in the nucleus becoming prestressed, so that it balances contractile forces of the cytoskeleton with condensation forces of chromatin (Mazumder and Shivashankar, 2007; Mazumder et al., 2008; Mazumder and Shivashankar, 2010). Importantly, such a mechanism of nuclear mechanotransduction in response to forces that act on the cell surface is much faster than the propagation of signaling ions and molecules (Li et al., 2007). Nuclear prestress might also be crucial to determine the cell fate (Fig. 3), because stem cell differentiation leads to the dynamic reorganization of connections between the cytoskeleton and the nuclear membrane, thereby progressively leading to a stiffer nucleus as lineage-committed cells emerge (Mazumder and Shivashankar, 2010; Pajerowski et al., 2007).
Interestingly, the structure of the nucleus also appears to be crucial for transcriptional reprogramming of pluripotency. For example, transplantation of somatic nuclei into eggs or oocytes is sufficient to reactivate silenced pluripotent genes such as that encoding OCT4 (Pou5f1) (Kim et al., 2009; Stadtfeld et al., 2008). Moreover, this is a more-efficient way to reprogram cells into pluripotent stem cells than the forced induction of OCT4, SOX2, MYC and KLF4 protein expression in somatic cells (Kim et al., 2010; Pasque et al., 2010). Although the mechanism by which the physical structure of the nucleus induces gene reprogramming at the level of transcription is unclear, it is known that nuclear actin can regulate gene transcription through changes in cytoskeletal actin dynamics (Sotiropoulos et al., 1999) or by modulating the assembly of transcriptional regulatory complexes in the nucleus (Grummt, 2006). Nuclear actin polymerization also has an essential role in transcriptional reactivation of the gene encoding OCT4 in transplanted nuclei (Miyamoto et al., 2011), and decreased cytoskeletal tension destabilizes transcriptional regulation of pluripotency and, hence, compromises long-term survival of ESCs (Li et al., 2010). The different effects of pluripotency on transcriptional control in ESCs might, therefore, be regulated by mechanical forces that are exerted on the cell surface and transmitted to the nucleus across transmembrane adhesion receptors (e.g. integrins and cadherins) and the cytoskeletal filaments associated with these receptors, which result in tension-dependent changes in nuclear structure (Maniotis et al., 1997a; Maniotis et al., 1997b; Sims et al., 1992; Wang et al., 1993; Wang et al., 2009).
Mechanical forces can also regulate gene expression directly by altering the transport of transcription factors or other molecules into the nucleus. For example, nuclear transport of MKL1 is regulated by prestress in fibroblasts (McGee et al., 2011). Nuclear transport of NF-κB, which activates expression of the genes encoding PDGFA and PDGFB in response to fluid shear stress, is also specifically sensitive to mechanical perturbation in endothelial cells (Khachigian et al., 1995; Meiler et al., 2002). The levels of nuclear translocation of the two transcription factors TFII-I and GATA2, which control angiogenesis by regulating the expression of VEGFR2, have been shown to be sensitive to ECM mechanics in endothelial cells (Mammoto et al., 2009). In spreading cells, nuclear membrane distortion – as a result of cytoskeletal distortion – also stimulates cytoskeletal tension-dependent Ca2+ entry through nuclear ion channels (Fig. 3) (Prat and Cantiello, 1996) and induces gene transcription (Itano et al., 2003). Because nuclear envelope proteins, such as lamin A and emerin, bind to transcription factors and because these proteins are directly linked to the cytoskeleton through nesprin (Mejat and Méjat, 2010; Wang et al., 2009), any forces that are transferred through these molecules might also alter gene expression directly (Dreuillet et al., 2002; Haraguchi et al., 2004).
Taken together, these findings suggest that the micromechanical environment of the nucleus modulates the transcriptional activities that are crucial to control cell fate determination, and that are sensitive to the microenvironment of living embryonic and adult tissues. Furthering our understanding of these mechanisms will also be crucial for the development of new strategies to reprogram cells and to treat congenital diseases that are caused by perturbed nuclear structure and mechanics, such as Hutchinson–Gilford progeria syndrome, Emery–Dreifuss muscular dystrophy, dilated cardiomyopathy and familial partial lipodystrophy (Worman et al., 2009).
Conclusions
In this Commentary, we have reviewed multiple findings that demonstrate the central role of mechanosensitive control mechanisms of transcription that determine stem cell fate, embryogenesis and that control organ homeostasis. It is now clear that both external stresses and intracellular cytoskeletal tension can be driving forces behind changes in transcriptional regulation. These mechanical signals influence transcription by altering intracellular signaling pathways or mediating changes in cytoskeletal and nuclear structure or mechanics. The extent of this response and the efficiency with which the mechanical signal is transferred through the cell and to the nucleus depend on the level of isometric tension or prestress in the cytoskeleton. Cell-generated tensile forces not only alter the chemical signals that are conveyed by cells, but also produce distortion of neighboring cells and ECM molecules that propagate mechano-chemical signaling over long distances. This process drives tissue patterning and organ formation on the embryonic and tissue scale. These findings also raise the possibility that a better understanding of these mechanotranscriptional control mechanisms will increase our chance of reversing developmental defects or treating diseases by restoring normal mechanical loading conditions or correcting abnormal transcriptional activities.
Despite a great effort to engineer 3D organs from ESCs, so far this approach has not been fully successful because of the lack of a comprehensive understanding about how tissues develop regarding a specific size, shape and material property during organogenesis. The necessity to integrate mechanosensitivity into existing models of transcriptional control to fully understand tissue and organ development is becoming clear. Advances in this area will require new methods in order to measure endogenous mechanical forces and local variations of the mechanical properties of cells, the ECM and entire tissues within forming organs, and a way to analyze how mechanical cues and chemical signals dynamically interact to regulate transcriptional activities during important morphogenetic and patterning events. Because cellular mechanotransduction is central to the control of developmental processes and tissue homeostasis, a deeper understanding of mechanosensitive transcriptional control mechanisms in embryonic and adult organs might also lead to new forms of therapeutic intervention for various diseases, as well as new strategies for tissue engineering and regenerative medicine.
Supplementary Material
Acknowledgments
We thank E. Jiang and A. Jiang for the figures. The authors declare no competing financial interests.
Footnotes
Funding
This work was supported by the National Institutes of Health [grant numbers CA45548 and DE019023], the Department of Defense [grant number BC074986], AHA, the Hearst Foundation and ABTA. Deposited in PMC for release after 12 months.
This article is part of a Minifocus on Mechanotransduction. For further reading, please see related articles: ‘Deconstructing the third dimension – how 3D culture microenvironments alter cellular cues’ by Brendon M. Baker and Christopher S. Chen (J. Cell Sci. 125, 3015-3024). ‘Finding the weakest link – exploring integrin-mediated mechanical molecular pathways’ by Pere Roca-Cusachs et al. (J. Cell Sci. 125, 3025-3038). ‘Signalling through mechanical inputs – a coordinated process’ by Huimin Zhang and Michel Labouesse (J. Cell Sci. 125, 3039-3049). ‘United we stand – integrating the actin cytoskeleton and cell–matrix adhesions in cellular mechanotransduction’ by Ulrich S. Schwarz and Margaret L. Gardel (J. Cell Sci. 125, 3051-3060). ‘Molecular force transduction by ion channels – diversity and unifying principles’ by Sergei Sukharev and Frederick Sachs (J. Cell Sci. 125, 3075-3083).
References
- Aarnes G. T., Steen H., Ludvigsen P., Kristiansen L. P., Reikerås O. (2002). High frequency distraction improves tissue adaptation during leg lengthening in humans. J. Orthop. Res. 20, 789–792 10.1016/S0736-0266(01)00175-9 [DOI] [PubMed] [Google Scholar]
- Adamo L., Naveiras O., Wenzel P. L., McKinney–Freeman S., Mack P. J., Gracia–Sancho J., Suchy–Dicey A., Yoshimoto M., Lensch M. W., Yoder M. C.et al. (2009). Biomechanical forces promote embryonic haematopoiesis. Nature 459, 1131–1135 10.1038/nature08073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S., Deschner J., Long P., Verma A., Hofman C., Evans C. H., Piesco N. (2004). Role of NF-kappaB transcription factors in antiinflammatory and proinflammatory actions of mechanical signals. Arthritis Rheum. 50, 3541–3548 10.1002/art.20601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad M., Theofanidis P., Medford R. M. (1998). Role of activating protein-1 in the regulation of the vascular cell adhesion molecule-1 gene expression by tumor necrosis factor-alpha. J. Biol. Chem. 273, 4616–4621 10.1074/jbc.273.8.4616 [DOI] [PubMed] [Google Scholar]
- Alenghat F. J., Tytell J. D., Thodeti C. K., Derrien A., Ingber D. E. (2009). Mechanical control of cAMP signaling through integrins is mediated by the heterotrimeric Galphas protein. J. Cell. Biochem. 106, 529–538 10.1002/jcb.22001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allport V. C., Slater D. M., Newton R., Bennett P. R. (2000). NF-kappaB and AP-1 are required for cyclo-oxygenase 2 gene expression in amnion epithelial cell line (WISH). Mol. Hum. Reprod. 6, 561–565 10.1093/molehr/6.6.561 [DOI] [PubMed] [Google Scholar]
- Almendro N., Bellón T., Rius C., Lastres P., Langa C., Corbí A., Bernabéu C. (1996). Cloning of the human platelet endothelial cell adhesion molecule-1 promoter and its tissue-specific expression. Structural and functional characterization. J. Immunol. 157, 5411–5421 [PubMed] [Google Scholar]
- Assoian R. K., Klein E. A. (2008). Growth control by intracellular tension and extracellular stiffness. Trends Cell Biol. 18, 347–352 10.1016/j.tcb.2008.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badri K. R., Zhou Y., Schuger L. (2008). Embryological origin of airway smooth muscle. Proc. Am. Thorac. Soc. 5, 4–10 10.1513/pats.200704-049VS [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balligand J. L., Feron O., Dessy C. (2009). eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol. Rev. 89, 481–534 10.1152/physrev.00042.2007 [DOI] [PubMed] [Google Scholar]
- Bartman T., Walsh E. C., Wen K. K., McKane M., Ren J., Alexander J., Rubenstein P. A., Stainier D. Y. (2004). Early myocardial function affects endocardial cushion development in zebrafish. PLoS Biol. 2, E129 10.1371/journal.pbio.0020129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann P., Body J. J., Boonen S., Boutsen Y., Devogelaer J. P., Goemaere S., Kaufman J., Reginster J. Y., Rozenberg S. (2010). Loading and skeletal development and maintenance. J. Osteoporos. 2011, 786752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. E., Lindahl G. (1999). Regulation of cardiovascular collagen synthesis by mechanical load. Cardiovasc. Res. 42, 27–44 10.1016/S0008-6363(99)00021-8 [DOI] [PubMed] [Google Scholar]
- Boutahar N., Guignandon A., Vico L., Lafage–Proust M. H. (2004). Mechanical strain on osteoblasts activates autophosphorylation of focal adhesion kinase and proline-rich tyrosine kinase 2 tyrosine sites involved in ERK activation. J. Biol. Chem. 279, 30588–30599 10.1074/jbc.M313244200 [DOI] [PubMed] [Google Scholar]
- Bruneau B. G. (2002). Transcriptional regulation of vertebrate cardiac morphogenesis. Circ. Res. 90, 509–519 10.1161/01.RES.0000013072.51957.B7 [DOI] [PubMed] [Google Scholar]
- Buxboim A., Ivanovska I. L., Discher D. E. (2010). Matrix elasticity, cytoskeletal forces and physics of the nucleus: how deeply do cells ‘feel’ outside and in? J. Cell Sci. 123, 297–308 10.1242/jcs.041186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaruzza M., Eberhardt I., Hecker M. (2001). Mechanosensitive transcription factors involved in endothelin B receptor expression. J. Biol. Chem. 276, 36999–37003 10.1074/jbc.M105158200 [DOI] [PubMed] [Google Scholar]
- Chan M. W., Arora P. D., Bozavikov P., McCulloch C. A. (2009). FAK, PIP5KIgamma and gelsolin cooperatively mediate force-induced expression of alpha-smooth muscle actin. J. Cell Sci. 122, 2769–2781 10.1242/jcs.044008 [DOI] [PubMed] [Google Scholar]
- Chaqour B., Goppelt–Struebe M. (2006). Mechanical regulation of the Cyr61/CCN1 and CTGF/CCN2 proteins. FEBS J. 273, 3639–3649 10.1111/j.1742-4658.2006.05360.x [DOI] [PubMed] [Google Scholar]
- Chen C. S., Mrksich M., Huang S., Whitesides G. M., Ingber D. E. (1997). Geometric control of cell life and death. Science 276, 1425–1428 10.1126/science.276.5317.1425 [DOI] [PubMed] [Google Scholar]
- Chowdhury F., Na S., Li D., Poh Y. C., Tanaka T. S., Wang F., Wang N. (2010). Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat. Mater. 9, 82–88 10.1038/nmat2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R. H., Kalinichenko V. V., Lim L. (2001). Transcription factors in mouse lung development and function. Am. J. Physiol. Lung Cell. Mol. Physiol. 280, L823–L838 [DOI] [PubMed] [Google Scholar]
- Culver J. C., Dickinson M. E. (2010). The effects of hemodynamic force on embryonic development. Microcirculation 17, 164–178 10.1111/j.1549-8719.2010.00025.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Addario M., Arora P. D., Ellen R. P., McCulloch C. A. (2002). Interaction of p38 and Sp1 in a mechanical force-induced, beta 1 integrin-mediated transcriptional circuit that regulates the actin-binding protein filamin-A. J. Biol. Chem. 277, 47541–47550 10.1074/jbc.M207681200 [DOI] [PubMed] [Google Scholar]
- Dahmann C., Oates A. C., Brand M. (2011). Boundary formation and maintenance in tissue development. Nat. Rev. Genet. 12, 43–55 10.1038/nrg2902 [DOI] [PubMed] [Google Scholar]
- Davis M. E., Grumbach I. M., Fukai T., Cutchins A., Harrison D. G. (2004). Shear stress regulates endothelial nitric-oxide synthase promoter activity through nuclear factor kappaB binding. J. Biol. Chem. 279, 163–168 10.1074/jbc.M307528200 [DOI] [PubMed] [Google Scholar]
- Dawes–Hoang R. E., Parmar K. M., Christiansen A E., Phelps C B., Brand A H., Wieschaus E. F. (2005). Folded gastrulation, cell shape change and the control of myosin localization. Development 132, 4165–4178 [DOI] [PubMed] [Google Scholar]
- Descot A., Rex–Haffner M., Courtois G., Bluteau D., Menssen A., Mercher T., Bernard O. A., Treisman R., Posern G. (2008). OTT-MAL is a deregulated activator of serum response factor-dependent gene expression. Mol. Cell. Biol. 28, 6171–6181 10.1128/MCB.00303-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desprat N., Supatto W., Pouille P. A., Beaurepaire E., Farge E. (2008). Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev. Cell 15, 470–477 10.1016/j.devcel.2008.07.009 [DOI] [PubMed] [Google Scholar]
- Dick F. A., Mymryk J. S. (2011). Sweet DREAMs for Hippo. Genes Dev. 25, 889–894 10.1101/gad.2050411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dike L. E., Chen C. S., Mrksich M., Tien J., Whitesides G. M., Ingber D. E. (1999). Geometric control of switching between growth, apoptosis, and differentiation during angiogenesis using micropatterned substrates. In Vitro Cell. Dev. Biol. Anim. 35, 441–448 10.1007/s11626-999-0050-4 [DOI] [PubMed] [Google Scholar]
- Dolmetsch R. E., Pajvani U., Fife K., Spotts J. M., Greenberg M. E. (2001). Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science 294, 333–339 10.1126/science.1063395 [DOI] [PubMed] [Google Scholar]
- Dong J., Feldmann G., Huang J., Wu S., Zhang N., Comerford S. A., Gayyed M. F., Anders R. A., Maitra A., Pan D. (2007). Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130, 1120–1133 10.1016/j.cell.2007.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreuillet C., Tillit J., Kress M., Ernoult–Lange M. (2002). In vivo and in vitro interaction between human transcription factor MOK2 and nuclear lamin A/C. Nucleic Acids Res. 30, 4634–4642 10.1093/nar/gkf587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A. J., Griffin M. A., Sen S., Bönnemann C. G., Sweeney H. L., Discher D. E. (2004). Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J. Cell Biol. 166, 877–887 10.1083/jcb.200405004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A. J., Sen S., Sweeney H. L., Discher D. E. (2006). Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 10.1016/j.cell.2006.06.044 [DOI] [PubMed] [Google Scholar]
- Ezzell R. M., Goldmann W. H., Wang N., Parashurama N., Ingber D. E. (1997). Vinculin promotes cell spreading by mechanically coupling integrins to the cytoskeleton. Exp. Cell Res. 231, 14–26 10.1006/excr.1996.3451 [DOI] [PubMed] [Google Scholar]
- Farge E. (2003). Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr. Biol. 13, 1365–1377 10.1016/S0960-9822(03)00576-1 [DOI] [PubMed] [Google Scholar]
- Farge E. (2011). Mechanotransduction in development. Curr. Top. Dev. Biol. 95, 243–265 10.1016/B978-0-12-385065-2.00008-6 [DOI] [PubMed] [Google Scholar]
- Fernandez–Gonzalez R., Simoes S. M., Röper J. C., Eaton S., Zallen J. A. (2009). Myosin II dynamics are regulated by tension in intercalating cells. Dev. Cell 17, 736–743 10.1016/j.devcel.2009.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foker J. E., Linden B. C., Boyle E. M., Jr, Marquardt C. (1997). Development of a true primary repair for the full spectrum of esophageal atresia. Ann. Surg. 226, 533–541 10.1097/00000658-199710000-00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friday B. B., Horsley V., Pavlath G. K. (2000). Calcineurin activity is required for the initiation of skeletal muscle differentiation. J. Cell Biol. 149, 657–666 10.1083/jcb.149.3.657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friday B. B., Mitchell P. O., Kegley K. M., Pavlath G. K. (2003). Calcineurin initiates skeletal muscle differentiation by activating MEF2 and MyoD. Differentiation 71, 217–227 10.1046/j.1432-0436.2003.710303.x [DOI] [PubMed] [Google Scholar]
- Gayer C. P., Basson M. D. (2009). The effects of mechanical forces on intestinal physiology and pathology. Cell. Signal. 21, 1237–1244 10.1016/j.cellsig.2009.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Topper J. N., Nagel T., Anderson K. R., Garcia–Cardeña G. (2000). Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann. N. Y. Acad. Sci. 902, 230–240 10.1111/j.1749-6632.2000.tb06318.x [DOI] [PubMed] [Google Scholar]
- Groenendijk B. C., Van der Heiden K., Hierck B. P., Poelmann R. E. (2007). The role of shear stress on ET-1, KLF2, and NOS-3 expression in the developing cardiovascular system of chicken embryos in a venous ligation model. Physiology (Bethesda) 22, 380–389 10.1152/physiol.00023.2007 [DOI] [PubMed] [Google Scholar]
- Grummt I. (2006). Actin and myosin as transcription factors. Curr. Opin. Genet. Dev. 16, 191–196 10.1016/j.gde.2006.02.001 [DOI] [PubMed] [Google Scholar]
- Gutierrez J. A., Ertsey R., Scavo L. M., Collins E., Dobbs L. G. (1999). Mechanical distention modulates alveolar epithelial cell phenotypic expression by transcriptional regulation. Am. J. Respir. Cell Mol. Biol. 21, 223–229 [DOI] [PubMed] [Google Scholar]
- Haraguchi T., Holaska J. M., Yamane M., Koujin T., Hashiguchi N., Mori C., Wilson K. L., Hiraoka Y. (2004). Emerin binding to Btf, a death-promoting transcriptional repressor, is disrupted by a missense mutation that causes Emery-Dreifuss muscular dystrophy. Eur. J. Biochem. 271, 1035–1045 10.1111/j.1432-1033.2004.04007.x [DOI] [PubMed] [Google Scholar]
- Harding R., Hooper S. B. (1996). Regulation of lung expansion and lung growth before birth. J. Appl. Physiol. 81, 209–224 [DOI] [PubMed] [Google Scholar]
- Harding R., Bocking A. D., Sigger J. N. (1986). Influence of upper respiratory tract on liquid flow to and from fetal lungs. J. Appl. Physiol. 61, 68–74 [DOI] [PubMed] [Google Scholar]
- Hautala N., Tokola H., Luodonpää M., Puhakka J., Romppanen H., Vuolteenaho O., Ruskoaho H. (2001). Pressure overload increases GATA4 binding activity via endothelin-1. Circulation 103, 730–735 [DOI] [PubMed] [Google Scholar]
- Hautala N., Tenhunen O., Szokodi I., Ruskoaho H. (2002). Direct left ventricular wall stretch activates GATA4 binding in perfused rat heart: involvement of autocrine/paracrine pathways. Pflugers Arch. 443, 362–369 10.1007/s004240100699 [DOI] [PubMed] [Google Scholar]
- Hemsley A. L., Hernandez D., Mason C., Pelling A. E., Veraitch F. S. (2011). Precisely delivered nanomechanical force induce blebbing in undifferentiated mouse embryonic stem cells. Cell Health and Cytoskeleton 3, 23–34 [Google Scholar]
- Hirokawa M., Miura S., Kishikawa H., Yoshida H., Nakamizo H., Higuchi H., Nakatsumi R. C., Suzuki H., Saito H., Ishii H. (2001). Loading of mechanical pressure activates mitogen-activated protein kinase and early immediate gene in intestinal epithelial cells. Dig. Dis. Sci. 46, 1993–2003 10.1023/A:1010607819842 [DOI] [PubMed] [Google Scholar]
- Hong S. Y., Jeon Y. M., Lee H. J., Kim J. G., Baek J. A., Lee J. C. (2010). Activation of RhoA and FAK induces ERK-mediated osteopontin expression in mechanical force-subjected periodontal ligament fibroblasts. Mol. Cell. Biochem. 335, 263–272 10.1007/s11010-009-0276-1 [DOI] [PubMed] [Google Scholar]
- Hooper S. B., Wallace M. J. (2006). Role of the physicochemical environment in lung development. Clin. Exp. Pharmacol. Physiol. 33, 273–279 10.1111/j.1440-1681.2006.04358.x [DOI] [PubMed] [Google Scholar]
- Hove J. R., Köster R. W., Forouhar A. S., Acevedo–Bolton G., Fraser S. E., Gharib M. (2003). Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421, 172–177 10.1038/nature01282 [DOI] [PubMed] [Google Scholar]
- Huang C., Ogawa R. (2010). Mechanotransduction in bone repair and regeneration. FASEB J. 24, 3625–3632 10.1096/fj.10-157370 [DOI] [PubMed] [Google Scholar]
- Huang S., Ingber D. E. (2002). A discrete cell cycle checkpoint in late G(1) that is cytoskeleton-dependent and MAP kinase (Erk)-independent. Exp. Cell Res. 275, 255–264 10.1006/excr.2002.5504 [DOI] [PubMed] [Google Scholar]
- Huang Y., Jia X., Bai K., Gong X., Fan Y. (2010). Effect of fluid shear stress on cardiomyogenic differentiation of rat bone marrow mesenchymal stem cells. Arch. Med. Res. 41, 497–505 10.1016/j.arcmed.2010.10.002 [DOI] [PubMed] [Google Scholar]
- Hufnagel L., Teleman A. A., Rouault H., Cohen S. M., Shraiman B. I. (2007). On the mechanism of wing size determination in fly development. Proc. Natl. Acad. Sci. USA 104, 3835–3840 10.1073/pnas.0607134104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilizarov G. A. (1989a). The tension-stress effect on the genesis and growth of tissues: Part II. The influence of the rate and frequency of distraction. Clin. Orthop. Relat. Res. (239), , 263–285 [PubMed] [Google Scholar]
- Ilizarov G. A. (1989b). The tension-stress effect on the genesis and growth of tissues. Part I. The influence of stability of fixation and soft-tissue preservation. Clin. Orthop. Relat. Res. (238), , 249–281 [PubMed] [Google Scholar]
- Inanlou M. R., Baguma–Nibasheka M., Kablar B. (2005). The role of fetal breathing-like movements in lung organogenesis. Histol. Histopathol. 20, 1261–1266 [DOI] [PubMed] [Google Scholar]
- Ingber D. E. (1993). Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J. Cell Sci. 104, 613–627 [DOI] [PubMed] [Google Scholar]
- Ingber D. E. (2003). Mechanosensation through integrins: cells act locally but think globally. Proc. Natl. Acad. Sci. USA 100, 1472–1474 10.1073/pnas.0530201100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D. E. (2006). Cellular mechanotransduction: putting all the pieces together again. FASEB J. 20, 811–827 10.1096/fj.05-5424rev [DOI] [PubMed] [Google Scholar]
- Ishida T., Peterson T. E., Kovach N. L., Berk B. C. (1996). MAP kinase activation by flow in endothelial cells. Role of beta 1 integrins and tyrosine kinases. Circ. Res. 79, 310–316 [DOI] [PubMed] [Google Scholar]
- Itano N., Okamoto S., Zhang D., Lipton S. A., Ruoslahti E. (2003). Cell spreading controls endoplasmic and nuclear calcium: a physical gene regulation pathway from the cell surface to the nucleus. Proc. Natl. Acad. Sci. USA 100, 5181–5186 10.1073/pnas.0531397100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon Y. M., Kook S. H., Son Y. O., Kim E. M., Park S. S., Kim J. G., Lee J. C. (2009). Role of MAPK in mechanical force-induced up-regulation of type I collagen and osteopontin in human gingival fibroblasts. Mol. Cell. Biochem. 320, 45–52 10.1007/s11010-008-9897-z [DOI] [PubMed] [Google Scholar]
- Kahn J., Shwartz Y., Blitz E., Krief S., Sharir A., Breitel D. A., Rattenbach R., Relaix F., Maire P., Rountree R. B.et al. (2009). Muscle contraction is necessary to maintain joint progenitor cell fate. Dev. Cell 16, 734–743 10.1016/j.devcel.2009.04.013 [DOI] [PubMed] [Google Scholar]
- Kanno T., Takahashi T., Tsujisawa T., Ariyoshi W., Nishihara T. (2007). Mechanical stress-mediated Runx2 activation is dependent on Ras/ERK1/2 MAPK signaling in osteoblasts. J. Cell. Biochem. 101, 1266–1277 10.1002/jcb.21249 [DOI] [PubMed] [Google Scholar]
- Khachigian L. M., Resnick N., Gimbrone M. A., Jr, Collins T. (1995). Nuclear factor-kappa B interacts functionally with the platelet-derived growth factor B-chain shear-stress response element in vascular endothelial cells exposed to fluid shear stress. J. Clin. Invest. 96, 1169–1175 10.1172/JCI118106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatau S. B., Kim D. H., Hale C. M., Bloom R. J., Wirtz D. (2010). The perinuclear actin cap in health and disease. Nucleus 1, 337–342 10.4161/nucl.1.4.12331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara T., Haghparast S. M., Shimizu Y., Yuba S., Miyake J. (2011). Physical properties of mesenchymal stem cells are coordinated by the perinuclear actin cap. Biochem. Biophys. Res. Commun. 409, 1–6 10.1016/j.bbrc.2011.04.022 [DOI] [PubMed] [Google Scholar]
- Kim J. B., Sebastiano V., Wu G., Araúzo–Bravo M. J., Sasse P., Gentile L., Ko K., Ruau D., Ehrich M., van den Boom D.et al. (2009). Oct4-induced pluripotency in adult neural stem cells. Cell 136, 411–419 10.1016/j.cell.2009.01.023 [DOI] [PubMed] [Google Scholar]
- Kim K., Doi A., Wen B., Ng K., Zhao R., Cahan P., Kim J., Aryee M. J., Ji H., Ehrlich L. I.et al. (2010). Epigenetic memory in induced pluripotent stem cells. Nature 467, 285–290 10.1038/nature09342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishikawa H., Miura S., Yoshida H., Hirokawa M., Nakamizo H., Higuchi H., Adachi M., Nakatsumi R. C., Suzuki H., Saito H.et al. (2002). Transmural pressure induces IL-6 secretion by intestinal epithelial cells. Clin. Exp. Immunol. 129, 86–91 10.1046/j.1365-2249.2002.01895.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein E. A., Yung Y., Castagnino P., Kothapalli D., Assoian R. K. (2007). Cell adhesion, cellular tension, and cell cycle control. Methods Enzymol. 426, 155–175 10.1016/S0076-6879(07)26008-2 [DOI] [PubMed] [Google Scholar]
- K�lsch V., Seher T., Fernandez–Ballester G J., Serrano L., Leptin M. (2007). Control of Drosophila gastrulation by apical localization of adherens junctions and RhoGEF2. Science 315, 384–386 [DOI] [PubMed] [Google Scholar]
- Komuro I., Katoh Y., Kaida T., Shibazaki Y., Kurabayashi M., Hoh E., Takaku F., Yazaki Y. (1991). Mechanical loading stimulates cell hypertrophy and specific gene expression in cultured rat cardiac myocytes. Possible role of protein kinase C activation. J. Biol. Chem. 266, 1265–1268 [PubMed] [Google Scholar]
- Kook S. H., Hwang J. M., Park J. S., Kim E. M., Heo J. S., Jeon Y. M., Lee J. C. (2009). Mechanical force induces type I collagen expression in human periodontal ligament fibroblasts through activation of ERK/JNK and AP-1. J. Cell. Biochem. 106, 1060–1067 10.1002/jcb.22085 [DOI] [PubMed] [Google Scholar]
- Kumar A., Lnu S., Malya R., Barron D., Moore J., Corry D. B., Boriek A. M. (2003). Mechanical stretch activates nuclear factor-kappaB, activator protein-1, and mitogen-activated protein kinases in lung parenchyma: implications in asthma. FASEB J. 17, 1800–1811 10.1096/fj.02-1148com [DOI] [PubMed] [Google Scholar]
- Landsberg K. P., Farhadifar R., Ranft J., Umetsu D., Widmann T. J., Bittig T., Said A., Jülicher F., Dahmann C. (2009). Increased cell bond tension governs cell sorting at the Drosophila anteroposterior compartment boundary. Curr. Biol. 19, 1950–1955 10.1016/j.cub.2009.10.021 [DOI] [PubMed] [Google Scholar]
- Lanyon L. E. (1992). Control of bone architecture by functional load bearing. J. Bone Miner. Res. 7 Suppl. 2, S369–S375 10.1002/jbmr.5650071403 [DOI] [PubMed] [Google Scholar]
- Lee J. S., Yu Q., Shin J. T., Sebzda E., Bertozzi C., Chen M., Mericko P., Stadtfeld M., Zhou D., Cheng L.et al. (2006). Klf2 is an essential regulator of vascular hemodynamic forces in vivo. Dev. Cell 11, 845–857 10.1016/j.devcel.2006.09.006 [DOI] [PubMed] [Google Scholar]
- Lele T. P., Pendse J., Kumar S., Salanga M., Karavitis J., Ingber D. E. (2006). Mechanical forces alter zyxin unbinding kinetics within focal adhesions of living cells. J. Cell. Physiol. 207, 187–194 10.1002/jcp.20550 [DOI] [PubMed] [Google Scholar]
- Li D., Zhou J., Wang L., Shin M. E., Su P., Lei X., Kuang H., Guo W., Yang H., Cheng L.et al. (2010). Integrated biochemical and mechanical signals regulate multifaceted human embryonic stem cell functions. J. Cell Biol. 191, 631–644 10.1083/jcb.201006094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Chen G., Zheng L., Luo S., Zhao Z. (2007). Osteoblast cytoskeletal modulation in response to compressive stress at physiological levels. Mol. Cell. Biochem. 304, 45–52 10.1007/s11010-007-9484-8 [DOI] [PubMed] [Google Scholar]
- Lichtinger M., Hoogenkamp M., Krysinska H., Ingram R., Bonifer C. (2010). Chromatin regulation by RUNX1. Blood Cells Mol. Dis. 44, 287–290 10.1016/j.bcmd.2010.02.009 [DOI] [PubMed] [Google Scholar]
- Londhe V. A., Nguyen H. T., Jeng J. M., Li X., Li C., Tiozzo C., Zhu N., Minoo P. (2008). NF-kB induces lung maturation during mouse lung morphogenesis. Dev. Dyn. 237, 328–338 10.1002/dvdy.21413 [DOI] [PubMed] [Google Scholar]
- Mack C. P., Owens G. K. (1999). Regulation of smooth muscle alpha-actin expression in vivo is dependent on CArG elements within the 5′ and first intron promoter regions. Circ. Res. 84, 852–861 [DOI] [PubMed] [Google Scholar]
- Mammoto A., Ingber D. E. (2009). Cytoskeletal control of growth and cell fate switching. Curr. Opin. Cell Biol. 21, 864–870 10.1016/j.ceb.2009.08.001 [DOI] [PubMed] [Google Scholar]
- Mammoto A., Huang S., Moore K., Oh P., Ingber D. E. (2004). Role of RhoA, mDia, and ROCK in cell shape-dependent control of the Skp2-p27kip1 pathway and the G1/S transition. J. Biol. Chem. 279, 26323–26330 10.1074/jbc.M402725200 [DOI] [PubMed] [Google Scholar]
- Mammoto A., Huang S., Ingber D. E. (2007). Filamin links cell shape and cytoskeletal structure to Rho regulation by controlling accumulation of p190RhoGAP in lipid rafts. J. Cell Sci. 120, 456–467 10.1242/jcs.03353 [DOI] [PubMed] [Google Scholar]
- Mammoto A., Mammoto T., Ingber D. E. (2008). Rho signaling and mechanical control of vascular development. Curr. Opin. Hematol. 15, 228–234 10.1097/MOH.0b013e3282fa7445 [DOI] [PubMed] [Google Scholar]
- Mammoto A., Connor K. M., Mammoto T., Yung C. W., Huh D., Aderman C. M., Mostoslavsky G., Smith L. E., Ingber D. E. (2009). A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature 457, 1103–1108 10.1038/nature07765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto T., Ingber D. E. (2010). Mechanical control of tissue and organ development. Development 137, 1407–1420 10.1242/dev.024166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammoto T., Mammoto A., Torisawa Y. S., Tat T., Gibbs A., Derda R., Mannix R., de Bruijn M., Yung C. W., Huh D.et al. (2011). Mechanochemical control of mesenchymal condensation and embryonic tooth organ formation. Dev. Cell 21, 758–769 10.1016/j.devcel.2011.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniotis A. J., Bojanowski K., Ingber D. E. (1997a). Mechanical continuity and reversible chromosome disassembly within intact genomes removed from living cells. J. Cell. Biochem. 65, 114–130 [DOI] [PubMed] [Google Scholar]
- Maniotis A. J., Chen C. S., Ingber D. E. (1997b). Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl. Acad. Sci. USA 94, 849–854 10.1073/pnas.94.3.849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. C., Kaschube M., Wieschaus E. F. (2009). Pulsed contractions of an actin-myosin network drive apical constriction. Nature 457, 495–499 10.1038/nature07522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T., Cardarelli P. M., Parry G. C., Felts K. A., Cobb R. R. (1997). Cytokine induction of monocyte chemoattractant protein-1 gene expression in human endothelial cells depends on the cooperative action of NF-kappa B and AP-1. Eur. J. Immunol. 27, 1091–1097 10.1002/eji.1830270508 [DOI] [PubMed] [Google Scholar]
- Martinac B. (2004). Mechanosensitive ion channels: molecules of mechanotransduction. J. Cell Sci. 117, 2449–2460 10.1242/jcs.01232 [DOI] [PubMed] [Google Scholar]
- Marttila M., Hautala N., Paradis P., Toth M., Vuolteenaho O., Nemer M., Ruskoaho H. (2001). GATA4 mediates activation of the B-type natriuretic peptide gene expression in response to hemodynamic stress. Endocrinology 142, 4693–4700 10.1210/en.142.11.4693 [DOI] [PubMed] [Google Scholar]
- Matthews B. D., Thodeti C. K., Tytell J. D., Mammoto A., Overby D. R., Ingber D. E. (2010). Ultra-rapid activation of TRPV4 ion channels by mechanical forces applied to cell surface beta1 integrins. Integr. Biol. (Camb) 2, 435–442 10.1039/c0ib00034e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder A., Shivashankar G. V. (2007). Gold-nanoparticle-assisted laser perturbation of chromatin assembly reveals unusual aspects of nuclear architecture within living cells. Biophys. J. 93, 2209–2216 10.1529/biophysj.106.102202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder A., Shivashankar G. V. (2010). Emergence of a prestressed eukaryotic nucleus during cellular differentiation and development. J. R. Soc. Interface 7 Suppl. 3, S321–S330 10.1098/rsif.2010.0039.focus [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder A., Roopa T., Basu A., Mahadevan L., Shivashankar G. V. (2008). Dynamics of chromatin decondensation reveals the structural integrity of a mechanically prestressed nucleus. Biophys. J. 95, 3028–3035 10.1529/biophysj.108.132274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBeath R., Pirone D. M., Nelson C. M., Bhadriraju K., Chen C. S. (2004). Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483–495 10.1016/S1534-5807(04)00075-9 [DOI] [PubMed] [Google Scholar]
- McCain M. L., Parker K. K. (2011). Mechanotransduction: the role of mechanical stress, myocyte shape, and cytoskeletal architecture on cardiac function. Pflugers Arch. 462, 89–104 10.1007/s00424-011-0951-4 [DOI] [PubMed] [Google Scholar]
- McGee K. M., Vartiainen M. K., Khaw P. T., Treisman R., Bailly M. (2011). Nuclear transport of the serum response factor coactivator MRTF-A is downregulated at tensional homeostasis. EMBO Rep. 12, 963–970 10.1038/embor.2011.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvinsky A., Dzierzak E. (1996). Definitive hematopoiesis is autonomously initiated by the AGM region. Cell 86, 897–906 10.1016/S0092-8674(00)80165-8 [DOI] [PubMed] [Google Scholar]
- Meiler S. E., Hung R. R., Gerszten R. E., Gianetti J., Li L., Matsui T., Gimbrone M. A., Jr, Rosenzweig A. (2002). Endothelial IKK beta signaling is required for monocyte adhesion under laminar flow conditions. J. Mol. Cell. Cardiol. 34, 349–359 10.1006/jmcc.2001.1519 [DOI] [PubMed] [Google Scholar]
- Méjat A., Misteli T. (2010). LINC complexes in health and disease. Nucleus 1, 40–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson C. R. (2000). Role of transcription factors in fetal lung development and surfactant protein gene expression. Annu. Rev. Physiol. 62, 875–915 10.1146/annurev.physiol.62.1.875 [DOI] [PubMed] [Google Scholar]
- Minoo P. (2000). Transcriptional regulation of lung development: emergence of specificity. Respir. Res. 1, 109–115 10.1186/rr20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K., Pasque V., Jullien J., Gurdon J. B. (2011). Nuclear actin polymerization is required for transcriptional reprogramming of Oct4 by oocytes. Genes Dev. 25, 946–958 10.1101/gad.615211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier B., Pelissier–Monier A., Brand A. H., Sanson B. (2010). An actomyosin-based barrier inhibits cell mixing at compartmental boundaries in Drosophila embryos. Nat. Cell Biol. 12, 60–65; Suppl. 1–9 10.1038/ncb2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. A., Huang S., Kong Y., Sunday M. E., Ingber D. E. (2002). Control of embryonic lung branching morphogenesis by the Rho activator, cytotoxic necrotizing factor 1. J. Surg. Res. 104, 95–100 10.1006/jsre.2002.6418 [DOI] [PubMed] [Google Scholar]
- Moore K. A., Polte T., Huang S., Shi B., Alsberg E., Sunday M. E., Ingber D. E. (2005). Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Dev. Dyn. 232, 268–281 10.1002/dvdy.20237 [DOI] [PubMed] [Google Scholar]
- Morawietz H., Ma Y. H., Vives F., Wilson E., Sukhatme V. P., Holtz J., Ives H. E. (1999). Rapid induction and translocation of Egr-1 in response to mechanical strain in vascular smooth muscle cells. Circ. Res. 84, 678–687 [DOI] [PubMed] [Google Scholar]
- Morita T., Mayanagi T., Sobue K. (2007). Reorganization of the actin cytoskeleton via transcriptional regulation of cytoskeletal/focal adhesion genes by myocardin-related transcription factors (MRTFs/MAL/MKLs). Exp. Cell Res. 313, 3432–3445 10.1016/j.yexcr.2007.07.008 [DOI] [PubMed] [Google Scholar]
- Moskowitz I. P., Wang J., Peterson M. A., Pu W. T., Mackinnon A. C., Oxburgh L., Chu G. C., Sarkar M., Berul C., Smoot L.et al. (2011). Transcription factor genes Smad4 and Gata4 cooperatively regulate cardiac valve development. [corrected]. Proc. Natl. Acad. Sci. USA 108, 4006–4011 10.1073/pnas.1019025108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraoka R. S., Bushdid P. B., Brantley D. M., Yull F. E., Kerr L. D. (2000). Mesenchymal expression of nuclear factor-kappaB inhibits epithelial growth and branching in the embryonic chick lung. Dev. Biol. 225, 322–338 10.1006/dbio.2000.9824 [DOI] [PubMed] [Google Scholar]
- Nelson C. M., Jean R. P., Tan J. L., Liu W. F., Sniadecki N. J., Spector A. A., Chen C. S. (2005). Emergent patterns of growth controlled by multicellular form and mechanics. Proc. Natl. Acad. Sci. USA 102, 11594–11599 10.1073/pnas.0502575102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning Q. M., Wang X. R. (2007). Activations of mitogen-activated protein kinase and nuclear factor-kappaB by mechanical stretch result in ventilation-induced lung injury. Med. Hypotheses 68, 356–360 10.1016/j.mehy.2006.06.053 [DOI] [PubMed] [Google Scholar]
- North T. E., Goessling W., Peeters M., Li P., Ceol C., Lord A. M., Weber G. J., Harris J., Cutting C. C., Huang P.et al. (2009). Hematopoietic stem cell development is dependent on blood flow. Cell 137, 736–748 10.1016/j.cell.2009.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H., Irvine K. D. (2008). In vivo regulation of Yorkie phosphorylation and localization. Development 135, 1081–1088 10.1242/dev.015255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H., Irvine K. D. (2009). In vivo analysis of Yorkie phosphorylation sites. Oncogene 28, 1916–1927 10.1038/onc.2009.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr A. W., Sanders J. M., Bevard M., Coleman E., Sarembock I. J., Schwartz M. A. (2005). The subendothelial extracellular matrix modulates NF-kappaB activation by flow: a potential role in atherosclerosis. J. Cell Biol. 169, 191–202 10.1083/jcb.200410073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr A. W., Helmke B. P., Blackman B. R., Schwartz M. A. (2006). Mechanisms of mechanotransduction. Dev. Cell 10, 11–20 10.1016/j.devcel.2005.12.006 [DOI] [PubMed] [Google Scholar]
- Ozawa N., Shichiri M., Iwashina M., Fukai N., Yoshimoto T., Hirata Y. (2004). Laminar shear stress up-regulates inducible nitric oxide synthase in the endothelium. Hypertens. Res. 27, 93–99 10.1291/hypres.27.93 [DOI] [PubMed] [Google Scholar]
- Pajerowski J. D., Dahl K. N., Zhong F. L., Sammak P. J., Discher D. E. (2007). Physical plasticity of the nucleus in stem cell differentiation. Proc. Natl. Acad. Sci. USA 104, 15619–15624 10.1073/pnas.0702576104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papachristou D. J., Papachroni K. K., Basdra E. K., Papavassiliou A. G. (2009). Signaling networks and transcription factors regulating mechanotransduction in bone. Bioessays 31, 794–804 10.1002/bies.200800223 [DOI] [PubMed] [Google Scholar]
- Park J., Puapong D. P., Wu B. M., Atkinson J. B., Dunn J. C. (2004). Enterogenesis by mechanical lengthening: morphology and function of the lengthened small intestine. J. Pediatr. Surg. 39, 1823–1827 10.1016/j.jpedsurg.2004.08.022 [DOI] [PubMed] [Google Scholar]
- Parsons S. A., Millay D. P., Wilkins B. J., Bueno O. F., Tsika G. L., Neilson J. R., Liberatore C. M., Yutzey K. E., Crabtree G. R., Tsika R. W.et al. (2004). Genetic loss of calcineurin blocks mechanical overload-induced skeletal muscle fiber type switching but not hypertrophy. J. Biol. Chem. 279, 26192–26200 10.1074/jbc.M313800200 [DOI] [PubMed] [Google Scholar]
- Pasque V., Miyamoto K., Gurdon J. B. (2010). Efficiencies and mechanisms of nuclear reprogramming. Cold Spring Harb. Symp. Quant. Biol. 75, 189–200 10.1101/sqb.2010.75.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peerani R., Rao B. M., Bauwens C., Yin T., Wood G. A., Nagy A., Kumacheva E., Zandstra P. W. (2007). Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J. 26, 4744–4755 10.1038/sj.emboj.7601896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polte T. R., Eichler G. S., Wang N., Ingber D. E. (2004). Extracellular matrix controls myosin light chain phosphorylation and cell contractility through modulation of cell shape and cytoskeletal prestress. Am. J. Physiol. Cell Physiol. 286, C518–C528 10.1152/ajpcell.00280.2003 [DOI] [PubMed] [Google Scholar]
- Pouille P. A., Ahmadi P., Brunet A. C., Farge E. (2009). Mechanical signals trigger Myosin II redistribution and mesoderm invagination in Drosophila embryos. Sci. Signal. 2, ra16 10.1126/scisignal.2000098 [DOI] [PubMed] [Google Scholar]
- Prat A. G., Cantiello H. F. (1996). Nuclear ion channel activity is regulated by actin filaments. Am. J. Physiol. 270, C1532–C1543 [DOI] [PubMed] [Google Scholar]
- Raab–Cullen D. M., Thiede M. A., Petersen D. N., Kimmel D. B., Recker R. R. (1994). Mechanical loading stimulates rapid changes in periosteal gene expression. Calcif. Tissue Int. 55, 473–478 10.1007/BF00298562 [DOI] [PubMed] [Google Scholar]
- Rauch C., Brunet A. C., Deleule J., Farge E. (2002). C2C12 myoblast/osteoblast transdifferentiation steps enhanced by epigenetic inhibition of BMP2 endocytosis. Am. J. Physiol. Cell Physiol. 283, C235–C243 [DOI] [PubMed] [Google Scholar]
- Ray A., Shakya A., Ray B. K. (2005). Inflammation-responsive transcription factors SAF-1 and c-Jun/c-Fos promote canine MMP-1 gene expression. Biochim. Biophys. Acta 1732, 53–61 [DOI] [PubMed] [Google Scholar]
- Ren F., Zhang L., Jiang J. (2010). Hippo signaling regulates Yorkie nuclear localization and activity through 14-3-3 dependent and independent mechanisms. Dev. Biol. 337, 303–312 10.1016/j.ydbio.2009.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y., Effler J. C., Norstrom M., Luo T., Firtel R. A., Iglesias P. A., Rock R. S., Robinson D. N. (2009). Mechanosensing through cooperative interactions between myosin II and the actin crosslinker cortexillin I. Curr. Biol. 19, 1421–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick N., Collins T., Atkinson W., Bonthron D. T., Dewey C. F., Jr, Gimbrone M. A., Jr (1993). Platelet-derived growth factor B chain promoter contains a cis-acting fluid shear-stress-responsive element. Proc. Natl. Acad. Sci. USA 90, 4591–4595 10.1073/pnas.90.10.4591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riveline D., Zamir E., Balaban N. Q., Schwarz U. S., Ishizaki T., Narumiya S., Kam Z., Geiger B., Bershadsky A. D. (2001). Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Biol. 153, 1175–1186 10.1083/jcb.153.6.1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robling A. G., Castillo A. B., Turner C. H. (2006). Biomechanical and molecular regulation of bone remodeling. Annu. Rev. Biomed. Eng. 8, 455–498 10.1146/annurev.bioeng.8.061505.095721 [DOI] [PubMed] [Google Scholar]
- Rossant J., Tam P. P. (2009). Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development 136, 701–713 10.1242/dev.017178 [DOI] [PubMed] [Google Scholar]
- Rubin C. T., Lanyon L. E. (1985). Regulation of bone mass by mechanical strain magnitude. Calcif. Tissue Int. 37, 411–417 10.1007/BF02553711 [DOI] [PubMed] [Google Scholar]
- Ruiz S. A., Chen C. S. (2008). Emergence of patterned stem cell differentiation within multicellular structures. Stem Cells 26, 2921–2927 10.1634/stemcells.2008-0432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoshima J., Izumo S. (1993). Mechanotransduction in stretch-induced hypertrophy of cardiac myocytes. J. Recept. Res. 13, 777–794 [DOI] [PubMed] [Google Scholar]
- Sandri M. (2008). Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 23, 160–170 10.1152/physiol.00041.2007 [DOI] [PubMed] [Google Scholar]
- Sansores–Garcia L., Bossuyt W., Wada K., Yonemura S., Tao C., Sasaki H., Halder G. (2011). Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 30, 2325–2335 10.1038/emboj.2011.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwank G., Basler K. (2010). Regulation of organ growth by morphogen gradients. Cold Spring Harb. Perspect. Biol. 2, a001669 10.1101/cshperspect.a001669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seher T. C., Narasimha M., Vogelsang E., Leptin M. (2007). Analysis and reconstitution of the genetic cascade controlling early mesoderm morphogenesis in the Drosophila embryo. Mech. Dev. 124, 167–179 [DOI] [PubMed] [Google Scholar]
- Shiu Y. T., Weiss J. A., Hoying J. B., Iwamoto M. N., Joung I. S., Quam C. T. (2005). The role of mechanical stresses in angiogenesis. Crit. Rev. Biomed. Eng. 33, 431–510 10.1615/CritRevBiomedEng.v33.i5.10 [DOI] [PubMed] [Google Scholar]
- Shraiman B. I. (2005). Mechanical feedback as a possible regulator of tissue growth. Proc. Natl. Acad. Sci. USA 102, 3318–3323 10.1073/pnas.0404782102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims J. R., Karp S., Ingber D. E. (1992). Altering the cellular mechanical force balance results in integrated changes in cell, cytoskeletal and nuclear shape. J. Cell Sci. 103, 1215–1222 [DOI] [PubMed] [Google Scholar]
- Small S., Blair A., Levine M. (1992). Regulation of even-skipped stripe 2 in the Drosophila embryo. EMBO J. 11, 4047–4057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiropoulos A., Gineitis D., Copeland J., Treisman R. (1999). Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell 98, 159–169 10.1016/S0092-8674(00)81011-9 [DOI] [PubMed] [Google Scholar]
- Stadtfeld M., Maherali N., Breault D. T., Hochedlinger K. (2008). Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell 2, 230–240 10.1016/j.stem.2008.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thodeti C. K., Matthews B., Ravi A., Mammoto A., Ghosh K., Bracha A. L., Ingber D. E. (2009). TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ. Res. 104, 1123–1130 10.1161/CIRCRESAHA.108.192930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidball J. G. (2005). Mechanical signal transduction in skeletal muscle growth and adaptation. J. Appl. Physiol. 98, 1900–1908 10.1152/japplphysiol.01178.2004 [DOI] [PubMed] [Google Scholar]
- Tseng C. P., Kim Y. J., Kumar R., Verma A. K. (1994). Involvement of protein kinase C in the transcriptional regulation of 12-O-tetradecanoylphorbol-13-acetate-inducible genes modulated by AP-1 or non-AP-1 transacting factors. Carcinogenesis 15, 707–711 10.1093/carcin/15.4.707 [DOI] [PubMed] [Google Scholar]
- Tzima E., Irani–Tehrani M., Kiosses W. B., Dejana E., Schultz D. A., Engelhardt B., Cao G., DeLisser H., Schwartz M. A. (2005). A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437, 426–431 10.1038/nature03952 [DOI] [PubMed] [Google Scholar]
- Urbich C., Stein M., Reisinger K., Kaufmann R., Dimmeler S., Gille J. (2003). Fluid shear stress-induced transcriptional activation of the vascular endothelial growth factor receptor-2 gene requires Sp1-dependent DNA binding. FEBS Lett. 535, 87–93 10.1016/S0014-5793(02)03879-6 [DOI] [PubMed] [Google Scholar]
- Vermot J., Forouhar A. S., Liebling M., Wu D., Plummer D., Gharib M., Fraser S. E. (2009). Reversing blood flows act through klf2a to ensure normal valvulogenesis in the developing heart. PLoS Biol. 7, e1000246 10.1371/journal.pbio.1000246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilos G. A., Liggins G. C. (1982). Intrathoracic pressures in fetal sheep. J. Dev. Physiol. 4, 247–256 [PubMed] [Google Scholar]
- Wang N., Butler J. P., Ingber D. E. (1993). Mechanotransduction across the cell surface and through the cytoskeleton. Science 260, 1124–1127 10.1126/science.7684161 [DOI] [PubMed] [Google Scholar]
- Wang N., Tytell J. D., Ingber D. E. (2009). Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 10, 75–82 10.1038/nrm2594 [DOI] [PubMed] [Google Scholar]
- Wang Y., Gilmore T. D. (2003). Zyxin and paxillin proteins: focal adhesion plaque LIM domain proteins go nuclear. Biochim. Biophys. Acta 1593, 115–120 10.1016/S0167-4889(02)00349-X [DOI] [PubMed] [Google Scholar]
- Wang Y., Riechmann V. (2007). The role of the actomyosin cytoskeleton in coordination of tissue growth during Drosophila oogenesis. Curr. Biol. 17, 1349–1355 10.1016/j.cub.2007.06.067 [DOI] [PubMed] [Google Scholar]
- Wang Y., Maciejewski B. S., Lee N., Silbert O., McKnight N. L., Frangos J. A., Sanchez–Esteban J. (2006). Strain-induced fetal type II epithelial cell differentiation is mediated via cAMP-PKA-dependent signaling pathway. Am. J. Physiol. Lung Cell. Mol. Physiol. 291, L820–L827 10.1152/ajplung.00068.2006 [DOI] [PubMed] [Google Scholar]
- Wheeler D. G., Barrett C. F., Groth R. D., Safa P., Tsien R. W. (2008). CaMKII locally encodes L-type channel activity to signal to nuclear CREB in excitation-transcription coupling. J. Cell Biol. 183, 849–863 10.1083/jcb.200805048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigglesworth J. S., Desai R., Hislop A. A. (1987). Fetal lung growth in congenital laryngeal atresia. Pediatr. Pathol. 7, 515–525 10.3109/15513818709161415 [DOI] [PubMed] [Google Scholar]
- Wirrig E. E., Yutzey K. E. (2011). Transcriptional regulation of heart valve development and disease. Cardiovasc. Pathol. 20, 162–167 10.1016/j.carpath.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert L. (1969). Positional information and the spatial pattern of cellular differentiation. J. Theor. Biol. 25, 1–47 10.1016/S0022-5193(69)80016-0 [DOI] [PubMed] [Google Scholar]
- Worman H. J., Fong L. G., Muchir A., Young S. G. (2009). Laminopathies and the long strange trip from basic cell biology to therapy. J. Clin. Invest. 119, 1825–1836 10.1172/JCI37679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak M. A., Chen C. S. (2009). Mechanotransduction in development: a growing role for contractility. Nat. Rev. Mol. Cell Biol. 10, 34–43 10.1038/nrm2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R., Nelson C. M., Muschler J. L., Veiseh M., Vonderhaar B. K., Bissell M. J. (2009). Sustained activation of STAT5 is essential for chromatin remodeling and maintenance of mammary-specific function. J. Cell Biol. 184, 57–66 10.1083/jcb.200807021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Im H. J., Wang J. H. (2005). Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene 363, 166–172 10.1016/j.gene.2005.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Beqaj S., Kemp P., Ariel I., Schuger L. (2000). Stretch-induced alternative splicing of serum response factor promotes bronchial myogenesis and is defective in lung hypoplasia. J. Clin. Invest. 106, 1321–1330 10.1172/JCI8893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Li L., Lei Q., Guan K. L. (2010). The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 24, 862–874 10.1101/gad.1909210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X. H., Laschinger C., Arora P., Szászi K., Kapus A., McCulloch C. A. (2007). Force activates smooth muscle alpha-actin promoter activity through the Rho signaling pathway. J. Cell Sci. 120, 1801–1809 10.1242/jcs.001586 [DOI] [PubMed] [Google Scholar]
- Ziros P. G., Basdra E. K., Papavassiliou A. G. (2008). Runx2: of bone and stretch. Int. J. Biochem. Cell Biol. 40, 1659–1663 10.1016/j.biocel.2007.05.024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.