Abstract
Objective
To compare population-based metrics for assessing progress toward the U.S. National HIV/AIDS Strategy (NHAS) goals.
Design
Analysis of surveillance data from persons living with HIV/AIDS (PLWHA) in King County (KC), WA, 2005–2009.
Methods
We examined indicators of the timing of HIV diagnosis [intertest interval, CD4 count at diagnosis, and AIDS ≤1 year of diagnosis (late diagnosis)]; linkage to initial care [CD4 or viral load (VL) report ≤3 months after diagnosis] and sustained care [a laboratory report 3–9 months after linkage]; engagement in continuous care in 2009 [≥2 laboratory reports ≥3 months apart]; and virologic suppression.
Results
Thirty-two percent of persons had late HIV diagnoses, 31% of whom reported testing HIV negative in the 2 years preceding their HIV diagnosis. Linkage to sustained care, but not linkage to initial care, was significantly associated with subsequent virologic suppression. Among 6070 PLWHA in KC, 65% of those with ≥1 VL reported in 2009 and 53% of all PLWHA had virologic suppression. Although only 66% of all PLWHA were engaged in continuous care, 81% were defined as engaged using the denominator proposed in the NHAS (≥1 laboratory result reported in 2009 excluding persons establishing care in the second half of the year).
Conclusions
Proposed metrics for monitoring the HIV care continuum may not accurately measure late diagnoses or linkage to sustained care and are sensitive to assumptions about the size of the population of PLWHA. Monitoring progress toward achievement of NHAS goals will require improvements in HIV surveillance data and refinement of care metrics.
Keywords: Continuity of Patient Care, HIV Infections/diagnosis/drug therapy/prevention & control, HIV Infections/diagnosis/epidemiology, Health Services/utilization, Population Surveillance/methods
INTRODUCTION
The U.S. National HIV/AIDS Strategy (NHAS) proposes a population-based approach to HIV prevention that centers on efforts to ensure that persons living with HIV/AIDS (PLWHA) are diagnosed soon after acquiring HIV, successfully link to sustained medical care, and begin effective antiretroviral therapy (ART) in accordance with national guidelines [1]. Several metrics have been used to evaluate late HIV diagnoses, engagement in care, and virologic suppression on the population level [2–7], and the NHAS outlines specific targets in each of these areas to achieve by 2015 [8]. Laboratory-based reporting of CD4 and HIV RNA [viral load (VL)] results to health departments make population-based monitoring of virologic suppression and engagement in care feasible. However, variation in state reporting requirements currently prohibits a nationwide assessment using these data [9], and to date, we are not aware of published state or local reports providing a comprehensive view of the data that will be used to measure the outcomes envisioned in the NHAS. We used surveillance data from King County, Washington to evaluate metrics of HIV testing, linkage to care, engagement in care, and virologic suppression. Here we present data on the state of the HIV care continuum in one U.S. urban area and evaluate metrics designed to assess the care continuum. We sought to assess how varying assumptions affect estimates of the population's receipt of care in order to identify gaps in existing knowledge and deficiencies in the HIV surveillance system that might be addressed as the U.S. develops a population-based approach to monitoring HIV prevention and care.
METHODS
Data Sources
We examined data from two sources: Public Health – Seattle & King County's (PHSKC) enhanced HIV/AIDS reporting system (eHARS) [10] and, separately, the electronic database of the PHSKC One-on-One program. We limited analysis to events that occurred prior to January 2010 and were reported through December 2010. In September 2006, WA State implemented name-based HIV case reporting and requirements that laboratories report all CD4 and VL results to public health [11]. Prior to that time, WA State had name-based AIDS reporting and a name-to-code HIV case reporting system. Variables in eHARS are defined according to CDC criteria [3]. HIV testing history data, collected through case reports and the CDC Testing and Treatment History Questionnaire, are predominately self-reported. PHSKC staff attempt to provide partner services to all persons diagnosed with HIV in King County and attempt to ensure the completion of the questionnaire in that process.
PHSKC conducts efforts to improve the quality of King County HIV surveillance data. Deaths are ascertained through monthly evaluation of death records that include HIV on the death certificate and annual matching of data with the WA State Center for Health Statistics and the Social Security Death Index. Beginning in 2000, PHSKC staff attempted to collect initial CD4 count results on all persons newly diagnosed with HIV by calling reporting providers and through medical records review. Baseline CD4 data are complete for 95% of cases diagnosed in King County in 2000–2006, after which laboratory-based reporting of all CD4 results was mandatory. Since, 2007, PHSKC has conducted investigations of cases without CD4 or VL results reported in the preceding year in order to identify persons who are out of HIV care and to ascertain outmigration and incomplete data reporting [12].
Because the completeness and validity of HIV surveillance data is not well defined, we sought to separately evaluate measures of timely HIV diagnosis through another source, the PSHKC One-on-One program. Participation in One-on-One is open to all persons with newly diagnosed HIV in King County, and is routinely offered to all persons diagnosed with HIV through PHSKC-funded programs. Persons diagnosed elsewhere can be referred to One-on-One by medical providers or disease intervention specialists providing HIV partner services. The program involves meeting with a physician for counseling, referral for HIV medical care, CD4 count and VL testing and partner services. In 2009, 34% of persons diagnosed with HIV in King County were diagnosed at a PHSKC-funded testing site [13], 82% of whom participated in One-on-One.
Outcome Measures and Populations
The measures of HIV testing, linkage to care, engagement in continuous care, and virologic suppression that we examined are defined in Table 1, which distinguishes outcome measures included in the NHAS from those derived from other sources. We restricted analyses of the timing of HIV diagnosis and linkage to care to cases diagnosed in King County in 2005–2009, the most recent 5 years for which we had complete data. For analysis of linkage to and engagement in care we used the dates of CD4 or VL results as proxy measures for medical visits. We restricted the analysis of linkage to sustained care to HIV cases diagnosed in King County in 2007–09 because 2007 was the first complete year of mandatory CD4 and VL reporting in WA State.
Table 1.
Outcome measure definitions and data sources
| Metric | Definition | Reference | Data Sources | Numerator Population | Denominator Population |
|---|---|---|---|---|---|
| Timing of HIV diagnosis | |||||
| Intertest interval | Median number of months between the last negative and the first positive HIV test | Helms, et al [2] | Surveillance case reports, testing history questionnaires, One-on-One charts. | NA | All persons newly diagnosed with HIV in King County, 2005–2009 |
| CD4 count at diagnosis | Median CD4 count at the time of HIV diagnosis | CDC [3] | First CD4 count reported to surveillance within 6 months after HIV diagnosis, One-on-One charts. | NA | |
| Late HIV diagnosis | Proportion of persons diagnosed with AIDS within one year of HIV diagnosis | CDC [4] | Surveillance case report forms, CD4 count reports | Persons reported with an AIDS diagnosis or laboratory surveillance evidence of a CD4 count <200 or CD4 percentage <14% within 12 month of HIV diagnosis date | |
| AIDS and death within one year of HIV diagnosis | Proportion of persons who were diagnosed with AIDS and died in the first year after HIV diagnosis | Death reports, death ascertainment | Persons with AIDS diagnosis and date of death <12 months after HIV diagnosis date | ||
| Linkage to HIV care | |||||
| Linkage to initial care | Proportion of persons who completed a medical visit within 3 months after HIV diagnosis | NHAS [8] | Dates of CD4 and VL results reported to surveillance | Persons with a CD4 or VL result reported ≤ 3 months after HIV diagnosis date | |
| Linkage to sustained care | Proportion of persons who linked to initial care and completed a second visit 3–9 months after linkage | NA | Persons with a CD4 or VL result reported ≤ 3 months after HIV diagnosis date and second CD4 or VL result reported ≤ 9 months after the first | All persons newly diagnosed with HIV in King County, 2007–2008 | |
| Engagement in continuous HIV care | |||||
| NHAS engagement in care | Proportion of persons with ≥ 2 medical visits ≥ 3 months apart in a calendar year | NHAS [8] and the US Health Resources and Services Administration [5] | Dates of CD4 and VL results reported to surveillance | Persons with ≥ 2 CD4 or VL results ≥ 3 months apart in 2009 (excluding those whose first CD4 or VL was reported in the second half of 2009) | Persons with ≥ 1 laboratory result in 2009 (excluding those whose first CD4 or VL was reported in the second half of 2009) |
| Population-based engagement in care | NA | Persons with ≥ 2 CD4 or VL results ≥ 3 months apart in 2009 (excluding those whose first CD4 or VL was reported in the second half of 2009) | All PLWHA presumed to reside in King County as of December 31, 2009a (excluding those whose first CD4 or VL was reported in the second half of 2009) | ||
| Virologic parameters | |||||
| Population-level virologic suppression | Proportion of persons with virologic suppression (HIV RNA <48 copies/mL) | NHAS [8] Montaner, et al [6] | VL results reported to surveillance | Persons with undetectable VL at the time of last report | Persons with ≥ 1 VL result reported in 2009 |
| Mean community viral load | Mean viral load in the population | Das, et al [7] | NA |
Empty cells indicate that the data source, numerator definition or denominator definition is the same as the completed cell above
All persons known to have an HIV diagnosis in King County, regardless of their place of diagnosis, who were not known to have died or relocated
NA, not applicable; CDC, U.S. Centers for Disease Control and Prevention; NHAS, U.S. National HIV/AIDS Strategy; PLWHA, persons living with HIV/AIDS; VL, viral load (HIV RNA)
We examined indicators of engagement in continuous care and virologic suppression in the population of PLWHA presumed to reside in King County as of December 31, 2009. This included all persons known to have an HIV diagnosis in King County, regardless of their place of diagnosis, who were not known to have died or relocated. We defined virologic suppression as VL ≤48 copies/mL at last report in 2009, and we calculated mean community VL as the average of the last VL reported in 2009 among all persons with ≥1 VL reported in 2009 [7].
Data Analysis
We defined outcomes for each metric in Table 1, analyzing and reporting data from eHARS and the One-on-One program separately. Laboratory results obtained through the One-on-One Program are included in eHARS. We did not attempt to exclude those results from the analysis of CD4 count at diagnosis because we wanted to evaluate this measure for the entire county, and we were unable to exclude them from analysis of linkage to care because the ordering source for some laboratory data in eHARS was not available. For analysis of intertest intervals (ITI), we assumed that cases with a complete year but missing month of the last negative HIV test were tested in July, unless the test year was the same as the diagnosis year, in which case we assumed the last negative test was midway between January and the diagnosis month. We conducted a subset analysis of ITI limited to persons with a laboratory-confirmed date of last HIV negative test in the PHSKC STD Clinic records. We excluded initial CD4 counts that were reported >6 months after the HIV diagnosis date from analyses of CD4 count at diagnosis. Analyses of virologic suppression and CD4 stratification used the last VL and CD4 count reported in 2009. CD4 strata (≤200, 200–350, 351–500, and >500 cells/mm3) reflect thresholds used clinically in decisions regarding ART initiation [14]. For analyses comparing MSM vs. non-MSM, we classified injection drug using (IDU) MSM as MSM and excluded persons with unknown transmission risk factors.
We used linear regression to assess associations between independent variables and continuous outcomes and logistic regression to assess associations between independent variables and binary outcomes. When evaluating non-normally distributed continuous outcomes, we compared the results of non-parametric statistical tests (Kruskal-Wallis, Wilcoxan rank-sum and Spearman's correlation) with parametric tests (t-tests, one-way ANOVA, and linear regression); when results were similar, we report the results of parametric tests. We used Stata 10 for all analyses.
RESULTS
HIV diagnosis metrics
A total of 1590 persons were diagnosed with HIV/AIDS in King County and 368 persons completed the One-on-One program in 2005–2009. Table 2 summarizes results for indicators of the timing of HIV diagnosis countywide (using eHARS data) and in the One-on-One program (see Table 1 for metric definitions). Countywide, over the five year period, 15% of newly diagnosed persons had never previously tested for HIV, the median ITI among those who had previously tested was 13 months, and 32% of persons had an AIDS diagnosis within one year of HIV diagnosis (late HIV diagnosis). Compared to non-MSM, MSM were more likely to report having previously tested HIV negative [91% vs. 61%, OR 6.38 (95% CI: 4.33 – 9.41)], had higher CD4 counts at diagnosis (median 399 vs. 220 cells/mm3, p<0.001), lower odds of late HIV diagnosis [26% vs. 46%, OR 0.41 (95% CI: 0.33 – 0.51)], and lower odds of dying with AIDS within one year [1% vs. 4%, OR 0.31 (95% CI: 0.15 – 0.63)]. There were no statistically significant changes in the metrics of timely HIV diagnosis during 2005–2009 countywide or in the One-on-One Program (data not shown).
Table 2.
Metrics of the timing of HIV diagnosis and linkage to HIV care among persons diagnosed with HIV in King County, 2005–2009
| HIV Surveillance | One-on-One Program | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 2005 (n = 325) | 2006 (n = 314) | 2007 (n = 324) | 2008 (n = 316) | 2009 (n = 311) | Combined (n = 1590) | 2005–09 (n = 368) | |
| Completed HIV testing history, No. (% total) | 138 (42) | 196 (62) | 203 (63) | 200 (63) | 187 (60) | 925 (58) | 314 (85) |
| Never previously tested, No. (% of those with testing history) | 13 (9) | 34 (17) | 34 (17) | 33 (17) | 26 (14) | 140 (15) | 34 (11) |
| Intertest interval, median (IQR), monthsa | 11 (5–27) | 12 (5–36) | 11 (6–31) | 16 (8–36) | 15 (6–33) | 13 (6–33) | 12 (6–28) |
| Completed CD4 count within 6 months of diagnosis, No. (%) | 224 (69) | 234 (75) | 279 (86) | 281 (89) | 286 (92) | 1304 (82) | 348 (95) |
| CD4 count, median (IQR), cells/mm3 | 349 (155–553) | 328 (161–549) | 383 (224–555) | 355 (158–565) | 342 (171–545) | 357 (172–555) | 453 (321–629) |
| AIDS within one year of HIV diagnosis, No. (%) | 94 (29) | 102 (32) | 86 (27) | 121 (38) | 105 (34) | 508 (32) | NA |
| AIDS and death within one year of HIV diagnosis, No. (%) | 3 (1) | 7 (2) | 4 (1) | 10 (3) | 7 (2) | 31 (2) | NA |
| Linked to initial HIV care within 3 months, No. (%) | 268 (82) | 271 (86) | 291 (90) | 287 (91) | 282 (91) | 1399 (88) | NA |
| Linked to sustained HIV care, No. (%) | NA | NA | 230 (71) | 234 (74) | 227 (73) | 691 (73)b | NA |
IQR, interquartile range; NA, not applicable
Among persons who previously tested HIV negative
Denominator restricted to cases diagnosed between 2007 and 2009 (N=951)
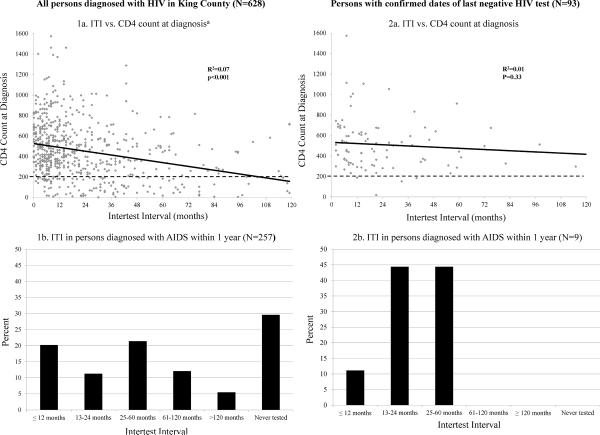
As shown in Figure 1, the ITI was inversely correlated with CD4 count at diagnosis (p<0.001), and longer ITI was associated with higher odds of late diagnosis [OR 1.21 (95% CI: 1.15 – 1.28) per 12 month testing interval]. However, ITI appeared to explain little of the variance in CD4 count at time of diagnosis (R2=0.07), even among cases with confirmed dates of last negative HIV test (Figure 1). Moreover, of the 257 persons defined as having a late HIV diagnosis with available testing history data, 80 (31%) reported testing HIV negative in the two years prior to their HIV diagnosis. Of 508 persons with late diagnosis, 311 (61%) had AIDS at the time of HIV diagnosis, and 31 (6%) died within the first year. Among the 93 persons with a laboratory-confirmed date of last negative HIV test, nine (10%) had a late HIV diagnosis, five (56%) of whom had tested negative for HIV in the two years preceding diagnosis. Of 1304 persons with complete CD4 data at the time of diagnosis, 894 (69%) had CD4 counts ≤500 cells/mm3 and 638 (49%) had CD4 counts ≤350 cells/mm3.
Figure 1. Correlation between intertest interval (ITI) and CD4 count at diagnosis, and ITI in persons diagnosed with AIDS within one year.
Box 1a. Correlation between intertest interval (ITI) and CD4 count at diagnosis among all persons diagnosed with HIV/AIDS in King County 2005–2009.
Box 2a. Correlation between ITI and CD4 count at diagnosis among persons with laboratory-confirmed dates of last negative HIV test.
Box 1b. Distribution of ITI in persons diagnosed with AIDS within one year among all persons diagnosed with HIV/AIDS in King County 2005–09
Box 2b. Distribution of ITI in persons diagnosed with AIDS within one year among persons with laboratory-confirmed dates of last negative HIV test.
aLimited to the >95% of persons with ITI <120 months.
Linkage to care metrics
Between 2005 and 2009, 88% of 1590 persons with newly diagnosed HIV in King County linked to care within 3 months of diagnosis (Table 1), and that proportion increased over the analysis period (p<0.001). Of the 191 who did not link within 3 months, 125 (65%) had a CD4 count or VL reported 4–56 months after diagnosis. Of the 860 persons diagnosed in 2007–2009 who successfully linked to initial HIV care, 169 (20%) had no additional results reported in the 3–9 months after linkage. Younger age was associated with lower odds of linking to sustained care [OR 0.89 per each 5 year decrease (95% CI: 0.84 – 0.95)], as was having a CD4 count >350 cells/mm3 at diagnosis [77% vs. 84% in those with CD4 ≤350 cells/mm3, OR 0.64 (95% CI: 0.45 – 0.90)]. There were no significant differences in linkage to sustained care by gender, race, or HIV risk factor (data not shown).
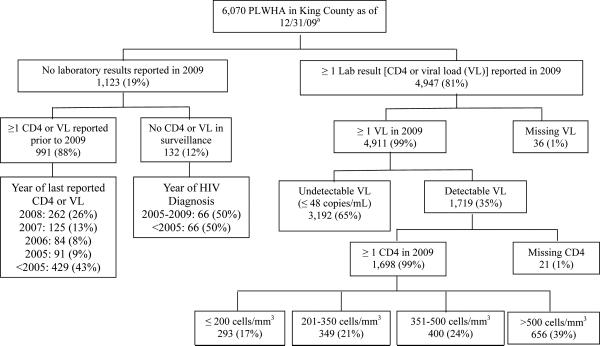
At the end of 2009, 6070 diagnosed PLWHA were presumed to reside in King County. Figure 2 shows the completeness of laboratory reporting, virologic suppression, and CD4 count distribution of these persons. Laboratory data were reported in 2009 for 81% of all PLWHA presumed to reside in the area. Among 1123 persons for whom no data were available in 2009, 495 (44%) had not had any data reported to surveillance for over five years (As Figure 2 shows, 429 persons last had a CD4 or VL result reported prior to 2005; 66 persons had no CD4 or VL reported and were diagnosed with HIV prior to 2005). Compared to whites, African-Americans and Latinos were less likely to have had at least one VL reported in 2009 [80% vs. 83%, OR 0.81 (95% CI: 0.68 – 0.96) and 76% vs. 83%, OR 0.66 (95% CI: 0.54 – 0.81), respectively]. The proportion of PLWHA without laboratory data in 2009 did not vary significantly by age, gender or HIV transmission risk factor (data not shown).
Figure 2. Virologic suppression and CD4 count distribution of persons living with HIV/AIDS (PLWHA) presumed to be residing in king County at the end of 2009.
aCases determined through investigation to have died or relocated were excluded from the total population of PLWHA in King County.
Engagement in continuous care
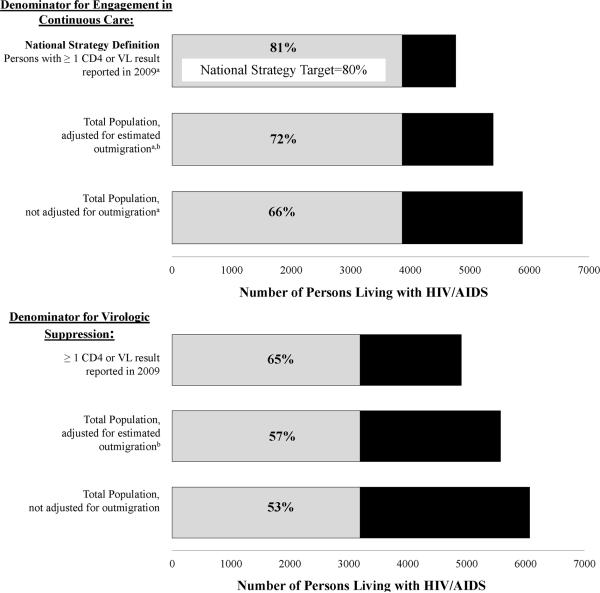
The proportion of PLWHA engaged in continuous care (≥2 visits ≥3 months apart in 2009) depended substantially on the denominator we used to define engagement in care (Figure 3). Using the denominator of persons who had at least one visit in 2009, excluding those who established care in the second half of the year, which is the Health Resources and Services Administration (HRSA) definition used in the NHAS, 81% (3864/4762) were engaged in continuous care. Using a less restrictive denominator, the total number of PLWHA presumed to reside in King County at the end of 2009 (excluding those who established care in the second half of the year) 66% (3864/5885) were engaged in continuous care. Finally, assuming that persons with no data reported to HIV surveillance for 5 years were no longer in King County at the end of 2009 and thus eliminating them from the denominator, 72% (3864/5390) were engaged in continuous care.
Figure 3. Variation in estimates of engagement in continuous care and viral suppression based on differing denominator definitions.
aExcludes persons who established HIV care in the second half of 2009
bAdjusted estimate based on the assumption that persons whose last lab was reported prior to 2005 have left king County. Persons determined through case investigation to have died or moved have been removed from the total denominator of PLWHA in king County.
VL, viral load
Viral suppression
Of persons with at least one VL reported in 2009, 65% had undetectable VL at the time of last report. However, like estimates of engagement in care, estimates of virologic suppression varied substantially based on the denominator used. Including either all PLWHA not known to have died or left the area or all PLWHA for whom laboratory data were reported in the prior 5 years, 53% and 57%, respectively, had an undetectable VL. Among persons with at least one VL reported, compared to whites, African-Americans had lower odds of virologic suppression [62% vs. 66%, OR 0.85 (95% CI: 0.73 – 0.99)], and virologic suppression among Latinos was similar (67% vs. 66%, OR 1.06 (95% CI: 0.86 – 1.30)]. The mean community VL was significantly higher among African-Americans compared to whites (41,497 vs. 17,874 copies/mL, p=0.008), but did not differ significantly between Latinos and whites (14,408 vs. 17,874 copies/mL, p=0.87). While the mean community VL was higher among MSM than non-MSM (20,618 vs. 15,627 copies/mL; p=0.01), the proportions of MSM and non-MSM with virologic suppression did not significantly differ [65% vs. 63%, OR 1.11 (0.94 – 1.30)].
Associations between linkage, engagement, and viral suppression
Of persons diagnosed with HIV in 2007, those who linked to sustained care in the year following their diagnosis were more likely than those who did not link to sustained care to be engaged in continuous care in 2009 [91% vs. 74%, OR 3.65 (95% CI: 1.55 – 8.59)] and more likely to have an undetectable VL at the time of last report [63% vs. 44% of persons with at least one VL reported, OR 2.19 (95% CI: 1.12 – 4.29)]. In contrast, engagement in continuous care and virologic suppression did not differ significantly by linkage status when linkage was defined as completion of a single visit [88% vs. 87%, OR 1.16 (95% CI: 0.25 – 5.46); and 60% vs. 53%, OR 1.32 (95% CI: 0.46 – 3.78, respectively]. Among persons with at least one VL reported in 2009, those engaged in continuous care were more likely to have virologic suppression [69% vs. 58%, OR 1.56 (95% CI: 1.34 – 1.81)] and had a lower mean viral load (14,158 vs. 29,623, p<0.001) than those not engaged in continuous care. Despite this, most persons with only one VL reported to surveillance in 2009 had an undetectable VL.
DISCUSSION
We investigated surveillance measures for HIV testing, linkage to and engagement in care, and treatment in order to assess the current state of HIV care in King County, WA and to evaluate metrics that public health agencies can monitor as they pursue the goals of the NHAS. As in other areas of the U.S., we observed that a large proportion of persons diagnosed with HIV had AIDS within one year of HIV diagnosis [4]. However, we also found that the ITI, a metric used to estimate the time between HIV acquisition and diagnosis, yielded disparate results. Linkage to care was high, but it was not associated with subsequent virologic suppression, and many PLWHA appeared to fall out of care in the first year, suggesting that it may be important to monitor linkage to sustained HIV care. Finally, we found that estimates of engagement in care and virologic suppression were highly dependent on how we defined the population of PLWHA, highlighting the need for health departments to have up-to-date data on which PLWHA reside in their area if they propose to monitor engagement in care and viral suppression.
Our findings suggest that current metrics of late HIV diagnosis may yield incomplete information. The CD4 count at diagnosis was only weakly correlated with ITI (R2=0.07), and nearly one-third of persons diagnosed with AIDS within one year of HIV diagnosis reported testing HIV negative in the prior 2 years. Some persons with low CD4 counts and recent negative HIV tests may have had transient drops in CD4 count associated with acute infection, and prior studies have observed substantial variation in the rate of CD4 depletion that might explain the poor correlation we observed between CD4 count and ITI [15, 16]. Another possibility is that the HIV testing history collected through our surveillance system is invalid. This possibility is supported by our observation that the risk of AIDS diagnosis within one year of HIV diagnosis was much lower among persons for whom we had confirmed dates of last negative HIV tests (10%) - most of whom had tested relatively recently - than among all persons for whom we had testing history data. However, we had confirmed testing history for a relatively small number of persons. If AIDS diagnosis within one year of HIV diagnosis is a nonspecific indicator of late diagnosis, it might not be a sensitive metric for detecting improvements in testing frequency in the population. Given these uncertainties, we propose to continue monitoring both ITI and AIDS diagnosis within one year of HIV diagnosis and are attempting to more consistently collect laboratory-confirmed testing history data for each new HIV case.
We also found that linkage to sustained care, but not linkage to initial care, was associated with virologic suppression, a clinically meaningful endpoint. These findings are consistent with previous clinic-based studies of linkage to and retention in HIV care [17, 18], and argue for the importance of ensuring that persons with newly diagnosed HIV attend more than a single visit. Based on our findings, we believe that health departments should include explicit plans to monitor linkage to sustained care and should consider modifying outreach activities to ensure linkage beyond an initial clinical visit.
Our findings also highlight inadequacies in and difficulties using HIV surveillance data that affect our ability to monitor progress toward the NHAS goals. Approximately one-fifth of PLWHA presumed to reside in King County at the end of 2009 had no VL results reported in 2009. This proportion is lower than that reported from San Francisco (26% missing viral load alone) [7], Washington, DC (52% missing) [19] and New York (43% missing) [20]. The population missing laboratory results reflects a combination of persons who are out of care, incomplete capture of laboratory data, and unascertained outmigration and death. Our estimates of care engagement and virologic suppression were highly dependent on assumptions we adopted about the status of persons with no recent data in surveillance. The implementation plan for the NHAS focuses on engagement in care among Ryan White clients and uses the HRSA definition of engagement in care, which considers only persons with at least one medical visit in a year. The advantage of this approach is that it represents a reproducible metric for a definable population and should allow comparisons across jurisdictions. However, the metric is not population-based and is liable to overestimate the health system's success in providing care to PLWHA because it disregards persons out of care. It also disregards those receiving care outside of the Ryan White program, a sizeable population from which few data are available regarding patterns of engagement in care. Surveillance data have the substantial advantage of being population-based, but the use of such data is currently hampered by uncertainty about which PLWHA reside in an area, the population in which such estimates must be derived. The U.S. HIV surveillance system is evolving from its past focus on counting and describing new HIV/AIDS diagnoses to a broader focus that includes monitoring the receipt of health services and guiding interventions to improve health outcomes [21]. Doing this successfully will require ongoing efforts to ascertain migration between jurisdictions, which in turn requires uniformity in state requirements for CD4 and VL reporting.
This study had important limitations. We had incomplete testing history data for a substantial portion of the population. In WA, as in other states with comprehensive reporting requirements for CD4 and VL results, collection of these data has not been robustly validated. Finally, the accuracy of laboratory result dates as proxies for medical visit dates has not been validated, and we may have overestimated retention in care by including tests sent from settings other than primary HIV care visits.
The release of the NHAS was a critical step in advancing a coordinated, population-based plan to prevent HIV infections and provide effective medical care to PLWHA in the U.S. Our findings highlight the fact that many PLWHA are diagnosed after their infection is clinically advanced, linkage to sustained care is inadequate, and many PLWHA appear not to be engaged in continuous care. Efforts to monitor progress toward the achievement of the goals defined by the NHAS will require substantial improvements in HIV surveillance and efforts to refine the metrics used to measure HIV testing, engagement in care, and virologic suppression.
ACKNOWLEDGEMENTS
The authors thank Hanne Thiede for reviewing an initial draft of the manuscript and Amy Bennett, Fred Koch, and Keith Okita for data management.
Funding Sources: This work was funded by the Public Health – Seattle & King County HIV/STD Program and an NIH grant to J.C.D. (5K23MH090923-01).
Footnotes
Conflicts of Interest: None
These findings were presented in part at the 18th Conference on Retroviruses and Opportunistic Infections
Author contributions: All authors participated in conception and design of the analysis and critically revised the manuscript. J.C.D. and J.B.K analyzed the data. J.C.D. and M.R.G. drafted the manuscript.
REFERENCES
- 1.White House Office of National AIDS Policy . National HIV/AIDS Strategy for the United States. The White House; Washington, DC: Jul 13, 2010. [Accessed June 1, 2011]. Available at: http://www.whitehouse.gov/sites/default/files/uploads/NHAS.pdf2. [Google Scholar]
- 2.Helms DJ, Weinstock HS, Mahle KC, Bernstein KT, Furness BW, Kent CK, et al. HIV testing frequency among men who have sex with men attending sexually transmitted disease clinics: implications for HIV prevention and surveillance. J Acquir Immune Defic Syndr. 2009;50:320–326. doi: 10.1097/QAI.0b013e3181945f03. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention and Council of State and Territorial Epidemiologists . Technical Guidance for HIV/AIDS Surveillance Programs, Volume I: Policies and Procedures. Centers for Disease Control and Prevention; Atlanta, Georgia: 2005. [Google Scholar]
- 4.Centers for Disease Control and Prevention Vital signs: HIV testing and diagnosis among adults--United States, 2001–2009. MMWR Morb Mortal Wkly Rep. 2010;59:1550–1555. [PubMed] [Google Scholar]
- 5.Health Resources and Services Administration . The HIV/AIDS Bureau HIV Core Clinical Performance Measures for Adult/Adolescent Clients: Group 1. Department of Health and Human Services; Washington, D.C.: [Accessed June 1, 2011]. Available at: ftp://ftp.hrsa.gov/hab/habGrp1PMs08.pdf. [Google Scholar]
- 6.Montaner JS, Lima VD, Barrios R, Yip B, Wood E, Kerr T, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376:532–539. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das M, Chu PL, Santos GM, Scheer S, Vittinghoff E, McFarland W, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 5:e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White House Office of National AIDS Policy . Federal Implementation Plan. The White House; Washington, DC: [Accessed June 1, 2011]. Available at: http://aids.gov/federal-resources/policies/national-hiv-aids-strategy/nhas-implementation.pdf3. [Google Scholar]
- 9.Fagan JL, Bertolli J, McNaghten AD. Understanding people who have never received HIV medical care: a population-based approach. Public Health Rep. 2010;125:520–527. doi: 10.1177/003335491012500406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention . Surveillance Brief: Surveillance Systems Supported by the Division of HIV/AIDS Prevention. Centers for Disease Control and Prevention; Atlanta, Georgia: [Accessed April 14, 2011]. Available at: http://www.cdc.gov/hiv/topics/surveillance/resources/factsheets/surveillance.htm. [Google Scholar]
- 11.HIV/AIDS Epidemiology Unit, Public Health – Seattle & King County and the Infectious Disease and Reproductive Health Assessment Unit, Washington State Department of Health [Accessed May 31, 2011];HIV/AIDS Epidemiology Report, Second Half 2010: Volume 77. Available at http://www.kingcounty.gov/healthservices/health/communicable/hiv/epi/reports.aspx.
- 12.Buskin S, Barash E, Bauer A, Kent J, Garcia-Smith H, Wood R. HIV infected individuals presumed to not be receiving HIV medical care: a surveillance program evaluation for investigations and referrals in Seattle, Washington State, USA. [Accessed June 16, 2011];jHASE – Journal of HIV/AIDS Surveillance & Epidemiology, North America. 2011 3:3.1–3.8. Available at: http://www.ieph.org/hase/j-current-issue.htm. [Google Scholar]
- 13.Golden MR, Kerndt PR. Improving Clinical Operations: Can We and Should We Save Our STD Clinics? Sex Transm Dis. 2010;37:264–265. doi: 10.1097/OLQ.0b013e3181d5e01e. [DOI] [PubMed] [Google Scholar]
- 14.Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Washington, DC: Dec 1, 2009. [Accessed April 12, 2010]. Panel on Antiretroviral Guidelines for Adults and Adolescents. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 15.Mellors JW, Margolick JB, Phair JP, Rinaldo CR, Detels R, Jacobson LP, et al. Prognostic value of HIV-1 RNA, CD4 cell count, and CD4 Cell count slope for progression to AIDS and death in untreated HIV-1 infection. JAMA. 2007;297:2349–2350. doi: 10.1001/jama.297.21.2349. [DOI] [PubMed] [Google Scholar]
- 16.Mussini C, Cossarizza A, Sabin C, Babiker A, DeLuca A, Bucher HC, et al. Decline of CD4+ T-cell count before start of therapy and immunological response to treatment in antiretroviral-naive individuals. AIDS. 2011;25:1041–1049. doi: 10.1097/QAD.0b013e3283463ec5. [DOI] [PubMed] [Google Scholar]
- 17.Mugavero MJ, Lin HY, Willig JH, Westfall AO, Ulett KB, Routman JS, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis. 2009;48:248–256. doi: 10.1086/595705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giordano TP, Gifford AL, White AC, Jr., Suarez-Almazolr ME, Rabeneck L, Harman C, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44:1493–1499. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- 19.Castel A, Befus M, West-Ojo T, Griffin A, Hader S, Kamanu-Elias N, et al. Community viral load as a population-based biomarker of HIV, Washington, DC, 2004 to 2008. Presented at: 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2011. [Google Scholar]
- 20.Terzian A, Bodach S, Wiewel E, Braunstein S, Sepkowitz K, Peters V. Characterizing HIV viral load trajectories among HIV-infected New Yorkers, 2006 to 2007. Presented at: 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2011. [Google Scholar]
- 21.Fairchild AL, Bayer R. HIV surveillance, public health, and clinical medicine – will the walls come tumbling down? N Engl J Med. 2011;365:685–687. doi: 10.1056/NEJMp1107294. [DOI] [PubMed] [Google Scholar]