Abstract
A failure of neural changes initiated by the estrogen surge in late pregnancy to reverse the valence of infant stimuli from aversive to rewarding is associated with dysfunctional maternal behavior in nonhuman mammals. Estrogen receptor-α plays the crucial role in mediating these neural effects of estrogen priming. This preliminary study examines associations between estrogen receptor-α gene polymorphisms and human maternal behavior. Two polymorphisms were associated with human negative maternal parenting. Furthermore, hemodynamic responses in functional magnetic resonance imaging to child stimuli in neural regions associated with social cognition fully mediated the association between genetic variation and negative parenting. This suggests testable hypotheses regarding a biological pathway between genetic variants and dysfunctional human maternal parenting.
Keywords: Estrogen receptor-α, ESR1, maternal parenting, functional magnetic resonance imaging
Introduction
Nulliparous small-brain mammals are unprepared for motherhood; they typically find neonates to be aversive, often avoiding or attacking them [26]. Neural functioning must change dramatically during pregnancy to reverse the valence of infant stimuli to rewarding. Rising estrogen in late pregnancy binds to estrogen receptors, allowing adaptive maternal behaviors [9, 27] . This “estrogen priming” [7, 29] influences serotonergic [28] and dopaminergic circuits [32], and increases oxytocin OT receptor proliferation and synthesis in brain [29]. After estrogen priming, pups are approached [5, 16], rejection and infanticide are reduced, and aggression toward threatening intruders is increased [6]. If this process fails, pups are rejected or killed [15].
The estrogen alpha receptor (ESR1) is critical to estrogen priming [7, 29]. ESR1 knockout mice exhibit reduced estrogen sensitivity and abnormal maternal behavior [7]. Notably, the effect of low maternal licking and grooming of pups on later deficits in the offspring’s maternal behavior is mediated by reductions of ESR1 expression due to methylation [7] and reduced transcription in offspring [8].
It is not yet clear, however, if estrogen priming plays a similar role in humans. Most nulliparous human females like infants, but because this process is highly conserved in mammals [5], a role in humans is plausible. Therefore, we conducted a preliminary test of associations between variants in the ESR1 gene and maternal parenting in humans using genetic functional magnetic resonance imaging (fMRI). Understanding dysfunctional maternal parenting in humans is a priority because abusive parenting is a potent risk factor for mental and physical disorders that increase premature mortality [36].
Previous fMRI studies revealed that infant faces and cries activate human maternal cortical and limbic regions [33] and regions involved in reward [2, 22, 35]. In mothers of older children, amygdala and insula activations are not observed, but cortical regions involved in social cognition are activated [21, 34]. In this study, we examine individual differences in neural responses to child stimuli to determine if they are associated with both polymorphisms in ESR1 and variations in human maternal behavior.
Methods
As previously described [18], 121 4–6 year olds were recruited from a child psychiatry clinic in 1994–1995 for a longitudinal study of children with attention-deficit/hyperactivity disorder (ADHD) and matched controls [10, 18, 20]. In wave 1, mother-child interactions were videotaped in a room with furniture, toys, and other objects [11]. Mothers were invited to play freely with their children then conduct assigned tasks with them (Supplemental Materials). Mother-child interactions were coded from videotapes using a standard system [31]. Two measures of parenting were defined by averaging standardized scores in the structured and play situations: Positive parenting (praise, positive affect, and physical positive) and negative parenting (negative commands, critical statements, and physical negatives) were coded. Inter-coder agreement was >.90 for positive parenting, negative parenting, and child disruptive behavior. Such parenting measures predicted later mental health outcomes of children in this sample [11, 19].
Sixteen years later, we used extreme group sampling [23] based on scores on previously observed positive and negative maternal parenting to conduct the present study. From 90 selected mothers, 3 declined and 29 could not be contacted. Eighteen mothers could not be scanned due to metal implants, claustrophobia, or medication. Forty mothers were scanned, but data for 5 mothers were lost to programming errors (Table 1).
Table 1.
Demographics of sample.
| Scanned N = 35* | Genotyped N = 36* | Scanned and Genotyped N = 31* | |
|---|---|---|---|
| Mother | |||
| Age at scan (mean years, SD) | 47.31 (5.18) | 47.42 (5.03) | 47.39 (5.27) |
| Number of live births (mean, SD) | 2.34 (0.72) | 2.36 (0.68) | 2.39 (0.72) |
| Child | |||
| Age in wave 1 (mean years, SD) | 5.23 (0.77) | 5.25 (0.77) | 5.19 (0.79) |
| % Male | 85.7 | 86.1 | 87.1 |
| % African American | 45.7 | 41.7 | 41.9 |
| Prematurity (mean weeks, SD) | 0.46 (1.01) | 0.44 (1.00) | 0.52 (1.06) |
| % Caesarian | 25.7 | 22.2 | 25.8 |
| % ADHD in wave 1 | 48.6 | 50.0 | 48.4 |
| % First born | 57.1 | 52.8 | 51.6 |
One missing parenting data.
Neuroimaging
Photographs of the child at 4–6 years and photos of unrelated children of the same age, sex, and race-ethnicity were used as stimuli. Mothers also viewed dynamic visual stimuli depicting inappropriate (dishonest or aggressive) or neutral behaviors. Each consisted of 3 600 × 480 pixel color photographs presented successively for 1000, 200, and 1000 ms to imply motion. Forty-eight stimuli portrayed inappropriate behaviors (e.g., child intentionally kicking an adult) and 48 portrayed neutral behaviors (e.g., child standing by an adult) without showing faces. These stimuli were matched on race, complexity, and situations.
Stimuli were presented with E-prime 1.2 [30] by back-projection. A black fixation cross was presented in 16 18 s baseline blocks and dynamic child stimuli were presented in 16 19.8 s blocks. Stimuli were blocked by type (appropriate/inappropriate behavior), each consisting of 6 stimuli (2200 ms each) with 6 1100ms inter-stimulus intervals, during which the fixation cross was presented against a gray background. Participants were shown the stimuli in 2 sessions (8 active blocks per session). In one session, immediately preceding every stimulus block, mothers were presented with the photograph of their own child for 6 s and instructed to “imagine this is your child.” In the other session, each stimulus block was preceded by a photograph of the matched unfamiliar child for 6 s and mothers were instructed to “imagine this is not your child.” Order was counterbalanced across sessions and participants.
MRI was performed on a 3-T Philips Achieva Quasar scanner. Pulse sequence parameters included time repetition/time echo (TR/TE) 2000/25, flip angle = 77, 32 contiguous slices with 4 mm thickness, slice gap 0.5mm, 224 × 224 mm2 field of view (FOV), approximately 64 × 64 matrix. High resolution structural images were acquired in the sagittal plane using a T1-weighted 3D Turbo Field Echo (TFE/MP-RAGE) anatomical scan with the following parameters: TR = 8.1 ms, TE = 3.7 ms, FOV = 224 × 224 × 160 mm3, isotropic voxel size 1 × 1 × 1 mm3, matrix size 224 × 224. During anatomical scans after the stimulation paradigms, participants watched a movie about tropical beaches.
Image preprocessing in SPM8 [37] in MATLAB 7.0 [24] included correction for head motion, normalization to the SPM8 echo-planar imaging template, and smoothing using a 6-mm full-width half-maximum isotropic Gaussian kernel. Images were realigned and normalized using standard SPM procedures. All participants had less than 0.5 voxels of in-plane motion throughout scanning.
Genotyping
Saliva was collected using Oragene kits [12] from 36 mothers. DNA was checked for quality by OD ratio of 260/280 and concentration. We genotyped two intronic ESR1 SNPs (rs1884051 and rs3020377) associated with dysphoria in women [14]. TaqMan SNP genotyping assays [1] PCR reactions were conducted in 3ul containing 10 ng genomic DNA, 1.5 ul 2XTaqMan universal PCR master mix [1], 0.075 ul 40X SNP genotyping assay. After 95°C 10 min, 40 cycles consisting of 15 sec at 92°C and 1 min at 60°C annealing temperature were performed. After PCR amplification, an endpoint plate read using a 7900 Real-Time PCR System [1] was performed. Both ESR1 SNPs were in Hardy-Weinberg equilibrium: rs3020377, X2 = 0.00. N. S.; rs1884051, X2 = 0.65, N. S. There were no ancestry group differences in allele frequencies for rs1884051, but the A allele of rs3020377 was more common in European (64.3%) than African American mothers (30.0%), Fisher’s Exact Test, p = 0.0080, requiring ancestry to be covaried.
Results
Whole-Brain Tests
A voxel-by-voxel multiple regression analysis of signal changes for the child photographs and the 2 block categories was applied to preprocessed images using the SPM8 hemodynamic response function. Individual data were analyzed using a fixed-effects model; group data were analyzed using a random-effects model. Condition effects at the subject level were modeled by box-car regressors representing type of child photograph and blocks.
Only regions identified in previous studies of maternal neural responses to child stimuli [33] were selected a priori. ROIs in these regions were tested for associations with external variables if a voxel in a selected region was activated at p < .005, uncorrected, or p < .05, small-volume corrected with k=10 [38]. Small volumes consisted of 5mm spheres centered at the most significant activated voxel of the clusters at p < 0.001 uncorrected in the whole brain analysis. Data extraction for 5-mm spherical ROIs was performed using the rfxplot toolbox [13] in SPM8. The contrasts were: Own/other child and inappropriate/neutral child behavior in the own-child session. Activations were overlaid on a representative high-resolution structural T1-weighted image from one subject from the SPM8 canonical image set, coregistered to Montreal Neurological Institute (MNI) space. Coordinates were based on results from the whole-brain analyses and neuroanatomical atlases.
Tests of Association
Only ROIs significant in whole-brain analyses were used in independent tests of association using generalized linear models with robust standard errors [25], controlling child’s sex, race-ethnicity, birth order, age in wave 1, mother’s age at scanning, delivery (Caesarian or vaginal), and parity. To adjust for child effects on parenting [3], the child’s diagnosis of ADHD and disruptive behavior during mother-child interaction were covariates.[19] Three sequenced sets of prioritized models were conducted:
Additive terms coded -1, 0, and 1 tested differences in numbers of minor alleles and a term coded -1, 2, and -1 captured nonadditive associations. Omnibus 4-DF tests of the association of these terms for the two SNPs with maternal parenting were conducted. These did not detect a significant association of ESR1 SNPs with positive parenting, χ2 = 7.42, p = 0.1151, but revealed a significant joint association with negative parenting, χ2 = 18.25, p < 0.0011. A significant interaction between ancestry and the additive term for rs3020377 (β = -0.1543, χ2 = 17.97, p < 0.0001), reflecting stronger association between rs3020377 and negative parenting for African American mothers was found, but not a significant ancestry-by- rs1884051 interaction.
Separate models regressed parenting on each ROI significantly activated in whole-brain analyses, with the same covariates plus time of scanning.
Associations with ER-α SNPs were separately tested for each ROI that was significantly associated with parenting in step 2.
Photographs of own > another child and depictions of inappropriate > appropriate behavior evoked hemodynamic responses in social cognition areas [33] (Figure 1). Eight of the 36 ROIs that were significantly activated in the own > other child contrast were nominally associated with negative parenting, exceeding the 1.8 associations expected at P ≤. 05 by chance (none significant at corrected α = .05/36 = .0014). Five of 39 associations between ROIs activated/deactivated in the inappropriate > appropriate youth behavior contrast were significant (1.95 expected by chance; none significant at α = .05/39 = .0013) (see Supplemental Materials).
Figure 1.
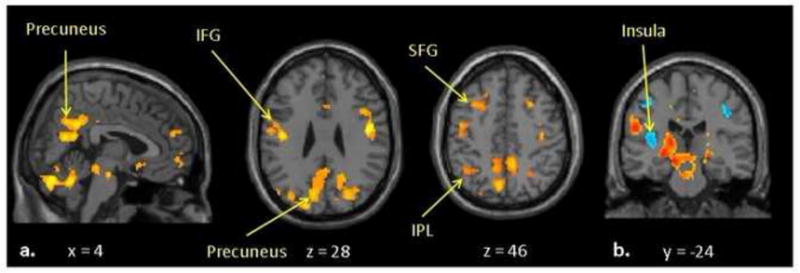
Significant hemodynamic responses in (a) own > other child, and in (b) inappropriate > appropriate behavior contrasts in fMRI.
Notably, 4 of 8 4-DF tests were significant for the association of ESR1 SNPs with the ROIs that were both significantly activated in the own > other child contrast and associated with negative parenting. Precuneus and superior and inferior frontal gyri activations (all left side) were each related to negative parenting at P <.05/4 = .0125. Individually, rs1884051 A alleles were additively associated with these 4 ROIs, with rs3020377 associated with one ROI. In the inappropriate > appropriate contrast, ESR1 SNPs were jointly related to right insula activation at corrected P < .05/4 = .0125. Both rs1884051 and rs3020377 were additively associated with this ROI (Table 2).
Table 2.
Omnibus and individual tests of associations between ER-α polymorphisms and negative maternal parenting (N = 35) and neural regions of interest that were both significantly activated and significantly associated with negative parenting (N = 30), with all covariates in models.
| Negative Maternal Parenting | ||
|---|---|---|
| χ2 | p < | |
| rs1884051 (additive) | 9.74 | 0.0018 |
| rs1884051 (nonadditive) | 0.02 | 0.8907 |
| rs3020377 (additive) | 7.02 | 0.0081 |
| rs3020377 (nonadditive) | 2.54 | 0.1109 |
| Own Child > Other Child fMRI Contrast | ||
| Right medial prefrontal cortex (omnibus 4-DF test: χ2 = 2.39, p = 0.6636) | ||
| χ2 | p < | |
| rs1884051 (additive) | 0.12 | 0.7319 |
| rs1884051 (nonadditive) | 0.41 | 0.5222 |
| rs3020377 (additive) | 0.02 | 0.8765 |
| rs3020377 (nonadditive) | 0.04 | 0.8389 |
| Left dorsolateral prefrontal cortex (omnibus 4-DF test: χ2 = 5.94, p = 0.2034) | ||
| rs1884051 (additive) | 0.65 | 0.4213 |
| rs1884051 (nonadditive) | 0.92 | 0.3383 |
| rs3020377 (additive) | 0.02 | 0.8843 |
| rs3020377 (nonadditive) | 0.05 | 0.8250 |
| Right dorsolateral prefrontal cortex (omnibus 4-DF test: χ2 = 2.87, p = 0.5803) | ||
| rs1884051 (additive) | 0.02 | 0.8787 |
| rs1884051 (nonadditive) | 0.55 | 0.4579 |
| rs3020377 (additive) | 0.66 | 0.4162 |
| rs3020377 (nonadditive) | 0.18 | 0.6721 |
| Left superior frontal gyrus (omnibus 4-DF test: χ2 = 16.65, p < 0.0023) | ||
| rs1884051 (additive) | 10.10 | 0.0015 |
| rs1884051 (nonadditive) | 0.92 | 0.3379 |
| rs3020377 (additive) | 2.27 | 0.1315 |
| rs3020377 (nonadditive) | 0.03 | 0.8622 |
| Left inferior frontal gyrus (omnibus 4-DF test: χ2 = 17.16, p = 0.0018) | ||
| rs1884051 (additive) | 8.81 | 0.0030 |
| rs1884051 (nonadditive) | 5.13 | 0.0236 |
| rs3020377 (additive) | 0.06 | 0.8125 |
| rs3020377 (nonadditive) | 4.64 | 0.0312 |
| Left fusiform gyrus (omnibus 4-DF test: χ2 = 6.01, p = 0.1980) | ||
| rs1884051 (additive) | 1.16 | 0.2806 |
| rs1884051 (nonadditive) | 2.66 | 0.1029 |
| rs3020377 (additive) | 0.04 | 0.8419 |
| rs3020377 (nonadditive) | 5.24 | 0.0220 |
| Left inferior parietal lobule (omnibus 4-DF test: χ2 = 10.88, p = 0.0280) | ||
| rs1884051 (additive) | 5.51 | 0.0189 |
| rs1884051 (nonadditive) | 0.78 | 0.3772 |
| rs3020377 (additive) | 3.10 | 0.0785 |
| rs3020377 (nonadditive) | 0.00 | 0.9606 |
| Left precuneus (omnibus 4-DF test: χ2 = 15.11, p = 0.0045) | ||
| rs1884051 (additive) | 13.69 | 0.0002 |
| rs1884051 (nonadditive) | 1.48 | 0.2236 |
| rs3020377 (additive) | 2.25 | 0.1332 |
| rs3020377 (nonadditive) | 0.79 | 0.3729 |
| Inappropriate > Neutral Behavior fMRI Contrast | ||
| Right insula (omnibus 4-DF test: χ2 = 14.76, p < 0.0052) | ||
| χ2 | p < | |
| rs1884051 (additive) | 10.69 | 0.0011 |
| rs1884051 (nonadditive) | 5.90 | 0.0151 |
| rs3020377 (additive) | 18.67 | 0.0001 |
| rs3020377 (nonadditive) | 1.79 | 0.1812 |
| Right posterior superior temporal sulcus (omnibus 4-DF test: χ2 = 3.08, p = 0. 5441) | ||
| rs1884051 (additive) | 0.01 | 0.9416 |
| rs1884051 (nonadditive) | 0.01 | 0.9119 |
| rs3020377 (additive) | 0.24 | 0.6267 |
| rs3020377 (nonadditive) | 1.33 | 0.2488 |
| Left precuneus (omnibus 4-DF test: χ2 = 5.25, p = 0.2625) | ||
| rs1884051 (additive) | 0.13 | 0.7163 |
| rs1884051 (nonadditive) | 3.10 | 0.0784 |
| rs3020377 (additive) | 0.08 | 0.7823 |
| rs3020377 (nonadditive) | 1.79 | 0.1806 |
| Right caudate (omnibus 4-DF test: χ2 = 7.53, p < 0.1103) | ||
| rs1884051 (additive) | 1.64 | 0.1997 |
| rs1884051 (nonadditive) | 0.95 | 0.3296 |
| rs3020377 (additive) | 2.46 | 0.1166 |
| rs3020377 (nonadditive) | 3.33 | 0.0680 |
Structural Equation Modeling
To prepare for future tests of neural pathways from genetic variants through neural functioning to maternal behavior it is necessary to establish that each variable is significantly associated all others, then determine if neural variation statistically mediates the association between gene and behavior. Structural equation modeling (SEM) [4] with robust maximum likelihood estimation estimated associations among the 5 ROIs associated with both rs1884051 and with negative parenting [17]. The latent neural construct estimates concurrent activations in ROIs in the contrasts. All covariates used in regression models were included in these SEMs. The latent neural construct was significantly associated with both the additive term for rs1884051 (standardized coefficient = -0.65, p < .0001) and negative parenting (standardized coefficient = 0.26, p < .04). In Figure 2, the scatterplot shows the association of the latent neural construct with negative parenting residualized on the covariates; removing the two outliers increased the correlation to r = .48. In the full SEM, the path between the rs1884051 and the neural construct was significant and the path between the neural construct and negative parenting was significant, but the direct path between rs1884051 and negative parenting was no longer significant, indicating mediation by the neural construct.
Figure 2.
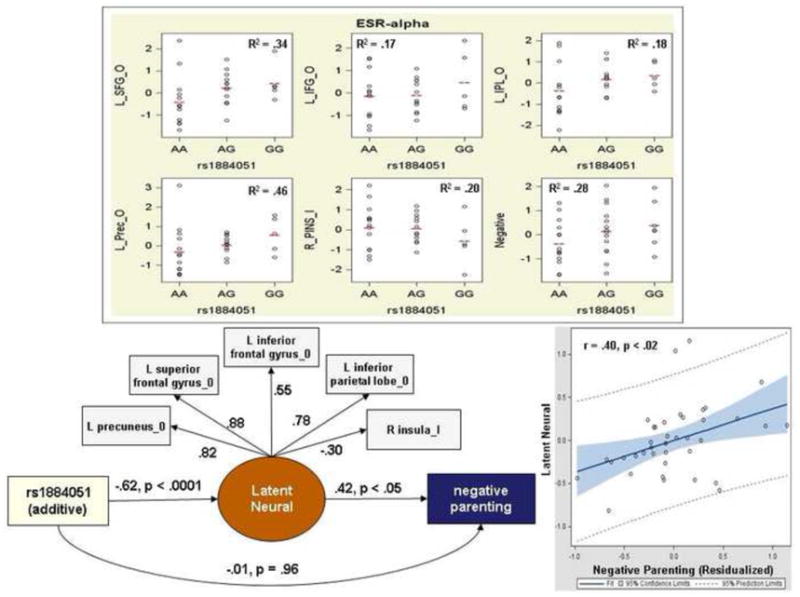
Scatter plots with group means for hemodynamic responses in own/other child (O) and inappropriate/appropriate behavior (I) contrasts in neural regions significantly related to both negative parenting and ESR1 rs1884051 (top). Activations are expressed as normalized z-scores residualized on covariates. SEM revealed mediation of the genetic association with negative parenting by the ROIs (bottom left). Scatter plot of negative parenting residualized on covariates with the latent neural construct (bottom right).
Discussion
The present preliminary findings are internally consistent and suggest the testable hypothesis that genetic variations in ESR1 are associated with hemodynamic responses to child stimuli in several neural regions, which are themselves positively correlated with negative parenting, and in the right insula, which is inversely related to negative parenting. Because previous studies also found activations to child stimuli in these cortical regions in mothers of children [21, 34], but not infants [2, 22, 35], these findings may be specific to mothers of older children.
These preliminary novel findings do not support any conclusions, however. The sample was small and heterogeneous, not all sampled mothers participated, and neuroimaging was not contemporaneous with parenting assessments. Nonetheless, if the present findings are confirmed in future studies using more extensive genotyping of ESR1, the hypothesis yielded by these findings could improve understanding of the role of estrogen in maternal parenting in humans and could ultimately lead to child abuse prevention.
Conclusions
Hemodynamic neural responses to preschool child stimuli in cortical regions were correlated with both tag SNPs in ESR1 and with observed negative maternal parenting. Furthermore, a latent construct defined by these correlated regions fully mediated the association between a SNPs in ESR1 negative parenting. This suggests testable hypotheses regarding a role of genetic variants in ESR1 in dysfunctional human maternal parenting.
Supplementary Material
Highlights.
Harsh parenting is a risk factor for mental and physical health problems in humans.
The ERα receptor is critical to adaptive maternal behavior in nonhuman mammals.
ERα genotype predicted neural responses to children and human maternal parenting.
Future study of ERα, neural responses to child stimuli, and child abuse is warranted.
Acknowledgments
Supported by the Brain Research Foundation and the National Institutes of Health (R01- MH53554).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.AppliedBiosystems. Foster City, CA: [Google Scholar]
- 2.Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Bell RQ, Chapman M. Child effects in studies using experimental or brief longitudinal approaches to socialization. Develop Psychol. 1986;22:595–603. [Google Scholar]
- 4.Bollen KA. Structural equations with latent variables. John Wiley; New York: 1989. [Google Scholar]
- 5.Brunton PJ, Russell JA. Endocrine induced changes in brain function during pregnancy. Brain Research. 2010;1364:198–215. doi: 10.1016/j.brainres.2010.09.062. [DOI] [PubMed] [Google Scholar]
- 6.Campbell A. Attachment, aggression and affiliation: The role of oxytocin in female social behavior. Biol Psychology. 2008;77:1–10. doi: 10.1016/j.biopsycho.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Champagne FA. Maternal imprints and the origins of variation. Hormones and Behavior. 2011;60:4–11. doi: 10.1016/j.yhbeh.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Champagne FA, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. PNAS. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choleris E, Ogawa S, Kavaliers M, Gustafsson J-A, Korach KS, Muglia LJ, Pfaff DW. Involvement of estrogen receptor alpha, beta, oxytocin in social discrimination: a detailed behavioral analysis with knockout female mice. Genes, Brain, and Behavior. 2006;5:528–539. doi: 10.1111/j.1601-183X.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- 10.Chronis-Tuscano A, Molina BSG, Pelham WE, Applegate B, Dahlke A, Overmyer M, Lahey BB. Very early predictors of adolescent depression and suicide attempts in children with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2010;67:1044–1051. doi: 10.1001/archgenpsychiatry.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chronis AM, Lahey BB, Pelham WE, Williams SH, Baumann BL, Kipp H, Jones HA, Rathouz PJ. Maternal depression and early positive parenting predict future conduct problems in young children with attention-deficit/hyperactivity disorder. Develop Psychol. 2007;43:70–82. doi: 10.1037/0012-1649.43.1.70. [DOI] [PubMed] [Google Scholar]
- 12.DNAGenotek. Oragene DNA self-collection kit. DNAGenotek, Inc; Ottawa: 2010. [Google Scholar]
- 13.Glascher J. Visualization of group inference data in functional neuroimaging. Neuroinformatics. 2009;7:73–82. doi: 10.1007/s12021-008-9042-x. [DOI] [PubMed] [Google Scholar]
- 14.Huo L, Straub RE, Roca C, Schmidt PJ, Shi K, Vakkalanka R, Weinberger DR, Rubinow DR. Risk for premenstrual dysphoric disorder is associated with genetic variation in ESR1, the estrogen receptor alpha gene. Biol Psychiatry. 2007;62:925–933. doi: 10.1016/j.biopsych.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kendrick KM. The neurobiology of social bonds. J Neuroendocrin. 2004;16:1007–1008. doi: 10.1111/j.1365-2826.2004.01262.x. [DOI] [PubMed] [Google Scholar]
- 16.Kendrick KM, DaCosta APC, Broad KD, Ohkura S, Guevara R, Levy F, Keverne EB. Neural control of maternal behaviour and olfactory recognition of offspring. Brain Res Bull. 1997;44:383–395. doi: 10.1016/s0361-9230(97)00218-9. [DOI] [PubMed] [Google Scholar]
- 17.Lahey BB, McNealy K, Knodt A, Zald DH, Sporns O, Manuck SB, Flory JD, Applegate B, Rathouz PJ, Hariri AR. Using confirmatory factor analysis to measure contemporaneous activation of defined neuronal networks in functional magnetic resonance imaging. NeuroImage. 2012;60:1982–1991. doi: 10.1016/j.neuroimage.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lahey BB, Pelham WE, Loney J, Lee SS, Willcutt E. Instability of the DSM-IV subtypes of ADHD from preschool through elementary school. Arch Gen Psychiatry. 2005;62:896–902. doi: 10.1001/archpsyc.62.8.896. [DOI] [PubMed] [Google Scholar]
- 19.Lahey BB, Rathouz PJ, Lee SS, Chronis-Tuscano A, Pelham WE, Waldman ID, Cook EH. Interactions between early parenting and a polymorphism of the child's dopamine transporter gene in predicting future child conduct disorder symptoms. J Abnorm Psychol. 2011;120:33–45. doi: 10.1037/a0021133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SS, Chronis-Tuscano A, Keenan K, Pelham WE, Loney J, Van Hulle CA, Cook EH, Lahey BB. Association of maternal dopamine transporter genotype with negative parenting: Evidence for gene × environment interaction with child disruptive behavior. Mol Psychiatry. 2010;15:548–558. doi: 10.1038/mp.2008.102. [DOI] [PubMed] [Google Scholar]
- 21.Leibenluft E, Gobbini MI, Harrison T, Haxby JV. Mothers' neural activation in response to pictures of their children and other children. Biol Psychiatry. 2004;56:225–232. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, Bohning DE, George MS. A potential role for thalamocingulate circuitry in human maternal behavior. Biol Psychiatry. 2002;51:431–445. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- 23.Makowsky R, Beasley TM, Gadbury GL, Albert JM, Kennedy RE, Allison DB. Validity and power of missing data imputation for extreme sampling and terminal measures designs in mediation analysis. Fron Genet. 2011;2:75. doi: 10.3389/fgene.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsh AA, Finger EC, Mitchell DGV, Reid ME, Sims C, Kosson DS, Towbin KE, Leibenluft E, Pine DS, Blair RJR. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry. 2008;165:712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- 25.Nelder J, Wedderburn R. Generalized linear models. J Royal Stat Soc Series A. 1972;135:370–384. [Google Scholar]
- 26.Numan M, Insel TR. Neurobiology of parental behavior. Springer; New York: 2003. [Google Scholar]
- 27.Ogawa S, Choleris E, Pfaff D. Genetic influences on aggressive behaviors and arousability in animals. Annals NY Acad Sci. 1036:257–266. doi: 10.1196/annals.1330.016. [DOI] [PubMed] [Google Scholar]
- 28.Osterlund MK, Overstreet DH, Hurd YL. The Flinders Sensitive Line rats, a genetic model of depression, show abnormal serotonin receptor mRNA expression in the brain that is reversed by 17 beta-estradiol. Mol Brain Res. 1999;74:158–166. doi: 10.1016/s0169-328x(99)00274-0. [DOI] [PubMed] [Google Scholar]
- 29.Pfaff D, Waters E, Khan Q, Zhang XT, Numan M. Minireview: Estrogen receptor-initiated mechanisms causal to mammalian reproductive behaviors. Endocrinology. 2011;152:1209–1217. doi: 10.1210/en.2010-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.PsychologySoftwareTools, E-prime. Pittsburgh, PA: 2011. [Google Scholar]
- 31.Robinson EA, Eyberg SM. The Dyadic Parent-Child Interaction Coding System: Standardization and validation. J Consult Clin Psychol. 1981;49:245–250. doi: 10.1037//0022-006x.49.2.245. [DOI] [PubMed] [Google Scholar]
- 32.Stolzenberg DS, Numan M. Hypothalamic interaction with the mesolimbic DA system in the control of the maternal and sexual behaviors in rats. Neuroscience and Biobehav Rev. 2011;35:826–847. doi: 10.1016/j.neubiorev.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Swain JE. The human parental brain: In vivo neuroimaging. Progress in Neuro-Psychopharm Biol Psychiatry. 2011;35:1242–1254. doi: 10.1016/j.pnpbp.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swain JE, Leckman JF, Mayes LC, Feldman R, Constable RT, Schultz RT. Neural substrates and psychology of human parent – infant attachment in the postpartum. Biol Psychiatry. 2004;55:153S. [Google Scholar]
- 35.Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent-infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. J Child Psychol Psychiatry. 2007;48:262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wegman HL, Stetler C. A meta-analytic review of the effects of childhood abuse on medical outcomes in adulthood. Psychosom Med. 2009;71:805–812. doi: 10.1097/PSY.0b013e3181bb2b46. [DOI] [PubMed] [Google Scholar]
- 37.Wellcome Department of Imaging Neuroscience. Statistical parametric mapping. London, U.K: 2009. [Google Scholar]
- 38.Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


