Abstract
Background
Schizophrenia patients have deficits across a broad range of important cognitive and clinical domains. Synchronization of oscillations in the gamma frequency range (~40 Hz) is associated with many normal cognitive functions and underlies at least some of the deficits observed in schizophrenia patients. Recent studies have demonstrated that gamma oscillations are modulated by the phase of theta waves, and this cross-frequency coupling indicates that a complex and hierarchical organization governs neural oscillatory dynamics. The aims of the present study were to determine if schizophrenia patients have abnormalities in the amplitude, synchrony, and cross-frequency coupling of gamma and theta oscillations in response to gamma-frequency steady-state stimulation and if abnormal neural oscillatory dynamics are associated with cognitive deficits in schizophrenia.
Methods
Schizophrenia patients (n = 234) and healthy control subjects (n = 188) underwent EEG testing in response to 40-Hz auditory steady-state stimulation. Cognitive functions were assessed with a battery of neuropsychological tests.
Results
Schizophrenia patients had significantly reduced gamma intertrial phase coherence, increased theta amplitude, and intact cross-frequency coupling relative to healthy control subjects. In schizophrenia patients, increased theta amplitude was associated with poor verbal memory performance.
Conclusions
Results suggest that schizophrenia patients have specific alterations in both gamma and theta oscillations but these deficits occur in the context of an intact hierarchical organization of their cross-frequency modulation in response to 40 Hz steady-state stimulation. Cortical oscillatory dynamics may be useful for understanding the neural mechanisms that underlie the disparate cognitive and functional impairments of schizophrenia.
Keywords: cross-frequency coupling, neural oscillations, gamma oscillations, theta oscillations, auditory steady-state response, schizophrenia
Introduction
Schizophrenia patients have deficits in many domains ranging from abnormalities in basic sensory registration to impairments in higher cognitive operations (1) which are associated with poor long-term functional outcome (2–3). Deficits in early sensory processing have also been extensively documented in schizophrenia using a variety of neurophysiological and neuroimaging techniques (4). These deficits serve as endophenotypes in genetic studies (5) and biomarkers in pharmacologic studies (6).
Neural oscillations in gamma band (30 – 80 Hz) have been proposed to play an important role in information processing (7). Gray and Singer (8) reported that in the cat visual cortex, the firing probability of neurons in response to visual stimuli oscillated in gamma frequency range. The neuronal firing pattern was tightly correlated with oscillatory activity of local field potential (LFP). Tallon-Baudry and colleagues (9) reported that gamma oscillations in human scalp EEG reflected visual perception. These findings led to the suggestion that the oscillatory pattern of neuronal firing represents information processing associated with not only visual perception but also other cognitive domains and reflected in electroencephalography (EEG). Recent studies have shown that gamma oscillations in neuronal firing, LFP, intracranial EEG, and scalp EEG are associated with a variety of sensory and cognitive processes including perception (9–10), attention (11–12), memory (13–14), and working memory (15) --all domains in which schizophrenia patients exhibit deficits (1, 16–17). In addition, schizophrenia patients show abnormal gamma oscillations in perception (18–19), sensory gating (20), backward masking (21), selective attention (22), working memory (23), and cognitive control (24). While some studies have found reduced power and phase synchronization of gamma oscillations in schizophrenia, others reported increased power of gamma oscillations in schizophrenia (25–26). These discrepancies suggest that the type of abnormal gamma oscillations depends upon the cognitive tasks and oscillatory parameters under investigation.
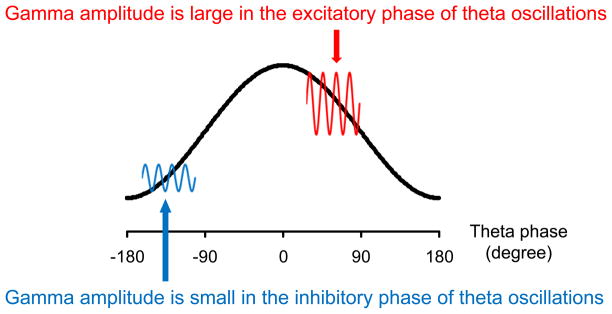
Since gamma oscillations are associated with important cognitive operations and are generated by interneurons and pyramidal cells in cortical networks (27–29), it is possible that abnormalities in the ability of neural circuits to support this critical frequency range might represent a fundamental deficit of schizophrenia (30–31). It is important to note, however, that gamma oscillations interact with neural oscillations in other frequency bands (32). For example, emerging evidence has shown that gamma oscillations are modulated by neural oscillations in lower (e.g., theta) frequency bands (33–34). This is termed “cross-frequency coupling.” While there are several types of cross-frequency coupling, phase-amplitude cross-frequency coupling has been proposed to play an important role in information processing (35). Phase-amplitude cross-frequency coupling indicates that the phase of lower-frequency oscillations modulates the amplitude of higher-frequency oscillations (Figure 1). In particular, phase-amplitude cross-frequency coupling between theta and gamma oscillations has been observed in LFP (34), intracranial EEG (33), and scalp EEG (36). Since theta oscillations have large temporal and spatial scales and gamma oscillations have small temporal and spatial scales (37), cross-frequency coupling may represent the integration of information processed across different temporal and spatial scales and has been observed during visual perception (36) and working memory (38) in nonpsychiatric subjects.
Figure 1.
Heuristic model of phase-amplitude cross-frequency coupling. Gamma oscillations (red and blue lines) are largest in the excitatory vs. inhibitory phase of ongoing theta oscillations (black line). Note that excitatory and inhibitory phase may vary according to tasks and neural sources.
Several models of cross-frequency coupling have been proposed, although the underlying neural mechanisms remain to be elucidated. Because the phase of neural oscillations in LFP modulates the probability of neuronal firings (34), cross-frequency coupling indicates that the phase of low-frequency oscillations modulates the excitability of high-frequency oscillations. In this context, low-frequency oscillations are entrained by rhythmic external stimuli and align the excitatory phase with attended stimuli (39). This “oscillatory selection” (40) model might explain the association between cross-frequency coupling and sensory processing. In contrast, the “phase coding” (41) model suggest that each memory is represented by a gamma cycle, whereas a sequence of memories is represented by several gamma cycles nested within one theta cycle (42). This phase coding model might explain the association between cross-frequency coupling and working memory.
Cross-frequency coupling indicates that neural oscillations have a complex and hierarchical organization. Accordingly, abnormal gamma oscillations in schizophrenia patients may represent only a part of the complex constellation of deficits in neural oscillatory dynamics that give rise to deficits in cognitive functions. Spencer and colleagues reported abnormal cross-frequency interactions between delta phase and gamma phase locking factor (43), but did not investigate phase-amplitude cross-frequency coupling. Conversely, Allen and colleagues reported abnormal phase-amplitude cross-frequency coupling between different frequency bands (44) but did not investigate other aspects of neural oscillations such as power and intertrial phase coherence (ITC) that are impaired in schizophrenia (45–46).
In this study, we investigated gamma and theta oscillations as well as theta-gamma phase-amplitude cross-frequency coupling in schizophrenia in response to 40 Hz (i.e., gamma-frequency) auditory steady-state stimuli. This paradigm assesses the capacity to support stimulus-driven, gamma oscillations. Auditory steady-state responses (ASSRs) are largest in response to 40 Hz stimulation (47) and are suitable for cross-species translational studies since rodents demonstrate homologous responses (48). Many studies have demonstrated that schizophrenia patients have robust deficits of gamma oscillations in this paradigm (43, 46, 49–50), although theta oscillations and cross-frequency coupling have not been previously examined in this context. We therefore hypothesized that schizophrenia patients would exhibit separate abnormalities in both gamma and theta oscillations as well as decreased theta-gamma cross-frequency coupling. In the present study, amplitude and ITC were selected as the primary measures of neural oscillations. Amplitude and ITC reflect complementary aspects of neural oscillatory dynamics and represent distinct alterations in schizophrenia (51). We also hypothesized that abnormalities in these oscillations would be associated with cognitive deficits in schizophrenia patients.
Methods and Materials
Subjects
Subjects included 234 schizophrenia patients and 188 healthy control subjects (Table 1). Evoked gamma power and ITC from a subset of these participants were previously published (46). All participants were assessed on their capacity to provide informed consent and after detailed description of study procedures were provided, written consent was obtained per University of California, San Diego Institutional Review Board (IRB)-approved forms (IRB#030510). All subjects received a urine toxicology screen to rule out recent drug use. In addition, all subjects were carefully screened with the use of the Structured Clinical Interview for DSM-IV to ensure that they did not have an Axis I diagnosis other than schizophrenia (52) and had not experienced a neurologic insult, such as significant head trauma and/or loss of consciousness. Audiometric testing was used to ensure that all participants could detect 1000 Hz tones at 40 dB.
Table 1.
Demographic, Clinical, and Cognitive Characteristics of the Subjects
| Characteristic | HC (n = 188) | SZ (n = 234) | Statistic | p | |
|---|---|---|---|---|---|
| Sex (Male/Female) | 94/94 | 182/52 | χ2=35.55 | < .001 | |
| Age (years) | 43.9 (11.1) | 44.5 (8.8) | F(1,418) = 1.13 | .29 | |
| Duration of Illness (years) | 22.7 (9.9) | ||||
| SAPS score | 8.7 (4.1) | ||||
| SANS score | 13.7 (4.5) | ||||
| GAF Scale score | 41.2 (7.1) | ||||
| WRAT-3 | Reading Total Score | 51.0 (4.8) | 44.2 (7.1) | F(1,418) = 105.61 | < .001 |
| CVLT-2 | List A trial 1–5 | 51.7 (10.9) | 35.3 (10.8) | F(1,418) = 188.09 | < .001 |
| Long-delay free recall | 11.6 (3.2) | 7.2 (3.4) | F(1,418) = 150.52 | < .001 | |
| WCST-64 | Perseverative responses | 11.0 (7.8) | 21.6 (17.6) | F(1,418) = 46.69 | < .001 |
| Categories completed | 3.2 (1.5) | 2.0 (1.5) | F(1,418) = 52.48 | < .001 | |
| LNS | Forward | 14.0 (2.9) | 11.7 (3.1) | F(1,418) = 56.97 | < .001 |
| Reorder | 10.8 (2.4) | 8.0 (2.5) | F(1,418) = 127.41 | < .001 | |
Abbreviations: CVLT-2, California Verbal Learning Test 2nd edition; GAF, Global Assessment of Functioning; HC, Healthy control subjects; LNS, Letter-Number Sequencing; SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms; SZ, schizophrenia patients; WCST-64, Wisconsin Card Sorting Test; WRAT-3, Wide Range Achievement Test 3rd edition
Healthy control subjects were recruited through newspaper and internet advertisements. Schizophrenia patients were recruited from community residential facilities and via physician referral. Antipsychotic medications were prescribed for 218 schizophrenia patients. In schizophrenia patients, clinical symptoms were assessed with the Scale for the Assessment of Negative Symptoms (SANS) (53) and the Scale for the Assessment of Positive Symptoms (SAPS) (54). The current level of functioning was assessed with the modified Global Assessment of Functioning (GAF) (55).
Cognitive Tasks
The Wide Range Achievement Test 3 Reading subtest was used to estimate premorbid verbal abilities (56). Verbal memory was assessed via the California Verbal Learning Test-2 (CVLT-2) list A 1–5 total score and delayed free recall indices (57). Perseverative responses and number of categories completed on the Wisconsin Card Sorting Test were used to assess concept formation and conceptual flexibility (58). Performance on the Letter-Number Sequencing test was used to assess the immediate online storage and repetition of auditory information (forward condition) as well as working memory via manipulation and retrieval of stored information (reordering condition) (59–60).
Stimuli
Auditory steady-state stimuli were presented to subjects by means of foam insert earphones (Model 3A; Aearo Company Auditory Systems, Indianapolis, Indiana). The stimuli were 1 millisecond, 93 dB clicks presented in 500 milliseconds trains varying in rate of presentation (20, 30, and 40 Hz) in each of 3 blocks (order fixed). Blocks contained 200 trains of clicks with 500 milliseconds intervals.
EEG Recording
EEG recordings were acquired with a Neuroscan Nuamp system (Neuroscan Laboratories, El Paso, Texas). The EEG was recorded from the scalp through 34 sintered Ag/AgCl electrodes with the use of an electrode cap (EasyCap; Falk Minow Services, Herrshing-Breitbrunn, Germany). Electrodes placed at the tip of the nose and at Fpz served as the reference and ground, respectively. Four additional electrodes placed above and below the left eye and at the outer canthi of both eyes were used for monitoring blinks and eye movements. All impedances were below 4 kΩ. Signals were digitized at a rate of 1000 Hz with system acquisition filter settings at 0.5–100 Hz. EEG and stimulus markers were recorded continuously. Subjects did not smoke for at least 60 minutes before EEG testing, and were instructed to minimize eye movements and muscle artifact during the recording. During testing, subjects were observed through a one-way mirror. In addition, signal quality and the number of sweeps free of gross artifacts (defined as ±100 μV across the 0–512 milliseconds after stimuli) were closely monitored.
EEG Analyses
EEG preprocessing was performed offline with BrainVision Analyzer (Brain Products GmbH, Gilching, Germany). Continuous data in response to 40 Hz stimulation were segmented relative to the onset of stimuli (−1.5 to 1.5 seconds). Segmented data were mathematically corrected for eye movement artifact with an established method (61). After blink correction, epochs containing > ±100 μV were automatically rejected. Epochs were also manually reviewed to reject EEG epochs with other artifacts (e.g., muscle activity).
Time-frequency analyses were performed with EEGLAB (62) and MATLAB (Mathworks, Natick, Massachusetts). Data at Fz were used for further analyses because this is the electrode with maximal responses (46, 49). We selected 100 artifact-free epochs randomly from each subject and used them for further analyses since the number of epochs can impact signal-to-noise ratios and ITC measures. First, the raw signals were filtered with central frequencies from 6 to 50 Hz, in 2 Hz steps with 4 Hz bandwidth. A two-way least squares FIR filter (eegfilt.m in EEGLAB) was used because this filtering method does not distort phase (63). Second, a Hilbert transform was applied to the filtered signals. Third, amplitude, phase, and ITC were calculated from Hilbert transformed signals. Amplitude indicates the total amplitude including prestimulus baseline activity and event-related spectral perturbations. ITC indicates phase consistency across trials and ranges from 0 (random phase across trials) to 1 (identical phase across trials). Amplitude and ITC during stimulation were averaged and used for statistical analyses (Figure S1 in the Supplement).
For analysis of cross-frequency coupling, we focused on theta (4–8 Hz) and gamma oscillations (38–40Hz) since previous studies (33–34, 36, 38) showed that phase of theta oscillations modulated amplitude of gamma oscillations. Theta phase and gamma amplitude during stimulation from all epochs were concatenated in each subject. Gamma amplitude was sorted according to theta phase. Theta phase was divided into 6 bins of 60 degrees (−180 to −120, −120 to −60, −60 to 0, 0 to 60, 60 to 120, 120 to 180). Mean gamma amplitude was calculated in each bin. We compared gamma amplitude in each bin of theta phase using analysis of variance (ANOVA). A modulation index (33) was computed to quantitatively assess the strength of cross-frequency coupling. Gamma amplitude was divided by the mean gamma amplitude to obtain an index of relative gamma amplitude in each subject since large gamma amplitude produces large modulation indices regardless of cross-frequency coupling strength (64). We composed a complex-valued signal z by combining relative gamma amplitude AGA with theta phase θTH: z = AGA eiθTH. A complex value of z at each time point was plotted in the complex plane (Figure S2 in the Supplement). The average of z at all time points was calculated and shown as a mean vector. The modulation index is the length of the mean vector. Identical procedures were used for surrogate data that was created by pairing theta phase with gamma amplitude from randomly shuffled trials. We used the modulation index from surrogate data to differentiate true cross-frequency coupling with spurious cross-frequency coupling. Log transformation of the modulation index was used for statistical analysis as reported by Penny and colleagues (65).
Statistical Analyses
Statistical analyses were performed with PASW Statistic (IBM Corporation, Somers, New York). Chi-square tests were used to assess differences in sex distribution. Age effects were assessed using 2-way (group-by-sex) ANOVA. Differences in clinical symptoms between sexes in schizophrenia patients were assessed with t-tests. Differences in neuropsychological performances and amplitude and ITC of neural oscillations were assessed with 2-way (group-by-sex) ANOVA. Cross-frequency coupling was analyzed in a repeated-measures ANOVA with group and sex as between-subjects factors and theta phase as a within-subjects factor with Greenhouse-Geisser epsilon adjustment. The modulation index was analyzed in a repeated-measures ANOVA with group and sex as between-subjects factors and data (observed data and surrogate data) as a within-subjects factor. Spearman correlations were performed to assess the relationships of EEG measures to cognitive measures. Differences in correlation coefficients between groups were assessed with Fisher’s z transformation. All statistical comparisons were two-tailed with α-level = 0.05. We used Bonferroni correction to correct for the effect of multiple comparisons in neural oscillations (6 ANOVAs (theta amplitude, theta ITC, gamma amplitude, gamma ITC, cross-frequency coupling, modulation index): p = .05/6 = .008), neuropsychological performances (7 ANOVAs: p = .05/7 = .007), and their correlations (7 cognitive measures × 5 EEG measures (theta amplitude, theta ITC, gamma amplitude, gamma ITC, modulation index) = 0.05/35 = 0.001).
Results
Sample Characteristics
Table 1 shows demographic, clinical, and cognitive characteristics of the subjects. There were a greater proportion of males among schizophrenia patients than among healthy control subjects. Analyses of age revealed no significant effects of group or sex, or no significant group-by-sex interaction [F(1,418) < 1.71, p > .19]. There were no significant differences in age, duration of illness, SAPS, SANS, GAF (−1.20 < t232 < .82, p > .23) between male and female schizophrenia patients.
Compared to healthy control subjects, schizophrenia patients had significantly worse performance in all of the neuropsychological tests. Analyses of performance in neuropsychological tests revealed significant effects of sex in CVLT-2 List A trial 1–5 total score (Female > Male) [F(1,418) = 8.93, p = .003]. There were no other significant effects of sex or no significant group-by-sex interactions in neuropsychological tests [F(1,418) < 3.82, p > .05].
Theta and Gamma Oscillations
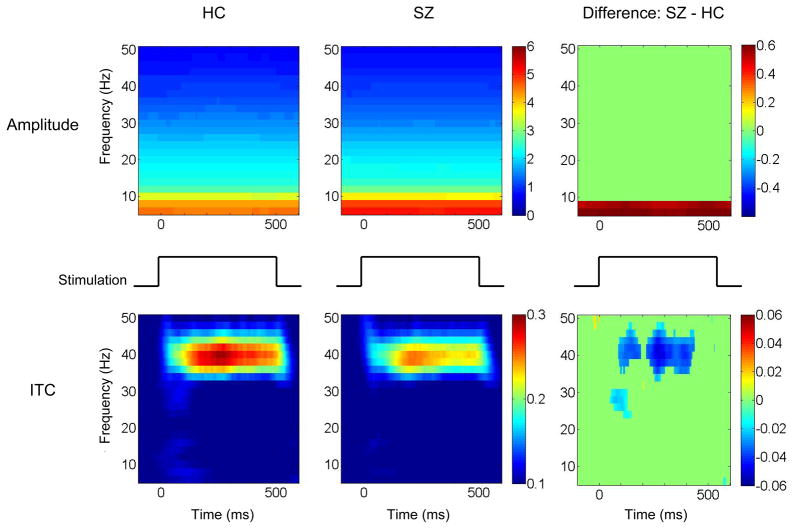
Figure 2 shows time-frequency maps. In the exploratory analysis, t-tests revealed differences (p < .01) between groups in both theta and gamma frequency responses. Thus, theta oscillations (4–8 Hz) and gamma oscillations (38–42 Hz) served as the focus of further analyses.
Figure 2.
Schizophrenia patients have increased theta amplitude and decreased gamma synchrony. The left column shows time-frequency maps from healthy control subjects and the middle column shows time-frequency maps from schizophrenia patients. The x-axis indicates time in milliseconds and the y-axis indicates frequency. Color indicates amplitude in the top row and intertrial phase coherence (ITC) in the bottom row. The right column shows difference between schizophrenia patients and healthy control subjects. Difference maps show only time-frequency points at p < 0.01. Abbreviations: HC, healthy control subjects; SZ, schizophrenia patients.
Schizophrenia patients had significantly larger theta amplitude and smaller gamma ITC compared to healthy control subjects (Table 2). There were no significant group differences in either theta ITC or gamma amplitude. Analysis of theta and gamma oscillations revealed significant effects of sex in theta amplitude (Female > Male) [F(1,418) = 19.48, p < .001] and in gamma amplitude (Female > Male) [F(1,418) = 17.45, p < .001]. There were no significant sex differences or no significant group-by-sex interaction in theta ITC and gamma ITC [F(1,418) < 3.67, p > .06].
Table 2.
Amplitude and intertrial phase coherence of theta and gamma oscillations
| EEG variables | HC (n = 188) | SZ (n = 234) | F1,418 | P |
|---|---|---|---|---|
| Theta amplitude (μV) | 4.493 (1.483) | 5.091 (2.240) | 22.09 | < .001 |
| Theta ITC | 0.100 (0.032) | 0.095 (0.031) | 1.58 | .21 |
| Gamma amplitude (μV) | 1.050 (0.260) | 1.054 (0.321) | 1.34 | .25 |
| Gamma ITC | 0.251 (0.101) | 0.220 (0.093) | 10.00 | .002 |
Data are given as mean (SD). Abbreviations: HC, Healthy control subjects; SZ, schizophrenia patients; ITC, intertrial phase coherence
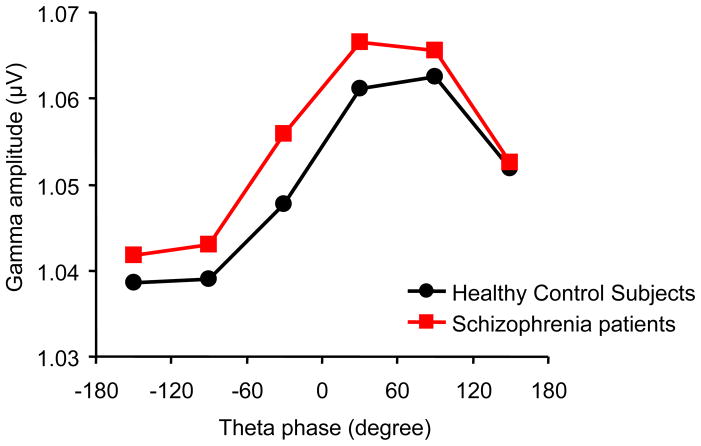
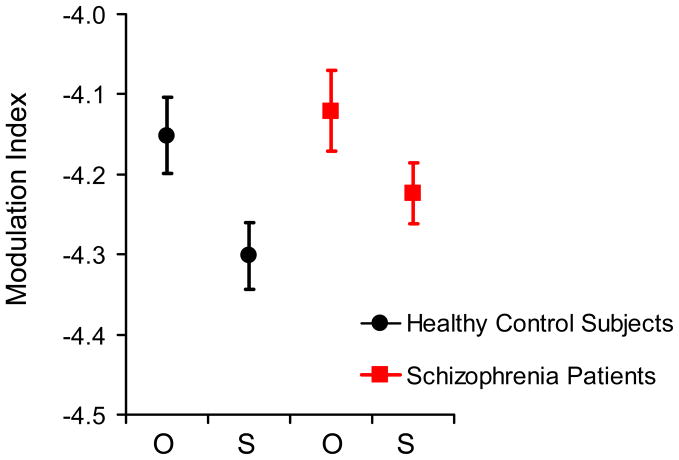
Analysis of cross-frequency coupling (Figure 3) revealed a significant main effect of theta phase on gamma amplitude [F(5,2090) = 39.66, p < .001]. Theta phase showed no significant interactions with group, sex, or group-by-sex [F(5,2090) < 1.31, p > .27]. These results indicate that theta phase modulates gamma amplitude in both healthy control subjects and schizophrenia patients. Because we found significant cross-frequency coupling, we used a modulation index to analyze the strength of cross-frequency coupling. The modulation index from the observed data was significantly larger than the modulation index from surrogate data [F(1,418) = 7.04, p = .008], indicating significant cross-frequency coupling (Figure 4). Analysis of the modulation index revealed no significant effects of group or sex, or no significant interactions [F(1,418) < 2.23, p > .14]. These results indicate that there is significant cross-frequency coupling in both healthy control subjects and schizophrenia patients and that there is no group difference in strength of cross-frequency coupling.
Figure 3.
The amplitude of stimulus-driven gamma oscillations is modulated by the phase of ongoing theta oscillations. This cross-frequency coupling indicates a hierarchical organization of cortical oscillatory dynamics in both healthy control subjects (black line) and schizophrenia patients (red line). The x-axis indicates theta phase. The y-axis indicates gamma amplitude.
Figure 4.
Schizophrenia patients have normal theta-phase/gamma-amplitude cross-frequency coupling. The modulation index demonstrates the relative strength of cross-frequency coupling via comparison of observed (O) vs. resampled or “surrogate” (S) EEG data in healthy control subjects (black circle) and schizophrenia patients (red squares). The y-axis indicates log transform of modulation index.
Neural Oscillations and Cognitive Measures
Theta amplitude was significantly correlated with CVLT-2 List A trial 1–5 total score in schizophrenia patients (rs = −.36, p < .001) but not in the healthy control subjects (rs = −.10, p = .19). The difference in this correlation coefficient between groups was significant (z = −4.79, p < .001). Because there were sex differences in theta amplitude and CVLT-2 List A trial 1–5 total score, correlations were separately analyzed for each sex. Theta amplitude was significantly correlated with CVLT-2 List A trial 1–5 total score in both male (rs = −.38, p < .001) and female (rs = −.46, p < .001) schizophrenia patients but in neither male (rs = .05, p = .64) nor female (rs = .06, p = .58) healthy control subjects (Figure S3). The differences in these correlation coefficients between groups were significant in both male (z = −3.45, p < .001) and female subjects (z = −3.09, p = .002). There were no other significant correlations between EEG measures and cognitive measures.
Discussion
The results of the present study demonstrate that schizophrenia patients exhibit increased theta amplitude and reduced gamma intertrial phase coherence during auditory steady-state stimulation. In addition, theta phase modulates gamma amplitude, and theta amplitude correlates with verbal memory performance. The finding of normal theta-gamma cross-frequency coupling indicates that schizophrenia patients have an intact hierarchical organization of neural oscillatory dynamics that is similar to healthy control subjects, but that schizophrenia patients have alterations in several components of this organization, augmenting previous findings that schizophrenia patients have alterations in gamma oscillations (43, 46, 49–50). To our knowledge, this is the first report of cross-frequency coupling between theta phase and gamma amplitude during auditory steady-state stimulation in schizophrenia patients.
The finding that theta phase modulates gamma amplitude is consistent with previous studies of LFP (34), intracranial EEG (33, 38), and scalp EEG (36). Cross-frequency coupling between theta phase and gamma amplitude has been observed in hippocampus (38) and cerebral cortex (33) including auditory cortex (34) where ASSRs are generated (43, 66). Gamma amplitude was largest at approximately 60 degrees of theta phase, consistent with some (34, 36), but not all studies which also reported that gamma amplitude was largest in 0 degree (38) or 180 degrees (33) of the theta phase. Such discrepancies suggest that the excitatory phase for surface-recorded gamma oscillations may vary according to the task demands and neural generators of these waveforms.
In contrast to our expectations, schizophrenia patients had no alterations in cross-frequency coupling. This result is unexpected because theta-gamma phase-amplitude cross-frequency coupling is thought to play a critical role in sensory and cognitive operations. Nonetheless, schizophrenia patients did exhibit abnormalities in both theta and gamma components in the context of an apparently intact hierarchical organization of neural oscillations. Because several factors such as signal-to-noise ratio can influence the measurement of cross-frequency coupling (64–65), it is possible that abnormalities in theta and/or gamma oscillations may affect the finding of normal cross-frequency coupling in schizophrenia. The inability to detect significant deficits in cross-frequency coupling, however, does not appear to be due to methodological limitations since two different methods revealed a similar pattern of results – normal cross-frequency coupling in schizophrenia patients. In contrast, the failure to detect abnormalities in cross-frequency coupling may be due to the simple and passively elicited ASSR task used in this study, which does not require the substantial engagement of neural circuits associated with higher-order and integrative cognitive mechanisms. Thus, this paradigm may not be optimal for revealing cross-frequency coupling deficits since the present study of 422 individuals was adequately powered to detect even small effect-size differences. Since cross-frequency coupling may be task-dependent, the present results do not preclude the possibility that schizophrenia patients may have cross-frequency coupling abnormalities in tasks that depend upon more distributed neural systems.
The finding of increased theta amplitude in schizophrenia patients is consistent with previous studies that showed augmented resting state theta power in schizophrenia (45). In the present study, increased theta amplitude was associated with worse verbal memory performance in schizophrenia patients. Increased theta amplitude in schizophrenia patients is present in the temporal lobe (67) including the auditory cortex (68–69) where schizophrenia patients show decreased gray matter volume (70). Therefore, abnormal theta amplitude may reflect pathological processes that are associated with verbal memory deficits in schizophrenia.
Important sex differences were also revealed in the present study. Female subjects showed increased theta amplitude relative to males in both schizophrenia patients and healthy control subjects. Female subjects also showed better verbal memory performance relative to males in both schizophrenia patients and healthy control subjects. The sex difference in theta amplitude, however, do not account for the observed sex differences in verbal memory performance since theta amplitude was not associated with verbal memory performance in healthy control subjects, and increased theta amplitude was associated with worse verbal memory performance in schizophrenia patients. Additional studies of potential sex difference in neural activity associated with theta oscillations are necessary to fully explain the present pattern of results.
The finding of reduced gamma ITC in schizophrenia patients is consistent with previous studies (43, 46, 50). Reduced gamma ITC indicates that gamma phase at a given latency is inconsistent across trials. Imprecise gamma phase synchronization in schizophrenia may affect various sensory and cognitive processes since gamma phase modulates firing rates of neurons (71) and neuronal interactions (72). In contrast to our expectations and previous results (46), no significant correlations were observed between reduced gamma ITC and cognitive functions in this study. As noted above, this may be due to the task used in this study. Gamma ITC during auditory steady-state stimulation may not fully engage neural networks associated with higher cognitive functions because the auditory steady-state task is a passive task, and ASSRs are predominantly generated in the auditory cortex (43, 66). Gamma amplitude did not differ between groups, but showed a difference between sexes with female subjects producing larger gamma amplitude compared to male subjects, consistent with a previous study (73).
There are several limitations in this study. First, medications were not experimentally controlled in the current study. Schizophrenia patients were treated with a variety of antipsychotic and other psychiatric medications, which may affect neural oscillations (74). Prospective studies are needed to clarify potential medication effects on neural oscillations and to further validate the use of oscillatory measures as biomarkers of drug response. Second, it is possible that small eye movements during testing (i.e., microsaccades) may have influenced the results of this study. Saccades can generate spurious gamma band activity in scalp EEG (75). Microsaccade artifact, however, results in broadband and transient increases in power whereas auditory steady-state stimulation occurs in response to a narrow band of stimulation and results in continuous increases in power and ITC. Nevertheless, gamma amplitude and cross-frequency coupling may be affected by saccades. To account for this possibility, we performed the same analyses on the horizontal electrooculogram--no significant main effects or interactions in gamma amplitude or cross-frequency coupling (all F < 2.02, all p > .13) were present, highly reducing the likelihood of prominent saccade-induced artifact contamination oscillatory dynamics. Third, delta oscillations were not analyzed in this study since we could not apply the methods used in this study to delta oscillations, given the long epochs (i.e., >9 seconds) of artifact-free EEG segments required to measure delta activity with confidence in the current recordings. Delta phase may also be coupled with gamma amplitude since delta oscillations are entrained by rhythmic external stimuli and align the excitatory phase with attended stimuli (39). Thus, future studies are needed to assess delta-gamma cross-frequency coupling in schizophrenia.
In conclusion, schizophrenia patients had intact cross-frequency coupling, increased theta amplitude, and reduced gamma intertrial phase coherence. These findings suggest that schizophrenia patients have alterations in gamma and theta oscillations. Despite the deficits in gamma and theta oscillations, a hierarchical organization of neural oscillations is relatively preserved in schizophrenia patients in response to gamma frequency stimulation. Neural oscillations in different frequency bands are associated with distinct aspects of cognitive information processing. The interactions among different frequency bands appear to serve integrative cognitive functions. Future studies are needed to disentangle potential frequency-specific neural oscillatory alternations in schizophrenia under a variety of cognitive challenges and across the course of schizophrenia.
Supplementary Material
Acknowledgments
This study was supported by grants from the Department of Veterans Affairs (VISN 22 Mental Illness Research, Education, and Clinic Center) and grants MH079777,MH065571, and MH042228 from the National Institute of Mental Health, and the National Alliance for Research on Schizophrenia and Depression.
The part of this study was presented at the Society of Biological Psychiatry 66th Annual Meeting at San Francisco on May 13, 2011.
Footnotes
Financial Disclosures
Dr. Light has received financial compensation from Astellas for consulting unrelated to this project. All other authors report no biomedical financial interest or potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Braff DL, Light GA. Preattentional and attentional cognitive deficits as targets for treating schizophrenia. Psychopharmacology (Berl) 2004;174:75–85. doi: 10.1007/s00213-004-1848-0. [DOI] [PubMed] [Google Scholar]
- 2.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 3.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 4.Rissling AJ, Makeig S, Braff DL, Light GA. Neurophysiologic markers of abnormal brain activity in schizophrenia. Curr Psychiatry Rep. 2010;12:572–578. doi: 10.1007/s11920-010-0149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophr Bull. 2007;33:69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajos M. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7:68–83. doi: 10.1038/nrd2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 8.Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci U S A. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tallon-Baudry C, Bertrand O, Delpuech C, Pernier J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J Neurosci. 1996;16:4240–4249. doi: 10.1523/JNEUROSCI.16-13-04240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez E, George N, Lachaux JP, Martinerie J, Renault B, Varela FJ. Perception’s shadow: long-distance synchronization of human brain activity. Nature. 1999;397:430–433. doi: 10.1038/17120. [DOI] [PubMed] [Google Scholar]
- 11.Tiitinen H, Sinkkonen J, Reinikainen K, Alho K, Lavikainen J, Naatanen R. Selective attention enhances the auditory 40-Hz transient response in humans. Nature. 1993;364:59–60. doi: 10.1038/364059a0. [DOI] [PubMed] [Google Scholar]
- 12.Debener S, Herrmann CS, Kranczioch C, Gembris D, Engel AK. Top-down attentional processing enhances auditory evoked gamma band activity. Neuroreport. 2003;14:683–686. doi: 10.1097/00001756-200304150-00005. [DOI] [PubMed] [Google Scholar]
- 13.Gruber T, Tsivilis D, Montaldi D, Muller MM. Induced gamma band responses: an early marker of memory encoding and retrieval. Neuroreport. 2004;15:1837–1841. doi: 10.1097/01.wnr.0000137077.26010.12. [DOI] [PubMed] [Google Scholar]
- 14.Herrmann CS, Lenz D, Junge S, Busch NA, Maess B. Memory-matches evoke human gamma-responses. BMC Neurosci. 2004;5:13. doi: 10.1186/1471-2202-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced gamma-band activity during the delay of a visual short-term memory task in humans. J Neurosci. 1998;18:4244–4254. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woo TU, Spencer K, McCarley RW. Gamma oscillation deficits and the onset and early progression of schizophrenia. Harv Rev Psychiatry. 2010;18:173–189. doi: 10.3109/10673221003747609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Y, Farzan F, Barr MS, Kirihara K, Fitzgerald PB, Light GA, et al. Gamma oscillations in schizophrenia: Mechanisms and clinical significance. Brain Res. 2011;1413:98–114. doi: 10.1016/j.brainres.2011.06.065. [DOI] [PubMed] [Google Scholar]
- 18.Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uhlhaas PJ, Linden DE, Singer W, Haenschel C, Lindner M, Maurer K, et al. Dysfunctional long-range coordination of neural activity during Gestalt perception in schizophrenia. J Neurosci. 2006;26:8168–8175. doi: 10.1523/JNEUROSCI.2002-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clementz BA, Blumenfeld LD, Cobb S. The gamma band response may account for poor P50 suppression in schizophrenia. Neuroreport. 1997;8:3889–3893. doi: 10.1097/00001756-199712220-00010. [DOI] [PubMed] [Google Scholar]
- 21.Wynn JK, Light GA, Breitmeyer B, Nuechterlein KH, Green MF. Event-related gamma activity in schizophrenia patients during a visual backward-masking task. Am J Psychiatry. 2005;162:2330–2336. doi: 10.1176/appi.ajp.162.12.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallinat J, Winterer G, Herrmann CS, Senkowski D. Reduced oscillatory gamma-band responses in unmedicated schizophrenic patients indicate impaired frontal network processing. Clin Neurophysiol. 2004;115:1863–1874. doi: 10.1016/j.clinph.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Haenschel C, Bittner RA, Waltz J, Haertling F, Wibral M, Singer W, et al. Cortical oscillatory activity is critical for working memory as revealed by deficits in early-onset schizophrenia. J Neurosci. 2009;29:9481–9489. doi: 10.1523/JNEUROSCI.1428-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barr MS, Farzan F, Tran LC, Chen R, Fitzgerald PB, Daskalakis ZJ. Evidence for excessive frontal evoked gamma oscillatory activity in schizophrenia during working memory. Schizophr Res. 2010;121:146–152. doi: 10.1016/j.schres.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 26.Basar-Eroglu C, Brand A, Hildebrandt H, Karolina Kedzior K, Mathes B, Schmiedt C. Working memory related gamma oscillations in schizophrenia patients. Int J Psychophysiol. 2007;64:39–45. doi: 10.1016/j.ijpsycho.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Daskalakis ZJ, Fitzgerald PB, Christensen BK. The role of cortical inhibition in the pathophysiology and treatment of schizophrenia. Brain Res Rev. 2007;56:427–442. doi: 10.1016/j.brainresrev.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Gandal MJ, Edgar JC, Klook K, Siegel SJ. Gamma synchrony: Towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 30.Green MF, Nuechterlein KH. Cortical oscillations and schizophrenia: timing is of the essence. Arch Gen Psychiatry. 1999;56:1007–1008. doi: 10.1001/archpsyc.56.11.1007. [DOI] [PubMed] [Google Scholar]
- 31.Light GA. Probing cortico-cortical interactions that underlie the multiple sensory, cognitive, and everyday functional deficits in schizophrenia. Behav Brain Sci. 2004;27:799. [Google Scholar]
- 32.Moran LV, Hong LE. High vs Low Frequency Neural Oscillations in Schizophrenia. Schizophr Bull. 2011;37:659–663. doi: 10.1093/schbul/sbr056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, et al. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, Schroeder CE. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J Neurophysiol. 2005;94:1904–1911. doi: 10.1152/jn.00263.2005. [DOI] [PubMed] [Google Scholar]
- 35.Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci. 2010;14:506–515. doi: 10.1016/j.tics.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demiralp T, Bayraktaroglu Z, Lenz D, Junge S, Busch NA, Maess B, et al. Gamma amplitudes are coupled to theta phase in human EEG during visual perception. Int J Psychophysiol. 2007;64:24–30. doi: 10.1016/j.ijpsycho.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 37.von Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophysiol. 2000;38:301–313. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- 38.Axmacher N, Henseler MM, Jensen O, Weinreich I, Elger CE, Fell J. Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc Natl Acad Sci U S A. 2010;107:3228–3233. doi: 10.1073/pnas.0911531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- 40.Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32:9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lisman J, Buzsaki G. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophr Bull. 2008;34:974–980. doi: 10.1093/schbul/sbn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lisman JE, Idiart MA. Storage of 7 +/−2 short-term memories in oscillatory subcycles. Science. 1995;267:1512–1515. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- 43.Spencer KM, Niznikiewicz MA, Nestor PG, Shenton ME, McCarley RW. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC Neurosci. 2009;10:85. doi: 10.1186/1471-2202-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allen EA, Liu J, Kiehl KA, Gelernter J, Pearlson GD, Perrone-Bizzozero NI, et al. Components of cross-frequency modulation in health and disease. Front Syst Neurosci. 2011;5:59. doi: 10.3389/fnsys.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boutros NN, Arfken C, Galderisi S, Warrick J, Pratt G, Iacono W. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophr Res. 2008;99:225–237. doi: 10.1016/j.schres.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, Swerdlow NR, et al. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry. 2006;60:1231–1240. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 47.Azzena GB, Conti G, Santarelli R, Ottaviani F, Paludetti G, Maurizi M. Generation of human auditory steady-state responses (SSRs). I: Stimulus rate effects. Hear Res. 1995;83:1–8. doi: 10.1016/0378-5955(94)00184-r. [DOI] [PubMed] [Google Scholar]
- 48.Vohs JL, Chambers RA, Krishnan GP, O’Donnell BF, Berg S, Morzorati SL. GABAergic modulation of the 40 Hz auditory steady-state response in a rat model of schizophrenia. Int J Neuropsychopharmacol. 2010;13:487–497. doi: 10.1017/S1461145709990307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwon JS, O’Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brenner CA, Krishnan GP, Vohs JL, Ahn WY, Hetrick WP, Morzorati SL, et al. Steady state responses: electrophysiological assessment of sensory function in schizophrenia. Schizophr Bull. 2009;35:1065–1077. doi: 10.1093/schbul/sbp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr Bull. 2008;34:907–926. doi: 10.1093/schbul/sbn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I, Version 2.0) New York, NY: New York State Psychiatric Institute; 1995. [Google Scholar]
- 53.Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- 54.Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- 55.Hall RC. Global assessment of functioning. A modified scale. Psychosomatics. 1995;36:267–275. doi: 10.1016/S0033-3182(95)71666-8. [DOI] [PubMed] [Google Scholar]
- 56.Wilkinson GS. WRAT3 Wide Range Achievement Test, Administration Manual. Wilmington, DE: Wide Range Inc; 1993. [Google Scholar]
- 57.Delis DC, Kramer JH, Kaplan E, Ober BA. CVLT-II California Verbal Learning Test. 2. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 58.Heaton RK. Wisconsin Card Sorting Test Manual - Revised and Expanded. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- 59.Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry. 1997;54:159–165. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- 60.Perry W, Heaton RK, Potterat E, Roebuck T, Minassian A, Braff DL. Working memory in schizophrenia: transient “online” storage versus executive functioning. Schizophr Bull. 2001;27:157–176. doi: 10.1093/oxfordjournals.schbul.a006854. [DOI] [PubMed] [Google Scholar]
- 61.Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- 62.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 63.Cohen MX. Assessing transient cross-frequency coupling in EEG data. J Neurosci Methods. 2008;168:494–499. doi: 10.1016/j.jneumeth.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 64.Tort AB, Komorowski R, Eichenbaum H, Kopell N. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol. 2010;104:1195–1210. doi: 10.1152/jn.00106.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Penny WD, Duzel E, Miller KJ, Ojemann JG. Testing for nested oscillation. J Neurosci Methods. 2008;174:50–61. doi: 10.1016/j.jneumeth.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hamm JP, Gilmore CS, Picchetti NA, Sponheim SR, Clementz BA. Abnormalities of neuronal oscillations and temporal integration to low- and high-frequency auditory stimulation in schizophrenia. Biol Psychiatry. 2011;69:989–996. doi: 10.1016/j.biopsych.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siekmeier PJ, Stufflebeam SM. Patterns of spontaneous magnetoencephalographic activity in patients with schizophrenia. J Clin Neurophysiol. 2010;27:179–190. doi: 10.1097/WNP.0b013e3181e0b20a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Canive JM, Lewine JD, Edgar JC, Davis JT, Torres F, Roberts B, et al. Magnetoencephalographic assessment of spontaneous brain activity in schizophrenia. Psychopharmacol Bull. 1996;32:741–750. [PubMed] [Google Scholar]
- 69.Ishii R, Shinosaki K, Ikejiri Y, Ukai S, Yamashita K, Iwase M, et al. Theta rhythm increases in left superior temporal cortex during auditory hallucinations in schizophrenia: a case report. Neuroreport. 2000;11:3283–3287. doi: 10.1097/00001756-200009280-00047. [DOI] [PubMed] [Google Scholar]
- 70.Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Onitsuka T, Spencer MH, et al. Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2003;60:766–775. doi: 10.1001/archpsyc.60.8.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Womelsdorf T, Schoffelen JM, Oostenveld R, Singer W, Desimone R, Engel AK, et al. Modulation of neuronal interactions through neuronal synchronization. Science. 2007;316:1609–1612. doi: 10.1126/science.1139597. [DOI] [PubMed] [Google Scholar]
- 73.Jausovec N, Jausovec K. Resting brain activity: differences between genders. Neuropsychologia. 2010;48:3918–3925. doi: 10.1016/j.neuropsychologia.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 74.Hong LE, Summerfelt A, McMahon R, Adami H, Francis G, Elliott A, et al. Evoked gamma band synchronization and the liability for schizophrenia. Schizophr Res. 2004;70:293–302. doi: 10.1016/j.schres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 75.Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY. Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron. 2008;58:429–441. doi: 10.1016/j.neuron.2008.03.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.