Abstract
BACKGROUND:
Apolipoprotein E (ApoE) gene encodes an important protein in reforming injuries of central nervous system (CNS). It is assumed that various ApoE alleles may be functionally different. The purpose of this study was to investigate the distribution of ApoE genotypes in multiple sclerosis (MS) patients in a small cohort of Iranians.
METHODS:
In this case-control study, blood samples of patients and healthy volunteers were collected (n = 40) from Neurology Clinic of Alzahra Medical Complex. The ApoE genotypes were determined using DNA extracted from the samples by polymerase chain reaction (PCR) techniques followed by digestion with HhaI restriction enzyme. The results were adjusted for age of MS onset, sex, expanded disability status scale (EDSS), and type of MS (primary or secondary progressive). Results were statistically analyzed using chi-square test.
RESULTS:
The ApoE3/E3 genotype was detected in the majority of MS patients and the control group. Frequency distribution of E4 allele did not differ significantly between the two groups. There was no difference between ApoE allele and age of disease onset, sex, expanded disability status, or type of multiple sclerosis.
CONCLUSIONS:
We found no significant differences in genotype frequency between patients with multiple sclerosis and the control group. Despite the fact that small sample size was a limitation for our study, it seems that ApoE polymorphism may not be useful as a marker for screening patients with multiple sclerosis.
KEYWORDS: Apolipoprotein E, Allele, Multiple Sclerosis, Polymorphism, Gene
Multiple sclerosis (MS) is the most common disorder of central nervous system (CNS) which exerts chronic inflammatory attack to CNS leading to demyelization and thus nerve degeneration in brain and spinal cord. MS is a heterogeneous disease causing multiple disturbances in a number of body functions.1–3 It predominantly affects the young population aging 16-35 years.4 In Asia, MS frequency varies from 3 to 5 cases in 100000 individuals. However, it has been reported as 25 to 30 cases in 100000 in Isfahan.5
A large number of studies have investigated the relationship between MS and genomic factors. One of the gene regions that may be influenced in MS is chromosome 19 where Apolipoprotein E (ApoE) gene is located.6–10 ApoE is one of the most important carriers of cholesterol and phospholipids in brain and nervous system.11
ApoE gene is located on the long arm of chromosome 19 and encodes a 299 amino acid polypeptide. It has three functionally distinct isoforms called ApoE2, ApoE3, and ApoE4. Such variation is caused by single nucleotide polymorphism (SNP) resulting in amino acid substitutions in codons 112 and 158.12–14 Brain is an important producer of ApoE after liver.7 Recent studies have shown that ApoE may have a role in regeneration of axons and myelin after injuries and lesions to central and peripheral nervous tissues. In vitro studies have recognized ApoE as a nerve growth factor.9 In regard to the role of ApoE in brain repair, most studies have focused on its immunomodulatory effect. It is assumed that ApoE suppresses the release of cytokines which are responsible for development of chronic inflammatory process in progression of MS. These inflammatory processes are also important in pathogenesis of MS.15
ApoE isoforms have been shown to act differently in modulation of inflammatory responses. According to a study by Michikawa et al., promotion of lipid efflux from cultured astrocytes and neurons depends on genotypic properties of ApoE alleles.16 Many studies have shown that +E4 patients have a greater risk for developing CNS disabilities,17,18 earlier onset of the disease, more frequent relapses,10 and poorer recovery following relapses9 compared to - E4 patients. ApoE polymorphism is also an important risk factor in development and progression of Alzheimer's disease.7
Although many investigators have studied the role of ApoE polymorphism in patients with MS, there are still some controversies.7,9 Some studies have suggested ApoE polymorphism as a risk factor for severity and progression of MS.7,9,10 However, others could not find a relation between ApoE polymorphism and risk of MS progression or severity.8,14,15,19,20 The aim of this study was to investigate the frequency of various ApoE alleles in patients with MS. The answer to this question may help us know whether ApoE alleles could be used as a screening factor for patients with MS.
Methods
Sample collection:
In this case-control study, whole blood samples were collected from 20 normal people and 20 patients (16 female and 4 male) from Isfahan, Iran whom their affliction with MS had been diagnosed by a consultant neurologist. Patients were selected by simple convenient method in a period of seven months. Eligible patients or their legally eligible companions were informed by one of the investigators about the whole study. Participation in the study was optional. Written informed consents were signed by all the included patients after the interview. Discontinuing participation in the study was permitted, without any fear of losing routine medical care. The study protocol was approved by Board of Human Studies, Isfahan University of Medical Sciences. The patients included in this study aged 20 to 40 (35.03 ± 8.2) years. and unrelated ethnically matched healthy blood donors with no history of MS or any other diseases related to the function of ApoE alleles (such as Alzheimer, atherosclerosis and stroke). Disability of patients was assessed using Kurtzke Expanded Disability Status Scale (EDSS).7 Clinical data of patients (including the age of onset of disease, sex, EDSS and type of MS) and blood samples were collected by ZM with the assistance of a third party health care professional.
DNA extraction:
Peripheral blood was obtained during regular follow-up visits. Whole blood samples were collected in ethylenediaminetetraacetic acid (EDTA) stained test tubes and the corpuscular fraction was frozen and stored at -20°C until the time of DNA extraction. Isolation of DNA from blood samples was performed by means of a standard DNA extraction kit (Roche, Germany). The extracted DNA was used as the template for polymerase chain reaction (PCR).21
PCR:
The PCR mixture consisted of 200 ng of genomic DNA, 1X reaction buffer (Bio Ran), 10 pmol of each primer, including forward (5’-ACAGAATTCGCCCCGGCCTGGTACAC-3’) and reverse (5’-TAAGCTTGGCACGGCTGTCCAAGGA-3’), 0.8 mM deoxyribonucleotide triphosphate (dNTP) (Boiron, Poland), 0.5 unit Taq DNA polymerase (Boiron, Poland), and 1.5 ml 1, 2-propandiol (to enhance the efficacy of PCR reaction) in a total volume of 25 μl.
PCR was performed (Bio-Rad thermal cycler, USA) for 35 cycles of denaturation at 94°C for 1 minute. Annealing temperature was set to 64.2°C for 2 minutes and extension temperature was 72°C for 3 minutes. A final extension step of 72°C for 5 minutes was also included. The PCR products were separated by 1% agarose gel electrophoresis and visualized by ethidium bromide staining.
Amplified DNA was then analyzed by restriction pattern of ApoE alleles. For this purpose, 8 ml of the PCR product was digested with 10 units HhaI enzyme (Fermentas, Poland) for 4 hours. The digested fragments were separated by electrophoresis on a 15% polyacrylamide gel at 100 volt for 3 hours. Finally, polymorphic patterns were visualized by ethidium bromide.21,22
Statistical analysis:
The allele distribution in the control group versus the MS group was compared using Pearson's chi-square test. This statistical analysis was performed using SPSS11.0 (Chicago, USA) and the odds-ratio for difference in proportion, standard error and 95% confidence intervals were calculated.
Results
In this study, 20% of patients were men and 80% were women. Table 1 shows the frequency (percent) of different ApoE genotypes in normal controls versus clinical subtypes of MS.
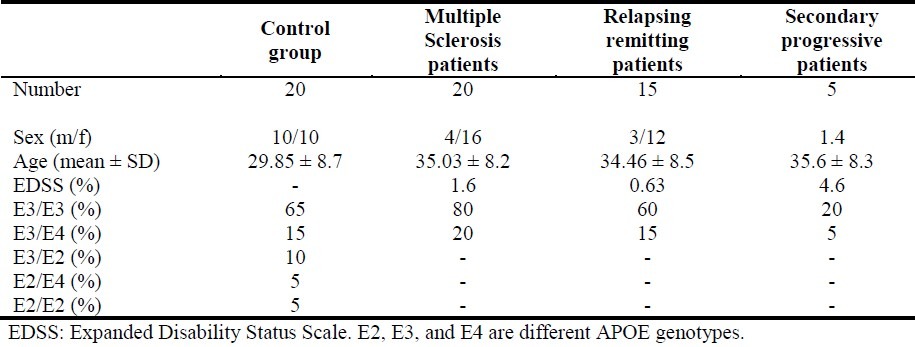
Table 1.
Patients’ demographic characteristics and distribution of ApoE genotypes
The ApoE genotyping was determined from the band pattern of restriction fragments. Besides bands composed of only a few base pairs, the ApoE2 allele gave clearly visible fragments of 104 and 91 base pairs. In addition, ApoE3 fragments were of 91, 48 and 53 base pairs. Finally, ApoE4 gave 72, 48 and 53 base pair fragments (Figure 1).
Figure 1.
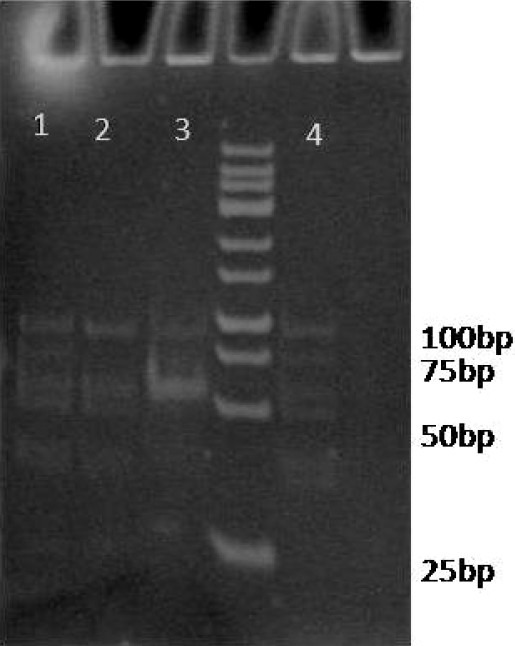
Digestion of PCR amplified ApoE gene with HhaI restriction enzyme
Lanes 1 and 4 show heterozygote E3/E4 genotype (91, 72, 48, and 53 bp bands). Lanes 2 and 3 show homozygote E3/E3 genotype (91, 48, and 53 bp bands).
Percentages of patients with MS with E3/E3 and E3/E4 genotypes were 80% and 20%, respectively. In these patients, E2 allele did not exist. In the control group, the frequency of E3/E3, E3/E4, E3/E2, E2/E4, and E2/E2 genotypes were 65%, 15%, 10%, 5%, and 5%, respectively.
The results indicated that the frequency of E3 allele was greater than other alleles in both patients and controls. However, the ApoE genotype frequency distribution in MS population was not significantly different from the controls. Moreover, the frequency of E4 allele was similar in both groups.
The relapsing remitting (RR) patients showed lower degree of EDSS factor compared to secondary progressive (SP) patients, but this difference was not statistically significant.
Figure 2 shows the relationship of patients’ E3/E4 genotype and the type of MS (RR or SP).
Figure 2.
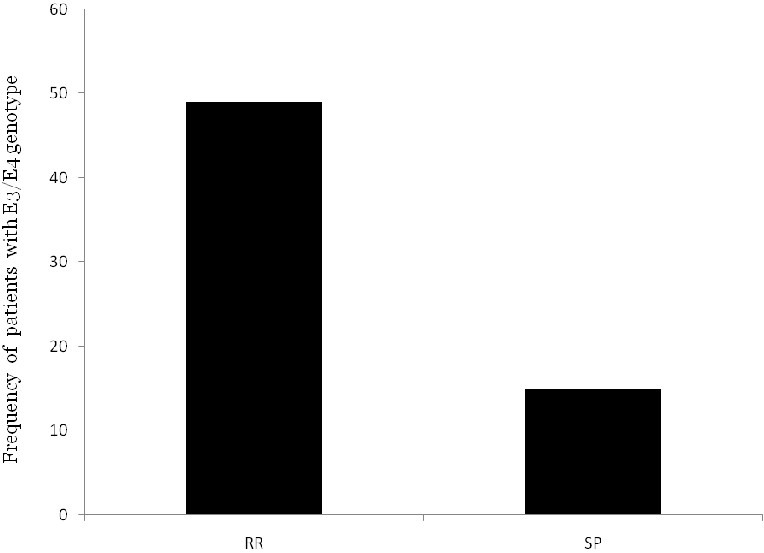
The frequency of E3/E4 genotype of the patients in different types of MS (RR and SP)
Discussion
MS is an inflammatory neurodegenerative disorder. Although the etiology of MS is not clear, MS pathology is suggested to affect genetically predisposed individuals. On the other hand, infection, injuries, diet, and stress may trigger the onset of this disease. Amongst various hypotheses put forward to explain the pathology of MS, involvement of immune system has been vastly documented. In this process, activation of T-cells outside the CNS and their migration into the brain parenchyma would lead to the synthesis of anti-myelin antibodies. It ultimately forms a site of inflammatory demyelination.4
Many studies have investigated the correlation between MS and various genes, amongst which the role of HLA allele system in pathogenesis of MS has been documented. Other target genes such as ApoE are under investigation.4
In an attempt to find a genetic marker to screen patients who are more susceptible to MS, ApoE alleles were investigated in the present study. Our results demonstrated that E3 allele was the most common isoform, in both the controls and patients with MS. Rall et al. also reported similar findings.23
We found the frequency of +E4 allele to be identical in patients with MS and the control group. EDSS factor in the +E4 patients was not statistically different from the –E4 patients. This is in contrast with some published research showing a correlation between MS and ApoE polymorphism.7,9,10
Although SP patients showed a higher degree of EDSS compared to the RR group, the difference was not statically significant (Figure 2). Other investigators also have reported similar results.8,14,15,19
In this study, the prevalence of MS was greater in women than in men (80% vs. 20%) which might be related to the effect of estrogen on cytokines production.24 However, this hypothesis should be examined in future experiments.
Conclusion
We found no significant relationship between ApoE genotypes and MS. Therefore, it seems that E4 allele cannot be used for screening patients with MS in Iran. However, further studies with larger sample sizes are needed to fully confirm these findings. Moreover, quantification of ApoE mRNA by real-time PCR in cerebro spinal fluid and blood can be helpful in finding the relationship between ApoE and MS. Finally, as mentioned above, based on our findings, it seems that ApoE gene may not be useful for screening patients with MS but its contribution in severity or progression of MS needs to be studied in future studies.
Authors’ Contributions
HMMS, AMS, MS, and SS participated in designing this study and supervising the research project. All the experiments were carried out by ZM as a part of her PharmD thesis. FM provided necessary technical support in the laboratory and helped in data interpretation.
Acknowledgments
This research was the result of an approved PharmD student (Zeinab Mousavian) thesis project (No. 388146) and was designed by the Isfahan Clinical Toxicology Research Center (http://ctrc.mui.ac.ir). The project was financially supported by the vice-chancellery of research of Isfahan University of Medical Sciences. The authors would like to thank Dr Kianoush Dormiani and Miss Farnaz Barneh for their help and support.
Footnotes
Conflict of Interests Authors have no conflict of interests.
References
- 1.Penkowa M, Hidalgo J. Treatment with metallothionein prevents demyelination and axonal damage and increases oligodendrocyte precursors and tissue repair during experimental autoimmune encephalomyelitis. J Neurosci Res. 2003;72(5):574–86. doi: 10.1002/jnr.10615. [DOI] [PubMed] [Google Scholar]
- 2.Oksenberg JR, Barcellos LF. Multiple sclerosis genetics: leaving no stone unturned. Genes Immun. 2005;6(5):375–87. doi: 10.1038/sj.gene.6364237. [DOI] [PubMed] [Google Scholar]
- 3.Dyment DA, Ebers GC, Sadovnick AD. Genetics of multiple sclerosis. Lancet Neurol. 2004;3(2):104–10. doi: 10.1016/s1474-4422(03)00663-x. [DOI] [PubMed] [Google Scholar]
- 4.Mustafina OE, Bakhtiiarova KZ, Mikhailova AM, Tuktarova IA, Khusainova AN, Nasibullin TR, et al. Analysis of the association of allelic variants of apolypoprotein E and interleukin 1 beta genes with multiple sclerosis in ethnic Tatars. Genetika. 2008;44(3):407–13. [PubMed] [Google Scholar]
- 5.Kurtzke JF. Multiple sclerosis in time and space--geographic clues to cause. J Neurovirol. 2000;6(Suppl 2):S134–S140. [PubMed] [Google Scholar]
- 6.Burwick RM, Ramsay PP, Haines JL, Hauser SL, Oksenberg JR, Pericak-Vance MA, et al. APOE epsilon variation in multiple sclerosis susceptibility and disease severity: some answers. Neurology. 2006;66(9):1373–83. doi: 10.1212/01.wnl.0000210531.19498.3f. [DOI] [PubMed] [Google Scholar]
- 7.Hogh P, Oturai A, Schreiber K, Blinkenberg M, Jorgensen OS, Ryder L, et al. Apoliprotein E and multiple sclerosis: impact of the epsilon-4 allele on susceptibility, clinical type and progression rate. Mult Scler. 2000;6(4):226–30. doi: 10.1177/135245850000600403. [DOI] [PubMed] [Google Scholar]
- 8.Ballerini C, Campani D, Rombola G, Gran B, Nacmias B, Amato MP, et al. Association of apolipoprotein E polymorphism to clinical heterogeneity of multiple sclerosis. Neurosci Lett. 2000;296(2-3):174–6. doi: 10.1016/s0304-3940(00)01646-3. [DOI] [PubMed] [Google Scholar]
- 9.Chapman J, Vinokurov S, Achiron A, Karussis DM, Mitosek-Szewczyk K, Birnbaum M, et al. APOE genotype is a major predictor of long-term progression of disability in MS. Neurology. 2001;56(3):312–6. doi: 10.1212/wnl.56.3.312. [DOI] [PubMed] [Google Scholar]
- 10.Parmenter BA, Denney DR, Lynch SG, Middleton LS, Harlan LM. Cognitive impairment in patients with multiple sclerosis: association with the APOE gene and promoter polymorphisms. Mult Scler. 2007;13(1):25–32. doi: 10.1177/1352458506070682. [DOI] [PubMed] [Google Scholar]
- 11.Zong Y, Liu Z, Bi H, Yao Y, Guo J, Qu S. Cloning and expression of apolipoprotein E3 and its variant apoE2 and apoE4. (16).J Huazhong Univ Sci Technolog Med Sci. 2006;26(1):1–3. doi: 10.1007/BF02828023. [DOI] [PubMed] [Google Scholar]
- 12.Rall SC, Jr, Weisgraber KH, Mahley RW. Human apolipoprotein E. The complete amino acid sequence. J Biol Chem. 1982;257(8):4171–8. [PubMed] [Google Scholar]
- 13.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240(4852):622–30. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 14.Pirttila T, Haanpaa M, Mehta PD, Lehtimaki T. Apolipoprotein E (APOE) phenotype and APOE concentrations in multiple sclerosis and acute herpes zoster. Acta Neurol Scand. 2000;102(2):94–8. doi: 10.1034/j.1600-0404.2000.102002094.x. [DOI] [PubMed] [Google Scholar]
- 15.Mustafina OE, Mikhailova AM, Bakhtiiarova KZ, Nasibulin TR, Tuktarova IA, Makarycheva OI, et al. Polymorphism of APOE gene and risk of development of the multiple sclerosis at ethnic Russians. Mol Biol (Mosk) 2008;42(6):957–64. [PubMed] [Google Scholar]
- 16.Michikawa M, Fan QW, Isobe I, Yanagisawa K. Apolipoprotein E exhibits isoform-specific promotion of lipid efflux from astrocytes and neurons in culture. J Neurochem. 2000;74(3):1008–16. doi: 10.1046/j.1471-4159.2000.0741008.x. [DOI] [PubMed] [Google Scholar]
- 17.Masterman T, Zhang Z, Hellgren D, Salter H, Anvret M, Lilius L, et al. APOE genotypes and disease severity in multiple sclerosis. Mult Scler. 2002;8(2):98–103. doi: 10.1191/1352458502ms787oa. [DOI] [PubMed] [Google Scholar]
- 18.Enzinger C, Ropele S, Strasser-Fuchs S, Kapeller P, Schmidt H, Poltrum B, et al. Lower levels of N-acetylaspartate in multiple sclerosis patients with the apolipoprotein E epsilon4 allele. Arch Neurol. 2003;60(1):65–70. doi: 10.1001/archneur.60.1.65. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt S, Barcellos LF, DeSombre K, Rimmler JB, Lincoln RR, Bucher P, et al. Association of polymorphisms in the apolipoprotein E region with susceptibility to and progression of multiple sclerosis. Am J Hum Genet. 2002;70(3):708–17. doi: 10.1086/339269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Finch CE. Increased synaptic sprouting in response to estrogen via an apolipoprotein E-dependent mechanism: implications for Alzheimer's disease. J Neurosci. 1998;18(9):3180–5. doi: 10.1523/JNEUROSCI.18-09-03180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh MJ, Chung EK, Shin YM, Lee KO, Park YS. The apolipoprotein E genotyping using the PCR-RFLP was useful to linkage analysis of Alzheimer's disease families. Exp Mol Med. 1997;29(3):161–4. [Google Scholar]
- 22.Zhang Z, Yang X, Meng L, Liu F, Shen C, Yang W. Enhanced amplification of GC-rich DNA with two organic reagents. Biotechniques. 2009;47(3):775–9. doi: 10.2144/000113203. [DOI] [PubMed] [Google Scholar]
- 23.Rall SC, Jr, Weisgraber KH, Innerarity TL, Mahley RW. Structural basis for receptor binding heterogeneity of apolipoprotein E from type III hyperlipoproteinemic subjects. Proc Natl Acad Sci U S A. 1982;79(15):4696–700. doi: 10.1073/pnas.79.15.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Utermann G, Langenbeck U, Beisiegel U, Weber W. Genetics of the apolipoprotein E system in man. Am J Hum Genet. 1980;32(3):339–47. [PMC free article] [PubMed] [Google Scholar]


