Abstract
BACKGROUND:
Lichen planus is recognized as an inflammatory disease of the skin with different morphologic patterns. Different treatment modalities, including topical and systemic corticosteroids, methotrexate, cyclosporine, azathioprine, topical calcineurin inhibitors, and psoralen plus UVA (PUVA), have been suggested for lichen planus. Although the efficacy of narrowband UVB (NBUVB) for treatment of lichen planus has been shown, no randomized clinical trial has compared NBUVB versus systemic corticosteroids for treatment of the disease. In the current study, we evaluated the efficacy of NBUVB versus systemic corticosteroids in the treatment of the lichen planus.
METHODS:
Forty-six patients with confirmed diagnosis of lichen planus were randomly selected. The subjects were randomized into two groups of 23 to be treated with either systemic corticosteroids or NBUVB. All of the selected cases had generalized lichen planus that involved at least 20% of the body area and their pruritus was resistant to antihistamine drugs. Patients in the systemic corticosteroids group were treated with prednisolon 0.3 mg/kg for 6 weeks. NBUVB was performed three times a week for 6 weeks. The maximum dose of NBUVB was 9 J/cm2. Data regarding demographic characteristics of the patients was also collected. All collected data was analyzed using SPSS15 and statistical tests including analysis of variance (ANOVA), chi-square, and t-test.
RESULTS:
46 patients (23 patients in systemic steroid group and 23 patients in NBUVB group) were evaluated. There was a significant difference between the 2 groups regarding the efficacy of the treatment. According to chi-square test, NBUVB was significantly more effective than systemic steroid in treatment of generalized lichen planus (p = 0.008). According to the results, patient satisfaction was also significantly higher in the group treated with NBUVB as compared with the systemic corticosteroids (p = 0.012).
CONCLUSIONS:
Overall, the results of our study and other previous studies showed that NBUVB may be regarded as an effective treatment for generalized cutaneous lichen planus. This treatment may be especially utilized when there is contraindication for systemic corticosteroids or other immunosuppressive drugs.
KEYWORDS: Lichen Planus, Narrowband UVB, Systemic Corticosteroids
Lichen planus is recognized as an inflammatory disease of the skin with different morphologic patterns.1 Its exact prevalence is not known but estimated to be found among 0.2-1% of the adult population.1 The diagnosis of lichen planus is based on clinical findings and histological examination.1 Different treatment modalities have been suggested for the disease including topical and systemic corticosteroids, methotrexate, cyclosporine, azathioprine, topical calcineurin inhibitors, and psoralen plus UVA (PUVA).2
As lichen planus is recognized as an immunologic disorder, phototherapy can be regarded as an effective alternative treatment for this disorder.3–5 In narrow band UVB (NBUVB) therapy, fluorescent tubes emitting NBUVB in the range of 310-315 nm with the maximum emission at 312 nm are used.6 NBUVB reduces langerhans cells and induces the production and secretion of the cytokines and neuropeptides. It can also induce anti-inflammatory effects through Intercellular Adhesion Molecule 1 (ICAM-1) suppression.5 NBUVB is the first available light source with the advantage of selective wave length and fewer side effects.7 Therefore, this method can be used safely in pregnant patients and children.8
In contrast to PUVA therapy, there is no need for eye protection after NBUVB and the cost of treatment will be reduced.5 In addition, compared to PUVA, NBUVB is associated with less risk of side effects including non-melanoma skin cancers and premature aging.7
Although the efficacy of NBUVB for treatment of lichen planus has been shown, no randomized clinical trial has compare NBUVB versus systemic corticosteroids for treatment of the disease. Thus, the current study evaluated the efficacy of NBUVB versus systemic corticosteroids in the treatment of lichen planus.
Methods
This study was performed during 2008-2010 in Alzahra Hospital, Khorshid Hospital and Skin Diseases and Leishmaniasis Research Center, Isfahan University of Medical Sciences, Isfahan, Iran.
Ethics committee clearance was achieved and informed consents were obtained from all patients (Isfahan University of Medical Sciences registration number: 385413).
A total number of 46 patients with confirmed diagnosis of lichen planus were randomly selected. They were randomized using simple randomization to be treated either with systemic corticosteroids or NBUVB. The diagnosis of lichen planus was confirmed by biopsy. All selected cases had generalized lichen planus that involved at least 20% of the body area and their pruritus had been resistant to antihistamine drugs for 2 weeks. Patients with erosive oral lichen planus, severe nail involvement and lichen planopilaris were excluded from the study.
For performing NBUVB, Fitzpatrick skin type was determined for each patient. After selecting minimal erythema dose (MED), NBUVB was performed three times a week at 70% of the MED for 6 weeks. The maximum dose of NBUVB was 9 j/cm2.9 On the other hand, patients in the systemic corticosteroids group were treated with prednisolon 0.3 mg/kg for 6 weeks.
The severity of pruritus was determined using a visual analogue scale (VAS) with a range of 0-10. Moreover, the severity of elevation and erythema were assessed by the investigator and were rated 0-4.
At the end of the study, according to the response of the lesions to treatment (elimination of the pruritus, elevation and erythema of the lesions), patients were classified into 4 groups. Groups 1-4 included patients with complete response (more than 90%), partial response (50-90%), weak response (20-50%) and no response (less than 20%), respectively.
Patient satisfaction with improvement of the lesions was assessed using a 10-point (0-10) VAS in which 0-5 indicated poor and moderate response, 6-7 indicated good and very good response and 8-10 represented excellent response.
Data regarding demographic characteristics of the patients was also collected. Collected data was analyzed using statistical tests including t-test and analysis of variance (ANOVA) for quantitative variables and chi-square test for qualitative variables. All analyses were performed by SPSS15. The level of significance was set at 0.05 (p < 0.05).
Results
Overall, 46 patients (23 patients in each group) were evaluated. The mean age of patients in the systemic corticosteroids and NBUVB groups were 42.04 ± 2.46 and 36.13 ± 2.88, respectively. There was no significant difference regarding age between the two groups (p = 0.109).
There was no significant association between sex and duration of the disease (p = 0.763 for females and p = 0.738 for males) (Table 1).
Table 1.
Mean of disease duration in the two groups separated by sex.
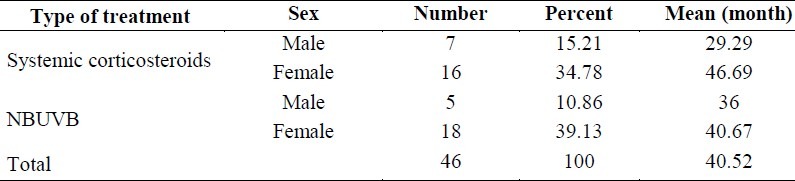
In addition, before initiation of the treatment, ANOVA did not show significant differences regarding duration of the disease between the two groups (p = 0.910).
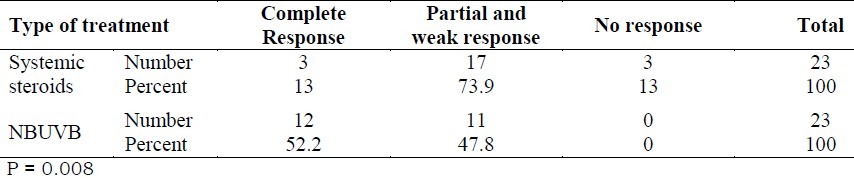
There was a significant difference in efficacy of treatment between the two groups. According to chi-square test, NBUVB was significantly more effective than systemic steroid in treatment of generalized lichen planus (p = 0.008) (Table 2).
Table 2.
The prevalence distribution of the response to treatment in the systemic steroid and NBUVB groups
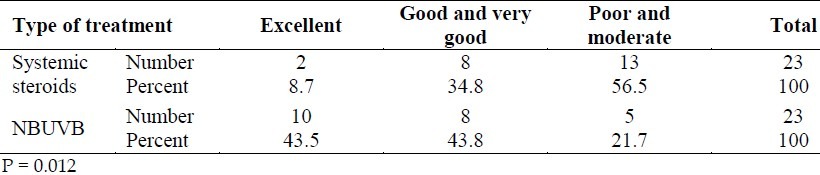
Patient satisfaction was also significantly higher in the group treated with NBUVB as compared with the systemic corticosteroids (p = 0.012) (Table 3). No significant side effects were observed in either group.
Table 3.
The prevalence distribution of the response to treatment as rated by the patients in the systemic steroid and NBUVB groups
Discussion
Corticosteroids have been used widely for a range of inflammatory and immune mediated disorders in the last fifty years.10 On the other hand, NBUVB radiation induces less erythema and carcinogenicity with lower cumulative doses than PUVA while the treatment response remains high. In the current study, we evaluated two different methods (NBUVB versus systemic corticosteroids) in the treatment of the lichen planus. Our results showed that NBUVB was significantly more effective than systemic corticosteroids in the treatment of generalized lichen planus.
Saricaoğlu et al. evaluated the efficacy of NBUVB in the treatment of 10 patients with lichen planus. They reported that five patients responded completely and four were partially responsive at the end of the 30th session. Moreover, 3 of the partially responsive cases responded completely at the 31st, 36th and 51st sessions, respectively. The authors suggested NBUVB as an inevitable treatment alternative for resistant cases of lichen planus.5
In another study, Habib et al. evaluated the efficacy of BUVB for the treatment of widespread lichen planus in a retrospective study among 20 patients. NBUVB was applied thrice a week. They considered 4 types of response including complete response, partial response, poor response and failure. Their results showed that complete and partial responses were obtained in 11 and 4 patients, respectively. Response was obtained with a median delay of 3 months (ranging: 2-6 months) following a median of 30 sessions (12 to 50) and an accumulated dose of UVB of 36 ± 4.8 j/cm2. The authors highlighted the positive effect of NBUVB for treatment of lichen planus.9
In a retrospective study by Pavlotsky et al. the efficacy of UVB (narrow band or broad band) for treatment of generalized lichen planus in 50 patients was evaluated. They treated 7 and 43 patients by broad and narrow band UVB, respectively. According to their results, while complete response was achieved in 70% of the patients, 85% of those were still in remission after a median of 34.7 months. The complete response rate and the need for higher cumulative exposure doses were not affected by sex, age, skin type, presence of additional diseases, failure of previous treatment or disease duration. The authors concluded that UVB was a safe and effective treatment option for generalized cutaneous lichen planus.11
Overall, the results of our study and previous research showed that NBUVB may be regarded as an effective treatment for generalized cutaneous lichen planus. This treatment may be especially regarded when there is contraindication for systemic corticosteroids or other immunosuppressive drugs.
We observed no significant side effects in either group. Therefore, NBUVB can be suggested as an ideal alternative for systemic steroids in patients with generalized lichen planus.
According to the literature, our study is the first randomized, controlled, clinical trial that evaluated the efficacy of NBUVB for treatment of lichen planus. More studies are recommended to better evaluate the efficacy of this treatment.
Authors’ Contributions
FI, GF, AA, and AHS provided the idea, performed the research, and prepared the manuscript. FTL and MA performed the research. All authors have read and approved the final manuscript.
Footnotes
Conflict of Interests Authors have no conflict of interests.
References
- 1.Shiohara T, Kano Y. Lichen planus and lichenoid dermatoses. In: Bolognia JL, Jorizzo JL, Rapini RP, editors. Dermatology. 2nd ed. New York: Mosby; 2008. p. 159. [Google Scholar]
- 2.Cribier B, Frances C, Chosidow O. Treatment of lichen planus.An evidence-based medicine analysis of efficacy. Arch Dermatol. 1998;134(12):1521–30. doi: 10.1001/archderm.134.12.1521. [DOI] [PubMed] [Google Scholar]
- 3.Wackernagel A, Legat FJ, Hofer A, Quehenberger F, Kerl H, Wolf P. Psoralen plus UVA vs.UVB-311 nm for the treatment of lichen planus. Photodermatol Photoimmunol Photomed. 2007;23(1):15–9. doi: 10.1111/j.1600-0781.2007.00261.x. [DOI] [PubMed] [Google Scholar]
- 4.Taneja A, Taylor CR. Narrow-band UVB for lichen planus treatment. Int J Dermatol. 2002;41(5):282–3. doi: 10.1046/j.1365-4362.2002.01499.x. [DOI] [PubMed] [Google Scholar]
- 5.Saricaoglu H, Karadogan SK, Baskan EB, Tunali S. Narrowband UVB therapy in the treatment of lichen planus. Photodermatol Photoimmunol Photomed. 2003;19(5):265–7. doi: 10.1034/j.1600-0781.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 6.Dogra S, Kanwar AJ. Narrow band UVB phototherapy in dermatology. Indian J Dermatol Venereol Leprol. 2004;70(4):205–9. [PubMed] [Google Scholar]
- 7.Snellman E, Klimenko T, Rantanen T. Randomized half-side comparison of narrowband UVB and trimethylpsoralen bath plus UVA treatments for psoriasis. Acta Derm Venereol. 2004;84(2):132–7. doi: 10.1080/00015550310022916. [DOI] [PubMed] [Google Scholar]
- 8.Tauscher AE, Fleischer AB, Jr, Phelps KC, Feldman SR. Psoriasis and pregnancy. J Cutan Med Surg. 2002;6(6):561–70. doi: 10.1007/s10227-001-0147-1. [DOI] [PubMed] [Google Scholar]
- 9.Habib F, Stoebner PE, Picot E, Peyron JL, Meynadier J, Meunier L. [Narrow band UVB phototherapy in the treatment of widespread lichen planus] Ann Dermatol Venereol. 2005;132(1):17–20. doi: 10.1016/s0151-9638(05)79189-4. [DOI] [PubMed] [Google Scholar]
- 10.Ghabanchi J, Bahri Najafi R, Haghnegahdar S. Treatment of oral inflammatory diseases with a new mucoadhesive prednisolone tablet versus triamcinolone acetonide paste. IRCMJ. 2009;11(2):155–9. [Google Scholar]
- 11.Pavlotsky F, Nathansohn N, Kriger G, Shpiro D, Trau H. Ultraviolet-B treatment for cutaneous lichen planus: our experience with 50 patients. Photodermatol Photoimmunol Photomed. 2008;24(2):83–6. doi: 10.1111/j.1600-0781.2008.00344.x. [DOI] [PubMed] [Google Scholar]


