Abstract
BACKGROUND:
The aim of this study was to compare the efficacy of azithromycin vs. fluticasone in treatment of adenotonsillar hypertrophy (AH).
METHODS:
In a clinical trial, 39 AH patients were selected using a convenient time-based sequential sampling method. The subjects were randomized into two treatment groups. Patients in group A (fluticasone) and B (azithromycin) were respectively treated with fluticasone spray and azithromycin suspension for a 6-week period. Data regarding the grade of obstruction (based on tonsillar size), level of adenotonsillar hypertrophy, and obstructive sleep apnea (OSA) symptoms (including mouth breathing, snoring, hyponasal speech, and sleep apnea) were collected by a self-administrated questionnaire before treatment, as well as 1 week and 8 weeks after treatment.
RESULTS:
Twenty AH patients in group A and 19 AH patients in group B were studied. AH related symptoms, including mouth breathing, snoring, hyponasal speech and sleep apnea, improved significantly in both groups (p < 0.05). We also found a statistically significant reduced grade of obstruction among patients in both groups. However, fluticasone was not effective on adenotonsillar hypertrophy. One week after treatment, outcomes related to apnea and hyponasal speech were better in group B than group A. Decreases in mouth breathing and snoring were not significantly different between group A and B.
CONCLUSIONS:
It could explain that though both of the improved and mentioned symptoms comparing within initial status, Azithromycin seems to be more effective than fluticasone in improving AH-related symptoms. Short term efficacy of the antibiotic is much significant than its long term effect.
KEYWORDS: Fluticasone, Azithromycin, Adenotonsillar Hypertrophy, Corticosteroid
Adenotonsillar hypertrophy (AH) is considered as the commonest disorder and cause of upper respiratory obstruction among children. It results in a spectrum of short-term and long-term symptoms. Short-term symptoms include mouth breathing, nasal congestion, hyponasal speech, snoring, obstructive sleep apnea (OSA), chronic sinusitis and recurrent otitis media.1 Long-term symptoms, on the other hand, are a series of serious complications related to OSA including growth failure, cardiovascular morbidity and neurocognitive abnormalities such as low intelligence quotient, learning and behavioral problems, hyperactivity and poor attention span.1
It was also found that children with resolution of OSA abnormalities experienced a change in the total and Low density lipoprotein (LDL)-cholesterol levels. This finding supports the hypothesis that reversal of OSA may also reverse the progression of dyslipidemia over time. Dyslipidemia is an important implication for the future cardiovascular disease (CVD) risk.2
OSA, characterized by repetitive increases in upper airway resistance and collapse, is considered a common problem in children with an incidence of 1-3%.2,3 It is actually the leading cause of huge morbidity, predominantly affecting the central nervous system (CNS) and cardiovascular system.4 AH remains the commonest cause of OSA in children and its association with OSA has been well documented in previous studies.5,6
Tonsillectomy and adenoidectomy are typical strategies for patients with AH. Although these procedures have an important role in relieving obstructive symptoms in patients with OSA, they may lead to some serious complications such as bleeding (4-5%) and postoperative respiratory compromise (27%) especially among younger children.7,8 Thus, regarding this potency, non-surgical therapies have attracted a lot of attention as the alternatives. In order to implement non-surgical treatments, understanding pathophysiology of AH is mandatory. Recently, several studies have investigated the etiology of the disease. According to these findings, a possible bacterial and inflammatory etiology has been suggested for AH.9,10 Children with OSA, experience a combination of oxidative stress, inflammation, autonomic activation, and disruption of sleep homeostasis.2 The bacterial involvement of AH was suggested based on an increase in lymphocytes within the tonsils and adenoids. It was confirmed by finding a greater number of pathogenic bacteria such as Haemophilus influenzae and other B-lactamase-producing organisms in hypertrophied tonsils and adenoids.11 Many studies have indicated the efficacy of broad-spectrum antibiotics in improvement of symptoms and complications of AH and OSA.12,13
The role of inflammatory factors in AH and OSA has been suggested by the increased expression of various mediators of inflammatory responses in tonsils and a proper response to anti-inflammatory agents such as corticosteroids.14,15 In addition, finding a lot of steroid receptors and mRNA in adenoid tissue has supported the involvement of inflammatory factors in AH.16 Nasal corticosteroids have been shown to reduce cellular proliferation and production of pro-inflammatory cytokines in a tonsil/adenoid mixed-cell culture system.15 Accordingly, some studies revealed the efficacy of nasal corticosteroids in treatment of AH and OSA.17,18
Considering the increasing rate of investigations on non-surgical alternatives for AH and the importance of the treatment, the current study aimed to compare the efficacy of AH treatment with azithromycin and fluticasone.
Methods
This clinical trial was conducted in Isfahan, Iran from May 2010 to April 2011. Children aged 2-10 years diagnosed with AH were selected.
Individuals with obstructive symptoms such as adenoidal hypertrophy (assessed on radiography as an adenoidal/nasopharyngeal index or 2+ or 3+ tonsillar hypertrophy on clinical evaluation) and signs and symptoms of OSA such as difficult breathing, hyponasal speech without any evidences of cleft palate, loud snoring or restless sleep and other related symptoms reported by parents for at least 2 months before the study were included. Patients were selected using a convenient time-based sequential sampling method. The subjects were then divided into two groups using a table of random numbers. Patients with craniofacial syndromes, anatomical abnormalities, neuromuscular diseases, acute upper respiratory infections, recurrent tonsillitis, allergic rhinitis, severe OSA requiring urgent surgery, recent use of any related medications, e.g. corticosteroids or antibiotics, within the past 4 weeks, and asthma were excluded. The protocol was approved by the Research Bureau and Medical Ethics Committee of Isfahan University of Medical Sciences.
Written consents were obtained from all parents and they were informed about their right to withdraw at anytime they wished. Three patients discontinued the treatment after a week of drug treatment. Moreover, four patients who had symptoms of upper respiratory infection were excluded from the study. Finally, 39 patients completed their treatment (20 in group A and 19 in group B).
Group A was treated with fluticasone nasal spray for 6 weeks (one spray per nostril, twice daily for one week followed by 1 puff per nostril per day until the end of the treatment).18 Group B received 12 mg/kg azithromycin suspension on days 1-5. This regimen was repeated with 5-day intervals, i.e. on days 11-15, 21-25, and 31-35, for 6 weeks.12 Complete instructions on how to use the drugs were given to all parents. In addition, to ensure proper use of medications patients were followed by phone calls.
All cases were examined by 2 otorhinolaryngologists for confirming the diagnosis and inclusion criteria. Data about demographic characteristics of the patients, grade of obstruction based on tonsillar size grade, grade of AH and the commonest related symptoms including mouth breathing, snoring, hyponasal speech and sleep apnea were gathered by a self-administrated questionnaire designed by 2 otorhinolaryngologists. Face validity of the questionnaire was evaluated by 10 patients with OSA aged 2-10 years. The questionnaire was completed by the parents (mostly mothers) and reviewed by an otorhinolaryngologist. The second part of the questionnaire was about the disease and its related symptoms and was completed 3 times, i.e. before the trial, and 1 and 8 weeks after it. Results of clinical and radiographic assessments of cases were recorded. Data of the two groups were compared for their efficacy in reducing the symptoms of the disease.
Grade of obstruction was evaluated based on the grade of tonsillar size by an otorhinolaryngologist in clinical assessment. The grading was based on the proportion of the distance between the anterior tonsillar pillars that was taken up by the tonsillar tissue. While a score of 0 indicated tonsils not extending beyond the pillars, and not obstructing the airway ,1+ scores 1+ to 4+ showed tonsils extended; and obstructed airway, 1+, 0% to 25%; 2+, 25% to 50%; 3+, 50% to 75%; and 4+, 75% to 100% repsectively.20
Lateral nasopharyngeal radiography was used to evaluate adenoid hypertrophy. The adenoidal/nasopharyngeal ratio and the diameter (mm) of the nasopharyngeal airway at its narrowest point were measured by a radiologist21 and expressed in terms of percent.
Collected data was analyzed by t-test, Wilcoxon, Friedman, and Mann Whitney tests in SPSS17. To compare the differences between the three measurements (before, and after 1 and 8 weeks) Friedman test was used. Moreover, Wilcoxon test was used for comparing the values before and 1 week after the treatment.
Results
In this trial, 39 patients with AH, aged 2-10 years, were studied in two groups (20 in group A and 19 in group B) (Figure 1). Mean (± SD) age of patients in the two groups was 6.23 ± 1.9 (range: 2.3-9.7) and 5.76 ± 1.7 (range: 2.7-9.8) years in groups A and B, respectively. Sex contribution was identical in the two groups.
Figure 1.
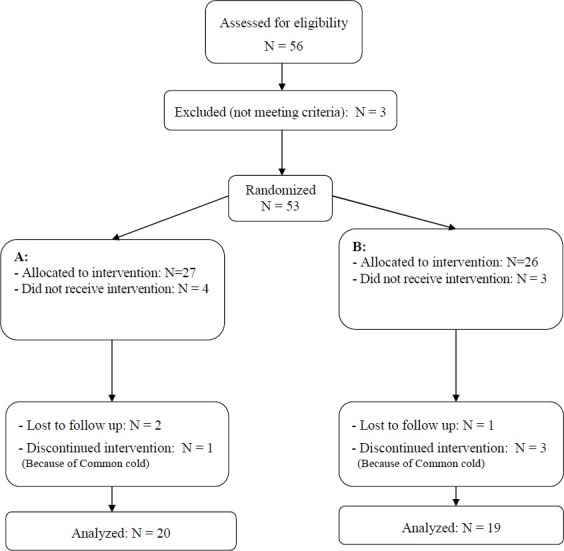
Participants’ retention vs. attrition
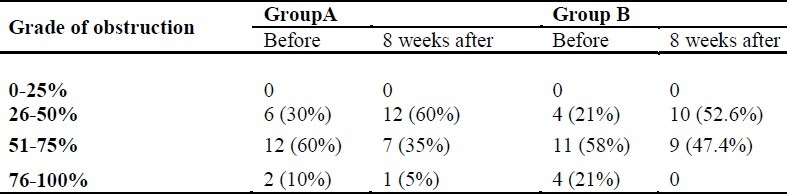
Percentages of obstruction in both groups before and after the intervention are presented in Table 1. Wilcoxon test revealed a significant difference between pre- and post-test steps regarding the percentage of obstruction in both groups (p = 0.004).
Table 1.
The grades of adenoid hypertrophy in groups A (fluticasone) and B (azithromycin) before and 8 weeks after the treatment (p = 0.004 for both groups).
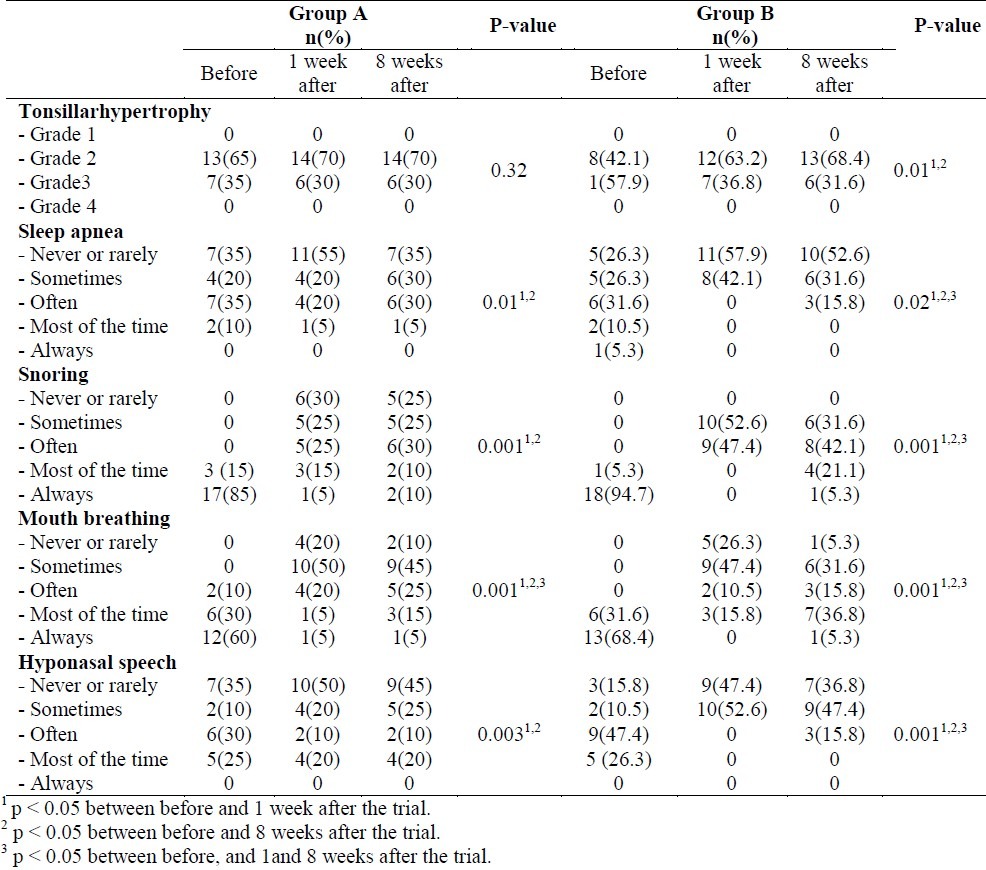
Different grades of tonsillar hypertrophy and OSA symptoms including mouth breathing, snoring, hyponasal speech and sleep apnea in the studied patients before the trial and 1 and 8 weeks after it are presented in Table 2. Comparing the results before and 1 week after the treatment, the rate of apnea was significantly lower in group B than group. The lowered rate for mouth breathing and snoring was not statistically significant between the 2 groups. The rate for hyponasal speech was significantly lower in group B than group A.
Table 2.
Different grades of obstructin and OSA (obstructive sleep apnea) symptoms in the studied patients in groups A (fluticasone) and B (azithromycin) before, and 1 and 8 weeks after the treatment
In addition, regarding the side effects of the drugs in the studied population, only one patient in group B reported short-term abdominal pain.
Discussion
In this trial, the efficacy of azithromycin and fluticasone in treatment of AH-related symptoms and grades of obstruction and AH were investigated among children with AH. The goal of our study was to assess and compare the efficacy of the two alternative non-aggressive methods to control AH symptoms in order to avoid surgery. Although fluticasone has more records in the literature, the effects of azithromycin have not been vastly studied. Findings of our research indicated that both medications improved AH-related symptoms including mouth breathing, snoring, hyponasal speech and sleep apnea. They also reduced obstruction grade among the studied population. However, fluticasone had not a significant effect on AH.
As mentioned, many studies have supported the role of inflammatory factors and bacterial involvements in the etiology of AH due to the effects of corticosteroids and antibiotics in improving symptoms and complications.12,13,17–19 In this study, we chose azithromycin as an antibacterial treatment. It is considered as a broad-spectrum antibiotic with appropriate activity against Streptococcus pneumoniae, Haemophilus influenzae, and other b-lactamase-producing organisms. It also maintains high tissue concentrations especially in the tonsil.22 Using azithromycin with our method would make bacterial resistance unlikely to happen.12
Although evidence support the safety of nasal corticosteroids in AH treatment among children due to limited or absent side effects and local anti-inflammatory activity on the upper airways, recent studies have indicated that long-term use of some corticosteroids may have suppressive effects on hypothalamic-pituitary-adrenal axis.23 In this trial, we used fluticasone because investigations have suggested no concerns about side effects of nasal corticosteroids with lower bioavailability such as fluticasone.24
Don et al. demonstrated azithromycin as useful in management of AH and OSA because of its modest effects. It can also cause temporary improvement of OSA symptoms in patients with severe AH who are candidate for surgical intervention. They concluded that it could not be a proper alternative to surgery.12
In this study, azithromycin had an appropriate effect on all AH-related variables 1 and 8 weeks after the treatment. However, compared to 1 week after the intervention, grades of some symptoms such as mouth breathing, snoring, hyponasal speech and sleep apnea were not reduced and even adversely increased in a significant way after 8 weeks. Therefore, although both post-treatment measurements showed improvements in symptoms compared to the initial status, short-term efficacy of the antibiotic was much more significant than its long-term effects. However, further studies with larger sample sizes and longer follow-up periods are needed for more accurate conclusion in this field.
Brouillette et al. investigated the efficacy of fluticasone nasal spray for pediatric OSA. They showed that a 6-week course of fluticasone administration decreased the severity of pediatric OSA symptoms. They concluded that nasal corticosteroids most likely affect the anatomic component of OSA by reducing the inspiratory upper airway resistance at the nasal, adenoidal, or tonsillar levels.18
Burton et al. published a review article about the efficacy of nasal corticosteroids for nasal airway obstruction in children with moderate to severe adenoidal hypertrophy. They reviewed five randomized trials and concluded that nasal corticosteroids may significantly improve nasal obstruction symptoms in this group of patients and the improvement may be associated with a reduction in adenoid size.25
Fluticasone in current research had an appropriate effect on improving AH-related symptoms and grade of obstruction, except for tonsillar hypertrophy. However, although the severity of mouth breathing improved 1 and 8 weeks after the intervention compared to initial measurements, significant improvements were not observed after 8 weeks compared to the first week. As mentioned for azithromycin, further studies with larger sample sizes and longer durations are needed for more accurate conclusion in this field.
In our study, improvements of symptoms were not accompanied with reduction of palatine tonsil size which might have been due to the short-term use of the drugs. In accordance with our study, Lepcha et al. reported beclomethasone, a nasal corticosteroid, not to be useful in treatment of adenoid hypertrophy in children.26 Ciprandi et al. evaluated the effects of intranasal flunisolide on AH and indicated that the treatment was associated with significant reduction of AH degree. They concluded that an 8-week treatment with intranasal flunisolide would prevent the rate of adenoidectomy.27 Varricchio et al. assessed the long-term (12 months) effects of intranasal flunisolide on AH. They similarly reported the drug to be useful in preventing adenoidectomy.28
Unfortunately, the present study had some limitations. First, data was collected according to the parental reports of the studied children with AH. This method may not be a reliable indicator of AH-related symptoms and complications and outcome measurement for a clinical trial. Second, we did not perform polysomnography as a pre- and post-trial tool for evaluating the efficacy of the drugs for the obstructive symptoms. Third, the small sample size and not including a placebo group to compare the results. However, since other studies compared the effects of each medication, i.e. azithromycin and fluticasone, with a placebo group, we did not include a placebo group. Finally, we studied the effects of drug therapy on AH. We could have more precise and relevant results if we performed our study in separate groups of adenoid hypertrophy and tonsillar hypertrophy.
Conclusion
Findings of this study indicated that azithromycin and fluticasone can be appropriately used for mild and moderate cases of AH. The drugs can also be administered for patients with severe AH for whom surgery is high risk. However, azithromycin seemed more effective than fluticasone nasal spray in improving AH-related symptoms. On the other hand, long-term administration of nasal corticosteroids with lower bioavailability would be more effective in this filed. Therefore, further studies with larger sample sizes and without the above mentioned limitations are suggested to define the usefulness of the two studied drugs in the management of AH and OSA.
Authors’ Contributions
All the authors have carried out the study, participated in the design of the study and data colection, performed the statistical analysis and wrote the manuscript. All authors have read and approved the final manuscript.
Acknowledgments
This study was financially supported by the Vice Chancellor for Research, Isfahan University of Medical Sciences (Thesis No. 389282)
Footnotes
Conflict of Interests Authors have no conflict of interests.
References
- 1.Scadding G. Non-surgical treatment of adenoidal hypertrophy: The role of treating IgE-mediated inflammation. Pediatr Allergy Immunol. 2010;21(8):1095–106. doi: 10.1111/j.1399-3038.2010.01012.x. [DOI] [PubMed] [Google Scholar]
- 2.Kelishadi R, Nilforoushan N, Okhovat A, Amra B, Poursafa P, Rogha M. Effects of adenoidectomy on markers of endothelial function and inflammation in normal-weight and overweight prepubescent children with sleep apnea. J Res Med Sci. 2011;16(Special Issue):386–94. [PMC free article] [PubMed] [Google Scholar]
- 3.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):242–52. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capdevila OS, Kheirandish-Gozal L, Dayyat E, Gozal D. Pediatric obstructive sleep apnea: complications, management, and long-term outcomes. Proc Am Thorac Soc. 2008;5(2):274–82. doi: 10.1513/pats.200708-138MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenfeld M, Tauman R, DeRowe A, Sivan Y. Obstructive sleep apnea syndrome due to adenotonsillar hypertrophy in infants. Int J Pediatr Otorhinolaryngol. 2003;67(10):1055–60. doi: 10.1016/s0165-5876(03)00182-4. [DOI] [PubMed] [Google Scholar]
- 6.Katz ES, D’Ambrosio CM. Pathophysiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):253–62. doi: 10.1513/pats.200707-111MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterson J, Losek JD. Post-tonsillectomy hemorrhage and pediatric emergency care. Clin Pediatr (Phila) 2004;43(5):445–8. doi: 10.1177/000992280404300505. [DOI] [PubMed] [Google Scholar]
- 8.Stuck BA, Gotte K, Windfuhr JP, Genzwurker H, Schroten H, Tenenbaum T. Tonsillectomy in children. Dtsch Arztebl Int. 2008;105(49):852–60. doi: 10.3238/arztebl.2008.0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeong JH, Lee DW, Ryu RA, Lee YS, Lee SH, Kang JO, et al. Bacteriologic comparison of tonsil core in recurrent tonsillitis and tonsillar hypertrophy. Laryngoscope. 2007;117(12):2146–51. doi: 10.1097/MLG.0b013e31814543c8. [DOI] [PubMed] [Google Scholar]
- 10.Serpero LD, Kheirandish-Gozal L, Dayyat E, Goldman JL, Kim J, Gozal D. A mixed cell culture model for assessment of proliferation in tonsillar tissues from children with obstructive sleep apnea or recurrent tonsillitis. Laryngoscope. 2009;119(5):1005–10. doi: 10.1002/lary.20147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brook I, Shah K, Jackson W. Microbiology of healthy and diseased adenoids. Laryngoscope. 2000;110(6):994–9. doi: 10.1097/00005537-200006000-00021. [DOI] [PubMed] [Google Scholar]
- 12.Don DM, Goldstein NA, Crockett DM, Ward SD. Antimicrobial therapy for children with adenotonsillar hypertrophy and obstructive sleep apnea: a prospective randomized trial comparing azithromycin vs placebo. Otolaryngol Head Neck Surg. 2005;133(4):562–8. doi: 10.1016/j.otohns.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Sclafani AP, Ginsburg J, Shah MK, Dolitsky JN. Treatment of symptomatic chronic adenotonsillar hypertrophy with amoxicillin/clavulanate potassium: short- and long-term results. Pediatrics. 1998;101(4 Pt 1):675–81. doi: 10.1542/peds.101.4.675. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Bhattacharjee R, Dayyat E, Snow AB, Kheirandish-Gozal L, Goldman JL, et al. Increased cellular proliferation and inflammatory cytokines in tonsils derived from children with obstructive sleep apnea. Pediatr Res. 2009;66(4):423–8. doi: 10.1203/PDR.0b013e3181b453e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kheirandish-Gozal L, Serpero LD, Dayyat E, Kim J, Goldman JL, Snow A, et al. Corticosteroids suppress in vitro tonsillar proliferation in children with obstructive sleep apnoea. Eur Respir J. 2009;33(5):1077–84. doi: 10.1183/09031936.00130608. [DOI] [PubMed] [Google Scholar]
- 16.Goldbart AD, Veling MC, Goldman JL, Li RC, Brittian KR, Gozal D. Glucocorticoid receptor subunit expression in adenotonsillar tissue of children with obstructive sleep apnea. Pediatr Res. 2005;57(2):232–6. doi: 10.1203/01.PDR.0000150722.34561.E6. [DOI] [PubMed] [Google Scholar]
- 17.Demain JG, Goetz DW. Pediatric adenoidal hypertrophy and nasal airway obstruction: reduction with aqueous nasal beclomethasone. Pediatrics. 1995;95(3):355–64. [PubMed] [Google Scholar]
- 18.Brouillette RT, Manoukian JJ, Ducharme FM, Oudjhane K, Earle LG, Ladan S, et al. Efficacy of fluticasone nasal spray for pediatric obstructive sleep apnea. J Pediatr. 2001;138(6):838–44. doi: 10.1067/mpd.2001.114474. [DOI] [PubMed] [Google Scholar]
- 19.Berlucchi M, Salsi D, Valetti L, Parrinello G, Nicolai P. The role of mometasone furoate aqueous nasal spray in the treatment of adenoidal hypertrophy in the pediatric age group: preliminary results of a prospective, randomized study. Pediatrics. 2007;119(6):e1392–e1397. doi: 10.1542/peds.2006-1769. [DOI] [PubMed] [Google Scholar]
- 20.Fernbach SK, Brouillette RT, Riggs TW, Hunt CE. Radiologic evaluation of adenoids and tonsils in children with obstructive sleep apnea: plain films and fluoroscopy. Pediatr Radiol. 1983;13(5):258–65. doi: 10.1007/BF00973342. [DOI] [PubMed] [Google Scholar]
- 21.Brodsky L. Modern assessment of tonsils and adenoids. Pediatr Clin North Am. 1989;36(6):1551–69. doi: 10.1016/s0031-3955(16)36806-7. [DOI] [PubMed] [Google Scholar]
- 22.Nahata MC, Koranyi KI, Gadgil SD, Hilligoss DM, Fouda HG, Gardner MJ. Pharmacokinetics of azithromycin in pediatric patients after oral administration of multiple doses of suspension. Antimicrob Agents Chemother. 1993;37(2):314–6. doi: 10.1128/aac.37.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehle ME. Are nasal steroids safe? Curr Opin Otolaryngol Head Neck Surg. 2003;11(3):201–5. doi: 10.1097/00020840-200306000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Allen DB. Systemic effects of intranasal steroids: an endocrinologist's perspective. J Allergy Clin Immunol. 2000;106(4 Suppl):S179–S190. doi: 10.1067/mai.2000.110038. [DOI] [PubMed] [Google Scholar]
- 25.Burton MJ, Derkay CS, Rosenfeld RM. Extracts from The Cochrane Library: intranasal corticosteroids for moderate to severe adenoidal hypertrophy. Otolaryngol Head Neck Surg. 2009;140(4):451–4. doi: 10.1016/j.otohns.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 26.Lepcha A, Kurien M, Job A, Jeyaseelan L, Thomas K. Chronic adenoid hypertrophy in children Çö is steroid nasal spray beneficial? Indian Journal of Otolaryngology and Head & Neck Surgery. 2002;54(4):280–4. doi: 10.1007/BF02993743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciprandi G, Varricchio A, Capasso M, Varricchio AM, De LA, Ascione E, et al. Intranasal flunisolide treatment in children with adenoidal hypertrophy. Int J Immunopathol Pharmacol. 2007;20(4):833–6. doi: 10.1177/039463200702000420. [DOI] [PubMed] [Google Scholar]
- 28.Varricchio A, Tortoriello G, Capasso M, De LA, Marchisio P, Varricchio AM, et al. Prevention of surgery in children with adenoidal hypertrophy treated with intranasal flunisolide: a 12-month follow-up. J Biol Regul Homeost Agents. 2009;23(2):95–101. [PubMed] [Google Scholar]


