Abstract
BACKGROUND:
The growing interest in filler injection requires a more comprehensive knowledge about the complications of this procedure.
METHODS:
A total of 5 cases with debilitating chronic complications following filler injection referred to Al-Zahra hospital, Isfahan are presented in this report.
RESULTS:
The outcome of treatment for two of the cases was satisfactory. In one case the treatment led to failure. A case committed suicide, the remaining case had received vitamin E injection which caused severe necrosis and scaring.
CONCLUSIONS:
All fillers are considered foreign bodies and may provoke the immune system to varying degrees. Most complications are, however, caused by the technique of injection not the filler itself. Experience of physicians along with adequate knowledge about fillers and their complications can definitely guarantee a better outcome.
KEYWORDS: Filler Complications, Granuloma, Biofilms
The increasing number of individuals seeking esthetic improvement has given rise to the use of filler as a minimally invasive choice. Recently, an FDA conducted study reported serious complication of fillers specially when injected by untrained person.1 It's quite obvious that most of such complications are related to techniques of injection, and not the material itself.2,3 Therefore, knowledge and experience of doctors who inject fillers seem to put a great impact on the esthetic outcome. Filler injection complications can be divided into three categories as early, late and delayed complication.4 Here we report five cases of severe chronic complications of fillers leading to patient disability.
Methods
Five patients who developed complications following filler injection were referred to the plastic surgery clinic at Alzahra university hospital, Isfahan, Iran. Informed consent was taken from the subjects. These cases that all needed aggressive treatments are presented below:
Case 1: A 34-year old woman who had polymethylmethacrylate (PMMA) injection on both nasolabial folds about 1 month before by a general practitioner. She had developed induration around the site of injection besides multiple fistulas .Psychiatric examination revealed major depression. She received systemic antibiotics, intralesional and systemic steroids and NSAID which had no sensible effects. The culture of fistula secretion was reported negative. About 10 days later (40 days after injection) the patient committed a successful suicide (Figure 1).
Figure 1.
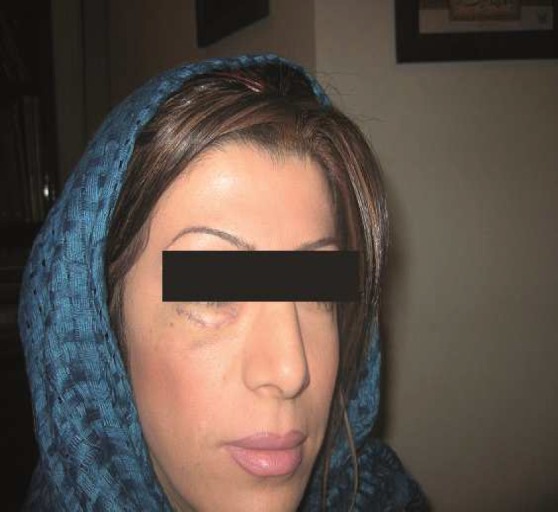
Case 1
Case 2: A 36-year old woman developed unilateral induration and fistulas 2 months following a hyaloronic acid injection on both her nasolabial folds by a dermatologist. Medical treatment including intralesional steroids did not help to improve the patient situation. Culture of fistula secretions was negative (Figure 2).
Figure 2.
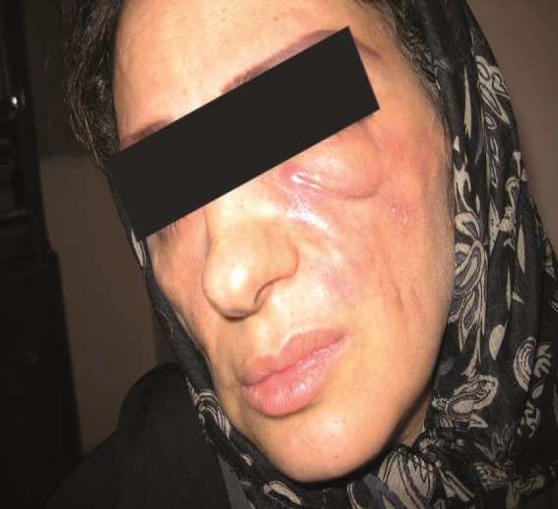
Case 2
A bilateral drainage was performed. Six months later, a dermofat graft was instituted into the left nasolabial fold through a face lift incision. Now the patient feels happy with the treatment.
Case 3: A 24-year old woman developed bilateral fistula on nasolabial folds and cheeks one month after the injection of hyaloronic acid by a general practitioner (Figure 3). The culture of discharge was negative. Secretions were drained and the patient was put on intralesional and oral steroid, antihistamines and NSAIDs. When inflammation decreased, subscission of adhesions in the left nasolabial fold was performed and proper amount of fat was transferred into the region. The lower eyelid was bulged. Suction did not help to correct the condition. The patient did not accept a surgical intervention.
Figure 3.
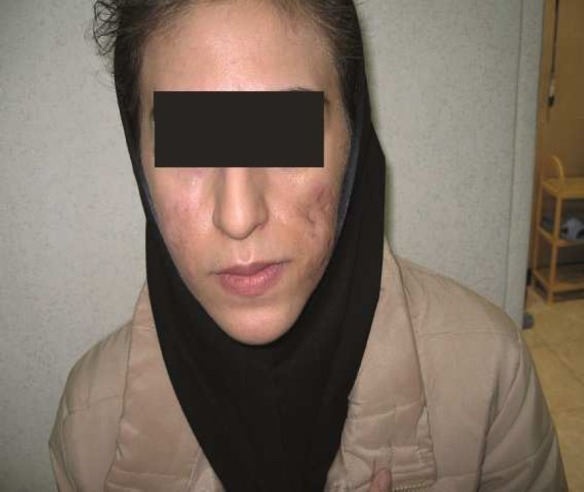
Case 3
Case 4: A 24-year old woman referred with a bulging of her right lower eyelid following a PMMA injection in her right cheek two months earlier by a general surgeon. Seemingly, the filler had migrated upwards. The patient reported a blow to her right face during a boxing exercise a week earlier. Ultrasonography showed filler localization within the right cheek and lower eyelid. Surgical exploration of affected side was performed through a blepharoplasty incision. The injected filler formed small granules over the orbital septum some of which penetrated into the septum and anterior periorbital fat. The field was cleared from granules as much as possible. The operation outcome was satisfactory.
Case 5: A 20-year old man was injected vitamin E as filler into both of his cheeks by an unauthorized medical employ. One week later, redness and induration developed over the injection area. The patient was hospitalized twice for cellulites and received intravenous antibiotics and anti-inflammatory drugs. External ultrasonic energy and suction did not help much. Unfortunately tissue necrosis developed bilaterally. One year later severe scar appeared.
Results
Treatment outcome was satisfactory in two cases. On the contrary, one case was not satisfied and one case committed suicide. It should be mentioned that vitamin E is not considered filler and thus should never be injected into the skin.
Discussion
Though quite uncommon, complications may occur with any kind of fillers.5,6 As fillers are recognized as non-self in the body the immune system respond to them to varying degrees.7 Complications of fillers are basically classified into three categories:
-
1-
Early complications (less than 14 days) including erythema, edema, necrosis due to intra-arterial injection8 and allergy and bumps and lumps following superficial injection.
-
2-
Late complications including chronic inflammation, late allergic reactions, nodules (granulomas) and filler migration, hypertrophic scar and telangectasia.
-
3-
Delayed complications which are largely due to biofilm formation.4
Granulomas are the product of chronic inflammation and are basically formed in response to foreign bodies and chronic infections. The rate of foreign body granuloma has been reported to range from 0.01- 0.1%.2
Diagnosis of foreign body granuloma is clinical. Patients usually do not show any sign during the first 6-24 months. However they may develop swelling, redness and discoloration afterwards.2,9 Causes of granuloma formation include massive injection of fillers at one time, filler impurities, filler surface irregularities, biofilms and repeated injection of filler at the same side. Nodules or lumps are commonly formed during the first four weeks. They have definite margins and do not grow. On the contrary granulomas appear later and usually have rapid growth. They show a dramatic response to intralesional steroid injection.2
In the first 3 cases of our study, the presentations were swelling and fistula which were probably the granuloma formation. Of course, definite diagnosis of granuloma requires microscopic examination that was not performed for our patients.
Biofilms are referred to a collection of microorganisms sticking to surfaces and are not recognized by the immune system. They are considered to be a 100 times more resistant to antibiotics.4 Biofilms are among the causes of the delayed formation of granuloma. To avoid biofilms aseptic measures should be fully observed during injection. Disinfecting the skin with chlorhexidine prior to injection seems to be more preferable than alcohol for its residual effects.4 Injection in patients with focal or systemic infections is contraindicated.10
Smaller needles naturally impose less trauma and bacterial penetration. Patients A therapeutic modality for the nodule was proposed by Rohrich et al.4 In this method, if the lesion is fluctuating, a needle aspiration should be performed; samples should be cultured for 21 days in order to identify the atypical species. In non-fluctuating lesions administration of at least two antibiotics (a quinoline pluss macrolide) should be started at the same time. If no improvement was achieved after two weeks, in case of hyaloronic acid fillers, hyaloronidase injection should be tried. For, non-hyaloronic acid fillers administration of high dose intralesional steroid is suggested. Surgical excision is always the last resort. Other modalities such as oral steroids, bleomycin, allopurinol, minocycline, isotretinoin, Imuran, topical tacrolimus, intralesional steroid pluss 5-Fluorouracil (5FU) have also been reported.2,11,12
External ultrasonic energy causes liquefaction of the fat by cellular fragmentation. This fatty emulsion can be extracted from the subcutaneous tissue spontaneously by means of suction cannula.13,14 As fillers are usually injected into subdermal area, this method can help sucking out the remaining fillers. Inappropriate injection is often the most common cause mistake in filler injection.4 Acne scars are the only lesions for which superficial (intra dermal) injection is suggested. Sub dermal injection is always more preferable for the rest of lesions.
Among fillers, natural ones such as hyaloronic acid (HA) and collagen are broken by enzymes thus forming little tissue reactions.2 Though very rare, acute hypersensitivity and foreign body granuloma reaction have been reported following hyaloronic acid injection.3 PMMA is commonly surrounded by connective tissue and remains unchanged.2 However, formation of delayed granuloma was reported to have occurred quite rarely with Artefill and Artecoll. Such granulomas often appear after repeated injections.3 Two of our patients had received PMMA injection. Two of our cases showed severe complications though relatively safe HA injection. There were two cases who had been injected by a non physician medical personal that developed serious complications. In general, compared to short acting fillers, long acting ones seem to cause more chronic and more severe complications. Moreover, experience and knowledge of the person injecting the filler may profoundly affect the outcome.
Authors’ Contributions
All authors planned and conducted the study procedure and wrote. All authors read and approved the final draft of the manuscript.
Footnotes
Conflict of Interests Authors have no conflict of interests.
References
- 1.Executive Summery Dermal Filler Devices. Rockville: FDA; 2008. Food and Drug Administration Center for Devices and Radiological Health. [Google Scholar]
- 2.Lemperle G, Rullan PP, Gauthier-Hazan N. Avoiding and treating dermal filler complications. Plast Reconstr Surg. 2006;118(3 Suppl):92S–107S. doi: 10.1097/01.prs.0000234672.69287.77. [DOI] [PubMed] [Google Scholar]
- 3.Cohen JL. Understanding, avoiding, and managing dermal filler complications. Dermatol Surg. 2008;34(Suppl 1):S92–S99. doi: 10.1111/j.1524-4725.2008.34249.x. [DOI] [PubMed] [Google Scholar]
- 4.Rohrich RJ, Monheit G, Nguyen AT, Brown SA, Fagien S. Soft-tissue filler complications: the important role of biofilms. Plast Reconstr Surg. 2010;125(4):1250–6. doi: 10.1097/PRS.0b013e3181cb4620. [DOI] [PubMed] [Google Scholar]
- 5.Lowe NJ, Maxwell CA, Patnaik R. Adverse reactions to dermal fillers: review. Dermatol Surg. 2005;31(11 Pt 2):1616–25. [PubMed] [Google Scholar]
- 6.Andre P, Lowe NJ, Parc A, Clerici TH, Zimmermann U. Adverse reactions to dermal fillers: a review of European experiences. J Cosmet Laser Ther. 2005;7(3-4):171–6. doi: 10.1080/14764170500344393. [DOI] [PubMed] [Google Scholar]
- 7.Beljaards RC, de Roos KP, Bruins FG. NewFill for skin augmentation: a new filler or failure? Dermatol Surg. 2005;31(7 Pt 1):772–6. [PubMed] [Google Scholar]
- 8.Glaich AS, Cohen JL, Goldberg LH. Injection necrosis of the glabella: protocol for prevention and treatment after use of dermal fillers. Dermatol Surg. 2006;32(2):276–81. doi: 10.1111/j.1524-4725.2006.32052.x. [DOI] [PubMed] [Google Scholar]
- 9.Gelfer A, Carruthers A, Carruthers J, Jang F, Bernstein SC. The natural history of polymethylmethacrylate microspheres granulomas. Dermatol Surg. 2007;33(5):614–20. doi: 10.1111/j.1524-4725.2007.33123.x. [DOI] [PubMed] [Google Scholar]
- 10.Winslow CP. The management of dermal filler complications. Facial Plast Surg. 2009;25(2):124–8. doi: 10.1055/s-0029-1220653. [DOI] [PubMed] [Google Scholar]
- 11.Lemperle G, Duffy DM. Treatment options for dermal filler complications. Aesthet Surg J. 2006;26(3):356–64. doi: 10.1016/j.asj.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Alam M, Dover JS. Management of complications and sequelae with temporary injectable fillers. Plast Reconstr Surg. 2007;120(6 Suppl):98S–105S. doi: 10.1097/01.prs.0000248859.14788.60. [DOI] [PubMed] [Google Scholar]
- 13.Maxwell GP, Gingrass MK. Ultrasound-assisted lipoplasty: a clinical study of 250 consecutive patients. Plast Reconstr Surg. 1998;101(1):189–202. doi: 10.1097/00006534-199801000-00034. [DOI] [PubMed] [Google Scholar]
- 14.Zocchi ML. Ultrasonic assisted lipoplasty.Technical refinements and clinical evaluations. Clin Plast Surg. 1996;23(4):575–98. [PubMed] [Google Scholar]


