Abstract
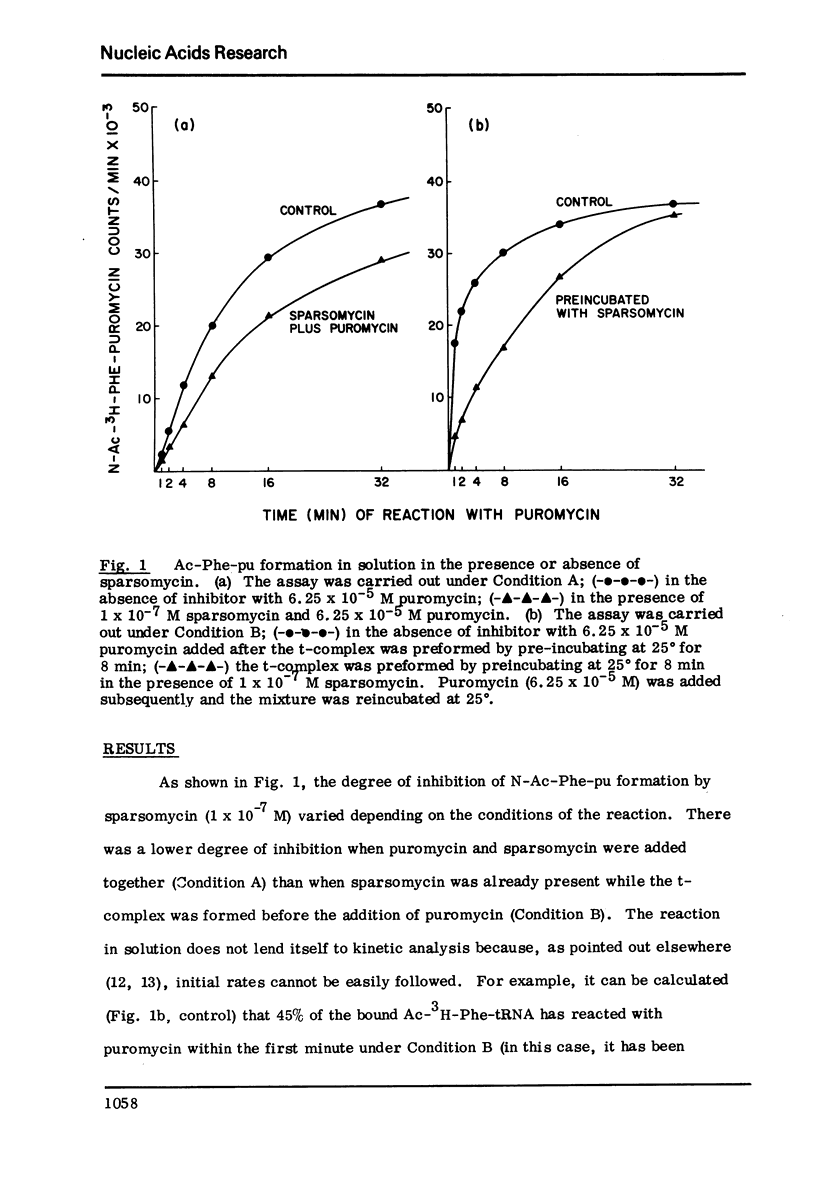
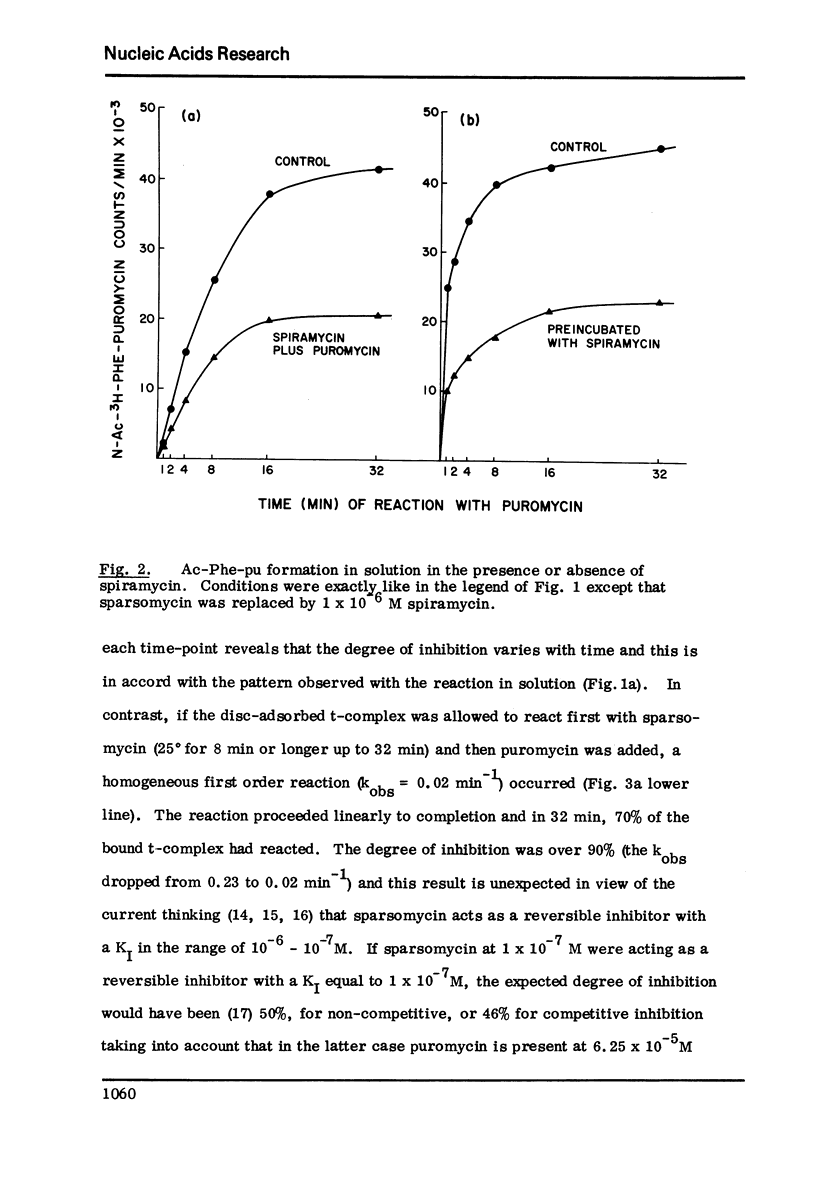
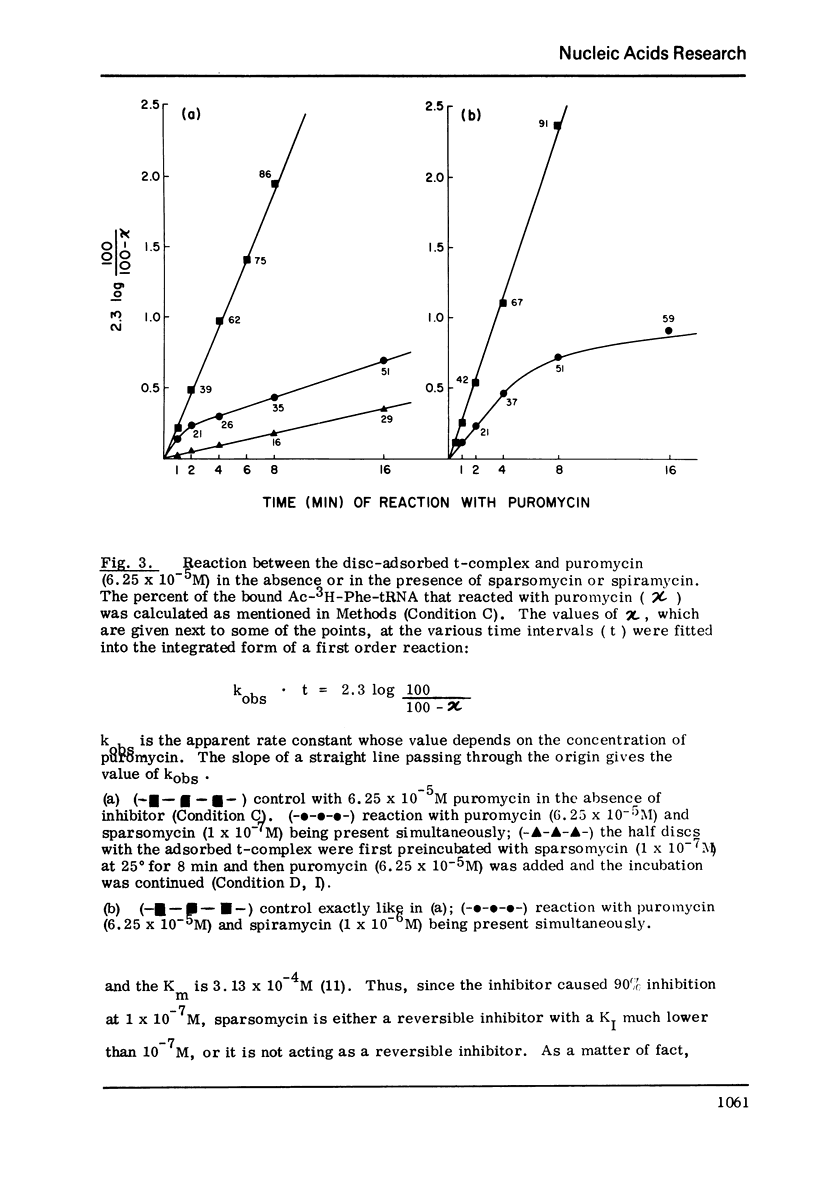
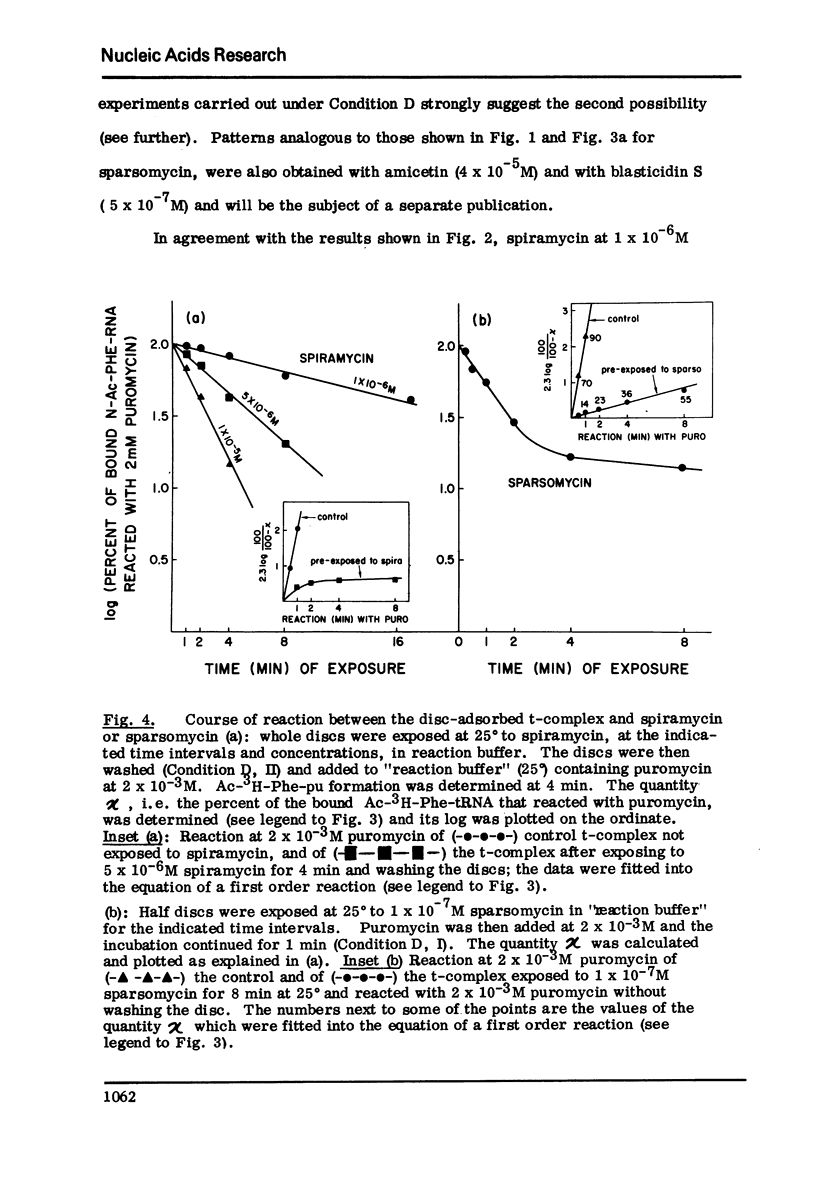
The initiation complex (t-complex) formed in a cell-free system (E. coli) from Ac-Phe-tRNA, poly(U) and washed ribosomes in the presence of initiation factors (ribosomal wash) and GTP, contains the Ac-Phe-tRNA bound quantitatively in a puromycin-reactive state. The t-complex is irreversibly inactivated by spiramycin with respect to its reactivity toward puromycin. The inactivated t-complex retains all of the Ac-Phe-tRNA bound, but it does not react with puromycin (2 x10-minus-3M) within 32 min at 25 degrees. In the case of another inhibitor protein synthesis, sparsomycin, the permanently "modified" t-complex not only retains all the bound Ac-Phe-tRNA but it can still react with puromycin. In the continuous presence of sparsomycin (1 x 10-minus-7M) the bound Ac-Phe-tRNA reacts quantitatively at a rate which is one-tenth the rate at which the t-complex reacts with puromycin, at low (6.25 x 10-minus-5M) or high (2 x 10-minus-3M) concentrations. These results are not in agreement with current views according to which aparsomycin binds to the ribosome reversibly at a single site with a KI in the range of 10-minus6-10-minus-7 M and according to which this stie is at the A'-site (puromycin site) of peptidyl transferase.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed A. Altered ribosomes in spiramycin-resistant mutants of Bacillus subtilis. Biochim Biophys Acta. 1968 Aug 23;166(1):218–228. doi: 10.1016/0005-2787(68)90505-4. [DOI] [PubMed] [Google Scholar]
- Bennett P. M., Maaloe O. The effects of fusidic acid on growth, ribosome synthesis and RNA metabolism in Escherichia coli. J Mol Biol. 1974 Dec 15;90(3):541–561. doi: 10.1016/0022-2836(74)90234-4. [DOI] [PubMed] [Google Scholar]
- Bernal S. D., Blumberg B. M., Nakamoto T. Requirement of initiation factor 3 in the initiation of polypeptide synthesis with N-acetylphenylalanyl-tRNA. Proc Natl Acad Sci U S A. 1974 Mar;71(3):774–778. doi: 10.1073/pnas.71.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutsogeorgopoulos C. Amino acylaminonucleoside inhibitors of protein synthesis. II. Effect on oligophenylalanine formation. Biochim Biophys Acta. 1971 Jun 17;240(1):137–150. doi: 10.1016/0005-2787(71)90519-3. [DOI] [PubMed] [Google Scholar]
- Coutsogeorgopoulos C., Fico R., Miller J. T. On the function of guanosine triphosphate in the formation of N-acetyl-phenylalanyl puromycin. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1056–1062. doi: 10.1016/0006-291x(72)90940-0. [DOI] [PubMed] [Google Scholar]
- Coutsogeorgopoulos C. On the accumulation of short peptides in the presence of certain inhibitors of protein synthesis. Arch Biochem Biophys. 1972 Nov;153(1):199–206. doi: 10.1016/0003-9861(72)90437-7. [DOI] [PubMed] [Google Scholar]
- Fernandez-Munoz R., Monro R. E., Torres-Pinedo R., Vazquez D. Substrate- and antibiotic-binding sites at the peptidyl-transferase centre of Escherichia coli ribosomes. Studies on the chloramphenicol. lincomycin and erythromycin sites. Eur J Biochem. 1971 Nov 11;23(1):185–193. doi: 10.1111/j.1432-1033.1971.tb01607.x. [DOI] [PubMed] [Google Scholar]
- Fico R., Coutsogeorgopoulos C. Peptidyl transferase: a new method for kinetic studies. Biochem Biophys Res Commun. 1972 May 12;47(3):645–651. doi: 10.1016/0006-291x(72)90927-8. [DOI] [PubMed] [Google Scholar]
- Goldberg I. H., Mitsugi K. Inhibition by sparsomycin and other antibiotics of the puromycin-induced release of polypeptide from ribosomes. Biochemistry. 1967 Feb;6(2):383–391. doi: 10.1021/bi00854a003. [DOI] [PubMed] [Google Scholar]
- Goldberg I. H., Mitsugi K. Sparsomycin inhibition of polypeptide synthesis promoted by synthetic and natural polynucleotides. Biochemistry. 1967 Feb;6(2):372–382. doi: 10.1021/bi00854a002. [DOI] [PubMed] [Google Scholar]
- Hansen M. T., Bennett P. M., von Meyenburg K. Intracistronic polarity during dissociation of translation from transcription in Escherichia coli. J Mol Biol. 1973 Jul 15;77(4):589–604. doi: 10.1016/0022-2836(73)90225-8. [DOI] [PubMed] [Google Scholar]
- Harris R., Pestka S. Studies on the formation of transfer ribonucleic acid-ribosome complexes. XXIV. Effects of antibiotics on binding of aminoacyl-oligonucleotides to ribosomes. J Biol Chem. 1973 Feb 25;248(4):1168–1174. [PubMed] [Google Scholar]
- Herner A. E., Goldberg I. H., Cohen L. B. Stabilization of N-acetylphenylalanyl transfer ribonucleic acid binding to ribosomes by sparsomycin. Biochemistry. 1969 Apr;8(4):1335–1344. doi: 10.1021/bi00832a006. [DOI] [PubMed] [Google Scholar]
- Innanen V. T., Nicholls D. M. Studies on peptidyl transferase in free ribosomes derived from rat liver. Biochim Biophys Acta. 1974 Aug 29;361(2):221–229. doi: 10.1016/0005-2787(74)90349-9. [DOI] [PubMed] [Google Scholar]
- Jimenez A., Monro R. E., Vazquez D. Interaction of Ac-Phe-tRNA with e. coli ribosomal subunits. 1. Sparsomycin-induced formation of a complex containing 50 S and 30 S subunits but not mRNA. FEBS Lett. 1970 Apr 2;7(2):103–108. doi: 10.1016/0014-5793(70)80131-4. [DOI] [PubMed] [Google Scholar]
- Leder P., Bursztyn H. Initiation of protein synthesis II. A convenient assay for the ribosome-dependent synthesis of N-formyl-C14-methionylpuromycin. Biochem Biophys Res Commun. 1966 Oct 20;25(2):233–238. doi: 10.1016/0006-291x(66)90586-9. [DOI] [PubMed] [Google Scholar]
- Lessard J. L., Pestka S. Studies on the formation of transfer ribonucleic acid-ribosome complexes. 23. Chloramphenicol, aminoacyl-oligonucleotides, and Escherichia coli ribosomes. J Biol Chem. 1972 Nov 10;247(21):6909–6912. [PubMed] [Google Scholar]
- Lodish H. F. Alpha and beta globin messenger ribonucleic acid. Different amounts and rates of initiation of translation. J Biol Chem. 1971 Dec 10;246(23):7131–7138. [PubMed] [Google Scholar]
- Pestka S. Antibiotics as probes of ribosome structure: binding of chloramphenicol and erythromycin to polyribosomes; effect of other antibiotics. Antimicrob Agents Chemother. 1974 Mar;5(3):255–267. doi: 10.1128/aac.5.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S. Inhibitors of ribosome functions. Annu Rev Microbiol. 1971;25:487–562. doi: 10.1146/annurev.mi.25.100171.002415. [DOI] [PubMed] [Google Scholar]
- Pestka S., Rosenfeld H., Harris R., Hintikka H. Studies on transfer ribonucleic acid-ribosome complexes. XXI. Effect of antibiotics on peptidyl-puromycin synthesis by mammalian polyribosomes. J Biol Chem. 1972 Nov 10;247(21):6895–6900. [PubMed] [Google Scholar]
- Pestka S. Studies on the formation of transfer ribonucleic acid-ribosome complexes. 8. Survey of the effect of antibiotics of N-acetyl-phenylalanyl-puromycin formation: possible mechanism of chloramphenicol action. Arch Biochem Biophys. 1970 Jan;136(1):80–88. doi: 10.1016/0003-9861(70)90329-2. [DOI] [PubMed] [Google Scholar]
- Pestka S. Studies on the formation of transfer ribonucleic acid-ribosome complexes. XI. Antibiotic effects on phenylalanyl-oligonucleotide binding to ribosomes. Proc Natl Acad Sci U S A. 1969 Oct;64(2):709–714. doi: 10.1073/pnas.64.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S. Studies on transfer ribonucleic acid-ribosome complexes. XIX. Effect of antibiotics on peptidyl puromycin synthesis on polyribosoms from Escherichia coli. J Biol Chem. 1972 Jul 25;247(14):4669–4678. [PubMed] [Google Scholar]
- Schneider J. A., Maxwell E. S. Peptidylpuromycin formation on mammalian polysomes. Studies on transpeptidation and translocation. Biochemistry. 1973 Jan 30;12(3):475–481. doi: 10.1021/bi00727a018. [DOI] [PubMed] [Google Scholar]
- Suttle D. P., Haralson M. A., Ravel J. M. Initiation factor 3 requirement for the formation of initiation complexes with synthetic oligonucleotides. Biochem Biophys Res Commun. 1973 Mar 17;51(2):376–382. doi: 10.1016/0006-291x(73)91268-0. [DOI] [PubMed] [Google Scholar]
- Tada K., Trakatellis A. C. Mechanism of action of sparsomycin on protein synthesis. Antimicrob Agents Chemother (Bethesda) 1970;10:227–230. [PubMed] [Google Scholar]
- Trakatellis A. C. Effect of sparsomycin on protein synthesis in the mouse liver. Proc Natl Acad Sci U S A. 1968 Mar;59(3):854–860. doi: 10.1073/pnas.59.3.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukioka M., Morisawa S. Enhancement of the phenylalanyl-oligonucleotide binding to the peptidyl recognition center of ribosomal peptidyltransferase and inhibition of the chloramphenicol binding to ribosomes. Biochim Biophys Acta. 1971 Dec 16;254(2):304–315. doi: 10.1016/0005-2787(71)90839-2. [DOI] [PubMed] [Google Scholar]



