Abstract
Epigenetic parental genetic effects are important in many biological processes but their roles in the evolution of adaptive traits and their consequences in naturally evolving populations remain to be addressed. By comparing two divergent blind cave-dwelling cavefish populations with a sighted surface-dwelling population (surface fish) of the teleost Astyanax mexicanus, we report here that convergences in vibration attraction behavior (VAB), the lateral line sensory receptors underlying this behavior, and the feeding benefits of this behavior are controlled by parental genetic effects, either maternal or paternal inheritance. From behavioral studies and mathematical evolutionary simulations, we further demonstrate that disparity in nuclear and mitochondrial DNA in one of these cavefish populations that has hybridized with surface fish can be explained by paternal inheritance of VAB. The results suggest that parental genetic effects in adaptive behaviors may be important factors in biasing mitochondrial DNA inheritance in natural populations that are subject to introgression.
Keywords: Cavefish, Vibration attraction behavior, Paternal genetic effects, Maternal genetic effects, Sensory neuromasts, Mitochondrial disparity
Introduction
Parental genetic effects are defined as the preferential inheritance of traits derived from a maternal or paternal parent and are often based on epigenetic processes (Mousseau and Fox 1998; Qvarnström and Price 2001). The recent discovery of about 1,300 loci showing parental bias of gene expression in the mouse brain (Gregg et al. 2010) suggests that epigenetic parental genetic effects could have important roles in many biological processes (Richards 2006; Chong et al. 2007). However, little is known about the relative impacts of maternal and paternal inheritance on the evolution of behavior.
The teleost Astyanax mexicanus is an excellent model organism for studying the evolution of behavior during adaptation to a novel environment: the perpetual darkness of caves (Mitchell et al. 1977; Wilkens 1988; Jeffery 2001, 2008, 2009). Within the past few million years, at least five independent colonizations by two different migrating waves of surface fish established 29 geographically isolated Astyanax cavefish populations in northeastern Mexico (Ornelas-García et al. 2008; Strecker et al. 2012; Bradic et al. 2012). After subsequent radiation underground, the founder cavefish populations became isolated in separate caves and evolved adaptive phenotypes to counteract the loss of vision, such as enhanced sensory systems and behavioral changes (Wilkens 1988; Jeffery 2005; Menuet et al. 2007; Varatharasan et al. 2009; Yamamoto et al. 2009). Despite this isolation, Astyanax surface fish and cavefish are completely interfertile, allowing the evolution of adaptive traits to be studied by genetic analysis.
Vibration attraction behavior (VAB), which is defined as the swimming of cavefish toward oscillating objects in water, is one of the constructive traits that have evolved to adapt cavefish to life in darkness (Yoshizawa et al. 2010). VAB is beneficial for feeding in the dark, heritable, and depends on an increase in the number and size (diameter) of superficial neuromasts (SN) (Yoshizawa et al. 2010), a type of sensory hair cell receptor in the teleost lateral line system. A small proportion of laboratory raised surface fish show a weak form of VAB, suggesting that this behavior may be subject to standing genetic variation in the lighted natural environment. In lighted environments, VAB may be risky because of predation, whereas in dark cave environments, which are devoid of macroscopic predators, it may be beneficial for feeding under conditions of food limitation. It has been proposed that the surface fish ancestors of cavefish became adapted to dark caves by positive selection for VAB, which was followed by continued selection for amplification of this behavior and its underlying sensory receptors, eventually resulting in the strong form of VAB exhibited by present-day cavefish (Yoshizawa and Jeffery 2011). VAB has converged in multiple cavefish populations (Yoshizawa et al. 2010), including those with different evolutionary histories in the Pachón and Los Sabinos caves (Dowling et al. 2002; Ornelas-García et al. 2008; Strecker et al. 2012; Bradic et al. 2012), but the genetic basis for VAB is unknown.
Pachón cavefish, but not Los Sabinos cavefish, exhibit a disparity between nuclear DNA (nDNA), which resembles the nDNA of other cavefish populations, and mitochondrial DNA (mtDNA), which groups with nearby surface fish mtDNA (Dowling et al. 2002; Strecker et al. 2003, 2012). The presence of surface fish mtDNA in Pachón cavefish is thought to be the result of a fairly recent episode of introgression with surface fish (Langecker et al. 1991) followed by differential fixation of maternally inherited surface fish mtDNA (Brown, 2008) in the hybrid cavefish population. Here we conduct genetic analysis to establish the mode of inheritance of VAB. We demonstrate that convergence of VAB and its underlying sensory receptors is controlled by different genetic mechanisms in Pachón and Los Sabinos cavefish, paternal and maternal inheritance respectively, and that paternal inheritance of this adaptive behavior can explain the disparity between nDNA and mtDNA in Pachón cavefish.
Methods
Biological materials
The experiments were performed on laboratory-raised Astyanax mexicanus cavefish and surface fish (Jeffery et al. 2000; Yamamoto et al. 2003; Yoshizawa and Jeffery 2008; Yamamoto et al. 2009). Fishes used in the assays ranged from 3 months to 2 years old and were fed living Artemia larvae. The number of individuals used in Fig. 1A to C and E to G were as follows, surface fish (Sf): n = 19; Pachón cavefish (Cf): n = 19; F1 hybrids from Sf ♀ × Pachón Cf ♂ cross (F1SC): n = 77 (11 independent families); F1 hybrids from Pachón Cf ♀ × Sf ♂ cross (F1CS): n = 56 (13 independent families); Los Sabinos Cf: n = 20; Los Sabinos F1SC: n = 53 (6 independent families; Los Sabinos F1CS: n = 70 (6 independent families). All hybrids were generated by in vitro fertilization. The procedures described below were approved by the University of Maryland Animal Care and Use Committee and conformed to NIH guidelines.
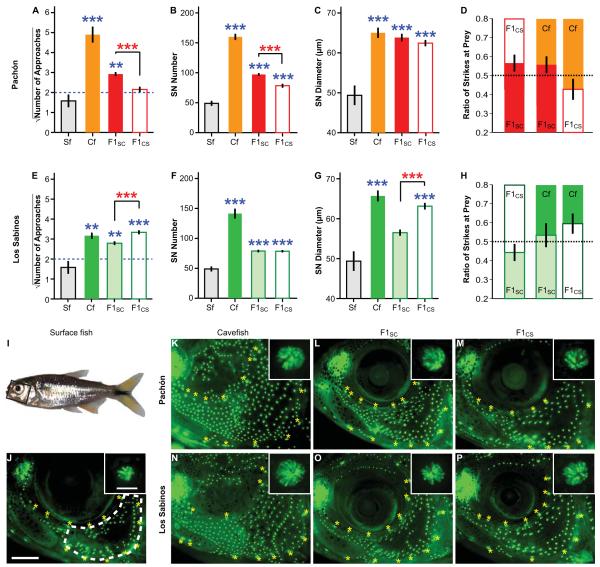
Figure 1.
(A-H). The role of parental genetic effects in the inheritance of VAB, SN number (B, F) and diameter (C, G), and VAB assisted prey capture (D, H) in Pachón (A to D) and Los Sabinos (E to H) cavefish. Cf: cavefish; Sf: surface fish. Blue asterisks: comparisons with surface fish; red asterisks: comparisons among hybrids (one-way ANOVA; ***P < 0.001; **P < 0.01). Blue dashed lines in (A) and (E) indicate the cutoff value for classifying fish with (square rooted NOA above 2) or without (square rooted NOA below 2) VAB (Yoshizawa et al. 2010; Yoshizawa and Jeffery 2011). The values on the Y axes are the square rooted means ± s.e.m. of NOA (A, E), the means ± s.e.m. of SN numbers and diameter sizes (B, C, F and G), or the mean ratios of strikes ± 95% confidence intervals of the mean (D, H). (I to P). DASPEI-stained neuromasts compared in surface fish, Pachón and Los Sabinos cavefish, and F1 hybrids. Scale bar in (J): 1.0 mm. Insets in (J) to (P) show a typical SN in the cranial third suborbital bone region. Scale bar in inset (J): 50 μm. Yellow asterisks indicate the position of canal neuromasts in the head lateral line. Black rectangles in (I) and the area enclosed by a white dotted line in (J) indicate the imaged third suborbital bone regions for reference to K to P.
Vibration attraction behavior assay
VAB was assayed as previously described (Yoshizawa et al. 2010). At 4 or 5 days before the beginning of an assay, individuals were acclimated in a cylindrical assay chamber (Pyrex 325 ml glass dish, 10 cm diameter × 5 cm high, Corning, Corning, NY) filled to 30-35 mm with conditioned water. During the assays, the VAB stimulus was created using a 7.5 mm-diameter glass rod inserted 15-18 mm into the water supported by a 1 mm thick and 95 mm long transparent plastic plate. Vibration stimuli were produced mechanically at 35 Hz by a Leader LG1301 function generator (Leader Instruments Corp., Cypress, CA) with an audio speaker (Pro Speakers, Apple, Cupertino, CA). Swimming movements were video recorded for a 3 min period under the infrared illumination (880 nm wave length, BL41192-880 black light, Advanced Illumination, Rochester, VT). An infrared CCD camera (Qicam IR, Qimaging, Surrey, Canada) with a zoom lens (Zoom 7000, Navitar, Rochester, NY) was used to capture images at 10 frames/sec using StreamPix 3.36.0 video recording software (NorPix, Montreal, Canada). ImageJ 1.35s software (NIH, Bethesda, MD) was used for video analysis.
Prey capture competition assay
F1 hybrids showing typical VAB levels (e. g. 3 square-rooted number of approaches (NOA) for F1SC and 2 NOA for F1CS; see Fig. 1A) were selected from different families for the prey competition assays and marked with an Alcian Blue tattoo near their dorsal fins. Five days prior to the assay, a pair of tattooed fish was transferred to a test aquarium (12 × 20 × 10 cm), deprived of food, and subjected to complete darkness. On the day of the assay, drops of living Artemia (brine shrimp) larvae were added to the aquarium, and strikes at prey were recorded for 1 min with the infrared camcorder (DCR-SR200C, Sony, Tokyo, Japan). The number of strikes at brine shrimp were counted for each fish using JWatcher + Video (Blumstein and Daniel 2007), and the total number of strikes for pairs was calculated by Microsoft Excel macro. Then, the ratio of strike numbers of a dominating fish was calculated. Each competition combination used 4-5 independent pairs (10 F1 hybrids from 6 independent families for Pachón Cf crosses and 10 F1 hybrids from 2 independent families for Los Sabinos Cf crosses), and the assay was repeated twice. The ratios of strike numbers were then averaged between the two trials.
Neuromast vital staining
Neuromasts were vitally stained with 2-(4-(dimethylamino)styryl)-N-ethylpyridinium iodide (DASPEI; Invitrogen, Eugene, OR) (Jørgensen 1989). Fish were immersed in 5 μg/ml DASPEI dissolved in conditioned water (conductivity approximately 600 μS) for 1 hr, followed by immersion in ice-cold 66.7 μg/ml Ethyl 3-aminobenzoate methane sulfonate salt (MS222, Sigma) in conditioned water, viewed with a fluorescence microscope (Axioskop 2 equipped with 2.5×Plan-Neofluar lens and a FITC filter set; Zeiss, Göttingen, Germany), and photographed with a Zeiss Axiocam CCD camera. Neuromasts were quantified on images of DASPEI-stained fish using ImageJ software. SN were counted in the cranial third suborbital bone region (Yamamoto et al. 2003). The longer diameter of elliptical SN was measured in the 10 largest stained neuromasts in the same area.
Sex determination
Sexes were distinguished by the shape and size of the gut. In males the gut turns at an acute angle toward the anus, whereas in females the gut makes larger angle (> 50°) to encompass the ovary. Females, in contrast to males, also show a swelled gut in the region where the ovary is located. In some cases, sex could not be determined by these criteria, and these fish were excluded from the analysis.
Mathematical simulation
Population genetics simulation software, simuPOP (version 1.0.4) (Peng and Kimmel 2005), was used to simulate parental genetic effects and conventional genetic models. Briefly, the size of a diploid population with one nuclear and one mitochondrial locus was set as 500 or 5000, based on the estimated population size of Pachón cavefish (1,300 ~ 18,200) and its effective size (220 ~ 990) (Mitchell et al. 1977; Panaram and Borowsky 2005). We assigned the populations sizes as 10, 50 or 250 for the number of introgressed surface fish. The selection coefficient (s) was surveyed from 0.0 to 0.5. Random mating was assumed (Şadoğlu 1979, Plath et al. 2006, and our observations). The simulations were repeated 400 ~ 2000 times and nuclear and mitochondrial loci were tracked for 10,000 generations. Perl 5.10 and Microsoft Excel macro were used for further data analyses.
Statistics
Statistical tests were conducted with IBM SPSS 18.0.3 software (IBM, Somers, NY). Non-parametric Mann-Whitney test was applied for tests with small sample sizes or when there were unequal numbers of individuals between two groups. Multiple comparisons with different sample sizes and unequal variance were adjusted by the Games-Howell test.
All data used for analyses, and PYTHON and Perl codes deposited in the Dryad repository: doi:10.5061/dryad.qn514810.
Results
To determine the mode of inheritance of VAB and SN enhancement, bi-directional crosses were conducted in which surface fish females (♀) were mated with cavefish males (♂) and cavefish ♀ were mated with surface fish ♂. We established 36 independent hybrid families and assayed up to 256 F1 progeny. The data in Figures 1 (A to C, E to G) and 2B represent the pooled results obtained from all of the offspring raised in similar laboratory conditions.
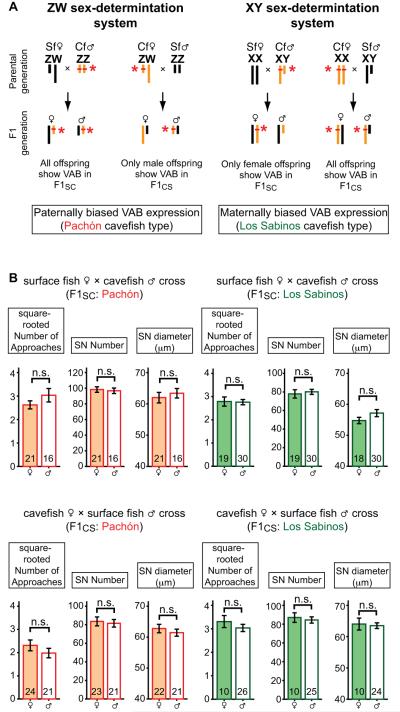
Figure 2.
Asymmetric VAB and SN inheritance is not related to sex linkage. (A). A possible mechanism for the inheritance of a parentally biased phenotype in offspring. Orange and black bars indicate putative ZW-type (left) or XY-type (right) sex chromosomes from cavefish and surface fish, respectively (Astyanax sex chromosomes have not been identified). Red ticks indicate an adaptive locus such as a VAB gene. If VAB were linked with ZW-type sex chromosome as shown, all cavefish-sired F1 hybrids would show the adaptive trait but the only half (males) of the F1CS offspring would show the adaptive trait. Similarly, in the case of the XY-type sex chromosome, only half (females) of the F1SC offspring would show VAB, providing a skewed expression of a parent-of-origin traits depending on the directions of the crosses. (B). A comparison of VAB and SN number and size in female and male Pachón F1SC and F1CS hybrids, and in female and male Los Sabinos F1SC and F1CS hybrids. There is no significant difference between sexes [n.s.; for Pachón F1SC (upper left panels): U = 126, P = 0.20 in NOA, U = 164, P = 0.91 in SN number, and U = 168, P = 1.00 in SN diameter size; for Pachón F1CS (lower left panels): U = 207, P = 0.31 in NOA, U = 223, P = 0.67 in SN number, and U = 212, P = 0.66 in SN size; for Los Sabinos F1SC (upper right panels): U = 276, P = 0.86 in NOA, U = 246, P = 0.43 in SN number, and U = 213, P = 0.23 in SN size; and for Los Sabinos F1CS (lower right panels): U = 107, P = 0.42 in NOA, U = 104, P = 0.46 in SN number, and U = 108, P = 0.67 in SN size]. The numbers on each bar indicate n used in each analysis.
We crossed 11 different pairs of surface fish ♀ and Pachón cavefish ♂ and 13 different pairs of Pachón cavefish ♀ and surface fish ♂ producing a total of 77 and 56 F1 hybrid progeny, respectively. The F1 hybrids sired by cavefish ♂, but not those sired by surface fish ♂, showed VAB (Fig. 1A). No significant differences were detected between different families in each bi-directional cross [One-way ANOVA test, F10, 66 = 1.3, P = 0.27, n = 77 in each family for F1 progeny from the surface fish ♀ × cavefish ♂ crosses (F1SC), and F12, 43 = 1.4, P = 0.22, n = 56 for F1 progeny from the cavefish ♀ × surface fish ♂ crosses (F1CS)], arguing against the possibility that the results could reflect genetic variation in the parental populations (Yoshizawa et al., 2010). To assess the possible role of sex linkage, VAB was examined in a total of 82 ♀ and ♂ from 24 different families, and no significant differences were observed in VAB between F1CS or F1SC males and females (Fig. 2A, B). Thus, the results strongly suggest that VAB is inherited as a paternal rather than a sex-linked genetic effect in Pachón cavefish.
The F1 hybrids resulting from the same Pachón cavefish × surface fish bi-directional crosses also showed asymmetric inheritance of SN. Based on measurements of SN in the cranial third suborbital bone region (Yamamoto et al. 2003), F1SC exhibited a significant increase in SN number (153 individuals in 24 families) compared to F1CS (One-way ANOVA planned-contrast test: t96.2 = 4.7, P < 0.001), although no differences in SN diameter size (151 individuals in 24 families) were found between the two types of hybrids (One-way ANOVA planned-contrast test: t108.3 = 1.1, P = 0.27; Fig. 1B, C, and K to M). Furthermore, no differences in the number of SN were detected when different sexes were examined in hybrids (Fig. 2B), implying a paternal genetic effect.
To determine whether the advantage in feeding related to VAB is also paternally inherited, prey-capture competition assays were conducted with10 F1 hybrids selected from 6 different families. The results showed that F1SC dominated in prey strikes over Pachón cavefish and F1CS (Fig. 1D), suggesting a paternal genetic effect in VAB assisted prey capture. The high competition capacity in F1 hybrids (middle bar in Fig. 1D, and right bar in Fig. 1H) could be the sum of parental inheritance of VAB and SN combined with aggression caused by high heterozygosity (Garten 1976; Tiira et al. 2003). In summary, the results revealed paternal inheritance of VAB, SN number, and VAB-related feeding advantage in Pachón cavefish, providing the first example of an adaptive behavior and its underlying sensory receptors controlled by paternal genetic effects.
We crossed 6 different pairs of surface fish ♀ and Los Sabinos cavefish ♂ and 6 different pairs of Los Sabinos cavefish ♀ and surface fish ♂ producing a total of 53 and 70 F1 hybrid progeny, respectively. The F1 progeny of bidirectional crosses between Los Sabinos cavefish and surface fish also showed a significant asymmetric distribution of VAB (Fig. 1E; One-way ANOVA planned-contrast test: t120.3 = −3.9, P < 0.001), which was independent of sex (Fig. 2A, B) and thus also interpreted as a parent of origin genetic effect. Instead of a paternal genetic effect, however, VAB in Los Sabinos cavefish showed a significant maternal genetic effect (Fig. 1E). Also in contrast to Pachón cavefish, SN size, but not number, showed an asymmetric distribution in F1 hybrids derived from bidirectional crosses of Los Sabinos cavefish and surface fish (One-way ANOVA planned-contrast test for SN number: t109.7 = 0.1, P = 0.91; for SN size: t115.5 = −6.0, P < 0.001; Fig. 1F, G, and N to P). Since SN number is more strongly correlated with VAB than SN size in Pachón cavefish (Yoshizawa et al, 2010), these results prompted us to investigate the relationship between SN number and size and VAB in Los Sabinos cavefish. The results showed that SN size is more strongly correlated with VAB than SN number in Los Sabinos cavefish (Fig. S1), providing further support for different mechanisms underlying VAB in the two cavefish populations. In prey-capture competition assays, F1 hybrids mothered by Los Sabinos cavefish dominated over those mothered by surface fish and over the parental Los Sabinos cavefish (Fig. 1H, total 10 F1 hybrids representing 2 different families). Therefore, the results show that VAB, SN enhancement, and VAB related feeding advantages are inherited maternally in Los Sabinos cavefish. The genetic analyses indicate that convergence of VAB in Pachón and Los Sabinos cavefish is controlled by different parental genetic mechanisms, paternal or maternal inheritance respectively.
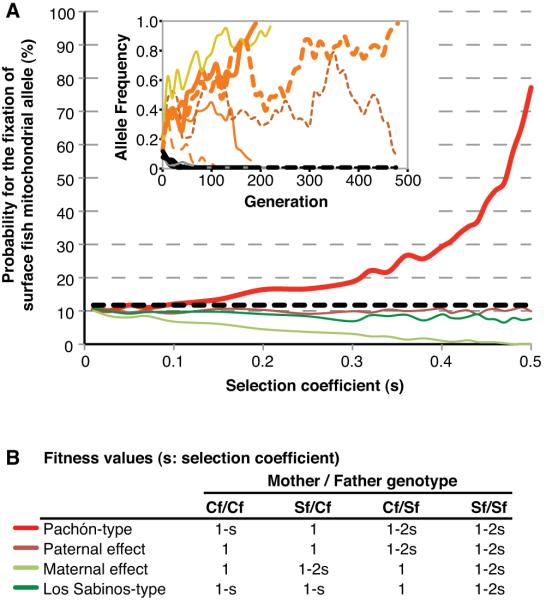
The disparity between mtDNA and nDNA in Pachón cavefish (Dowling et al. 2002; Strecker et al. 2003, 2012), which is also seen in many other hybridized species (Ballard and Whitlock 2004; Chan and Levin 2005), has been attributed to a hybridization event (Langecker et al. 1991) in which the original cavefish mtDNA was replaced by mtDNA from introgressing surface fish. In general, random genetic drift in small populations or positive selection for introgressed mitochondria have been proposed to explain mtDNA disparity (Ballard and Whitlock 2004), although there is no empirical evidence supporting either possibility in Pachón cavefish. Considering the mode of VAB inheritance revealed above, an alternative explanation could involve selection for an adaptive trait based on a paternal genetic effect. Accordingly, F1SC carrying surface fish mtDNA would show VAB and an advantage in prey capture, but F1CS carrying cavefish mtDNA would not be competitive and removed from the population (Fig. S2), resulting in disparity between mtDNA and nDNA. This hypothesis is supported by prey capture competition assays in which F1CS were subordinate to original Pachón cavefish (right bar in Fig. 1D) but F1SC showed significant dominance over cavefish (middle bar in Fig. 1D), suggesting that F1SC would have an advantage for survival in the cave environment. We further tested this hypothesis by genetic simulations (Peng and Kimmel 2005). Using a random mating scenario (Şadoğlu 1979, Plath et al. 2006, and our observations), we compared the following four possible genetic models: Pachón-type, typical paternal, Los Sabinos-type (as a control), and typical maternal genetic effect of an autosomal gene (Fig. 3A, B). In all the parameter combinations of population size and the number of hybridizing surface fish tested (see Methods), only the Pachón-type paternal genetic effect gave a higher probability for fixation of surface fish mtDNA than genetic drift (Fig. 3A Fig. S3).
Figure 3.
(A). Probabilities for fixation of surface fish mtDNA in cavefish during 2,000 repeated mathematical simulations. The black dashed line indicates the calculated fixation probability of surface fish mtDNA by random genetic drift. The parameters are population size (500), the number of introgressed surface fish (50), and the repeat number (2,000). Simulations were carried out until the 10,000th generation. Inset shows six examples of simulation with Pachón-type fitness. Yellow and orange lines indicate the frequency changes of surface fish mtDNA, and grey and black lines show those of surface fish nuclear DNA in the cavefish population up to the 500th generation. (B). Relative fitness values and color codes of lines used in (A). For example, in Pachón-type (red line at the first row), F1SC showed highest advantage in food capture (1: column of Sf/Cf), original cavefish showed intermediate values (1-s: Cf/Cf), and F1CS (1-2s: Cf/Sf) and surface fish (1-2s: Sf/Sf) showed the lowest advantage. s: selection coefficient.
Discussion
The results of the present investigation show that convergent evolution of VAB and its underlying sensory receptors in two divergent Astyanax cavefish populations is based on different parental genetic effects, either paternal or maternal inheritance. Furthermore, our studies demonstrate that paternal inheritance of an adaptive trait such as VAB can explain the disparity between mtDNA and nDNA in Pachón cavefish.
The finding of parental genetic effects in VAB, SN enhancement, and VAB related feeding benefits in different cavefish populations provides the first example of convergent evolution of adaptive traits via paternal or maternal inheritance. These results have several important implications. First, they show that different genetic mechanisms can lead to the same evolutionary consequences, in this specific case, a change in behavior that is adaptive for living in a dark cave environment. Second, they suggest that epigenetic processes may be important in the evolution of novel traits. Third, if epigenetic processes are indeed involved in this behavioral change, they imply that additional considerations, such as paternal imprinting, must be taken into account to completely understand the genes and mechanisms involved in cavefish evolution. Our results open a pathway to investigating the relative contributions of parental genetic effects on evolutionary processes.
Previous studies have shown that constructive evolution of SN is required for VAB (Yoshizawa et al. 2010). A key to understanding the basis of differential parental inheritance of VAB may be related to the contrasting roles of SN number and size in the two cavefish populations. In Pachón cavefish, paternal inheritance affected the number but not the size of SN, whereas in Los Sabinos cavefish, maternal inheritance was observed in SN size but not number. These results provide further support for differences in the mode of VAB inheritance in the two cavefish populations and also suggest that different epigenetic effects on SN number and size may be important factors in the convergent evolution of VAB in cavefish.
There are many examples of disparity between nDNA and mtDNA in natural populations (Ballard and Whitlock 2004; Chan and Levin 2005) but the ways in which these differences have evolved are not completely understood. Pachón cavefish are an excellent system to investigate this problem because there has been transfer and fixation of mtDNA from a nearby surface fish population, presumably during a recent introgression event (Langecker et al. 1991). Here we have shown that paternal inheritance of an adaptive behavioral trait such as VAB is an attractive explanation for this disparity. Two lines of evidence support this possibility. First, F1 hybrids produced from Pachón cavefish males and surface fish females (F1SC), which show VAB and carry surface fish mtDNA, are strong competitors of F1 hybrids produced by crosses in the opposite direction (F1CS) and of the original cavefish in prey-capture competition assays. Second, mathematical simulations show that only the Pachón type of paternal inheritance was able to drive surface fish mtDNA to fixation. Thus, we propose that paternal genetic effects in an adaptive behavior such as VAB could generate disparity in mtDNA and nDNA of Pachón cavefish (see Fig. S2) and other natural populations that are subject to introgression.
Supplementary Material
Acknowledgements
We thank K. L. Carlton and R. B. Roberts for valuable comments on the manuscript. This research was supported by a Japan Society for the Promotion of Science Postdoctoral Fellowship to M. Y. and NSF (IBN-05384) and NIH (R01-EY014619) grants to W. R. J. G. A. is supported by NIH grant (R01-DC00436) to C. E. Carr.
Literature cited
- Ballard JWO, Whitlock MC. The incomplete natural history of mitochondria. Mol. Ecol. 2004;13:729–744. doi: 10.1046/j.1365-294x.2003.02063.x. [DOI] [PubMed] [Google Scholar]
- Blumstein DT, Daniel JC. Quantifying behavior the JWatcher way. Sinauer Associates, Inc.; Sunderland, U.S.A: 2007. [Google Scholar]
- Bradic M, Beerli Garcia-de Leon, F. J., Esquivel-Bobadilla S, Borowsky RL. Gene flow and population structure in the Mexican blind cavefish complex (Astyanax mexicanus) BMC Evol. Biol. 2012;12 doi: 10.1186/1471-2148-12-9. doi:10.1186/1471-2148-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KH. Fish mitochondrial genomics: sequence, inheritance and functional variation. J. Fish Biol. 2008;72:355–374. [Google Scholar]
- Chan KMA, Levin SA. Leaky prezygotic isolation and porous genomes: Rapid introgression of maternally inherited DNA. Evolution. 2005;59:720–729. [PubMed] [Google Scholar]
- Chong S, Vickaryous N, Ashe A, Zamudio N, Youngson N, Hemley S, Stopka T, Skoultchi A, Matthews J, Scott HS, de Kretser D, O’Bryan M, Blewitt M, Whitelaw E. Modifiers of epigenetic reprogramming show paternal effects in the mouse. Nature Genet. 2007;39:614–622. doi: 10.1038/ng2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling TE, Martasian DP, Jeffery WR. Evidence for multiple genetic forms with similar eyeless phenotypes in the blind cavefish, Astyanax mexicanus. Mol. Biol. Evol. 2002;19:446–455. doi: 10.1093/oxfordjournals.molbev.a004100. [DOI] [PubMed] [Google Scholar]
- Garten CT. Relationships between aggressive-behavior and genic heterozygosity in oldfield mouse, Peromyscus polionotus. Evolution. 1976;30:59–72. doi: 10.1111/j.1558-5646.1976.tb00881.x. [DOI] [PubMed] [Google Scholar]
- Gregg C, Zhang J, Weissbourd B, Luo S, Schroth GP, Haig D, Dulac C. High-resolution analysis of parent-of-origin allelic expression in the mouse brain. Science. 2010;329:643–648. doi: 10.1126/science.1190830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery WR. Cavefish as a model system in evolutionary developmental biology. Dev. Biol. 2001;231:1–12. doi: 10.1006/dbio.2000.0121. [DOI] [PubMed] [Google Scholar]
- Jeffery WR. Adaptive evolution of eye degeneration in the Mexican blind cavefish. J. Hered. 2005;96:185–196. doi: 10.1093/jhered/esi028. [DOI] [PubMed] [Google Scholar]
- Jeffery WR. Emerging model systems in evo-devo: cavefish and microevolution of development. Evol. Dev. 2008;10:265–272. doi: 10.1111/j.1525-142X.2008.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery WR. Evolution and development in the cavefish Astyanax. Curr. Top. Dev. Biol. 2009;86:191–221. doi: 10.1016/S0070-2153(09)01008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery WR, Strickler AG, Guiney S, Heyser DG, Tomarev SI. Prox 1 in eye degeneration and sensory organ compensation during development and evolution of the cavefish Astyanax. Dev. Genes Evol. 2000;210:223–230. doi: 10.1007/s004270050308. [DOI] [PubMed] [Google Scholar]
- Jørgensen JM. Evolution of octavolateralis sensory cells. In: Coombs S, Görner P, Münz H, editors. The mechanosensory lateral line. Springer-Verlag; New York, U.S.A.: 1989. pp. 115–145. [Google Scholar]
- Langecker TG, Wilkens H, Junge P. Introgressive hybridization in the Pachón Cave population of Astyanax fasciatus (Teleostei Characidae) Ichthyol. Explor. Freshw. 1991;2:209–212. [Google Scholar]
- Menuet A, Alunni A, Joly JS, Jeffery WR, Rétaux S. Expanded expression of sonic hedgehog in Astyanax cavefish: multiple consequences on forebrain development and evolution. Development. 2007;134:845–855. doi: 10.1242/dev.02780. [DOI] [PubMed] [Google Scholar]
- Mitchell RW, Russell WH, Elliott WR. Mexican eyeless characin fishes, genus Astyanax: Environment, distribution, and evolution. Texas Tech Press; Texas, U.S.A.: 1977. [Google Scholar]
- Mousseau TA, Fox CW. Maternal effects as adaptation. Oxford University Press; Oxford. U.K.: 1998. [Google Scholar]
- Ornelas-García CP, Domínguez-Domínguez O, Doadrio I. Evolutionary history of the fish genus Astyanax Baird & Girard (1854) (Actinopterygii, Characidae) in Mesoamerica reveals multiple morphological homoplasies. BMC Evol. Biol. 2008;8:340. doi: 10.1186/1471-2148-8-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaram K, Borowsky R. Gene flow and genetic variability in cave and surface populations of the Mexican tetra, Astyanax mexicanus (Telcostei : Characidae) Copeia. 2005:409–416. [Google Scholar]
- Peng B, Kimmel M. simuPOP: a forward-time population genetics simulation environment. Bioinformatics. 2005;21:3686–3687. doi: 10.1093/bioinformatics/bti584. [DOI] [PubMed] [Google Scholar]
- Plath M, Rohde M, Schröder T, Taebel-Hellwig A, Schlupp I. Female mating preferences in blind cave tetras Astyanax fasciatus (Characidae, Teleostei) Behaviour. 2006;143:15–32. [Google Scholar]
- Qvarnström A, Price TD. Maternal effects, paternal effects and sexual selection. Trends Ecol. Evol. 2001;16:95–100. doi: 10.1016/s0169-5347(00)02063-2. [DOI] [PubMed] [Google Scholar]
- Richards EJ. Inherited epigenetic variation-revisiting soft inheritance. Nature Rev. Genet. 2006;7:395–401. doi: 10.1038/nrg1834. [DOI] [PubMed] [Google Scholar]
- Şadoğlu P. Breeding method for blind Astyanax-mexicanus based on annual spawning patterns. Copeia. 1979:369–371. [Google Scholar]
- Strecker U, Bernatchez L, Wilkens H. Genetic divergence between cave and surface populations of Astyanax in Mexico (Characidae, Teleostei) Mol. Ecol. 2003;12:699–710. doi: 10.1046/j.1365-294x.2003.01753.x. [DOI] [PubMed] [Google Scholar]
- Strecker U, Hausdorf B, Wilkens H. Parallel speciation in Astyanax cave fish (Teleostei) in Northern Mexico. Mol. Phylogenet. Evol. 2012;62:62–70. doi: 10.1016/j.ympev.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Tiira K, Laurila A, Peuhkuri N, Piironen J, Ranta E, Primmer CR. Aggressiveness is associated with genetic diversity in landlocked salmon (Salmo salar) Mol. Ecol. 2003;12:2399–2407. doi: 10.1046/j.1365-294x.2003.01925.x. [DOI] [PubMed] [Google Scholar]
- Varatharasan N, Croll RP, Franz-Odendaal T. Taste bud development and patterning in sighted and blind morphs of Astyanax mexicanus. Dev. Dyn. 2009;238:3056–3064. doi: 10.1002/dvdy.22144. [DOI] [PubMed] [Google Scholar]
- Wilkens H. Evolution and genetics of epigean and cave Astyanax-fasciatus (Characidae, Pisces) - Support for the neutral mutation theory. Evol. Biol. 1988;23:271–367. [Google Scholar]
- Yamamoto Y, Byerly MS, Jackman WR, Jeffery WR. Pleiotropic functions of embryonic sonic hedgehog expression link jaw and taste bud amplification with eye loss during cavefish evolution. Dev. Biol. 2009;330:200–211. doi: 10.1016/j.ydbio.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Espinasa L, Stock DW, Jeffery WR. Development and evolution of craniofacial patterning is mediated by eye-dependent and -independent processes in the cavefish Astyanax. Evol. Dev. 2003;5:435–446. doi: 10.1046/j.1525-142x.2003.03050.x. [DOI] [PubMed] [Google Scholar]
- Yoshizawa M, Jeffery WR. Shadow response in the blind cavefish Astyanax reveals conservation of a functional pineal eye. J. Exp. Biol. 2008;211:292–299. doi: 10.1242/jeb.012864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa M, Jeffery WR. Evolutionary tuning of an adaptive behavior requires enhancement of the neuromast sensory system. Commun. Integr. Biol. 2011;4:89–91. doi: 10.4161/cib.4.1.14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa M, Gorički Š, Soares D, Jeffery WR. Evolution of a behavioral shift mediated by superficial neuromasts helps cavefish find food in darkness. Curr. Biol. 2010;20:1631–1636. doi: 10.1016/j.cub.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.