SUMMARY
1. Adaptive maternal programming occurs when mothers alter their offspring's phenotype in response to environmental information such that it improves offspring fitness. When a mother's environment is predictive of the conditions her offspring are likely to encounter, such transgenerational plasticity enables offspring to be better-prepared for this particular environment. However, maternal effects can also have deleterious effects on fitness.
2. Here, we test whether female threespined stickleback fish exposed to predation risk adaptively prepare their offspring to cope with predators. We either exposed gravid females to a model predator or not, and compared their offspring's antipredator behaviour and survival when alone with a live predator. Importantly, we measured offspring behaviour and survival in the face of the same type of predator that threatened their mothers (Northern pike).
3. We did not find evidence for adaptive maternal programming; offspring of predator-exposed mothers were less likely to orient to the predator than offspring from unexposed mothers. In our predation assay, orienting to the predator was an effective antipredator behaviour and those that oriented, survived for longer.
4. In addition, offspring from predator-exposed mothers were caught more quickly by the predator on average than offspring from unexposed mothers. The difference in antipredator behaviour between the maternal predator-exposure treatments offers a potential behavioural mechanism contributing to the difference in survival between maternal treatments.
5. However, the strength and direction of the maternal effect on offspring survival depended on offspring size. Specifically, the larger the offspring from predator-exposed mothers, the more vulnerable they were to predation compared to offspring from unexposed mothers.
6. Our results suggest that the predation risk perceived by mothers can have long-term behavioural and fitness consequences for offspring in response to the same predator. These stress-mediated maternal effects can have nonadaptive consequences for offspring when they find themselves alone with a predator. In addition, complex interactions between such maternal effects and offspring traits such as size can influence our conclusions about the adaptive nature of maternal effects.
Keywords: fitness, Gasterosteus aculeatus, maternal effects, maternal programming, maternal stress, Northern pike, predator-exposure, prenatal stress, body size, transgenerational plasticity
INTRODUCTION
In many species, offspring are influenced by the environmental conditions experienced by their mother (Bernardo 1996; Mousseau & Fox 1998). In some cases, such `non-genetic maternal effects' are adaptive, in that they improve offspring fitness. Indeed, if mothers have reliable information about the future environment, then they might `programme' their offspring for the environment they are likely to encounter (Weaver et al. 2004; Gluckman et al. 2005; Uller 2008). For example, in several species, the offspring of mothers that are exposed to cues of predators are better-defended against predators compared to the offspring of unexposed mothers (Agrawal, Laforsch & Tollrian 1999; Shine & Downes 1999; Storm & Lima 2010). Such maternal effects can have important implications for population dynamics and predator-prey interactions (Sheriff, Krebs & Boonstra 2009, 2010).
However, not all maternal effects improve offspring fitness. A large literature in diverse vertebrate species suggests that maternal exposure to stressors can have deleterious effects on offspring that are mediated by glucocorticoid stress hormones (reviewed in: Weinstock 2008; Henriksen, Rettenbacher & Groothuis 2011; Schoech, Rensel & Heiss 2011). For example, prenatal exposure to stress hormones has negative consequences for embryo survival as well as offspring morphology and growth rate (birds: Hayward & Wingfield 2004; Love et al. 2005; Saino et al. 2005; fishes: McCormick 2006, 2009; mammals: Sheriff et al. 2009; lizards: Meylan & Clobert 2005; Meylan, Haussy & Voituron 2010). When lifetime maternal reproductive success or offspring survival are examined however, it often becomes less obvious whether we can divide up maternal effects into those with entirely negative consequences and those with entirely positive consequences (see Love & Williams 2008b). For example, stressed mothers might compensate for the fitness costs of producing low quality offspring by adjusting their reproductive investment (Love et al. 2005; Love & Williams 2008b; Monclús et al. 2011). Similarly, prenatal exposure to stress hormones can have a negative effect on one aspect of offspring phenotype but have a positive effect on a different aspect of offspring phenotype (Chin et al. 2009; Coslovsky & Richner 2011; Gagliano & McCormick 2009; Meylan & Clobert 2005).
Of particular importance are those maternal effects that have organisational effects on offspring development and result in lifelong consequences for offspring. For example, if prenatal exposure to stress hormones occurs during sensitive periods in development when the HPA (Hypothalamic-Pituitary-Adrenal) axis, or in fishes, HPI (Hypothalamic-Pituitary-Interrenal) axis, is developing, such maternal effects can impact an individual's stress response and many of their behaviours for life (Hayward & Wingfield 2004; Love & Williams 2008a; Brunton & Russell 2010; Green et al. 2011; reviewed in: Weinstock 2008; Matthews & Phillips 2010). Therefore, while it would be especially beneficial if mothers could `forewarn' their offspring about dangers such as predators in the environment, the possibility of such an adaptive maternal effect might be constrained if it is mediated by stress hormones that have organisational effects on the development of multiple offspring traits.
Exposure to predation risk has been shown to cause females of the threespined stickleback fish, Gasterosteus aculeatus (Fig. 1A), to produce eggs with higher concentrations of the stress hormone cortisol, and juvenile offspring with higher baseline levels of shoaling behaviour (Giesing et al. 2011). A plausible mechanism underlying these maternal effects is that exposure to predation risk triggers the release of cortisol in females (Bell et al. 2007), which then diffuses into the eggs, causing organisational effects on offspring development. Although it is possible that the maternal effect on behaviour is adaptive because shoaling is an effective predator defence in fishes (Pitcher 1993), it is unknown whether maternal exposure to predation risk results in improved offspring survival (fitness) when an individual encounters a real predator without the safety of a shoal.
Figure 1.
Study animals (line drawings by K.E. McGhee).
Here, we test the hypothesis that female sticklebacks adaptively manipulate their offspring in response to predation risk by comparing the survival of offspring from mothers exposed to a model predator (predator-exposed) to the survival of offspring from unexposed mothers. To prevent offspring from relying on the attentiveness of other individuals and the safety of a shoal, as well as to gain insights into individual antipredator behaviour, offspring encountered the predator alone. If predator-induced maternal effects are adaptive under these conditions, then the offspring of predator-exposed mothers should survive for longer during an encounter with a predator compared to the offspring of unexposed females. On the other hand, if these maternal effects are nonadaptive under these conditions, as suggested by the literature on maternal stress (Weinstock 2008; Matthews & Phillips 2010; Schoech et al. 2011), then the offspring of predator-exposed mothers should fare worse under the threat of real predation. Importantly, we measured the survival of juvenile sticklebacks when faced with the same predator (Northern pike, Esox lucius, Fig. 1B) that threatened their mothers, i.e. in the context where it is most likely that females might `program' their offspring (Gluckman et al. 2005; Uller 2008). In addition, maternal predator-exposure might affect offspring antipredator behaviour which in turn affects survival with a live predator. To gain insights into the possible behavioural mechanisms that contribute to differences in survival, we measured the behaviour of both the predator (pike) as well as the prey (stickleback) during their interaction.
MATERIALS AND METHODS
Adult sticklebacks were collected from Putah Creek, CA, in May 2010. While piscivorous predators (e.g. bass) are present in this population, Northern pike predators are absent (but see the multipredator hypothesis proposed by Blumstein 2006). Thus field-collected adults had not previously encountered a Northern pike and it was a novel predator for both adults and offspring. Breeding and treatment methods in this study are similar to those described elsewhere (Giesing et al. 2011). Briefly, in summer 2010, we randomly assigned females to either a predator-exposed or unexposed (control) treatment tank with five adult females per 37.8 L tank (36 × 33 × 24 cm, length × width × height). There were four predator-exposed tanks and two unexposed tanks. The tanks were covered with opaque plastic on all sides with a small closable “window” to allow us to monitor female reproductive state (i.e. gravidity) with minimal disturbance. Fish were maintained at 20 degrees C on a summer photoperiod schedule.
Predator-Exposure Protocol
We chased females in the predator-exposed tanks for approximately 30 sec once a day with a clay model of a natural predator, the Northern pike (23 cm in length and painted to match natural markings). Females were chased at a random time each day during the daylight hours so that exposure to predator cues was unpredictable to avoid habituation. Females were chased closely (~ 1 cm) by the pike model but not touched. Our rationale for this manipulation was two-fold. First, we wanted to mimic a high predation environment where females are exposed to high but unpredictable levels of predation risk every day. Similarly brief (30 sec) and unpredictable exposure to predator cues across only a few days is enough to alter juvenile growth (Bell et al. 2011). Second, we wanted to follow the same protocol as Giesing et al. (2011), which generated higher levels of cortisol in the eggs of predator-exposed females. Fish in the unexposed treatment were left undisturbed. When a female was obviously gravid, she was removed from the tank and stripped of eggs, and another female was added to the treatment tank in order to keep density constant. To estimate average egg mass (mg) for each female, we divided the mass of her entire clutch of eggs by the number of eggs in the clutch (manually counted).
To control for paternal effects (Tulley & Huntingford 1987), we artificially fertilised the eggs from each female and reared them without paternal care. We dissected out the testes of a sacrificed randomly selected male and macerated them with fine forceps to release sperm. For over half of the crosses, a single male fertilised the eggs of both a predator-exposed female and an unexposed female (N = 10 females). Due to differences among females in when they became gravid, in some crosses, a male fertilised the eggs of a single female (predator-exposed females: N = 3; unexposed females: N = 4). Successful fertilisation was confirmed by visual inspection for eyespots and unfertilised eggs were removed. We continued sampling for 11 weeks until we had collected clutches from eight different predator-exposed mothers and nine different control mothers.
Rearing Protocol
We incubated fertilised eggs from each clutch separately in a cup with a mesh screen bottom. To prevent fungus, we added one drop of methylene blue to each tank. A gently bubbling airstone was positioned under each cup. After hatching, fry from different families were kept separate from one another in initial fry tanks (~10 siblings per 9 L tank) with the fry from large clutches being separated into several initial fry-tanks. We fed fry Artemia nauplii and cyclopeez once a day. To maintain the juveniles for this study at a constant density, we randomly combined siblings from different initial fry-tanks into a single final juvenile tank (a maximum of 10 juveniles per 9 L tank) with a gravel bottom and an artificial plant when they were approximately 2 cm in length and safe to handle. Over the entire time period of fry rearing described above (several months), mortality was low and did not differ between the maternal treatments (number of fry deaths: unexposed mothers = 4.2 ± 1.8 fry (mean ± standard error), predator-exposed mothers = 5.2 ± 1.4 fry, t15 = −0.84, P = 0.412; analysis on ln(x+1) transformed mortality).
To reduce the possibility of bias, final juvenile rearing tanks were given random numbers unrelated to treatment and were arranged randomly across several racks of tanks. Juveniles were kept in these final rearing tanks until behavioural testing at approximately three cm in length. All fish from each final rearing tank were behaviourally tested (number of offspring behaviourally tested = 9.0 ± 0.5 offspring per mother; range 4 – 10 offspring per mother). Because all of the siblings that were behavioural tested came from the same final rearing tank, “rearing tank” and “mother” are synonymous. We accounted for this non-independence of siblings/tank-mates by including mother nested within treatment as a random effect in our statistical analyses. We fed juveniles a slurry of frozen adult Aretmia, mysis shrimp, bloodworms and cyclopeez once a day and they were maintained at 18 degrees C on a photoperiod schedule that matched seasonal changes.
Live Predator Assay
Pike were hatchery-reared (Spirit Lake Fish Hatchery, Iowa) and transported to the University of Illinois by car five weeks prior to being used in the live-predator assay. These pike were accustomed to eating only live prey. They were housed singly in large 83.3 L tanks (107 × 33 × 24 cm) on a separate water flow system and visually separated from the sticklebacks in the lab for five weeks. We used 12 pike that ranged in size from 18.2 to 22.3 cm in length. Water was cleaned in all tanks via a recirculating flow-through system with particulate, biological and UV filters (Aquaneering, San Diego, USA). Approximately 10% of the water volume in the tanks was replaced each day. Pike tanks had a gravel bottom, two artificial plants, two pieces of PVC pipe on the bottom and two PVC standpipes on the back wall for prey refuge (PVC pipes = 2 cm diameter).
The day before behavioural testing, a randomly-selected stickleback was netted from their rearing tank, measured for standard length and isolated in a novel temporary tank (37.8 L) overnight. The fish chosen for testing was chosen prior to netting and selection was not affected by fish size (e.g. larger fish were not tested sooner than smaller fish) (correlation between size and day of testing, r = 0.02, N = 146, P = 0.77). The next day between 1330 and 1600 hours, the stickleback was gently herded into a cup of water (no netting) and then immediately released into a pike's tank where the predation assays were conducted.
We tested stickleback singly in the predation assays for several reasons. First, we wanted to be able to record the detailed antipredator behaviour of each individual. Second, we did not want the attentiveness of other individuals and the location within a shoal to influence an individual's behaviour and/or survival. Third, field data indicates that juveniles are commonly found singly as well as in shoals in this population (S. Pearish, unpublished data). We did not test multiple siblings on the same day (i.e. maximum of one fish per rearing tank per day) and rearing tanks were tested in a random order. Therefore rearing tank (i.e. family) was not confounded with test day. Twelve stickleback were tested each day and randomly assigned to one of the 12 pike thus all pike were used equally and stickleback rearing tank was not confounded with pike identity. We continued rotating through the rearing tanks until all fish had been tested. In total, we behaviourally tested 74 offspring from unexposed mothers and 72 offspring from predator-exposed mothers.
To reduce the chances that a pike would capture the stickleback immediately upon release, we used two identical “feeding” cups: a decoy-cup containing only water and a cup containing water and the stickleback. The cups were on opposite sides of the tank and the one containing the stickleback was positioned so that it was the one furthest from the pike. The cups were partially submerged in the water and their contents were simultaneously poured out gently into the tank. The cups were removed and data recording began immediately. Data was recorded live by a single watcher (KEM, LMP, or D. Roche) using JWatcher (Blumstein & Daniel 2007) and trials lasted until the stickleback was successfully captured or a maximum of 10 minutes. All pike were tested once per day in a random order. On days when pike were not used in the experiment, they were fed in an identical way (two cups, one stickleback or goldfish).
During a trial, we recorded the survival time of the stickleback (i.e. the time until the pike captured the stickleback). We also simultaneously recorded the following pike and stickleback behaviours. For the pike, we recorded (1) latency to orient towards the stickleback for the first time and (2) latency to lunge at the stickleback for the first time. Survival time was often longer than latency to lunge because pike were not always successful in capturing the stickleback on the first attempt. For the stickleback, we recorded the time spent (1) frozen (holding still for > 2 seconds) and (2) oriented towards the pike. Since stickleback survived for variable amounts of time, each individual's behaviour was converted to proportions (time spent doing a particular behaviour divided by survival time). Survival time does not include the handling time required for the pike to manipulate and swallow the stickleback.
Offspring from the two treatments did not differ significantly in size or age at the time of behavioural testing (standard length: unexposed mothers = 30.7 ± 0.4 mm, predator-exposed mothers = 30.7 ± 0.4 mm, t144 = 0.027, P = 0.979; age: unexposed mothers = 223 ± 8 days old, predator-exposed mothers = 218 ± 8 days old, t15 = 0.438, P = 0.667).
Data Analysis
We used t-tests to compare the clutch and egg traits between maternal treatments. We excluded trials where the pike positioned itself directly under the stickleback cup and the stickleback was captured in less than 20 sec (five trials).
We used mixed models to examine how survival time and predator behaviour were influenced by maternal predator-exposure treatment with offspring size included as a covariate. For these analyses, we used the residuals of a regression of survival time (or predator behaviour) on day of testing as the dependent variable because the pike generally improved at capturing the stickleback over the course of the experiment.
We used a mixed model to examine how stickleback freezing behaviour was influenced by maternal predator-exposure treatment with offspring size and day of testing included as covariates. Because only half of the stickleback oriented to the predator (77 of 146 oriented to the predator), we examined stickleback orienting behaviour as a binomial response variable (orient – yes or no). We used a generalised linear mixed model in SAS GLIMMIX to examine whether orienting towards the predator was influenced by maternal predator-exposure treatment. For this analysis, we specified a binomial distribution with a probit link function and used the Laplace maximum likelihood approximation (Bolker et al. 2009). Again we included offspring size and day of testing as covariates in the initial model. To examine how orienting behaviour affected predator behaviour and survival, we used a mixed model to examine how predator behaviour (first time to orient to the prey and first time to lunge at the prey) and survival time were influenced by whether the stickleback did/did not orient to the predator. Again, to account for predator improvement, we conducted these analysis on the residuals of a regression between the dependent variable and day of testing.
For all the above analyses, the covariates and interactions with fixed effects were removed sequentially from the model if their F-values were nonsignificant and less than one. Because siblings are not necessarily independent of one another, we included (1) mother nested within maternal treatment and (2) father as random effects in the analyses. Since pike were repeatedly used, we included (3) pike identity as an additional random effect in the analyses. We assessed the significance of the random effects (mother(treatment), father and pike identity) in the models by removing them separately from the model and using log likelihood tests to compare the models with and without the random effect. Although term removal was necessary to assess statistical significance, all three random effects were included in final models regardless of statistical significance. Survival time, latency for the pike to first orient at the stickleback and latency for the pike to first lunge at the stickleback were natural log-transformed and the proportion of time that the stickleback spent frozen was arcsine squareroot transformed. We validated model assumptions by examining the residuals. We used REML in all mixed models due to the unbalanced design. We estimated the degrees of freedom using the Satterthwaite approximation for all mixed models. Because we are unable to use the Satterthwaite approximation with the Laplace method in the GLMM, we used the containment method. To examine the relationship between continuous traits we used Pearson correlations on transformed data. Means ± standard errors are used throughout. All analyses were conducted using SAS™ software, version 9.2 (SAS Institute Inc., Cary, N.C., U.S.A.).
Ethical Note
Allowing predators to interact freely with their prey was essential to quantify fitness. We did however provide numerous refuges in the predator tanks in order to give the sticklebacks an opportunity to hide or escape from the pike. In planning the study, we used power analyses based on results from a previous study (Giesing et al. 2011) to minimise the number of subjects used (ABS/ASAB 2003). This experiment was approved by the Animal Care and Use Committee of University of Illinois (protocol #09204).
RESULTS
Females of the two maternal treatments produced similar numbers of eggs (egg number: unexposed mothers = 109 ± 22, predator-exposed mothers = 117 ± 18, t15 = −0.46, P = 0.655; analysis on ln-transformed egg number). Consistent with previous findings (Giesing et al. 2011), the eggs of predator-exposed females were approximately 0.8 mg larger on average than the eggs of unexposed females although this was not a statistically significant difference (egg mass: unexposed mothers = 3.31 ± 0.28 mg, predator-exposed mothers = 4.14 ± 0.43 mg, t15 = −1.83, P = 0.086; analysis on ln-transformed egg mass).
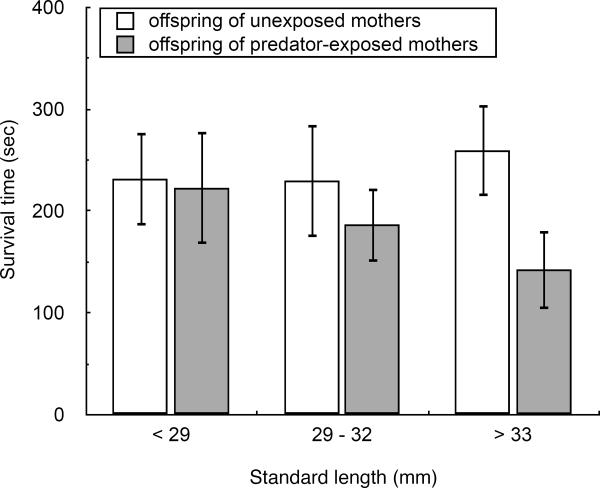
There was a negative effect of maternal exposure to predation risk on offspring fitness and this was particularly evident at certain sizes (Table 1). Offspring of predator-exposed mothers were captured more quickly than offspring of unexposed mothers (survival time: offspring of unexposed mothers = 241 ± 27 sec, offspring of predator-exposed mothers = 184 ± 24 sec). While the survival time of offspring from predator-exposed mothers decreased with offspring size, survival time of offspring from unexposed mothers seemed unrelated to offspring size (Fig. 2). In this analysis, both the random effects of mother(treatment) and pike identity were weakly statistically significant but the random effect of father was not (Mother(treatment): X2 = 2.6, P = 0.053; Father: X2 = 0.1, P = 0.376; Pike identity: X2 = 2.7, P = 0.050).
Table 1.
Offspring survival time (after controlling for the effect of the day of testing - see text) was influenced by maternal predator-exposure treatment and its interaction with stickleback size (covariate).
| Fixed effect | d.f. | F-value | P-value |
|---|---|---|---|
| Maternal treatment | 1, 139 | 4.24 | 0.041 |
| Stickleback standard length (SL) | 1, 133 | 0.84 | 0.361 |
| Maternal treatment * SL | 1, 136 | 5.01 | 0.027 |
Figure 2.
Survival time interacted with offspring size: for offspring of predator-exposed mothers, survival time decreased with offspring standard length. Shown are means ± SE with 24 ± 2 offspring contributing to each mean on average. Note that this figure is only illustrative - the analyses were conducted using standard length as a continuously distributed covariate.
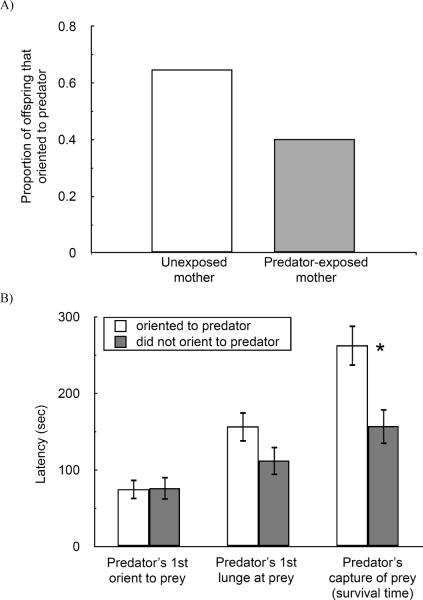
A closer look at the actual behavioural interaction between the pike and stickleback suggests that a stickleback's failure to orient to the predator may have contributed to its early death. Offspring from unexposed mothers were much more likely to orient towards the predator than offspring from predator-exposed mothers (Fig. 3A, Table 2). In this analysis, none of the random effects were statistically significant (Mother(treatment): X2 = 0, P = 0.5; Father: X2 = 0, P = 0.5; Pike identity: X2 = 0.21, P = 0.324), and offspring size did not affect the probability of orienting (standard length: oriented = 30.5 ± 0.4 mm, did not orient = 30.9 ± 0.4 mm, t144 = 0.844, P = 0.400). Regardless of maternal treatment, stickleback that oriented to the predator (N = 77) had a longer survival time compared to stickleback that did not orient (N = 69), suggesting that orienting towards the predator is an effective antipredator behaviour in our predation assay (Fig. 3B, survival time: oriented = 263 ± 27 sec, did not orient = 157 ± 23 sec, F1,140 = 5.98, P = 0.016; random effects of Mother(treatment): X2 = 1.8, P = 0.090; Father: X2 = 0.1, P = 0.376; Pike identity: X2 =2.3, P = 0.065). This survival difference was perhaps due to a weak trend for stickleback orienting behaviour to delay a predator's first lunge (Fig. 3B, predator's first lunge: oriented = 159 ± 20 sec, did not orient = 112 ± 19 sec, F1,141 =3.01, P = 0.085; random effects of Mother(treatment): X2 = 2.9, P = 0.044; Father: X2 = 0, P = 0.5; Pike identity: X2 = 2.5, P = 0.057). Whether the stickleback oriented towards the predator or not did not affect how quickly the predator first oriented to the stickleback (oriented = 75 ± 13 sec, did not orient = 76 ± 15 sec, F1,139 = 0.10, P = 0.753). How quickly a pike first oriented to the stickleback was strongly influenced by the random effect of pike identity (random effects of Mother(treatment): X2 = 2.2, P = 0.069; Father: X2 = 0, P = 0.5; Pike identity: X2 = 4.2, P = 0.020).
Figure 3.
Orienting at the pike was an effective antipredator behaviour and differed between maternal predator-exposure treatments. (A) A greater proportion of offspring from unexposed mothers oriented to the predator than did offspring from predator-exposed mothers. (B) Stickleback that oriented to the predator survived for longer than those that did not orient to the predator. Shown are means ± SE. * indicates P = 0.016.
Table 2.
Whether offspring oriented to the predator or not was influenced by maternal predator-exposure treatment but not day of testing (covariate). Stickleback size and the 2-way interactions with the covariates were removed sequentially because the F-values were <1 and non-significant. D.f. approximated using containment, Pearson Chi-squared / d.f. = 0.97.
| Fixed effect | d.f. | F-value | P-value |
|---|---|---|---|
| Maternal treatment | 1, 15 | 8.84 | 0.009 |
| Day of testing | 1, 117 | 2.31 | 0.131 |
Survival time was also positively correlated with the proportion of time sticklebacks spent frozen (r = 0.47, P < 0.0001, N = 146) suggesting that it too is an effective antipredator behaviour in our predation assay. However we did not detect a statistically significant difference in this behaviour between offspring from the two maternal treatments (Table 3; proportion of time alive spent frozen: unexposed mothers = 0.32 ± 0.03, predator-exposed mothers = 0.32 ± 0.03), and offspring size was not related to their freezing behaviour (correlation between proportion of time alive spent frozen and size: r = 0.05, P = 0.561; N = 146). The only effect that explained a significant amount of the variation in the proportion of time a stickleback spent frozen was the random effect of pike identity (random effects of Mother(treatment): X2 = 0.2, P = 0.327; Father: X2 = 0.5, P = 0.263; Pike identity: X2 = 5.5, P = 0.009).
Table 3.
The proportion of time before capture that offspring spent frozen was not influenced by maternal predator-exposure treatment or day of testing (covariate). Stickleback size and the 2-way interactions with the covariates were removed sequentially because the F-values were <1 and non-significant.
| Fixed effect | d.f. | F-value | P-value |
|---|---|---|---|
| Maternal treatment | 1, 6.43 | 0.01 | 0.927 |
| Day of testing | 1, 123 | 1.42 | 0.236 |
DISCUSSION
Female sticklebacks exposed to a model pike predator did not adaptively prepare their offspring for coping with a live pike predator. Indeed, larger offspring were more vulnerable to predation if their mother had been exposed to a predator compared to offspring from unexposed mothers. Additionally, we identified a potential behavioural mechanism for this difference in survival; offspring from predator-exposed mothers were less likely to orient towards the predator than offspring from unexposed mothers, and those that oriented had a survival advantage. Orienting towards the predator indicates that the stickleback has noticed the predator and can then modify its behaviour accordingly (e.g. by freezing, seeking cover, swimming away). Thus failure to pay attention specifically to the presence of a predator may have contributed to the greater vulnerability of offspring from predator-exposed mothers. Remaining motionless (i.e. freezing) was also an effective antipredator behaviour but this behaviour did not differ between maternal treatments. Unlike orienting, which required that the stickleback actually perceive the predator, a stickleback's freezing behaviour could be completely unrelated to the presence of the predator and instead reflect a reaction to the new tank environment for example. The survival advantage offered by freezing seems to be related to the fact that pike quickly noticed prey that were moving (i.e. not freezing), while the survival advantage offered by orienting seemed to be related to attention of the stickleback to the predator specifically.
The nonadaptive maternal effect found here is somewhat surprising because similar studies that have exposed offspring to the same predator as their mother have suggested that mothers might adaptively `programme' their offspring for the predators they are likely to encounter (e.g. Agrawal et al. 1999; Shine & Downes 1999; Storm & Lima 2010). In a previous study of predator-mediated maternal effects in sticklebacks, offspring of predator-exposed mothers shoaled more tightly than offspring of unexposed mothers prior to a mild disturbance (Giesing et al. 2011). Those results suggested that mothers might adaptively program their offspring in response to predation risk because shoaling is an effective antipredator defence (Pitcher 1993). However, differences in shoaling behaviour between the treatment groups disappeared after the offspring experienced a mild disturbance. In other words, when actually threatened (i.e. when shoaling is most important as an antipredator defence), offspring of predator-exposed mothers did not shoal any closer than offspring of unexposed mothers. Therefore, one interpretation of the Giesing et al. (2011) study is that the increased tendency to shoal pre-disturbance of offspring from predator-exposed mothers reflected their more fearful or stressed state and did not necessarily reveal how they would behave in a potentially dangerous situation. Alternatively, an increased tendency to shoal pre-disturbance might indeed reflect the effectiveness of their antipredator behaviour but because sticklebacks were alone with the predator in our study, offspring of predator-exposed mothers could not take advantage of this potentially beneficial maternal effect on shoaling behaviour. Both maternal treatments invested equally in their clutches which suggests that offspring from predator-exposed mothers are not of lower value in terms of maternal reproductive effort (see also McCormick 2006, 2009; Giesing et al. 2011). Measuring the survival of offspring from predator-exposed mothers and unexposed mothers under a variety of conditions, including while they are in groups and capable of shoaling, is an obvious topic for future work.
While inconsistent with adaptive maternal programming, the lower survival of offspring from predator-exposed mothers in this study is consistent with the idea that there might be constraints on adaptive maternal programming when it is mediated by prenatal stress. Developmental exposure to elevated stress hormones (i.e. glucocorticoids) can have a number of deleterious effects on offspring morphology, behaviour and survival (Hayward & Wingfield 2004; Saino et al. 2005; Weinstock 2008; Sheriff et al. 2009, 2010; Henriksen et al. 2011 Schoech et al. 2011). While studies in stickleback suggest that predator-exposure triggers the release of cortisol in females (Bell et al. 2007) and the higher concentrations of cortisol in eggs of predator-exposed mothers (Giesing et al. 2011) are probably maternally-derived, we do not know if differences in cortisol are the most important (or only) difference between predator-exposed and unexposed females because we did not manipulate cortisol directly. However, cortisol playing an important role in stickleback fits well with the scenario that females mount a cortisol stress response as a way of immediately coping with a predator, but this immediate survival benefit for mothers has long-term negative consequences for offspring (i.e. the developmental response is not predictively adaptive, sensu Gluckman et al. 2005), at least under the particular conditions examined here.
An alternative explanation for why we did not find evidence for adaptive maternal programming in this study is that the environment experienced by the mothers was not equivalent to that experienced by her offspring. In other words, mothers were preparing their offspring for an environment the offspring did not end up encountering. For example, mothers had repeated visual-only exposure to a model pike, which they may have viewed as an ineffective predator, while their offspring had a single encounter with a real pike and had both visual and chemical cues. In addition, mothers encountered the model predator in their home tank with other females and thus could shoal, while their offspring encountered the real predator in a novel environment (i.e. pike home tank) and were alone. Thus while we attempted to match a mother's experiences with the future experiences of her offspring, they were not identical. It remains unclear how similar the mother and offspring environments must be for us to infer a role for maternal adaptive programming.
Offspring survival was also influenced by offspring body size, but in an intriguing way. While the survival of offspring from unexposed mothers seemed unrelated to offspring size, the survival of offspring from predator-exposed mothers was negatively related to offspring size. Thus, large offspring were captured more quickly than small offspring if their mother had been exposed to a predator. That the relationship between offspring survival and size differs between the maternal treatments is particularly surprising because there was no difference in body size between maternal treatments and no relationship between body size and antipredator behavior. Additionally, the negative correlation between survival and size for offspring from predator-exposed mothers is in contrast to a number of studies that have found smaller sticklebacks are more vulnerable to piscivorous predators than larger sticklebacks (Reimchen 1990, 1991; Bell et al. 2011). While the mechanism behind this offspring survival-size interaction remains unclear, it can affect our conclusions about the adaptive nature of the maternal effect. Specifically, our conclusions about the survival differences between the maternal treatments depend on where along the size continuum that we compare survival. The survival difference between the maternal treatments is striking at larger sizes but not so at smaller sizes. If we had tested all individuals at a single size, we might have concluded that the maternal effect had no fitness consequences. A similar pattern of context dependence has been found in studies where the consequences of maternal effects depend on the sex of the offspring (Bian, Wu & Liu 2005; Love et al. 2005; Meylan & Clobert 2005; Love & Williams 2008a,b; Brunton & Russell 2010; Monclús, Tiulim & Blumstein 2011; Zohar & Weinstock 2011). If these types of interactions between maternal effects and offspring traits are common (Bernardo 1996), it might not be surprising that studies find conflicting patterns about the adaptive nature of maternal effects.
Are there generalisations we can draw from the empirical evidence regarding factors that might influence whether a predator-induced maternal effect is adaptive or maladaptive? Overlooking methodological differences among studies in how they induce maternal effects (e.g. maternal exposure to stressor vs. experimental elevation of cortisol in offspring) and what they measure in offspring (e.g. survival vs. morphological traits), the literature does suggest several relevant factors that could affect whether maternal experience with predators can have preparatory, adaptive effects on offspring (see also Bernardo 1996). For example, whether the stressor is a serious threat to the mother is likely to be important. Mothers that are fearful for their own survival might mount a different physiological response compared to mothers that are fearful for the survival of their future offspring, perhaps leading to different consequences for their offspring. Related to this is whether novel predators (as was the case with our study) induce the same physiological and behavioural response in mothers (and offspring) as predators with which mothers are familiar (Blumstein 2006). In addition to the type of threat posed by the stressor, the magnitude, predictability and timing of the stressor are likely to be important. Whether maternal exposure to predator cues is short and unpredictable (e.g. Sheriff et al. 2009; Coslovsky & Richner 2011; Giesing et al. 2011) or continuous over an extended time period (e.g. Agrawal et al. 1999; Shine & Downes 1999; Storm & Lima 2010) has implications for the likelihood of offspring encountering similar conditions. Finally, the scope for generating an adaptive maternal effect could be influenced by the mechanism underlying it (e.g. epigenetic, hormonal, chemical, etc.; Weaver et al. 2004; Groothuis et al. 2005; Groothuis & Schwabl 2008). If mothers have little control over the mechanism (e.g. passive transfer of stress hormones to eggs), there might be less opportunity for mothers to actively manipulate their offspring in response to the environment.
In this study we showed that maternal predator-exposure affects offspring antipredator behaviour and their survival prospects when encountering a live predator alone. However, instead of finding evidence for adaptive maternal effects, we found an interaction between maternal predator-exposure and offspring size that resulted in deleterious fitness consequences for offspring at particular sizes. Because maternal experience influenced offspring survival, our results underscore the evolutionary importance of maternal effects. However, our results also show that the fitness consequences of maternal effects are not always straightforward, and can be complicated by interactions between maternal effects and offspring traits.
ACKNOWLEDGEMENTS
We thank the Spirit Lake Fish Hatchery (Iowa) for providing the pike, the Jake Wolf Fish Hatchery (Illinois) for holding the pike, and B. Mommer and M. Schrader for help transporting the pike. We thank D. Roche for help collecting behavioural data. We thank M. Schrader, three anonymous reviewers and C. Fox for insightful comments that greatly helped improve the manuscript. K.E.M. was supported by an NIH/NICHD fellowship to the University of Illinois (T32 HD007333) and an NSF grant to A.M.B. and K.E.M. (IOS 1121980). L.M.P. and E.L.S. were supported by University of Illinois start-up funds to A.M.B.
REFERENCES
- ABS/ASAB Guidelines for the treatment of animals in behavioral research and teaching. Animal Behaviour. 2003;65:249–255. [Google Scholar]
- Agrawal AA, Laforsch C, Tollrian R. Transgenerational induction of defences in animals and plants. Nature. 1999;401:60–63. [Google Scholar]
- Bell AM, Backstrom T, Huntingford FA, Pottinger TG, Winberg S. Variable neuroendocrine responses to ecologically-relevant challenges in sticklebacks. Physiology and Behavior. 2007;91:15–25. doi: 10.1016/j.physbeh.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Bell AM, Dingemanse NJ, Hankison SJ, Langenhof MBW, Rollins K. Early exposure to nonlethal predation risk by size-selective predators increases somatic growth and decreases size at adulthood in threespined sticklebacks. Journal of Evolutionary Biology. 2011;24:943–953. doi: 10.1111/j.1420-9101.2011.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo J. Maternal effects in animal ecology. American Zoologist. 1996;36:83–105. [Google Scholar]
- Bian JH, Wu Y, Liu J. Effect of predator-induced maternal stress during gestation on growth in root voles Microtus oeconomus. Acta Theriologica. 2005;50:473–482. [Google Scholar]
- Blumstein DT. The multipredator hypothesis and the evolutionary persistence of antipredator behavior. Ethology. 2006;112:209–217. [Google Scholar]
- Blumstein DT, Daniel JC. Quantifying behavior the JWatcher Way. Sinauer; Sunderland, Massachusetts: 2007. [Google Scholar]
- Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J-SS. Generalized linear mixed models: a practical guide for ecology and evolution. Trends in Ecology and Evolution. 2009;24:127–135. doi: 10.1016/j.tree.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA. Prenatal social stress in the rat programmes neuroendocrine and behavioural responses to stress in the adult offspring: sex-specific effects. Journal of Neuroendocrinology. 2010;22:258–271. doi: 10.1111/j.1365-2826.2010.01969.x. [DOI] [PubMed] [Google Scholar]
- Chin EH, Love OP, Vespoor JJ, Williams TD, Rowley K, Burness G. Juveniles exposed to embryonic corticosterone have enhanced flight performance. Proceedings of the Royal Society of London. Series B. Biological Sciences. 2009;276:499–505. doi: 10.1098/rspb.2008.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coslovsky M, Richner H. Predation risk affects offspring growth via maternal effects. Functional Ecology. 2011;25:878–888. [Google Scholar]
- Gagliano M, McCormick MI. Hormonally mediated maternal effects shape offspring survival potential in stressful environments. Oecologia. 2009;160:657–665. doi: 10.1007/s00442-009-1335-8. [DOI] [PubMed] [Google Scholar]
- Giesing ER, Suski CD, Warner RE, Bell AM. Female sticklebacks transfer information via eggs: effects of maternal experience with predators on offspring. Proceedings of the Royal Society of London. Series B. Biological Sciences. 2011;278:1753–1759. doi: 10.1098/rspb.2010.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Spencer HG, Bateson P. Environmental influences during development and their later consequences for health and disease: implications for the interpretation of empirical studies. Proceedings of the Royal Society of London. Series B. Biological Sciences. 2005;272:671–677. doi: 10.1098/rspb.2004.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MK, Rani CSS, Joshi A, Soto-Piña AE, Martinez PA, Frazer A, Strong R, Morilak DA. Prenatal stress induces long term stress vulnerability, compromising stress response systems in the brain and impairing extinction of conditioned fear after adult stress. Neuroscience. 2011;192:438–451. doi: 10.1016/j.neuroscience.2011.06.041. [DOI] [PubMed] [Google Scholar]
- Groothuis TGG, Muller W, von Engelhardt N, Carere C, Eising C. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neuroscience and Biobehavioral Reviews. 2005;29:329–352. doi: 10.1016/j.neubiorev.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Groothuis TGG, Schwabl H. Hormone-mediated maternal effects in birds: mechanisms matter but what do we know of them? Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363:1647–1661. doi: 10.1098/rstb.2007.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward LS, Wingfield JC. Maternal corticosterone is transferred to avian yolk and may alter offspring growth and adult phenotype. General and Comparative Endocrinology. 2004;135:365–371. doi: 10.1016/j.ygcen.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Henriksen R, Rettenbacher S, Groothuis TGG. Prenatal stress in birds: pathways, function and perspectives. Neuroscience and Biobehavioral Reviews. 2011;35:1484–1501. doi: 10.1016/j.neubiorev.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Love OP, Chin EH, Wynne-Edwards KE, Williams TD. Stress hormones: A link between maternal condition and sex-biased reproductive investment. American Naturalist. 2005;166:751–766. doi: 10.1086/497440. [DOI] [PubMed] [Google Scholar]
- Love OP, Williams TD. Plasticity in the adrenocortical response of a free-living vertebrate: The role of pre- and post-natal developmental stress. Hormones and Behavior. 2008a;54:496–505. doi: 10.1016/j.yhbeh.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Love OP, Williams TD. The adaptive value of stress-induced phenotypes: Effects of maternally derived corticosterone on sex-biased investment, cost of reproduction, and maternal fitness. American Naturalist. 2008b;172:E135–E149. doi: 10.1086/590959. [DOI] [PubMed] [Google Scholar]
- Matthews SG, Phillips DIW. Minireview: Transgenerational inheritance of the stress response: a new frontier in stress research. Endocrinology. 2010;151:7–13. doi: 10.1210/en.2009-0916. [DOI] [PubMed] [Google Scholar]
- McCormick MI. Mothers matter: Crowding leads to stressed mothers and smaller offspring in marine fish. Ecology. 2006;87:1104–1109. doi: 10.1890/0012-9658(2006)87[1104:mmclts]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- McCormick MI. Indirect effects of heterospecific interactions on progeny size through maternal stress. Oikos. 2009;118:744–752. [Google Scholar]
- Meylan S, Clobert J. Is corticosterone-mediated phenotype development adaptive? - Maternal corticosterone treatment enhances survival in male lizards. Hormones and Behavior. 2005;48:44–52. doi: 10.1016/j.yhbeh.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Meylan S, Haussy C, Voituron Y. Physiological actions of corticostreone and its modulation by an immune challenge in reptiles. General and Comparative Endocrinology. 2010;169:158–166. doi: 10.1016/j.ygcen.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Monclús R, Tiulim J, Blumstein DT. Older mothers follow conservative strategies under predation pressure: the adaptive role of maternal glucocorticoids in yellow-bellied marmots. Hormones and Behavior. 2011;60:660–665. doi: 10.1016/j.yhbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Mousseau TA, Fox CW. Maternal effects as adaptations. Oxford University Press; New York: 1998. [Google Scholar]
- Pitcher TJ. Functions of shoaling behaviour in teleosts. In: Pitcher TJ, Parish JK, editors. Behaviour of teleost fishes. Chapman and Hall; New York: 1993. [Google Scholar]
- Reimchen TE. Size-structured mortality in a threespine stickleback (Gasterosteus aculeatus) cutthroat trout (Oncorhynchus clarki) community. Canadian Journal of Fisheries and Aquatic Sciences. 1990;47:1194–1205. [Google Scholar]
- Reimchen TE. Trout foraging failures and the evolution of body size in stickleback. Copeia. 1991:1098–1104. [Google Scholar]
- Saino N, Romano M, Ferrari RP, Martinelli R, Moller AP. Stressed mothers lay eggs with high corticosterone levels which produce low-quality offspring. Journal of Experimental Zoology. 2005;303A:998–1006. doi: 10.1002/jez.a.224. [DOI] [PubMed] [Google Scholar]
- Schoech SJ, Rensel MA, Heiss RS. Short- and long-term effects of developmental corticosterone exposure on avian physiology, behavioral phenotype, cognition, and fitness: A review. Current Zoology. 2011;57:514–530. [Google Scholar]
- Sheriff MJ, Krebs CJ, Boonstra R. The sensitive hare: sublethal effects of predator stress on reproduction in snowshoe hares. Journal of Animal Ecology. 2009;78:1249–1258. doi: 10.1111/j.1365-2656.2009.01552.x. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Krebs CJ, Boonstra R. The ghosts of predators past: population cycles and the role of maternal programming under fluctuating predation risk. Ecology. 2010;91:2983–2994. doi: 10.1890/09-1108.1. [DOI] [PubMed] [Google Scholar]
- Shine R, Downes SJ. Can pregnant lizards adjust their offspring phenotypes to environmental conditions? Oecologia. 1999;119:1–8. doi: 10.1007/s004420050754. [DOI] [PubMed] [Google Scholar]
- Storm JJ, Lima SL. Mothers forewarn offspring about predators: A transgenerational maternal effect on behavior. American Naturalist. 2010;175:382–390. doi: 10.1086/650443. [DOI] [PubMed] [Google Scholar]
- Tulley JJ, Huntingford FA. Paternal care and the development of adaptive variation in antipredator responses in sticklebacks. Animal Behaviour. 1987;35:1570–1572. [Google Scholar]
- Uller T. Developmental plasticity and the evolution of parental effects. Trends in Ecology and Evolution. 2008;23:432–438. doi: 10.1016/j.tree.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Weaver ICG, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weinstock M. The long-term behavioural consequences of prenatal stress. Neuroscience and Biobehavioral Reviews. 2008;32:1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Zohar I, Weinstock M. Differential effect of prenatal stress on the expression of cortiocotrophin-releasing hormone and its receptors in the hypothalamus and amygdala in male and female rats. Journal of Neuroendocrinology. 2011;23:320–328. doi: 10.1111/j.1365-2826.2011.02117.x. [DOI] [PubMed] [Google Scholar]