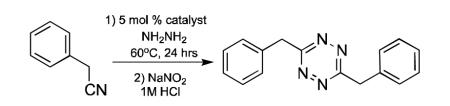
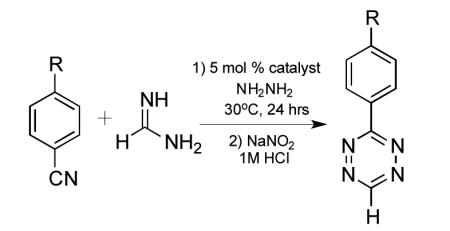
There is rapidly growing interest in the use of 1,2,4,5-tetrazines as bioorthogonal coupling agents.[1-3] Recent applications of tetrazine cycloadditions include intracellular small molecule imaging, genetically targeted protein tagging, post-synthetic DNA labeling, nanoparticle based clinical diagnostics, and in-vivo imaging.[4-7] In addition, tetrazines have seen significant use in materials science[8,9], coordination chemistry, [10,11] and specialty explosives research.[12,13] They are also valuable synthetic intermediates, and have been elegantly deployed on route to several natural product syntheses.[14-16] Despite the promise of tetrazines, the lack of convenient synthetic methods is a significant roadblock to their broader use and study by the scientific community.[17] Here we report that Lewis acid transition metal catalysts, most notably divalent nickel and zinc salts, can catalyze the formation of 1,2,4,5-tetrazines directly from nitriles. To our knowledge, this is the first method utilizing homogenous catalysis to directly synthesize tetrazines from a wide range of unactivated aliphatic nitriles and hydrazine. Symmetric and asymmetric tetrazines were conveniently prepared from multiple precursors including alkyl nitriles, aromatic nitriles, and formamidine salts. This methodology should greatly improve the accessibility of tetrazines and lead to further exploration of their applications, particularly with respect to bioorthogonal conjugations.
The most convenient route to 1,2,4,5-tetrazines is by addition of hydrazine to aromatic nitriles followed by oxidation of the 1,2-dihydrotetrazine product.[18] Unfortunately, this strategy is not viable for producing tetrazines from unactivated nitriles such as alkyl nitriles. Earlier reports claiming to access tetrazines directly from alkyl nitriles have proven difficult to reproduce, likely due to confusion between the intermediate 1,2-dihydrotetrazines and isomeric 4-amino-1,2,4 -triazoles.[19-21] There have been several reported methods to access dialkyl tetrazines from alternative precursors, such as imidates, amidine salts, and aldehydes, but these methods suffer from low yields, limited substrate scope, and the requirement of additional synthetic steps.[22-24] For these reasons, a general and robust method to prepare symmetric and asymmetric 1,2,4,5-tetrazines directly from unactivated nitriles would be highly desirable.
Though the mechanism of tetrazine synthesis has been debated, it is generally agreed that reaction begins with nucleophilic attack of the nitrile by hydrazine forming an amidrazone.[25,26] We speculated that the addition of Lewis acid catalysts might promote this reaction by binding to the nitrile and/or hydrazine. Metal ions have long been known to activate nitriles to nucleophilic addition.[27-29] However, to our knowledge, there has not been a report of using homogenous catalysis to promote the formation of 1,2,4,5-tetrazines.[21,30] We used the reaction of benzyl cyanide with neat hydrazine to survey a range of Lewis acid catalysts at 5 mol % loading (Table 1).[31] In the absence of catalyst, tetrazine products could not be isolated.[20] Remarkably, the addition of 5 mol % nickel triflate (Ni(OTf)2) led to near quantitative yield of 3,6-dibenzyl-1,2,4,5-tetrazine. Zinc salts also gave good yields, with addition of 5 mol % zinc triflate (Zn(OTf)2) leading to 70% yield of the desired tetrazine. Nickel and zinc salts possessing stronger coordinating anions gave lower yields, possible due to the lowered solubility of these salts in aprotic media and the decreased Lewis acid strength compared to the triflates.[32]
Table 1.
Survey of metal catalysts
| catalyst | yield[a] | catalyst | yield[a] | catalyst | yield[a] |
|---|---|---|---|---|---|
| none | 0% | Cu(OAc)2 | 59% | NiCI2 | 73% |
| Zn(OAc)2 | 38% | MnBr2 | 55% | Nil2 | 93% |
| ZnCI2 | 11% | CuBr2 | 23% | Ni(OTf)2 | 95% |
| ZnBr2 | 46% | CoCI2·6H20 | 13% | CuOAc | 53% |
| Znl2 | 68% | MgCI2 | 63% | CuCI | 12% |
| Zn(OTf)2 | 70% | Yb(OTf)3 | 31% | CuBr | 42% |
| Cu(OTf)2 | 11% | Sc(OTf)3 | 26% | Cul | 50% |
| MgBr2 | 15% | Ni(acac)2 | 10% | Cu(OTf) | 57% |
yields reported after isolation by silica flash chromatography
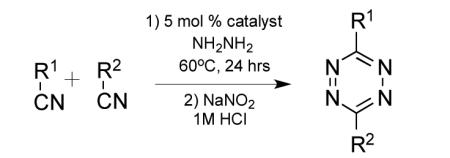
Given the high yields obtained with nickel and zinc triflates, we tested their effect on the yields of several other tetrazine syntheses where at least one component was an alkyl nitrile (Table 2). In each instance we tested either Ni(OTf)2 or Zn(OTf)2 for catalytic effect. In general we observed that zinc salts gave higher yields for less active nitriles such as those that were sterically hindered or affected by electron donating groups. On the other hand, more reactive nitriles benefited from the use of nickel salts. However, there were exceptions and it is suggested that both catalysts be tried when attempting synthesis of new tetrazines. For the synthesis of symmetric 3,6-dialkyl 1,2,4,5-tetrazines, yields ranged from 95% for 3,6-dibenzyl-1,2,4,5-tetrazine (entry 1) to 24% for the sterically hindered 3,6-di-tert-butyl-1,2,4,5-tetrazine (entry 3). The moderate yield for the latter tetrazine is impressive given that all previous attempts to synthesize the molecule have only led to trace isolated yield.[24,33]
Table 2.
Synthesis of 1,2,4,5-tetrazines directly from nitriles catalyzed by Ni(OTf)2 or Zn(OTf)2.
| entry | R1 | R2 | catalyst | product | yield[a] |
|---|---|---|---|---|---|
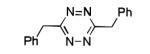
| 1 |
|
|
Ni |
|
95% |
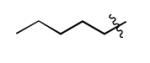
| 2 |
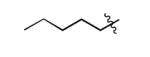
|
|
Zn |
|
59% |
| 3 |
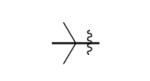
|
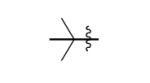
|
Zn |
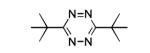
|
24% |
| 4 |
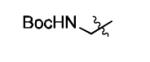
|
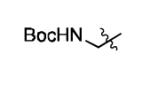
|
Zn |
|
32% |
| 5[b] |
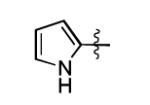
|
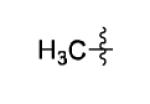
|
Ni |
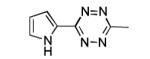
|
58% |
| 6 |
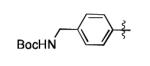
|
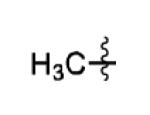
|
Ni |
|
68% |
| 7 |
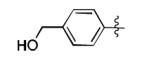
|
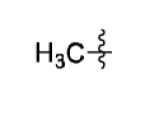
|
Ni |
|
66% |
| 8 |
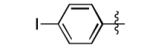
|
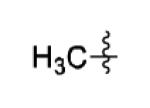
|
Ni |
|
41% |
| 9 |
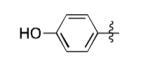
|
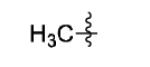
|
Zn |
|
43% |
| 10 |
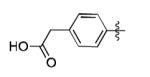
|
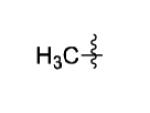
|
Ni |
|
70% |
| 11 |
|
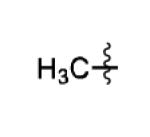
|
Zn |
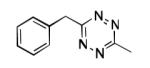
|
40% |
| 12[c] |
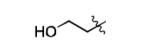
|
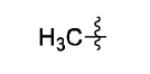
|
Zn |
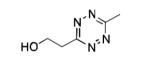
|
36% |
| 13 |
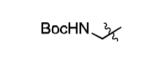
|
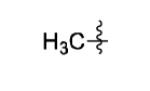
|
Ni |
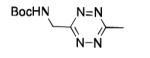
|
36% |
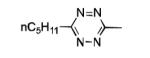
| 14 |
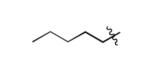
|
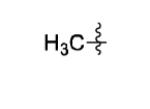
|
Zn |
|
40% |
| 15 |
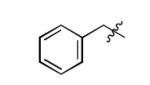
|
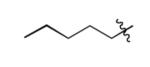
|
Zn |
|
12% |
| 16[c] |
|
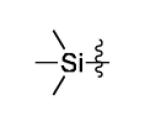
|
Zn |
|
30% |
yields reported after isolation by silica flash chromatography
the protective groups were lost during oxidative workup
reaction required 36 hours
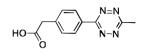
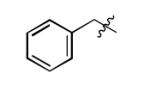
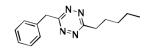
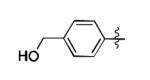
The scope of this method extends to asymmetric 3,6-disubstituted 1,2,4,5-tetrazines, which are among the most challenging tetrazines to synthesize.[17,25,34] Metal ions could readily promote the formation of 6-methyl terminated tetrazines from acetonitrile and aromatic nitriles in 40-70% yield (entries 5-10). Alkyl nitriles and acetonitrile could also combine with hydrazine to yield 6-methyl terminated alkyl tetrazines in 36-40% yield (entries 11-14). Several of these tetrazines possess functional group handles to facilitate their use in biological applications. For instance, it has recently been demonstrated that methyl terminated tetrazines are highly stable partners in bioorthogonal cycloadditions and can be used in a mutually orthogonal fashion with azide-alkyne cycloadditions.[6,35] 6-methyl terminated tetrazines were previously only accessible from reactive precursors such as imidates and amidine salts and in lower yield.[23,35]. Dialkyl asymmetric tetrazines with bulkier substituents are extremely difficult to isolate, even from imidates and amidines. In contrast, we were able to isolate 3-benzyl-6-pentyl-1,2,4,5-tetrazine (entry 15) from benzyl cyanide and excess hexanenitrile, albeit in lower relative yield (12%).
Several groups have measured the rate of cycloaddition between various tetrazines and strained dienophiles such as norbornene and trans-cyclooctene.[1,2,5,36] The substituents on the 3 and 6 positions of 1,2,4,5-tetrazines have a significant affect on the kinetics of the reaction.[35] While 6-methyl terminated tetrazines benefit from stability, tetrazines terminated with hydrogen at the 6 position react much faster and have proven utility in live cell and live animal applications where lowered concentrations of labeling agent are typically used.[2,7,36] With this in mind, we examined if metal catalysis could improve synthetic routes to hydrogen terminated monoaryl-tetrazines. We found that an excess of trimethylsilyl cyanide can be used along with an aromatic nitrile to yield a hydrogen terminated asymmetric tetrazine (entry 16). This is the first example of using trimethylsilyl cyanide to synthesize tetrazines and is possible due to the addition of nitrile activating metal catalysts. Additionally, we explored the affect of Ni(OTf)2 and Zn(OTf)2 on the synthesis of tetrazine from aromatic nitriles and formamidine salts (Table 3). Although these reactions do not require catalysis, yields are typically low, between 10-20%.[35] Interestingly, we found that metal ions could promote the reaction and significantly increase the yield of tetrazine, which was 60-74% depending on the precursors and catalyst used. This improved methodology will be highly useful to researchers interested in performing rapid bioorthogonal couplings.
Table 3.
Metal catalyzed synthesis of tetrazine from aromatic nitriles and formamidine.
| entry | R | catalyst | product | yield |
|---|---|---|---|---|
| 17 |
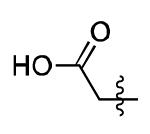
|
Ni |
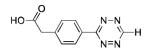
|
74% |
| 18 |
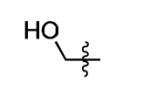
|
Ni |
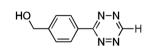
|
64% |
| 19[b] |
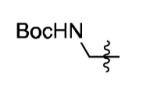
|
Zn |
|
70% |
yields reported after isolation by silica flash chromatography
required use of DMF as cosolvent and 36 hours of reaction
Given the lack of clarity on the mechanism of tetrazine formation, and the large number of metal coordinating species involved such as hydrazine, nitriles, and amidrazones, it is difficult to confidently propose the precise role of the metal ion. It is likely that the metal acts as a Lewis acid by coordinating to the nitrile and promoting nucleophilic addition by hydrazine.[28,29,37,38] It is also plausible that the metal binds both the nitrile and hydrazine, promoting synthesis of the amidrazone intermediate (Figure 1).[39] Recent elegant work has demonstrated that a similar mechanism is likely responsible for the nickel catalyzed addition of pyrazole to acetonitrile.[37] Clearly future studies are required to refine our understanding of tetrazine synthesis.
In conclusion we have discovered that metal salts such as nickel and zinc triflates can be used to catalyze the one-pot synthesis of 1,2,4,5-tetrazines from unactivated nitriles. Previously, these tetrazines were either not practically accessible or could be obtained in low yield after multistep syntheses. The ability to conveniently obtain a range of symmetric and asymmetric tetrazines directly from commercially available nitriles should encourage further use of tetrazines as bioorthogonal coupling agents. Furthermore, this finding will benefit important niche applications of tetrazines, for instance in coordination chemistry, explosives research, materials science, and total synthesis.[8,11,12,15,16,40] We believe that this methodology will increase the accessibility of substituted tetrazines to a broader range of researchers, allow syntheses of compounds that were previously unobtainable in useful quantities, and help remove a synthetic roadblock to studying the unique properties and applications of these
Supplementary Material
Scheme 1.
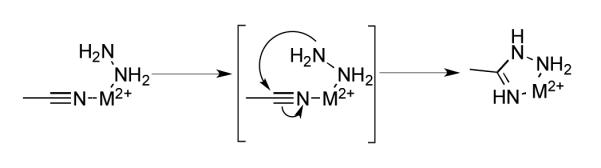
Plausible catalytic role of the metal during synthesis
Acknowledgments
[**] The authors gratefully acknowledge Ralph Mazitschek, Carlos Guerrero, and Scott Hilderbrand for helpful discussions. This material is based upon work supported in part by NIH grant K01EB010078, the University of California, San Diego, and the NSF under CHE-0741968.
Contributor Information
Dr. Jun Yang, Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA, 92093
Dr. Mark R. Karver, Massachusetts General Hospital, 185 Cambridge Street, Boston, MA, 02114
Weilong Li, Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA, 92093.
Swagat Sahu, Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA, 92093.
Prof. Neal. K. Devaraj, Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA, 92093.
References
- [1].Blackman ML, Royzen M, Fox JM. J. Am. Chem. Soc. 2008;130:13518–13519. doi: 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Devaraj NK, Weissleder R, Hilderbrand SA. Bioconjug. Chem. 2008;19:2297–2299. doi: 10.1021/bc8004446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wiessler M, Waldeck W, Kliem C, Pipkorn R, Braun K. Int. J. Med. Sci. 2009;7:19–28. doi: 10.7150/ijms.7.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liu DS, Tangpeerachaikul A, Selvaraj R, Taylor MT, Fox JM, Ting AY. J. Am. Chem. Soc. 2012;134:792–795. doi: 10.1021/ja209325n. [DOI] [PMC free article] [PubMed] [Google Scholar]; Reiner T, Keliher EJ, Earley S, Marinelli B, Weissleder R. Angew. Chem. Int. Ed. 2011;50:1922–1925. doi: 10.1002/anie.201006579. [DOI] [PMC free article] [PubMed] [Google Scholar]; Schoch J, Wiessler M, Jaschke A. J. Am. Chem. Soc. 2010;132:8846–8847. doi: 10.1021/ja102871p. [DOI] [PubMed] [Google Scholar]; Seitchik JL, Peeler JC, Taylor MT, Blackman ML, Rhoads TW, Cooley RB, Refakis CA, Fox JM, Mehl RA. J. Am. Chem. Soc. 2012;134:2898–2901. doi: 10.1021/ja2109745. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lang K, Davis L, Torres-Kolbus J, Chou C, Deiters A, Chin JW. Nature Chemistry. 2012;AOP doi: 10.1038/nchem.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]; Budin G, Yang KS, Reiner T, Weissleder R. Angew. Chem. Int. Ed. 2011;50:9378–9381. doi: 10.1002/anie.201103273. [DOI] [PMC free article] [PubMed] [Google Scholar]; Devaraj NK, Hilderbrand S, Upadhyay R, Mazitschek R, Weissleder R. Angew. Chem. Int. Ed. 2010;49:2869–2872. doi: 10.1002/anie.200906120. [DOI] [PMC free article] [PubMed] [Google Scholar]; Han HS, Devaraj NK, Lee J, Hilderbrand SA, Weissleder R, Bawendi MG. J. Am. Chem. Soc. 2010;132:7838–7839. doi: 10.1021/ja101677r. [DOI] [PMC free article] [PubMed] [Google Scholar]; Haun JB, Castro CM, Wang R, Peterson VM, Marinelli BS, Lee H, Weissleder R. Sci. Transl. Med. 2011;3:71ra16. doi: 10.1126/scitranslmed.3002048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rossin R, Verkerk PR, van den Bosch SM, Vulders RC, Verel I, Lub J, Robillard MS. Angew. Chem. Int. Ed. 2010;49:3375–3378. doi: 10.1002/anie.200906294. [DOI] [PubMed] [Google Scholar]
- [6].Karver MR, Weissleder R, Hilderbrand SA. Angew. Chem. Int. Ed. 2012;51:920–922. doi: 10.1002/anie.201104389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Haun JB, Devaraj NK, Hilderbrand SA, Lee H, Weissleder R. Nat. Nanotechnol. 2010;5:660–665. doi: 10.1038/nnano.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hansell CF, Espeel P, Stamenovic MM, Barker IA, Dove AP, Du Prez FE, O’Reilly RK. J. Am. Chem. Soc. 2011;133:13828–13831. doi: 10.1021/ja203957h. [DOI] [PubMed] [Google Scholar]
- [9].Audebert P, Sadki S, Miomandre F, Clavier G. Electrochem. Commun. 2004;6:144–147. [Google Scholar]; Li Z, Ding JF, Song NH, Lu JP, Tao Y. J. Am. Chem. Soc. 2010;132:13160–13161. doi: 10.1021/ja106052e. [DOI] [PubMed] [Google Scholar]
- [10].Kaim W. Coordin. Chem. Rev. 2002;230:127–139. [Google Scholar]; Xue M, Ma SQ, Jin Z, Schaffino RM, Zhu GS, Lobkovsky EB, Qiu SL, Chen BL. Inorg. Chem. 2008;47:6825–6828. doi: 10.1021/ic800854y. [DOI] [PubMed] [Google Scholar]
- [11].Xu Y, Duan L, Akermark T, Tong L, Lee BL, Zhang R, Akermark B, Sun L. Chemistry. 2011;17:9520–9528. doi: 10.1002/chem.201100274. [DOI] [PubMed] [Google Scholar]
- [12].Wei T, Zhu W, Zhang X, Li YF, Xiao H. J. Phys. Chem. A. 2009;113:9404–9412. doi: 10.1021/jp902295v. [DOI] [PubMed] [Google Scholar]
- [13].Chavez DE, Hiskey MA, Gilardi RD. Angew. Chem. Int. Ed. 2000;39:1791–1793. doi: 10.1002/(sici)1521-3773(20000515)39:10<1791::aid-anie1791>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- [14].Saracoglu N. Tetrahedron. 2007;63:4199–4236. [Google Scholar]; Benson SC, Lee L, Yang L, Snyder JK. Tetrahedron. 2000;56:1165–1180. [Google Scholar]
- [15].Hamasaki A, Zimpleman JM, Hwang I, Boger DL. J. Am. Chem. Soc. 2005;127:10767–10770. doi: 10.1021/ja0526416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Boger DL, Hong J. J. Am. Chem. Soc. 2001;123:8515–8519. doi: 10.1021/ja011271s. [DOI] [PubMed] [Google Scholar]
- [17].Clavier G, Audebert P. Chem. Rev. 2010;110:3299–3314. doi: 10.1021/cr900357e. [DOI] [PubMed] [Google Scholar]
- [18].Hofmann KA, Ehrhart O. Ber. Dtsch. Chem. Ges. 1912;45:2731–2740. [Google Scholar]; Muller E, Herrdegen L. J. Praktische Chemie. 1921;102:113–155. [Google Scholar]; Curtius T, Hess A. J. Praktische Chemie. 1930;125:40–53. [Google Scholar]
- [19].Erickson JG, Wiley PF, Wystrach VP. Intersciences Publishers. New York: 1956. The 1,2,3- and 1,2,4-Triazines, Tetrazines, and Pentazines; p. 179. [Google Scholar]
- [20].Bowie RA, Neilson DG, Watson KM, Gardner MD, Ridd V, Mahmood S. J. Chem. Soc. Perk. Trans. 1. 1972:2395–2399. [Google Scholar]
- [21].Abdel-Rahman MO, Kira MA, Tolba MN. Tetrahedron Lett. 1968:3871–3872. [Google Scholar]
- [22].Pinner A. Chem. Ber. 1893;26:2126. [Google Scholar]
- [23].Lang SA, Johnson BD, Cohen E. J. Heterocyclic Chem. 1975;12:1143–1153. [Google Scholar]
- [24].Skorianetz W, Kovats ES. Helv. Chim. Acta. 1971;54:1922–1939. [Google Scholar]
- [25].Neunhoeffer H, Wiley PF. Chemistry of 1,2,3-Triazines, Tetrazines, and Pentazines. John Wiley & Sons; New York: 1978. p. 1073. [Google Scholar]
- [26].Audebert P, Sadki S, Miomandre F, Clavier G, Vernieres MC, Saoud M, Hapiot P. New Journal of Chemistry. 2004;28:387–392. [Google Scholar]
- [27].Oxley P, Partridge MW, Short WF. J. Chem. Soc. 1947:1110–1116. [Google Scholar]; Siegl WO. J. Org. Chem. 1977;42:1872–1878. [Google Scholar]; Demko ZP, Sharpless KB. J. Org. Chem. 2001;66:7945–7950. doi: 10.1021/jo010635w. [DOI] [PubMed] [Google Scholar]; Wang JF, Xu F, Cai T, Shen Q. Org. Lett. 2008;10:445–448. doi: 10.1021/ol702739c. [DOI] [PubMed] [Google Scholar]
- [28].Kukushkin VY, Pombeiro AJL. Chem. Rev. 2002;102:1771–1802. doi: 10.1021/cr0103266. [DOI] [PubMed] [Google Scholar]
- [29].Rousselet G, Capdevielle P, Maumy M. Tetrahedron Lett. 1993;34:6395–6398. [Google Scholar]
- [30].Though there have been a few previous reports of using catalysts such as sulfur (see ref. [21]) and heterogenous metals to promote tetrazine formation, previous results were mostly limited to aromatic nitriles and suffer from irreproducibility. Zajac WW, Siuda JF, Nolan MJ, Santosusso TM. J. Org. Chem. 1971;36:3539–3541. Lim CL, Pyo SH, Kim TY, Yim ES, Han BH. Bull. Korean Chem. Soc. 1995;16:374–377.
- [31].Reaction conditions were optimized for catalyst loading, hydrazine equivalents, and temperature when we first realized that Lewis acids could be used to catalyze tetrazine synthesis. For most tetrazines, the optimum yields were obtained at 5% catalyst loading, 5 equivalents of hydrazine and a temperature of 60°C. Certain tetrazines required alternative conditions to achieve satisfactory yields and these are noted in the text and supplementary information.
- [32].Hertweck C. J. Prakt. Chem. 2000;342:316–321. [Google Scholar]
- [33].Larsen C, Binderup E, Moller J. Acta. Chem. Scand. 1967;21:2855–2858. [Google Scholar]
- [34].Hu WX, Xu F. J. Heterocyclic Chem. 2008;45:1745–1750. [Google Scholar]
- [35].Karver MR, Weissleder R, Hilderbrand SA. Bioconjug. Chem. 2011;22:2263–2270. doi: 10.1021/bc200295y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Devaraj NK, Upadhyay R, Haun JB, Hilderbrand SA, Weissleder R. Angew. Chem. Int. Ed. 2009;48:7013–7016. doi: 10.1002/anie.200903233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hsieh CC, Lee CJ, Horng YC. Organometallics. 2009;28:4923–4928. [Google Scholar]
- [38].Mason R, Thomas KM, Galbraith AR, Shaw BL, Elson CM. J. Chem. Soc. Chem. Comm. 1973:297–299. [Google Scholar]
- [39].Kukushkin VY, Pombeiro AJL. Inorg. Chim. Acta. 2005;358:1–21. [Google Scholar]
- [40].Audebert P, Miomandre F, Clavier G, Vernieres MC, Badre S, Meallet-Renault R. Chemistry. 2005;11:5667–5673. doi: 10.1002/chem.200401252. [DOI] [PubMed] [Google Scholar]; Bu XH, Morishita H, Tanaka K, Biradha K, Furusho S, Shionoya M. Chem. Commun. 2000:971–972. [Google Scholar]; Chen C, Allen CA, Cohen SM. Inorg. Chem. 2011;50:10534–10536. doi: 10.1021/ic2017598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


