Abstract
Purpose
T-cell depletion (TCD) reduces the incidence of graft-versus-host disease (GVHD) after hematopoietic cell transplantation (HCT). However, concerns about relapse, graft rejection, and variability in technique have limited the widespread application of this approach.
Patients and Methods
Outcomes of 44 patients receiving HLA-identical sibling TCD grafts using a uniform technique for CD34+ selection as the sole form of immune suppression were compared with outcomes of 84 patients receiving T-replete grafts and pharmacologic immune suppression therapy (IST).
Results
Groups were similar, except for fewer men (36% with TCD v 56% with IST) and more frequent use of radiation-containing regimens (100% with TCD v 50% with IST) in the CD34-selected TCD cohort. The proportion of patients with neutrophil engraftment at day 28 was similar (96% with IST and 100% with TCD grafts). The 100-day rates of grade 2 to 4 acute GVHD were 39% and 23% with IST and TCD grafts, respectively (P = .07). Corresponding 2-year rates of chronic GVHD were lower with TCD grafts than IST (19% v 50%, respectively; P < .001). There were no differences in rates of graft rejection, leukemia relapse, treatment-related mortality, and disease-free and overall survival rates. At 1 year, 54% and 12% of patients were still on immunosuppression in the IST and TCD cohorts, respectively. TCD was associated with a higher GVHD-free survival at 2 years compared with IST (41% v 19%, respectively; P = .006).
Conclusion
These results suggest that TCD via CD34 selection might lower long-term morbidity as a result of chronic GVHD without negatively impacting relapse rates in patients with acute myeloid leukemia. Additional prospective studies should be undertaken to definitively address the role of TCD in HCT.
INTRODUCTION
Hematopoietic cell transplantation (HCT) is a curative treatment for acute myeloid leukemia (AML). However, graft-versus-host disease (GVHD) after HCT remains a major challenge, leading to substantial morbidity and mortality. Interventions that reduce the number of donor T cells in the graft effectively decrease the risk of GVHD.1,2 However, in some early series, this strategy has been associated with graft rejection and disease relapse, particularly after transplantation for chronic myelogenous leukemia (CML).3 Additionally, ex vivo T-cell depletion (TCD) techniques vary widely, with different and sometimes inconsistent degrees of depletion. These observations have limited the enthusiasm for T-cell–depleted HCT in the last decade.
Most studies of TCD occurred in an era when bone marrow was the preferred stem-cell source and CML was the most common HCT indication. Techniques available at that time included negative selection with monoclonal antibodies, counterflow centrifugal elutriation, and soybean lectin agglutination with sheep erythrocyte rosette depletion.4–7 The role of TCD is not well studied in the current era, where mobilized peripheral-blood stem cells (PBSCs) are more commonly used as a graft source and AML is the most common indication.8 PBSC grafts contain substantially higher numbers of both T cells and hematopoietic progenitor cells. In the largest prospective randomized trial of ex vivo TCD, done in the setting of unrelated donor bone marrow transplantation, there was no increase in relapse rate among patients who received transplantation for AML.2
The availability of clinical-grade magnetic bead columns for cell separation has maximized the efficiency and accuracy of graft manipulation compared with early techniques. Recently, a phase II trial of HLA-identical sibling HCT with CD34-selected T-cell–depleted grafts in patients with early AML was conducted by the Blood and Marrow Transplant Clinical Trials Network (BMT CTN).9 That trial demonstrated that this technique provided consistent 4- to 5-log reduction in T-cell content in all participating centers.9,10 There are currently no randomized trials assessing CD34 selection as the sole form of GVHD prophylaxis. The purpose of this study was to compare outcomes of patients treated on the BMT CTN CD34+ selection TCD trial to outcomes of a similar cohort of patients enrolled onto a different but contemporaneous BMT CTN trial who received HCT with a T-replete PBSC blood graft and pharmacologic immune suppression therapy (IST) for GVHD prophylaxis.
PATIENTS AND METHODS
BMT CTN
The BMT CTN was established in 2001 to develop and conduct multicenter clinical trials addressing important issues in HCT.11 The BMT CTN is led by EMMES (Rockville, MD), the Center for International Blood and Marrow Transplant Research (CIBMTR), and the National Marrow Donor Program. The protocols for the two trials from which data for the current comparison were obtained were approved by the institutional review boards at each transplantation center, and written informed consent was obtained in accordance with the Declaration of Helsinki before the initiation of conditioning therapy. The BMT CTN uses a centralized electronic data entry system with data collection conducted under a common manual of procedure. Case report forms were the same for both studies including the BMT CTN–wide common collection of engraftment, GVHD, infection, relapse, and survival status. Definitions of outcomes were the same across the two cohorts. The clinical trial data were complimented with long-term outcomes extracted from the CIBMTR8 in accordance with an observational database protocol, which was also approved by institutional review boards at each participating transplantation center.8 Detailed cross-check queries were implemented to ensure consistent quality of data between the two data sources.
Eligibility
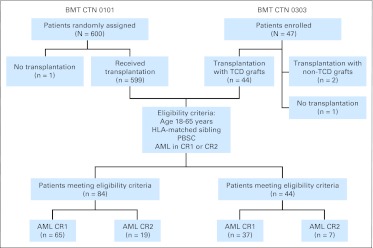
BMT CTN 0303 (ClinicalTrials.gov identifier: NCT00201240) was a phase II clinical trial designed to test rigorous TCD using a magnetic bead column12–16 as the sole form of GVHD prophylaxis in HLA-identical sibling HCT for treatment of patients with AML in first or second remission.9 The trial enrolled 47 patients from 2005 to 2008. BMT CTN 0101 (ClinicalTrials.gov identifier: NCT00075803) was a multicenter phase III trial that compared fluconazole with voriconazole as antifungal prophylaxis after allogeneic HCT.17 BMT CTN 0101 enrolled 600 patients from 2003 to 2006, and 576 of these patients received non-TCD grafts. The current comparative analysis included patients fulfilling the following eligibility criteria: enrolled onto BMT CTN 0101 and received a non-TCD PBSC graft or enrolled onto BMT CTN 0303; 18 years of age or older; transplantation for AML in first or second remission; and HLA-identical sibling donor (Fig 1).
Fig 1.
Patient selection from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0303 clinical trial of transplantation with T-cell–depleted (TCD) grafts in acute myeloid leukemia (AML) and the BMT CTN 0101 antifungal prophylaxis clinical trial (immune suppression therapy cohort). CR1, first complete remission; CR2, second complete remission; PBSC, peripheral-blood stem cell.
Cytogenetics at time of diagnosis were classified according to the Cancer and Leukemia Group B/Southwest Oncology Group criteria into favorable, intermediate, and unfavorable risk.18 Median follow-up times of survivors were 48 and 34 months for the IST and TCD cohorts, respectively.
Treatment
All patients enrolled onto the TCD cohort received myeloablative conditioning with total-body irradiation (days −9, −8, −7, and −6; total dose of 13.75 Gy), thiotepa (days −5 and −4 at a dose of 5 mg/kg per day), cyclophosphamide (days −3 and −2, at a dose of 60 mg/kg per day), and rabbit antithymocyte globulin (ATG; thymoglobulin, on day −4 at a dose of 2.5 mg/kg). Low-dose thymoglobulin was included in the regimen to promote engraftment. Graft manipulation was performed using the Miltenyi CliniMACS CD34 System (Miltenyi Biotec, Cologne, Germany).12–16 Target graft composition after TCD was CD34+ cells more than 5 × 106/kg and CD3+ cells less than 1 × 105/kg; median infusion doses were 7.9 × 106 CD34+ cells/kg (range, 2.4 to 31.3 × 106 CD34+ cells/kg) and 6.6 × 103 CD3+ cells/kg (range, 1.1 to 84.9 × 103 CD3+ cells/kg).10 All patients in the IST cohort received a myeloablative conditioning–specific regimen, which was left to the discretion of the transplantation center (Table 1). Approximately half of the patients in the IST cohort received total-body irradiation–based conditioning regimens; the remainder received busulfan-based conditioning regimens. GVHD prophylaxis with calcineurin inhibitor–based regimens was used in the IST cohort. Growth factor for neutrophil recovery was used according to institutional guidelines and used in 36% and 2% of patients in the IST and TCD cohorts, respectively.
Table 1.
Demographics and Clinical Characteristics of Patients With Acute Myeloid Leukemia in CR1 or CR2 Who Received HLA-Matched Sibling Donor Hematopoietic Cell Transplantation With Chronic Pharmacologic IST or TCD Graft
| Demographic or Clinical Characteristic | IST (n = 84) |
TCD (n = 44) |
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Transplantation centers | 18 | 8 | — | ||
| Male sex | 47 | 56 | 16 | 36 | .04 |
| Age, years | |||||
| Median | 45 | 48 | .14 | ||
| Range | 20-63 | 21-59 | |||
| ≤ 50 years | 57 | 68 | 25 | 57 | |
| > 50 years | 27 | 32 | 19 | 43 | |
| Karnofsky performance score ≥ 90 | 68 | 81 | 34 | 77 | .36 |
| Cytogenetic risk stratification | .34 | ||||
| Favorable | 9 | 11 | 2 | 4 | |
| Intermediate | 55 | 65 | 28 | 64 | |
| Unfavorable | 12 | 14 | 11 | 25 | |
| Unknown | 8 | 10 | 3 | 7 | |
| Disease status at transplantation | .37 | ||||
| CR1 | 65 | 77 | 37 | 84 | |
| CR2 | 19 | 23 | 7 | 16 | |
| Time from diagnosis to transplantation, months | .79 | ||||
| Median | 4 | 4 | |||
| Range | 2-36 | 2-23 | |||
| Time from CR1 to transplantation, months | .13 | ||||
| Median | 2 | 3 | |||
| Range | < 1-11 | 1-6 | |||
| Time from CR2 to transplantation, months | .49 | ||||
| Median | 1 | 1 | |||
| Range | < 1-4 | 1-4 | |||
| Conditioning regimen* | < .01 | ||||
| Cy+TBI+ATG+Thio | 0 | 0 | 44 | 100 | |
| Cy+TBI | 34 | 40 | 0 | 0 | |
| Cy+TBI+ATG | 1 | 1 | 0 | 0 | |
| Bu+Cy | 23 | 27 | 0 | 0 | |
| Bu+Cy+ATG | 3 | 4 | 0 | 0 | |
| Bu+Flu | 12 | 14 | 0 | 0 | |
| Bu+Flu+ATG | 2 | 2 | 0 | 0 | |
| TBI+etoposide | 8 | 10 | 0 | 0 | |
| Bu+ATG | 1 | 1 | 0 | 0 | |
| ATG† | 7 | 8 | 44 | 100 | < .01 |
| GVHD prophylaxis | < .01 | ||||
| TCD | 0 | 0 | 44 | 100 | |
| Tacrolimus-based regimen‡ | 48 | 57 | 0 | 0 | |
| Cyclosporine-based regimen§ | 37 | 43 | 0 | 0 | |
| Follow-up of survivors | < .01 | ||||
| Median | 48 | 34 | |||
| Range | 10-69 | 12-52 | |||
Abbreviations: ATG, antithymocyte globulin; Bu, busulfan; CR1, first complete remission; CR2, second complete remission; Cy, cyclophosphamide; Flu, fludarabine; GVHD, graft-versus-host disease; IST, immune suppression therapy; MTX, methotrexate; TBI, total-body irradiation; TCD, T-cell depletion; Thio, thiotepa.
Range of TBI dose in the IST cohort was 5.5 to 14 Gy, and the range of busulfan dose was 10 to 16 mg/kg.
ATG was reported as part of the conditioning regimen or as GVHD prophylaxis.
Tacrolimus-based GVHD prophylaxis included tacrolimus plus methotrexate (n = 39); tacrolimus plus mycophenolate mofetil (n = 4); tacrolimus plus corticosteroids (n = 1); tacrolimus, methotrexate, and mycophenolate mofetil (n = 1); or tacrolimus alone (n = 3).
Cyclosporine-based GVHD prophylaxis included cyclosporine plus methotrexate (n = 22), cyclosporine plus corticosteroids (n = 3), cyclosporine plus mycophenolate mofetil and methotrexate (n = 1), or cyclosporine alone (n = 11).
Outcomes
A detailed data analysis plan was prepared ahead of any analyses with prespecified outcomes including overall survival, disease-free survival, GVHD-free survival, platelet and neutrophil engraftment, treatment-related mortality (TRM), leukemia relapse, grade 2 to 4 and 3 to 4 acute GVHD, chronic GVHD, and infections. Neutrophil engraftment is defined as the achievement of an absolute neutrophil count greater than 0.5/μL for 3 consecutive days. Platelet engraftment is defined as achievement of a platelet count greater than 20,000/μL for 3 consecutive days without platelet transfusion support. Acute GVHD organ involvement and symptomatology were assessed on a weekly basis and graded according to the National Institutes of Health consensus conference criteria.19 The incidence of grade 2 to 4 and grade 3 to 4 acute GVHD by day 100 was described. Diagnosis of chronic GVHD was based on both clinical and histopathologic findings, with grading based on established guidelines. Relapse was considered on the first day that relapse was confirmed. Infectious complications were graded based on organism, site, and severity. Death by any cause was considered a competing risk for neutrophil engraftment, platelet engraftment, GVHD, and leukemia relapse, whereas leukemia relapse was considered a competing risk for TRM. The numbers of patients with infections in the first year after HCT were compared between the cohorts. Overall survival considers death from any cause, and patients are censored at time of last follow-up. Disease-free survival considers death or relapse, and GVHD-free survival considers death, grade 2 to 4 acute GVHD, and chronic GVHD as events of interest, and patients who were alive and disease free were censored on the date of last follow-up.
Statistical Analysis
Categorical variables were compared using the Pearson χ2 statistic, whereas comparisons with smaller numbers were analyzed using the Fisher's exact test. The t test was used to analyze continuous parameters between groups when normality was assumed, whereas the Wilcoxon signed rank test was considered for smaller sample sizes. The Kaplan-Meier estimate was used to analyze the probability of overall, disease-free, and GVHD-free survival defined as the time from transplantation to the event (death, GVHD, or relapse) or date of last follow-up.20 The log-rank statistic was used to compare covariates between groups. The cumulative incidence method was used to analyze the incidence of events in the presence of competing risks.21 Covariates in the cumulative incidence analyses were compared using Gray's K-sample test.22 All P values are reported as two-sided, and P < .05 was considered statistically significant. Cox proportional regression analysis for overall mortality and treatment failure (inverse of disease-free survival) was performed with graft manipulation as the main effect. Variables considered in the statistical model included age, sex, Karnofsky performance score, remission status, and cytogenetic status at time of transplantation. All analyses were univariate and conducted using SAS version 9.2 (SAS Institute, Cary, NC) or R version 2.8.0 (http://www.r-project.org/).
RESULTS
Demographics
Eighty-four patients from 18 US centers in the IST cohort and 44 patients from eight US centers in the TCD cohort fulfilled eligibility. Table 1 lists the characteristics of both cohorts, which were comparable except for a lower proportion of men in the TCD cohort. AML characteristics, such as disease status, cytogenetics at diagnosis, and disease and remission duration, were similar in both groups.
Engraftment
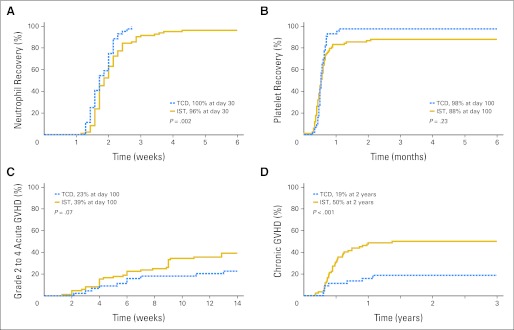
Cumulative incidences of neutrophil engraftment by day 28 were 96% (95% CI, 92% to 100%) and 100% (95% CI, 90% to 100%; P = .002) in the IST and TCD cohorts, respectively (Fig 2A); corresponding median times to neutrophil engraftment were 13 days (range, 8 to 30 days) and 12 days (range, 9 to 19 days), respectively. Day 100 cumulative incidences of platelet recovery were 88% (95% CI, 81% to 95%) and 98% (95% CI, 92% to 100%; P = .23) in the IST and TCD cohorts, respectively (Fig 2B); corresponding median times to platelet recovery were 15 days (range, 8 to 63 days) and 16 days (range, 8 to 33 days), respectively. Day 100 peripheral-blood donor chimerism of more than 90% was observed in 85% and 88% of patients in the IST and TCD cohorts, respectively.
Fig 2.
(A) Cumulative incidence of neutrophil engraftment by treatment arm. (B) Cumulative incidence of platelet engraftment by treatment arm. (C) Cumulative incidence of grade 2 to 4 acute graft-versus-host disease (GVHD) by treatment arm. (D) Cumulative incidence of chronic GVHD by treatment arm. IST, immune suppression therapy; TCD, T-cell depletion.
GVHD
Day 100 cumulative incidences of grade 2 to 4 acute GVHD were 39% (95% CI, 29% to 50%) and 23% (95% CI, 10% to 35%; P = .07) in the IST and TCD cohorts, respectively (Fig 2C); rates of grade 3 to 4 acute GVHD were lower among the TCD cohort (4.5%; 95% CI, 0% to 11%) than the IST cohort (9.5%; 95% CI, 3% to 16%; P = .31). Two-year cumulative incidences of chronic GVHD were 50% (95% CI, 39% to 61%) and 19% (95% CI, 7% to 31%) in the IST and TCD cohorts, respectively (P < .001; Fig 2D). Among 59 patients in the IST cohort alive at 1 year, 32 (54%) were still receiving IST compared with four (12%) of 34 patients in the TCD cohort.
Leukemia Relapse and TRM
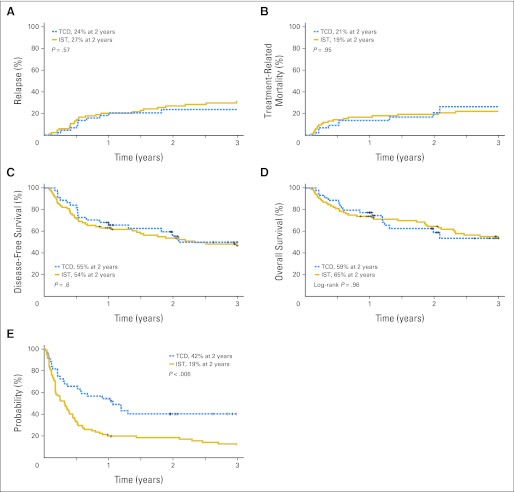
Two-year incidences of leukemia relapse were 27% (95% CI, 17% to 37%) and 24% (95% CI, 11% to 37%; P = .6) in the IST and TCD cohorts, respectively (Fig 3A); corresponding incidences were 24% (95% CI, 13% to 34%) and 17% (95% CI, 4% to 30%; P = .4), respectively, for patients who received transplantation in first remission and 39% (95% CI, 15% to 64%) and 57% (95% CI, 15% to 100%; P = .5) for patients who received transplantation in second remission. Two-year cumulative incidences of TRM were 19% (95% CI, 11% to 28%) and 21% (95% CI, 7% to 34%; P = .95) in the IST and TCD cohorts, respectively (Fig 3B).
Fig 3.
(A) Cumulative incidence of leukemia relapse by treatment arm. (B) Cumulative incidence of nonrelapse mortality by treatment arm. (C) Probability of disease-free survival by treatment arm. (D) Probability of overall survival by treatment arm. (E) Probability of graft-versus host disease–free survival by treatment arm. IST, immune suppression therapy; TCD, T-cell depletion.
Survival
Two-year probabilities of overall survival were 65% (95% CI, 53% to 74%) and 59% (95% CI, 41% to 73%; P = .97) in the IST and TCD cohorts, respectively (Fig 3D). In Cox regression analysis, the hazard ratio (HR) for mortality was 1.01 (95% CI, 0.57 to 1.81) for TCD versus IST. None of the other variables tested in the multivariate analysis were significantly associated with mortality. Two-year probabilities of disease-free survival were 54% (95% CI, 42% to 64%) and 55% (95% CI, 38% to 70%; P = .60) in the IST and TCD cohorts, respectively (Fig 3C). In Cox regression analyses, the HR for treatment failure with TCD versus IST was 0.86 (95% CI, 0.50 to 1.49). The only variable significantly associated with treatment failure was remission status. The HR for transplantation in second versus first remission was 2.0 (95% CI, 1.1 to 3.5; P = .02). When considering the composite end point of being alive and GVHD free, the 2-year probabilities of GVHD-free survival were 19% (95% CI, 11% to 28%) and 42% (95% CI, 25% to 55%; P = .006) in the IST and TCD cohorts, respectively (Fig 3E).
Infectious Complications
Fifty-seven patients (68%) in the IST cohort and 32 patients (73%) in the TCD cohort experienced at least one infectious complication within 1 year after transplantation. In the IST and TCD cohorts, 57% and 57% of patients had bacterial infections, 55% and 54% had viral infections, 10% and 11% had fungal infections, and 0% and 2% had protozoal infections, respectively. Severe infections were reported in 27% and 29% of patients and life-threatening or fatal infections in 4% and 9% of patients in the IST and TCD cohorts, respectively.
Causes of Death
Overall, 42 patients (50%) in the IST cohort (35% within 2 years) and 17 patients (39%) in the TCD cohort died (36% within 2 years). The most common cause of death was leukemia relapse, which occurred in 52% and 36% of patients who died during the study period in the IST and TCD cohorts, respectively. Table 2 lists causes of death by cohort.
Table 2.
Causes of Death
| Cause of Death | IST |
TCD |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Total deaths | 42 | 50 | 17 | 39 |
| Leukemia relapse | 22 | 52 | 6 | 36 |
| Infections | 6 | 14 | 4 | 23 |
| Bacterial infection | 5 | 1 | ||
| Fungal infection | 1 | 1 | ||
| Viral infection | 0 | 1 | ||
| Infection, organism unknown | 0 | 1 | ||
| Organ failure | 6 | 14 | 4 | 23 |
| Liver | 1 | 0 | ||
| Lung | 4 | 2 | ||
| Multiorgan failure | 1 | 1 | ||
| CNS | 0 | 1 | ||
| GVHD | 4 | 10 | 1 | 6 |
| Other causes | 4 | 10 | 2 | 12 |
| Hemorrhage | 1 | 0 | ||
| PTLD | 0 | 1 | ||
| Thromboembolic* | 1 | 0 | ||
| VOD† | 1 | 0 | ||
| Unknown‡ | 1 | 1 | ||
Abbreviations: GVHD, graft-versus-host disease; IST, immune suppression therapy; PTLD, post-transplantation lymphoproliferative disorder; TCD, T-cell depletion; VOD, hepatic veno-occlusive disease.
The thromboembolic event was a cerebral vascular accident.
The patient with VOD died as a result of multiorgan failure.
The patient in the TCD cohort had sudden death with cause unknown.
DISCUSSION
AML is the most common indication for allogeneic transplantation, with more than 2,000 procedures performed yearly in the United States.8 A recent meta-analysis of 6,000 patients with AML suggested a survival advantage for HCT compared with chemotherapy alone in patients with intermediate- or unfavorable-risk cytogenetics.23 There are several challenges to expanding the number of transplantations performed for this disease. In particular, the morbidity and mortality of GVHD remains a concern. A reduction in the incidence and severity of GVHD without compromising leukemia control would be an important advance in improving transplantation outcomes.
Among all techniques studied to reduce GVHD rates, manipulation of the T-cell dose in the graft is the most effective. Despite reduction of both acute and chronic GVHD, TCD transplantations often resulted in higher rates of graft failure, viral infections, and relapse, particularly for patients with CML. These observations in early TCD studies hampered widespread acceptance of the approach.
In contrast, our data show that ex vivo CD34+ selection using the CliniMACS CD34 System as the sole form of GVHD prophylaxis results in grafts with a high content of CD34+ cells with consistent and rigorous reduction of T cells in a multicenter setting.10 This leads to more consistent engraftment with a significantly lower incidence of chronic GVHD and consequently better chronic GVHD–free survival without higher relapse rates or infectious complications compared with non-TCD transplantation in patients undergoing related donor HCT for AML in first remission. Similarly, Wagner et al2 previously demonstrated in a prospective randomized trial that ex vivo TCD of unrelated bone marrow grafts could reduce GVHD rates without affecting leukemia relapse or disease-free survival. In contrast to Wagner et al,2 patients in the BMT CTN 0303 trial received peripheral blood as the stem-cell source and no additional pharmacologic agents for GVHD prophylaxis.9
Transplantation practices have evolved considerably since the early TCD studies. HCTs now commonly use mobilized PBSCs, patients are older, and CML is no longer the most common indication for HCT.24 In fact, PBSC HCT is associated with higher incidences of chronic GVHD compared with bone marrow grafts,25–27 and treatment of chronic GVHD after PBSC HCT is harder to control.28 Our study demonstrates a reduction of approximately 70% in the incidence of chronic GVHD at 2 years. Chronic GVHD has a significant adverse effect on quality of life and often prevents patients from resuming a normal lifestyle after HCT. Patients with extensive chronic GVHD must often deal with significant morbidity and chronic use of immune suppression, which further increases the risk of opportunistic infections. In the current study, more patients were still on IST in the IST cohort at 1 year after transplantation than TCD recipients.
Further evidence supporting TCD to reduce GVHD without compromising relapse rates in acute leukemia can be found in studies of in vivo TCD using antilymphocyte antibodies, including ATG and alemtuzumab, in the setting of myeloablative conditioning.1,29,30 Bacigalupo et al31 reported that ATG prevented chronic GVHD, chronic lung dysfunction, and late transplantation-related mortality as part of long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Socié et al1 reported that the addition of ATG to myeloablative regimens in a prospective randomized trial reduced the rate of chronic GVHD from 45% to 12% at 3 years after transplantation, without an increase in disease relapse. Long-term analysis of this trial revealed a higher likelihood of immune suppression–free survival in recipients of ATG.1 These studies using in vivo TCD are promising but require chronic pharmacologic GVHD prophylaxis after transplantation and lead to unpredictable levels of TCD. Furthermore, utilization of these antibodies in the setting of reduced-intensity conditioning might not result in the same benefits. Indeed, a retrospective registry analysis from the CIBMTR in more than 1,600 patients suggested that ATG preparations or alemtuzumab, although reducing chronic GVHD, were associated with higher relapse rates and inferior disease-free survival compared with pharmacologic prophylaxis alone.32 The TCD cohort used low-dose thymoglobulin on day −4 with the objective of promoting engraftment. This particular low dose has historically not provided benefit with regard to chronic GVHD reduction in prior studies.33 Moreover, Jakubowski et al34 reported durable engraftment in patients receiving similar myeloablative conditioning and CD34-selected TCD peripheral-blood cells after omission of low-dose ATG, suggesting that the low-dose ATG early in the conditioning regimen did little to affect outcome.
There are shortcomings to the current study. This was not a prospective randomized trial, and there were differences in patient sex and choice of preparative regimens. No definitive comparisons can be made for patients other than those in first complete remission and those receiving myeloablative preparative regimens. Nonetheless, the current analysis of clinical trial data does offer a unique opportunity to compare TCD with post-transplantation immune suppression as GVHD prophylaxis strategies after transplantation for AML in contemporary multicenter cohorts with similar data collection mechanisms and definitions. Despite its limitations, the data lend further support to the hypothesis that rigorous TCD can reduce GVHD without adversely affecting relapse and disease-free survival in patients with AML in first remission. This approach may prove to be a platform for future cellular therapies that target infectious agents or minimal residual disease, because the absence of immunosuppressive agents may offer a more appropriate in vivo milieu for these cellular products to thrive. More clinical research is needed to determine the precise settings in which TCD should be used.
Footnotes
Written on behalf of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN).
Supported in part by Grant No. U01HL069294 to the BMT CTN from the National Heart, Lung, and Blood Institute and the National Cancer Institute and by Miltenyi Biotec.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Steven Devine, Miltenyi Biotec (C); Richard J. O'Reilly, Miltenyi Biotec (C); Robert J. Soiffer, Miltenyi Biotec (C) Stock Ownership: None Honoraria: Marcelo C. Pasquini, Miltenyi Biotec; Steven Devine, Miltenyi Biotec; Carolyn A. Keever-Taylor, Miltenyi Biotec; Richard J. O'Reilly, Miltenyi Biotec Research Funding: Steven Devine, Miltenyi Biotec Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Marcelo C. Pasquini, Robert J. Soiffer
Administrative support: Marcelo C. Pasquini, Adam Mendizabal
Collection and assembly of data: Marcelo C. Pasquini, Adam Mendizabal, Shelly Carter
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Socié G, Schmoor C, Bethge WA, et al. Chronic graft-versus-host disease: Long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011;117:6375–6382. doi: 10.1182/blood-2011-01-329821. [DOI] [PubMed] [Google Scholar]
- 2.Wagner JE, Thompson JS, Carter SL, et al. Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell Depletion Trial): A multi-centre, randomised phase II-III trial. Lancet. 2005;366:733–741. doi: 10.1016/S0140-6736(05)66996-6. [DOI] [PubMed] [Google Scholar]
- 3.Marmont AM, Horowitz MM, Gale RP, et al. T-cell depletion of HLA-identical transplants in leukemia. Blood. 1991;78:2120–2130. [PubMed] [Google Scholar]
- 4.Drobyski WR, Hessner MJ, Klein JP, et al. T-cell depletion plus salvage immunotherapy with donor leukocyte infusions as a strategy to treat chronic-phase chronic myelogenous leukemia patients undergoing HLA-identical sibling marrow transplantation. Blood. 1999;94:434–441. [PubMed] [Google Scholar]
- 5.Papadopoulos EB, Carabasi MH, Castro-Malaspina H, et al. T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: Freedom from relapse in the absence of graft-versus-host disease. Blood. 1998;91:1083–1090. [PubMed] [Google Scholar]
- 6.Schattenberg A, De Witte T, Preijers F, et al. Allogeneic bone marrow transplantation for leukemia with marrow grafts depleted of lymphocytes by counterflow centrifugation. Blood. 1990;75:1356–1363. [PubMed] [Google Scholar]
- 7.Soiffer RJ, Fairclough D, Robertson M, et al. CD6-depleted allogeneic bone marrow transplantation for acute leukemia in first complete remission. Blood. 1997;89:3039–3047. [PubMed] [Google Scholar]
- 8.Pasquini MC, Wang Z, Horowitz MM, et al. 2010 report from the Center for International Blood and Marrow Transplant Research (CIBMTR): Current uses and outcomes of hematopoietic cell transplant for blood and bone marrow disorders, in Cecka JM, Terazaki PI (eds): Clinical Transplant 2010. Los Angeles, CA: Terasaki Foundation Laboratory; 2011. pp. 87–105. [PubMed] [Google Scholar]
- 9.Devine SM, Carter S, Soiffer RJ, et al. Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: Results of the Blood and Marrow Transplant Clinical Trials Network protocol 0303. Biol Blood Marrow Transplant. 2011;17:1343–1351. doi: 10.1016/j.bbmt.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keever-Taylor CA, Devine SM, Soiffer RJ, et al. Characteristics of CliniMACS® System CD34-enriched T cell-depleted grafts in a multicenter trial for acute myeloid leukemia: Blood and Marrow Transplant Clinical Trials Network (BMT CTN) protocol 0303. Biol Blood Marrow Transplant. 2012;18:690–697. doi: 10.1016/j.bbmt.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weisdorf D, Carter S, Confer D, et al. Blood and Marrow Transplant Clinical Trials Network (BMT CTN): Addressing unanswered questions. Biol Blood Marrow Transplant. 2007;13:257–262. doi: 10.1016/j.bbmt.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Barfield RC, Otto M, Houston J, et al. A one-step large-scale method for T- and B-cell depletion of mobilized PBSC for allogeneic transplantation. Cytotherapy. 2004;6:1–6. doi: 10.1080/14653240310004411. [DOI] [PubMed] [Google Scholar]
- 13.Bornhauser M, Platzbecker U, Theuser C, et al. CD34+-enriched peripheral blood progenitor cells from unrelated donors for allografting of adult patients: High risk of graft failure, infection and relapse despite donor lymphocyte add-back. Br J Haematol. 2002;118:1095–1103. doi: 10.1046/j.1365-2141.2002.03731.x. [DOI] [PubMed] [Google Scholar]
- 14.Elmaagacli AH, Peceny R, Steckel N, et al. Outcome of transplantation of highly purified peripheral blood CD34+ cells with T-cell add-back compared with unmanipulated bone marrow or peripheral blood stem cells from HLA-identical sibling donors in patients with first chronic phase chronic myeloid leukemia. Blood. 2003;101:446–453. doi: 10.1182/blood-2002-05-1615. [DOI] [PubMed] [Google Scholar]
- 15.Handgretinger R, Klingebiel T, Lang P, et al. Megadose transplantation of purified peripheral blood CD34(+) progenitor cells from HLA-mismatched parental donors in children. Bone Marrow Transplant. 2001;27:777–783. doi: 10.1038/sj.bmt.1702996. [DOI] [PubMed] [Google Scholar]
- 16.Aversa F, Terenzi A, Tabilio A, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: A phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23:3447–3454. doi: 10.1200/JCO.2005.09.117. [DOI] [PubMed] [Google Scholar]
- 17.Wingard JR, Carter SL, Walsh TJ, et al. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood. 2010;116:5111–5118. doi: 10.1182/blood-2010-02-268151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: A Southwest Oncology Group/Eastern Cooperative Oncology Group study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- 19.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 21.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 22.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 23.Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: Systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301:2349–2361. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasquini M. Current use and outcome of hematopoietic stem cell transplantation: Part I – CIBMTR summary slides, 2005. CIBMTR Newsletter. 2005;12:5–8. [Google Scholar]
- 25.Couban S, Simpson DR, Barnett MJ, et al. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood. 2002;100:1525–1531. doi: 10.1182/blood-2002-01-0048. [DOI] [PubMed] [Google Scholar]
- 26.Stem Cell Trialists' Collaborative Group. Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: An individual patient data meta-analysis of nine randomized trials. J Clin Oncol. 2005;23:5074–5087. doi: 10.1200/JCO.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anasetti C, Logan BR, Lee SJ, et al. Increased incidence of chronic graft-versus-host disease (GVHD) and no survival advantage with filgrastim-mobilized peripheral blood stem cells (PBSC) compared to bone marrow (BM) transplants from unrelated donors: Results of Blood and Marrow Transplant Clinical Trials Network (BMT CTN) protocol 0201, a phase III, prospective, randomized trial. Blood. 2011;118:1. (abstr) [Google Scholar]
- 28.Flowers MED, Parker PM, Johnston LJ, et al. Comparison of chronic graft-versus-host disease after transplantation of peripheral blood stem cells versus bone marrow in allogeneic recipients: Long-term follow-up of a randomized trial. Blood. 2002;100:415–419. doi: 10.1182/blood-2002-01-0011. [DOI] [PubMed] [Google Scholar]
- 29.Das-Gupta EP, Russell NH, Shaw BE, et al. Long-term outcome of unrelated donor transplantation for AML using myeloablative conditioning incorporating pretransplant alemtuzumab. Biol Blood Marrow Transplant. 2007;13:724–733. doi: 10.1016/j.bbmt.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 30.Marks DI, Bird JM, Vettenranta K, et al. T cell-depleted unrelated donor bone marrow transplantation for acute myeloid leukemia. Biol Blood Marrow Transplant. 2000;6:646–653. doi: 10.1016/s1083-8791(00)70031-0. [DOI] [PubMed] [Google Scholar]
- 31.Bacigalupo A, Lamparelli T, Barisione G, et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: Long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transplant. 2006;12:560–565. doi: 10.1016/j.bbmt.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 32.Soiffer RJ, LeRademacher J, Ho V, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117:6963–6970. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohty M, Bay J-O, Faucher C, et al. Graft-versus-host disease following allogeneic transplantation from HLA-identical sibling with antithymocyte globulin–based reduced-intensity preparative regimen. Blood. 2003;102:470–476. doi: 10.1182/blood-2002-12-3629. [DOI] [PubMed] [Google Scholar]
- 34.Jakubowski AA, Small TN, Young JW, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: Sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110:4552–4559. doi: 10.1182/blood-2007-06-093880. [DOI] [PMC free article] [PubMed] [Google Scholar]