Abstract
Purpose
HLA-mismatched unrelated donor (MMUD) hematopoietic stem-cell transplantation (HSCT) is associated with increased graft-versus-host disease (GVHD) and impaired survival. In reduced-intensity conditioning (RIC), neither ex vivo nor in vivo T-cell depletion (eg, antithymocyte globulin) convincingly improved outcomes. The proteasome inhibitor bortezomib has immunomodulatory properties potentially beneficial for control of GVHD in T-cell-replete HLA-mismatched transplantation.
Patients and Methods
We conducted a prospective phase I/II trial of a GVHD prophylaxis regimen of short-course bortezomib, administered once per day on days +1, +4, and +7 after peripheral blood stem-cell infusion plus standard tacrolimus and methotrexate in patients with hematologic malignancies undergoing MMUD RIC HSCT. We report outcomes for 45 study patients: 40 (89%) 1-locus and five (11%) 2-loci mismatches (HLA-A, -B, -C, -DRB1, or -DQB1), with a median of 36.5 months (range, 17.4 to 59.6 months) follow-up.
Results
The 180-day cumulative incidence of grade 2 to 4 acute GVHD was 22% (95% CI, 11% to 35%). One-year cumulative incidence of chronic GVHD was 29% (95% CI, 16% to 43%). Two-year cumulative incidences of nonrelapse mortality (NRM) and relapse were 11% (95% CI, 4% to 22%) and 38% (95% CI, 24% to 52%), respectively. Two-year progression-free survival and overall survival were 51% (95% CI, 36% to 64%) and 64% (95% CI, 49% to 76%), respectively. Bortezomib-treated HLA-mismatched patients experienced rates of NRM, acute and chronic GVHD, and survival similar to those of contemporaneous HLA-matched RIC HSCT at our institution. Immune recovery, including CD8+ T-cell and natural killer cell reconstitution, was enhanced with bortezomib.
Conclusion
A novel short-course, bortezomib-based GVHD regimen can abrogate the survival impairment of MMUD RIC HSCT, can enhance early immune reconstitution, and appears to be suitable for prospective randomized evaluation.
INTRODUCTION
Impaired survival and graft-versus-host disease (GVHD) remain significant barriers to allogeneic hematopoietic stem-cell transplantation (HSCT) in recipients lacking HLA-matched donors, in which standard-of-care calcineurin inhibitor (CNI) –based two-drug GVHD regimens appear inadequate.1–6 However, in reduced-intensity conditioning (RIC) transplantation, critically dependent on graft-versus-tumor (GVT) effect for cure, in vivo T-cell antibody–based GVHD prophylaxis (eg, antithymocyte globulin) can also impair survival.7 Novel T-cell-replete GVHD regimens would be of considerable utility.
The proteasome inhibitor bortezomib has immunomodulatory properties with the ability to selectively deplete proliferating alloreactive T lymphocytes, reduce T-helper Type 1 cytokines, and block antigen presenting cell activation.8,9 Bortezomib may also spare regulatory T cells (Treg) that may be relevant in GVHD control.10 We and others have shown that it can control GVHD in major histocompatibility complex–mismatched mouse HSCT while maintaining therapeutic GVT responses.11–13 Of note however, delayed or prolonged bortezomib administration can induce severe colonic toxicity in mice.12
We undertook a phase I/II trial to evaluate a bortezomib-based regimen for controlling GVHD after HLA-mismatched unrelated donor (MMUD) RIC HSCT. In the phase I segment, we documented minimal toxicity and preliminary evidence for acute GVHD control.14 We now report complete phase I/II results. We also compare the clinical and immune reconstitution data of bortezomib-MMUD transplantation with HLA-matched RIC HSCT.
PATIENTS AND METHODS
This prospective clinical trial was approved by the institutional review board of the Dana-Farber Cancer Institute/Harvard Cancer Center. Written informed consent was obtained before patient enrollment.
Inclusion and Exclusion Criteria
For the phase I/II clinical trial, patients with hematologic malignancies who did not have an HLA-matched donor available received MMUD grafts with one to two antigen/allele mismatches at HLA-A, -B, -C, or -DQB1 or a one to two antigen/allele mismatch at HLA-DRB1 or -DQB1. Patients with HIV infection, active hepatitis B or C, abnormal renal (serum creatinine > 2 mg/dL) or liver function (serum total bilirubin > 2 mg/dL, serum ALT > 90 U/L), Eastern Cooperative Oncology Group (ECOG) performance status more than 2, or peripheral neuropathy grade ≥ 2 within 21 days before transplantation were excluded. Transplantation time period was 2006 to 2010. RIC comprised fludarabine 30 mg/m2 intravenously (IV) and busulfan 0.8 mg/kg IV on days −5, −4, −3, and −2. The donor target peripheral blood stem-cell (PBSC) dose was ≥ 5 × 106 CD34+ cells/kg. GVHD prophylaxis comprised tacrolimus 0.05 mg/kg orally twice daily to achieve a target serum level of 5 to 10 ng/mL starting on day −3; methotrexate 5 mg/m2 IV on days +1, +3, +6, and +11; and bortezomib dose levels of 1, 1.3, or 1.5 mg/m2 IV administered on days +1, +4, and +7, in accordance with the standard 72-hour bortezomib dosing interval. Tacrolimus taper commenced after 9 weeks, with the goal of having the patient no longer receiving immune suppression by 6 months in the absence of GVHD. In the phase I portion of the study, we identified the maximum-tolerated dose (MTD) for bortezomib as 1.3 mg/m2.14 In phase II, efficacy was further assessed in 30 evaluable patients enrolled at the MTD. Eligibility criteria for phases I and II were identical, and outcomes were similar. We report the results of 45 patients in combined phases I and II.
Contemporaneous Comparison Cohort
Clinical outcomes were obtained for 176 consecutive RIC HSCT patients who received HLA-A, -B, -C, or -DRB1 matched unrelated donor (MUD) PBSC grafts and who received transplantations in 2006 to 2010, with sirolimus-based GVHD prophylaxis. In addition, immune reconstitution data were available for 139 consecutive MUD RIC HSCT patients who had transplantations from 2002 to 2008 with sirolimus-based GVHD prophylaxis. Transplantation eligibility criteria were similar for both groups. RIC comprised fludarabine 30 mg/m2 IV and busulfan 0.8 mg/kg IV once or twice daily on days −5, −4, −3, and −2. The donor target PBSC dose was ≥ 5 × 106 CD34+ cells/kg. GVHD prophylaxis comprised tacrolimus 0.05 mg/kg orally twice daily to attain a target serum level of 5 to 10 ng/mL starting on day −3; methotrexate 5 mg/m2 IV on days +1, +3, +6, and ±11; and sirolimus 12-mg loading dose and 4 mg daily thereafter to attain a target serum level of 5 to 10 ng/mL, starting on day −3. Tacrolimus and sirolimus taper were initiated around week 9 after RIC HSCT, with the goal of having the patient no longer receiving immune suppression by 6 months in the absence of GVHD.
Supportive Care
All patients received filgrastim at 5 μg/kg daily from day +1 until an absolute neutrophil count (ANC) of more than 1,000/μL was attained, with at least 12 months of Pneumocystis jiroveci and herpes simplex virus/varicella zoster virus prophylaxis. Antifungal prophylaxis was not routine.
Immune Reconstitution Assays
CD4+ T cells were defined as CD3+CD4+, CD8+ T cells as CD3+CD8+, Treg cells as CD3+CD4+CD25med-highCD127low, natural killer (NK) cells as CD56+CD3−, NK T cells as CD56+CD3+, and B cells as CD19+. Fifty microliters of whole blood (15% EDTA) in 5-mL polystyrene round-bottom reaction tubes was incubated with fluorophore-conjugated monoclonal antibodies: anti-CD3 V450 (clone UCHT1; BD Biosciences, Sparks, MD), anti-CD4 APC-H7 (clone RPA-T4; BD Biosciences), anti-CD8 Pacific Orange (clone 3B5; Invitrogen, Grand Island, NY), anti-CD25 PE-Cy7 (clone M-A251; BD Biosciences), anti-CD127 PE-Cy5 (clone eBioRDR5; eBioscience, San Diego, CA) for T-cell subsets, anti-CD56 PE (clone B159; BD Biosciences), anti-CD3 V450 (clone UCHT1; BD Biosciences) for NK cells/NK T cells, and anti-CD19 activated protein C (clone HIB19; BD Biosciences) for B cells. RBC lysis with 500 μL 1× BD Pharm Lyse followed. Immune reconstitution flow cytometry analysis used an FACSCanto II flow cytometer (BD Bioscience) and FACSDiva software (BD Biosciences).
Statistical Considerations
Neutrophil and platelet engraftment were assessed by the number of days to ANC ≥ 500 cells/μL and platelet count ≥ 20,000/μL, respectively, in the absence of transfusions, among patients who experienced a neutrophil or platelet nadir (ANC < 500 cells/μL; platelets < 20,000/μL) after conditioning. Acute GVHD was graded by using the consensus grading system.15 Progression-free survival (PFS) was measured from the date of transplantation to disease relapse/progression, death, or last contact. Overall survival (OS) was measured from the date of transplantation to death from any cause. PFS and OS were estimated by the Kaplan-Meier method. The cumulative incidence of acute and chronic GVHD was estimated with relapse and death as competing events (ie, GVHD that developed in the context of malignant relapse necessitating taper of immunosuppression counted as relapse) and were compared by using the Gray test.16 The cumulative incidence of nonrelapse mortality (NRM) and relapse were also estimated by considering these two events as competing risks. Comparisons were made of immune reconstitution data between groups by using a Wilcoxon rank sum test at each time point. All testing was two-sided at the significance level of 0.05, and multiple comparisons were not adjusted. The analyses were conducted by using SAS version 9.2 (SAS Institute, Cary, NC) and R version 2.11.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study Cohort
Forty-five patients were enrolled onto the phase I/II study. Baseline characteristics are presented in Table 1. The median age for patients was 59 years (range, 26 to 72 years); 36 patients (80%) were older than age 50 years. Forty patients (89%) received 1-locus and five patients (11%) received 2-locus HLA-mismatched grafts. The median follow-up of survivors was 36.5 months (range, 17.4 to 59.6 months).
Table 1.
Patient and Graft Characteristics of the Bortezomib-MMUD Study Patients
| Characteristic | No. of Patients(n = 45) | % |
|---|---|---|
| Patient age, years | ||
| Median | 59 | |
| Range | 26-72 | |
| Donor age, years | ||
| Median | 35 | |
| Range | 20-65 | |
| Female sex | 21 | 47 |
| Patient-donor sex match | ||
| F-F | 13 | 29 |
| F-M | 8 | 18 |
| M-F | 7 | 16 |
| M-M | 17 | 38 |
| HLA typing | ||
| Mismatched HLA-A only | 12 | 27 |
| Mismatched HLA-B only | 10 | 22 |
| Mismatched HLA-B and C | 4 | 9 |
| Mismatched HLA-C only | 14 | 31 |
| Mismatched HLA-DQB1 only | 2 | 4 |
| Mismatched HLA-DRB1 only | 2 | 4 |
| Mismatched HLA-DRB1 and DQB1 | 1 | 2 |
| 1-Antigen mismatched | 28 | 62 |
| 2-Antigen mismatched | 3 | 7 |
| 1-Allele mismatched | 12 | 27 |
| 2-Allele mismatched | 1 | 2 |
| 1-Antigen and 1-allele mismatched | 1 | 2 |
| Mismatched class I | 40 | 89 |
| Mismatched class II | 5 | 11 |
| Disease status at transplant | ||
| First CR/chronic phase | 16 | 36 |
| Second or later CR/chronic phase | 3 | 7 |
| Third or later CR/chronic phase | 1 | 2 |
| PR or accelerated phase | 19 | 42 |
| Relapse or blastic phase | 2 | 4 |
| Induction failure | 1 | 2 |
| Untreated | 3 | 7 |
| Diagnosis | ||
| AML | 14 | 31 |
| NHL | 11 | 24 |
| CLL/SLL/PLL | 8 | 18 |
| HL | 4 | 9 |
| MDS | 4 | 9 |
| ALL | 2 | 4 |
| MPD* | 2 | 4 |
| High-risk disease† | 30 | 67 |
| Patient or donor CMV positive | 31 | 69 |
| Prior transplantation | 8 | 18 |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; CMV, cytomegalovirus; CR, complete response; F, female; HL, Hodgkin's lymphoma; M, male; MDS, myelodysplastic syndromes; MMUD, mismatched unrelated donor; MPD, myeloproliferative disease; NHL, non-Hodgkin's lymphoma; PLL, prolymphocytic leukemia; PR, partial response; SLL, small lymphocytic lymphoma.
The protocol was subsequently amended to exclude patients with MPD.
Patients other than those with AML or ALL in first CR, CML in chronic phase, aplastic anemia, MDS with refractory anemia, or refractory anemia with ringed sideroblasts.
Only 20 patients experienced a neutrophil nadir, with a median engraftment time of 13 days (range, 6 to 29 days). Only 21 patients experienced a platelet nadir, with a median engraftment time of 20 days (range, 13 to 27 days). One patient died of intracranial hemorrhage during transfusion-refractory thrombocytopenia before engraftment. At the 1.5-mg/m2 bortezomib dose level, two patients had low donor chimerism by day +45, defined a priori as dose-limiting toxicity.14 One patient with chronic lymphocytic leukemia had 0% donor chimerism and persistent leukemia post-transplantation, and another patient with untreated myeloproliferative disease also experienced engraftment failure. Subsequent accrual was at bortezomib MTD of 1.3 mg/m2.
Nonhematologic toxicity was limited. There were seven episodes of Common Toxicity Criteria (CTC) grade 3 or 4 toxicity by day +45. One patient each had febrile neutropenia (grade 3), parainfluenza sinusitis (grade 3), cerebrovascular accident with paresthesias (grade 3), hyperglycemia (grade 3), deep venous thrombosis/pulmonary embolus (grade 3), or Clostridium difficile diarrhea (grade 3) plus coagulase-negative staphylococcal bacteremia (grade 3). These were deemed to be not attributable to bortezomib by the treating physician.
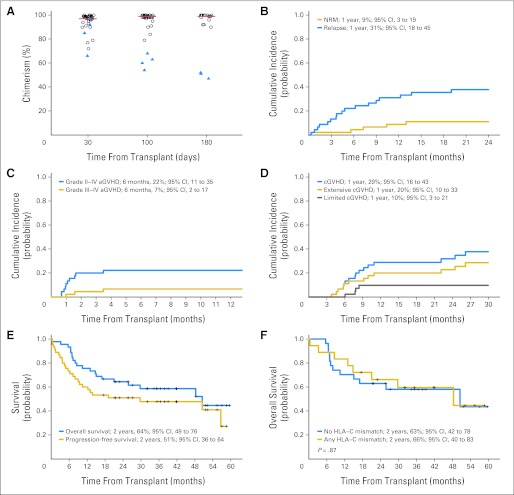
Median total donor cell chimerism in patients who did not experience failure to engraft or relapse or death before assessment was 97% (range, 66% to 100%; n = 37), 99% (range, 54% to 100%; n = 35), and 99% (range, 47% to 100%; n = 20) at days +30, +100, and +180, respectively (chimerism assays were not mandatory beyond day 100; Fig 1A). Four patients without disease relapse/progression did not sustain donor chimerism at ≥ 70% through day +100: two subsequently achieved full donor chimerism, and two underwent salvage transplantation for late graft failure.
Fig 1.
Bortezomib–mismatched unrelated donor reduced-intensity conditioning hematopoietic stem-cell transplantation. (A) Sustained total donor chimerism at days 30, 100, and 180 after transplantation in patients initially engrafted and without evidence of disease relapse/progression. Triangles indicate patients without sustained donor engraftment (≥ 70% total donor cell chimerism). Of note, donor chimerism analysis was not mandated beyond day 100. (B) Cumulative incidence of nonrelapse mortality (NRM; with relapse/progression as a competing event) and relapse/progression (with death as a competing event). (C) Cumulative incidence of grade 2 to 4 acute graft-versus-host disease (aGVHD) and grade 3 to 4 aGVHD (with relapse/progression and death as competing events). (D) Cumulative incidence of chronic GVHD (cGVHD; with relapse/progression and death as competing events). (E) Overall and progression-free survival. (F) Overall survival for HLA-C mismatched and non–HLA-C mismatched transplantation.
Eight patients died without disease relapse/progression, a 2-year cumulative incidence of NRM of 11% (95% CI, 4% to 22%; Fig 1B). Disease relapse/progression was observed in 17 patients, a 2-year cumulative incidence of 38% (95% CI, 24% to 52%; Fig 1B). Grade 2 to 4 acute GVHD occurred in 10 patients, with a median time to onset of 32 days (range, 21 to 106 days) and a 180-day cumulative incidence of 22% (95% CI, 11% to 35%; Fig 1C). Three patients had grade 3 acute GVHD (none had grade 4 acute GVHD), with a 180-day cumulative incidence of 7% (95% CI, 2% to 17%). Chronic GVHD occurred in 16 patients, with a median time to onset of 237 days (range, 115 to 796 days) and a 1-year cumulative incidence of 29% (95% CI, 16% to 43%; Fig 1D). Of these, four had limited and 12 had extensive chronic GVHD, with 1-year cumulative incidences of 10% (95% CI, 3% to 21%) and 20% (95% CI, 10% to 33%), respectively. The 2-year OS and PFS were 64% (95% CI, 49% to 76%) and 51% (95% CI, 36% to 64%), respectively (Fig 1E). Clinical outcomes of 18 patients with HLA-C locus mismatches versus the 27 patients with mismatches at other loci were similar with regard to end points of NRM (P = .94), relapse (P = .91), acute GVHD (P = .13) and chronic GVHD (P = 0.30), PFS (P = .99), or OS (P = .87; Fig 1F).
Comparison With MUD RIC Transplantation
The outcomes of 45 bortezomib-based MMUD (bortezomib-MMUD) RIC transplantations were compared with those in a contemporaneous cohort of 176 consecutive sirolimus-based MUD (sirolimus-MUD) patients who received transplantations from 2006 to 2010 with similar busulfan/fludarabine RIC, PBSC grafts, and GVHD prophylaxis based on a backbone of tacrolimus and methotrexate. They differed in the use of additional GVHD agent (bortezomib v sirolimus) and in the degree of HLA-match (MMUD v MUD). The cohorts did not differ regarding patient or donor age, sex, donor-recipient sex match, disease, disease risk, prior autologous transplantation, cytomegalovirus (CMV) serostatus, and median follow-up duration (Appendix Table A1, online only).
The bortezomib-MMUD and sirolimus-MUD RIC HSCT cohorts had similar clinical outcomes. There was no difference in OS, PFS, NRM, or grade 2 to 4 acute GVHD, with a marginally lower relapse incidence for the bortezomib-MMUD cohort compared with the sirolimus-MUD cohort (2-year cumulative incidence of 38% v 53%, respectively; P = .09; Table 2). The 1-year cumulative incidence of chronic GVHD was 29% (95% CI, 16% to 43%) for the bortezomib-MMUD versus 38% (95% CI, 31% to 46%) for the sirolimus-MUD cohort (P = .29).
Table 2.
Clinical Outcome Comparison of Bortezomib-MMUD and Sirolimus-MUD Cohorts
| Outcome | Bortezomib-MMUD(n = 45) |
Sirolimus-MUD(n = 176) |
P | ||
|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | ||
| Cumulative incidence of: | |||||
| 1-year NRM | 9 | 3 to 19 | 7 | 4 to 11 | |
| 2-year NRM | 11 | 4 to 22 | 8 | 4 to 13 | .29 |
| 1-year relapse | 31 | 18 to 45 | 46 | 39 to 54 | |
| 2-year relapse | 38 | 24 to 52 | 53 | 45 to 61 | .09 |
| 180-day grade 2 to 4 acute GVHD | 22 | 11 to 35 | 11 | 7 to 16 | .12 |
| 1-year chronic GVHD | 29 | 16 to 43 | 38 | 31 to 46 | .28 |
| PFS | |||||
| 1-year | 60 | 44 to 73 | 47 | 39 to 54 | |
| 2-year | 51 | 36 to 64 | 39 | 31 to 46 | .24 |
| OS | |||||
| 1-year | 76 | 60 to 86 | 64 | 56 to 70 | |
| 2-year | 64 | 49 to 76 | 55 | 47 to 63 | .24 |
NOTE. The cohorts received similar busulfan/fludarabine-based reduced-intensity conditioning, peripheral blood stem-cell grafts, and GVHD prophylaxis based on a backbone of tacrolimus and methotrexate. They differed in the use of additional GVHD agent (bortezomib v sirolimus) and in the degree of HLA-match (MMUD v MUD). Pre-transplantation variables were not different between the cohorts.
Abbreviations: GVHD, graft-versus-host disease; MUD, matched unrelated donor; MMUD, mismatched unrelated donor; NRM, nonrelapse mortality; OS, overall survival; PFS, progression-free survival.
Comparison of Immune Reconstitution in Both Cohorts
Bortezomib-MMUD RIC HSCT was compared with 139 sirolimus-MUD RIC transplantations with available immune reconstitution data. Having received transplantations from 2002 to 2008, the sirolimus-MUD cohort was otherwise similar with regard to busulfan/fludarabine-based RIC, PBSC grafts, and GVHD prophylaxis based on a backbone of tacrolimus and methotrexate. The cohorts differed with respect to use of bortezomib versus sirolimus and the degree of HLA-mismatch (MMUD v MUD). Pretransplantation immune parameters were similar between the cohorts.
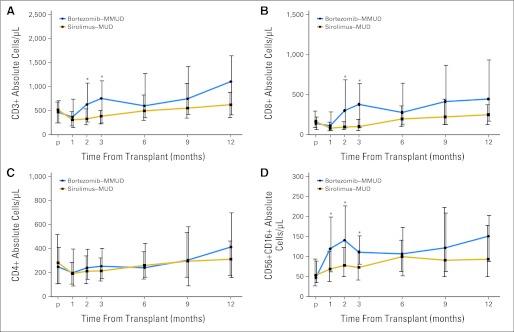
No immunologic reconstitution parameter was impaired for bortezomib-MMUD compared with sirolimus-MUD RIC HSCT. Indeed, immune reconstitution was more rapid in the bortezomib-MMUD cohort. Median peripheral WBC count was higher in the bortezomib-MMUD cohort (5.6 × 103 cells/L) versus the sirolimus-MUD cohort (3.7 × 103 cells/L [P = .001]) at 2 months post-transplantation. Median absolute lymphocyte count at 2 and 3 months post-transplantation was approximately two-fold higher in the bortezomib-MMUD cohort (1,232 and 1,368 cells/L, respectively) versus the sirolimus-MUD cohort (629 cells/L [P = .001] and 630 cells/L [P < .001], respectively), and it remained increased at 6 months post-transplantation (1,253 v 864 cells/μL [P = .03]).
With regard to immune subsets, median total CD3+ T-cell count at 2 and 3 months post-transplantation was approximately two-fold higher in the bortezomib-MMUD cohort (630 and 756 cells/L, respectively) versus the sirolimus-MUD cohort (331 cells/L [P < .001] and 386 cells/L [P < .001], respectively; Fig 2A). This was because of an approximately three-fold higher median CD8 T-cell count at 2 and 3 months post-transplantation in the bortezomib-MMUD cohort (302 and 379 cells/L, respectively) versus the sirolimus-MUD cohort (97 cells/L [P < .001] and 101 cells/L [P < .001], respectively; Fig 2B). In contrast, CD4 T-cell reconstitution was similar (Fig 2C). In addition, the median CD5616 NK-cell count at 1, 2, and 3 months after transplantation was approximately two-fold higher in the bortezomib-MMUD cohort (120, 141, and 111 cells/L, respectively) compared with the sirolimus-MUD cohort (69 cells/L [P = .003], 78 cells/L [P = .005], and 73 cells/μL [P = .004], respectively; Fig 2E). No difference was noted in median B-cell, NK–T-cell, or Treg-cell reconstitution between cohorts (data not shown).
Fig 2.
Bortezomib–mismatched unrelated donor (MMUD) and sirolimus–matched unrelated donor (MUD) cohorts. (A) CD3+ total T-cell reconstitution; (B) CD8+ T-cell reconstitution; (C) CD4+ T-cell reconstitution; (D) natural killer cell reconstitution. Median cell counts (with vertical bars indicating interquartile range). (*) Significant P value.
DISCUSSION
GVHD remains a major cause of morbidity and mortality after unrelated donor HSCT, especially in the HLA-mismatched setting, in which it is associated with higher NRM and impaired survival. There remains a great need for better prophylaxis regimens that can minimize GVHD without compromising GVT effect. GVHD regimens that improve HLA-mismatched transplantation outcomes would be a major advance and would likely also offer efficacy in HLA-matched transplantation.
T-cell depletion can control GVHD but may be associated with impaired immune reconstitution, increased graft rejection, infections, lymphoproliferative disease, and relapse.17 In RIC transplantation, in which success is critically dependent on immunologic GVT effect, even in vivo T-cell depletion with antithymocyte globulin or alemtuzumab can impair survival.7 In T-replete HLA-mismatched transplantation, usual GVHD prophylaxis regimens also appear inadequate.
For instance, we documented a 46% rate of grade 2 to 4 acute GVHD after CNI plus prednisone or CNI plus methotrexate prophylaxis in 52 patients with RIC HSCT mismatched at one or more HLA loci.18 Moreover, HLA-C antigen mismatch was associated with increased NRM (48% v 16%; P < .001) and worse 2-year OS (30% v 51%; P = .008) compared with HLA-matched RIC transplantation.4 A registry analysis also documented increased mortality in T-replete HLA-C antigen mismatched RIC HSCT (relative risk, 1.40; 95% CI, 1.01 to 1.95; P = .04).6 In a prospective phase I/II study of 1- to 2-loci HLA-mismatched RIC HSCT, CNI plus mycophenolate mofetil prophylaxis also appeared inadequate, with a grade 2 to 4 acute GVHD rate of 69%, NRM rate of 47%, and 2-year OS of only 29%.5
Given its immunomodulatory properties, we prospectively evaluated a GVHD regimen of short-course bortezomib plus CNI and methotrexate. Bortezomib, limited to three doses early after transplantation (on days +1, +4, and +7) appears to have little systemic toxicity. No patients developed toxicities that were associated with more prolonged bortezomib therapy (eg, neuropathy, colonic necrosis). Treatment-related toxicity after bortezomib-based HLA-mismatched RIC HSCT is in the range previously reported for HLA-matched transplantation.
The bortezomib-based regimen appears efficacious, with a 180-day cumulative incidence of grade 2 to 4 acute GVHD of 22%, which is in the range reported for HLA-matched transplantation.4 Moreover, MMUD transplantation survival appears better than that previously reported. For instance, 2-year OS at 64% appears better than the 30% survival we documented in HLA-C mismatched transplantation by similarly using PBSC and busulfan/fludarabine RIC, or the 29% survival prospectively reported for 1- to 2-loci HLA-mismatched transplantation by using PBSC and fludarabine/total body irradiation RIC.4,5 Importantly, bortezomib-MMUD transplantation outcomes of HLA-C mismatch were not different from mismatch at other loci. However, CNI-based, two-drug regimens may not represent an adequate standard of care in the context of T-replete HLA-mismatched transplantation, and improvements in supportive care in recent years also need to be considered.
Sirolimus is an immunosuppressive agent that we and others have used in allogeneic HSCT over the past decade.19 To place our findings in context, we therefore compared bortezomib-MMUD with a contemporaneous MUD HSCT cohort receiving similar busulfan/fludarabine RIC, a T-replete PBSC graft, and GVHD prophylaxis based on a backbone of CNI and methotrexate (plus sirolimus). The sirolimus-based cohorts were similar to the bortezomib-based cohort in patient and disease parameters, except that HLA-mismatch was greater in the bortezomib-based cohort. We document that bortezomib-MMUD and sirolimus-MUD cohorts had similar clinical outcomes regarding acute and chronic GVHD, NRM, relapse, and survival.
Another important measure is immunologic reconstitution, for which we also compared bortezomib-MMUD and sirolimus-MUD cohorts. Although the clinical relevance of the differences remains to be determined, it is notable that no measure of immune recovery appeared impaired in the bortezomib-MMUD cohort compared with sirolimus-based MUD transplantation. Although more rapid immune reconstitution in the bortezomib-MMUD cohort did not translate into better NRM compared with sirolimus-MUD transplantation, it did appear to abrogate the high NRM previously described in MMUD RIC transplantation.
Although these clinical and immunologic results are encouraging, we caution that retrospective comparisons have inherent limitations, being subject to bias and confounding, even in apparently well-matched cohorts such as those we have already described. However, they are useful in a hypothesis-generating context, hereby providing support for prospective randomized evaluation of bortezomib-based GVHD prophylaxis.
In conclusion, short-course, bortezomib-based GVHD prophylaxis appears safe and efficacious in HLA-mismatched RIC transplantation, with encouraging survival. Importantly, bortezomib-based MMUD transplantation achieved clinical outcomes comparable to HLA-matched transplantation, along with enhancement of various immune reconstitution parameters. Bortezomib appears to be an active immunomodulator in allogeneic HSCT and a candidate for prospective randomized evaluation. We are also evaluating the role of short-course bortezomib in the context of myeloablative conditioning for both HLA-matched and mismatched transplantation.
Acknowledgment
We thank clinical research nurses Susan Stephenson, RN, and Mildred Pasek, RN, for their help.
Appendix
Table A1.
Pretransplantation Variables for the Bortezomib-MMUD and Sirolimus-MUD Cohorts
| Variable | Bortezomib-MMUD(n = 45) |
Sirolimus-MUD(n = 176) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Patient age, years | .45 | ||||
| Median | 59 | 60 | |||
| Range | 26-72 | 20-74 | |||
| Donor age, years | .17 | ||||
| Median | 35 | 32 | |||
| Range | 20-65 | 19-56 | |||
| Female sex | 21 | 47 | 69 | 39 | .40 |
| Patient-donor sex match | |||||
| F-F | 13 | 29 | 26 | 15 | .19 |
| F-M | 8 | 18 | 43 | 24 | |
| M-F | 7 | 16 | 28 | 16 | |
| M-M | 17 | 38 | 79 | 45 | |
| Diagnosis | |||||
| AML | 14 | 31 | 46 | 26 | — |
| NHL | 11 | 24 | 28 | 16 | |
| CLL/SLL/PLL | 8 | 18 | 18 | 10 | |
| HL | 4 | 9 | 12 | 7 | |
| MDS | 4 | 9 | 28 | 16 | |
| ALL | 2 | 4 | 8 | 5 | |
| MPD | 2 | 4 | 13 | 7 | |
| MM | 0 | 0 | 14 | 8 | |
| CML | 0 | 0 | 4 | 2 | |
| Anemia/red cell disorder | 0 | 0 | 4 | 2 | |
| Other* | 0 | 0 | 1 | 1 | |
| Myeloid disease† | 20 | 44 | 91 | 52 | .41 |
| High-risk disease‡ | 30 | 67 | 141 | 80 | .07 |
| Patient or donor CMV positive | 31 | 69 | 118 | 67 | .86 |
| Prior transplantation | 8 | 18 | 50 | 28 | .15 |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; CML, chronic myelogenous leukemia; CMV, cytomegalovirus; F, female; HL, Hodgkin's lymphoma; M, male; MDS, myelodysplastic syndromes; MM, multiple myeloma; MPD, myeloproliferative disease; MUD, matched unrelated donor; MMUD, mismatched unrelated donor; NHL, non-Hodgkin's lymphoma; PLL, prolymphocytic leukemia; SLL, small lymphocytic lymphoma.
Hemophagocytic lymphohistiocytosis.
AML, MDS, MPD, and CML.
Patients other than those with AML or ALL in first complete response, CML in chronic phase, aplastic anemia, or MDS with refractory anemia, or refractory anemia with ringed sideroblasts.
Footnotes
Supported in part by Millennium Pharmaceuticals, the Jock and Bunny Adams Education and Research Endowment, the Ted and Eileen Pasquarello Research Fund, and by Grant No. P01 CA142106 from the National Institutes of Health (J.H.A.). Millennium Pharmaceuticals provided the study drug bortezomib free of charge.
Presented in part at the 51st Annual Meeting of the American Society of Hematology, New Orleans, LA, December 2-8, 2009; and the American Society of Blood and Marrow Transplantation/Center for International Blood and Marrow Transplantation Research Annual Tandem Meeting, Honolulu, HI, February 17-21, 2011.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00369226.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: John Koreth, Millennium Pharmaceuticals (C) Stock Ownership: None Honoraria: None Research Funding: John Koreth, Millennium Pharmaceuticals Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: John Koreth, Haesook T. Kim, Bruce R. Blazar, Jerome Ritz, Edwin P. Alyea III
Financial support: Joseph H. Antin
Collection and assembly of data: John Koreth, Kristen E. Stevenson, Haesook T. Kim, Sean M. McDonough, Bhavjot Bindra, Philippe Armand, Vincent T. Ho, Corey Cutler, Joseph H. Antin, Robert J. Soiffer, Jerome Ritz, Edwin P. Alyea III
Data analysis and interpretation: John Koreth, Kristen E. Stevenson, Haesook T. Kim, Jerome Ritz
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Flowers ME, Inamoto Y, Carpenter PA, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117:3214–3219. doi: 10.1182/blood-2010-08-302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 3.Petersdorf EW, Gooley T, Malkki M, et al. Clinical significance of donor-recipient HLA matching on survival after myeloablative hematopoietic cell transplantation from unrelated donors. Tissue Antigens. 2007;69(suppl 1):25–30. doi: 10.1111/j.1399-0039.2006.759_2.x. [DOI] [PubMed] [Google Scholar]
- 4.Ho VT, Kim HT, Liney D, et al. HLA-C mismatch is associated with inferior survival after unrelated donor non-myeloablative hematopoietic stem cell transplantation. Bone Marrow Transplant. 2006;37:845–850. doi: 10.1038/sj.bmt.1705315. [DOI] [PubMed] [Google Scholar]
- 5.Nakamae H, Storer BE, Storb R, et al. Low-dose total body irradiation and fludarabine conditioning for HLA class I-mismatched donor stem cell transplantation and immunologic recovery in patients with hematologic malignancies: A multicenter trial. Biol Blood Marrow Transplant. 2010;16:384–394. doi: 10.1016/j.bbmt.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woolfrey A, Klein JP, Haagenson M, et al. HLA-C antigen mismatch is associated with worse outcome in unrelated donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:885–892. doi: 10.1016/j.bbmt.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soiffer RJ, Lerademacher J, Ho V, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117:6963–6970. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nencioni A, Schwarzenberg K, Brauer KM, et al. Proteasome inhibitor bortezomib modulates TLR4-induced dendritic cell activation. Blood. 2006;108:551–558. doi: 10.1182/blood-2005-08-3494. [DOI] [PubMed] [Google Scholar]
- 9.Blanco B, Pérez-Simón JA, Sánchez-Abarca LI, et al. Bortezomib induces selective depletion of alloreactive T lymphocytes and decreases the production of Th1 cytokines. Blood. 2006;107:3575–3583. doi: 10.1182/blood-2005-05-2118. [DOI] [PubMed] [Google Scholar]
- 10.Kim JS, Lee JI, Shin JY, et al. Bortezomib can suppress activation of rapamycin-resistant memory T cells without affecting regulatory T-cell viability in non-human primates. Transplantation. 2009;88:1349–1359. doi: 10.1097/TP.0b013e3181bd7b3a. [DOI] [PubMed] [Google Scholar]
- 11.Sun K, Welniak LA, Panoskaltsis-Mortari A, et al. Inhibition of acute graft-versus-host disease with retention of graft-versus-tumor effects by the proteasome inhibitor bortezomib. Proc Natl Acad Sci U S A. 2004;101:8120–8125. doi: 10.1073/pnas.0401563101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun K, Wilkins DE, Anver MR, et al. Differential effects of proteasome inhibition by bortezomib on murine acute graft-versus-host disease (GVHD): delayed administration of bortezomib results in increased GVHD-dependent gastrointestinal toxicity. Blood. 2005;106:3293–3299. doi: 10.1182/blood-2004-11-4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vodanovic-Jankovic S, Hari P, Jacobs P, et al. NF-kappaB as a target for the prevention of graft-versus-host disease: Comparative efficacy of bortezomib and PS-1145. Blood. 2006;107:827–834. doi: 10.1182/blood-2005-05-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koreth J, Stevenson KE, Kim HT, et al. Bortezomib, tacrolimus, and methotrexate for prophylaxis of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation from HLA-mismatched unrelated donors. Blood. 2009;114:3956–3959. doi: 10.1182/blood-2009-07-231092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1994;15:825–828. [PubMed] [Google Scholar]
- 16.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 17.Ho VT, Soiffer RJ. The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood. 2001;98:3192–3204. doi: 10.1182/blood.v98.12.3192. [DOI] [PubMed] [Google Scholar]
- 18.Ho V, Kim H, Windawi S, et al. HLA mismatch and clinical outcome after unrelated donor (URD) non-myeloablative hematopoeitic stem cell transplantation (NST) Biol Blood Marrow Transplant. 2005;11(suppl 1):S14. [Google Scholar]
- 19.Cutler C, Antin JH. Sirolimus immunosuppression for graft-versus-host disease prophylaxis and therapy: An update. Curr Opin Hematol. 2010;17:500–504. doi: 10.1097/MOH.0b013e32833e5b2e. [DOI] [PubMed] [Google Scholar]