Abstract
Purpose
Major concerns surround combining chemotherapy with bevacizumab in patients with colon cancer presenting with an asymptomatic intact primary tumor (IPT) and synchronous yet unresectable metastatic disease. Surgical resection of asymptomatic IPT is controversial.
Patients and Methods
Eligibility for this prospective, multicenter phase II trial included Eastern Cooperative Oncology Group (ECOG) performance status 0 to 1, asymptomatic IPT, and unresectable metastases. All received infusional fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) combined with bevacizumab. The primary end point was major morbidity events, defined as surgical resection because of symptoms at or death related to the IPT. A 25% major morbidity rate was considered acceptable. Secondary end points included overall survival (OS) and minor morbidity related to IPT requiring hospitalization, transfusion, or nonsurgical intervention.
Results
Ninety patients registered between March 2006 and June 2009: 86 were eligible with follow-up, median age was 58 years, and 52% were female. Median follow-up was 20.7 months. There were 12 patients (14%) with major morbidity related to IPT: 10 required surgery (eight, obstruction; one, perforation; and one, abdominal pain), and two patients died. The 24-month cumulative incidence of major morbidity was 16.3% (95% CI, 7.6% to 25.1%). Eleven IPTs were resected without a morbidity event: eight for attempted cure and three for other reasons. Two patients had minor morbidity events only: one hospitalization and one nonsurgical intervention. Median OS was 19.9 months (95% CI, 15.0 to 27.2 months).
Conclusion
This trial met its primary end point. Combining mFOLFOX6 with bevacizumab did not result in an unacceptable rate of obstruction, perforation, bleeding, or death related to IPT. Survival was not compromised. These patients can be spared initial noncurative resection of their asymptomatic IPT.
INTRODUCTION
Colorectal cancer (CRC) is the third most commonly diagnosed cancer and is the third leading cause of cancer death. The American Cancer Society estimates that 142,570 individuals will have been diagnosed with CRC in 2010, and 51,370 will die from it in 2010 in the United States.1 Despite increasing use of CRC screening, 20% of patients with newly diagnosed CRC present with distant metastases.2 Patients may present with general symptoms of malaise, weight loss, or fatigue. Only a minority have symptoms related to the intact primary tumor (IPT) in the colon such as bowel obstruction, tumor perforation, or significant bleeding.3 Among patients presenting with synchronous distant metastases, approximately 80% have metastases that are unresectable for cure. Others are not medically fit to tolerate a major hepatectomy, and only a minority of patients require immediate surgery of the primary or metastatic lesions.
For patients with both unresectable metastatic disease and an asymptomatic IPT, the initial treatment strategy is controversial. Initial resection of the primary tumor has been advocated to prevent future complications of colonic obstruction, bleeding, or perforation.4–6 Recent retrospective series,10–17 however, have suggested that for patients treated with current chemotherapy regimens, the incidence of problems related to the IPT may be only 10% to 20%. Furthermore, the 30-day operative mortality of colon resection for patients with distant metastases is as high as 10%.11 This high operative mortality rate, likely attributable to increased disease burden, diminishes enthusiasm for surgical resection as a prevention strategy. Others advocate for initial surgical resection of IPT in this setting, suggesting a favorable impact on overall survival (OS).3
To date, no multicenter, prospective clinical trial has evaluated the role of systemic chemotherapy with an approved biologic agent as the initial treatment for patients presenting with unresectable stage IV colon cancer with an asymptomatic IPT. Irinotecan, fluorouracil, and leucovorin combined with the anti–vascular endothelial growth factor (anti-VEGF) monoclonal antibody bevacizumab has demonstrated an improvement in OS in patients with metastatic CRC.12 Use of bevacizumab has previously raised concerns of increased risk of tumor perforation for patients with IPT. The National Surgical Adjuvant Breast and Bowel Project C-10 (NSABP C-10) trial is a prospective multicenter phase II trial with the primary objective of determining the safety of nonoperative management by using fluorouracil, leucovorin, and oxaliplatin (FOLFOX) and bevacizumab in patients presenting with stage IV colon cancer, IPT, and metastases unresectable for cure.
PATIENTS AND METHODS
Objectives
The primary objective of this trial was to determine the rate of major morbidity resulting from the presence of the IPT in patients treated initially with FOLFOX plus bevacizumab. Major morbidity was defined as any event related to the IPT necessitating surgery or resulting in patient death. Specifically, colonic bleeding, perforation, bowel obstruction, or fistula formation requiring surgery or resulting in patient death was defined as the primary end point.
Secondary objectives were to determine the rate of other adverse events related to the IPT resulting in any intervention such as endoscopic stent placement, bleeding requiring a transfusion, or any related hospitalization. Serious adverse events (SAEs) related to systemic chemotherapy were also a secondary end point, as was OS.
Patients and Eligibility Criteria
Eligible patients were required to have histologically confirmed adenocarcinoma of the colon with unresectable metastatic disease. We defined colon cancer as tumor more than 12 cm from the anal verge. Metastatic disease burden was considered unresectable for cure by physicians at the participating center. Patients were required to be asymptomatic from the IPT: no evidence of bowel obstruction or perforation and no active bleeding requiring a transfusion. Specific colonoscopic criteria for relative degree of obstruction were not mandated, and obstruction remained on clinical assessment only. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 and to have adequate bone marrow, renal, and hepatic function. The protocol was approved by institutional review boards of all participating centers. All patients provided written informed consent before study entry.
Exclusion criteria consisted of the following: patients were not permitted to have prior chemotherapy, radiation therapy, or surgery. Patients with significant cardiac disease, uncontrolled hypertension (> 150/100), or history of transient ischemic attack (TIA) or stroke within 6 months were also excluded.
Study Design
This trial was designed as a prospective phase II trial, using a Simon two-step statistical design with a 0.05 type I error.13 If the true rate of major morbidity was 25%, this trial design had 85% power to rule out a rate of major morbidity of 40% or more. We would conclude that the rate of major morbidity was excessive if 10 or more cases of major morbidity were observed in the first 26 eligible patients or if 26 or more cases of major morbidity were observed in the first 81 eligible patients.
Statistical Methods
Other than the primary hypothesis test using the Simon procedure, all remaining analyses used the full cohort of eligible patients with clinical follow-up. The cumulative incidence of major morbidity was estimated by the method of Korn and Dorey.14 Competing events for the cumulative incidence analysis were potentially curative resections of the primary tumor, death due to causes unrelated to the primary tumor, and other resections of the primary tumor (neither curative nor in response to symptoms of the primary). The Kaplan-Meier method was used to estimate survival.15 Median follow-up was estimated by reverse censoring.16 P values less than .05 were considered significant and all CIs were 95%.
Treatment
Patients initiated treatment within 3 weeks of study enrollment. Chemotherapy consisted of fluorouracil 400 mg/m2 intravenous bolus on day 1, followed by continuous infusional fluorouracil 2,400 mg/m2 administered over 46 hours starting on day 1, leucovorin 400 mg/m2 on day 1, and oxaliplatin 85 mg/m2 on day 1 (modified FOLFOX6 [mFOLFOX6]) combined with bevacizumab given at 5 mg/kg on day 1, every 14 days. Treatment continued until excessive toxicity or disease progression. At disease progression, second-line systemic chemotherapy was at the discretion of the treating oncologist. If, in response to therapy, both the primary tumor and metastatic sites were resectable for curative intent, then resection of the primary tumor was allowed. Discontinuation of bevacizumab was recommended at least 28 days before elective surgery.
RESULTS
NSABP C-10 used a Simon two-step procedure for the primary test of hypothesis. The rule for stage I was to continue if nine or fewer cases of major morbidity were observed in the first 26 eligible patients with follow-up. We observed four cases of major morbidity, and the trial continued to stage II. The rule for stage II was to conclude that the rate of major morbidity was acceptable if 25 or fewer cases of major morbidity were observed in the first 81 eligible patients with follow-up. We observed 12 cases of major morbidity in stage II; thus, we rejected the hypothesis that the rate of major morbidity was 40% or higher and concluded that the rate of major morbidity was acceptable.
Ninety patients were registered for the study from 29 different institutions (range, 1 to 10 patients per institution). Three patients were determined to be ineligible, and one patient had no clinical follow-up, leaving 86 patients for these analyses. Median follow-up was 20.7 months. Patient demographic data are presented in Table 1. Patients were balanced by sex and had a median age of 58 years.
Table 1.
Patient Characteristics: NSABP C-10
| Characteristic | No. | % |
|---|---|---|
| Registered patients | ||
| Registered | 90 | 100.0 |
| Ineligible | 3 | 3.3 |
| With follow-up | 89 | 98.9 |
| Analysis cohort* | 86 | 95.6 |
| Median follow-up, months | 20.7 | N/A |
| Eligible patients with follow-up | ||
| Age, years | ||
| ≤ 59 | 46 | 53.5 |
| ≥ 60 | 40 | 46.5 |
| Median | 58 | |
| Sex | ||
| Male | 41 | 47.7 |
| Female | 45 | 52.3 |
| Race | ||
| White | 69 | 80.2 |
| Black | 10 | 11.6 |
| Asian | 5 | 5.8 |
| Native American | 2 | 2.3 |
| ECOG performance status | ||
| 0 | 56 | 65.1 |
| 1 | 30 | 34.9 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; N/A, not applicable; NSABP, National Surgical Adjuvant Breast and Bowel Project.
Eligible and with follow-up data.
Overall, there were 12 major events related to the IPT, with 10 surgeries for symptoms related to the primary tumor and two patient deaths attributed to complications of the intact primary, with 10 (83.3%) of 12 occurring within the first 12 months after study enrollment. Among 10 patients requiring surgery, indications were colonic obstruction in eight, perforation in one, and abdominal pain in one. Four surgeries were nonurgent and were performed with the patient not being treated with bevacizumab; six were urgent. A permanent ostomy was performed in three patients, and in all three of these, there were both extensive peritoneal disease and liver metastases. There was one postoperative death within 30 days among patients undergoing surgery for a primary end point, for an operative mortality of 10%. Outcomes for the primary end point of major morbidity are described in Table 2.
Table 2.
Outcomes for the Primary End Point of Major Morbidity
| Category | No. | % |
|---|---|---|
| Component events for major morbidity | ||
| Surgery for symptoms of primary tumor | 10 | 11.6 |
| Death with symptoms of primary tumor | 2 | 2.3 |
| Total with major morbidity | 12 | 14.0 |
| Attempted curative resection, primary tumor resected | 8 | 9.3 |
| Primary tumor resected for other reasons | 3 | 3.5 |
| Patient died with intact primary tumor | 28 | 32.6 |
| Patient alive at last follow-up with intact primary | 35 | 40.7 |
| Total | 86 | 100.0 |
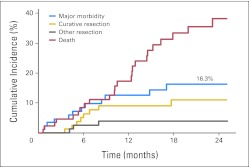
There were two patient deaths in which the IPT was likely a contributing cause. One patient presented with extensive liver metastases and pulmonary metastases and died after receiving the first cycle of chemotherapy. This patient presented to the emergency room as being unresponsive 2 weeks after treatment and was noted to have retroperitoneal air on an abdominal plain film. This may have represented a possible perforation at the IPT, and the patient was given palliative care without surgery. The second patient had both liver and lung metastases. After the second cycle of chemotherapy, a gastrograffin enema demonstrated bowel obstruction at the site of the IPT. Computed tomography (CT) imaging demonstrated significant progression of disease in the liver, and this patient was given palliative care without surgery. Taking into account competing events, such as patient death unrelated to the IPT, colon resection for attempted cure (in combination with metastectomy), and resections for reasons other than symptoms of the IPT or curative intent, the overall rate of major morbidity related to the IPT was 16.3% (95% CI, 7.6% to 25.1%) at 24 months (Fig 1).
Fig 1.
Cumulative incidence of major morbidity and competing events: National Surgical Adjuvant Breast and Bowel Project C-10 trial.
Complications at the Site of IPT Managed Without Surgery
Complications of the IPT requiring intervention short of surgery (minor morbidity) were a secondary end point. Two patients had minor morbidity events but no major morbidity: one with bowel obstruction requiring brief hospitalization and one with bowel obstruction requiring endoscopic colonic stent placement. Two additional patients had minor morbidity but also had major morbidity. Including all patients with major or minor morbidity, a total of 14 patients (16.3% of 86 evaluable) had complications related to the IPT meeting the definition of either the primary or secondary end point (local complications; Table 3).
Table 3.
Local Complications and SAEs
| Category | No. | % |
|---|---|---|
| Component events for local complications | ||
| Minor morbidity only | 2 | 2.3 |
| Minor and major morbidity | 2 | 2.3 |
| Major morbidity only | 10 | 11.6 |
| Total local complications | 14 | 16.3 |
| Patients without local complication (no morbidity) | 72 | 83.7 |
| Component events for SAEs | ||
| Grade 5, fatal | 8* | 9.3 |
| Grade 4, life threatening | 6 | 7.0 |
| Grade 3, serious | 13 | 15.1 |
| Total SAEs | 27 | 31.4 |
| Patients without SAEs | 59 | 68.6 |
| Total | 86 | 100.0 |
Abbreviation: SAE, serious adverse event.
Four were directly related to chemotherapy, four were unrelated (two died within 3 months of completing therapy from postoperative sepsis after curative resection, one from pain and anorexia, one from progressive chronic obstructive pulmonary disease).
Surgeries Performed on the Primary Tumor With Curative Intent
In total, eight surgeries were performed with intent to remove the IPT and all metastatic liver lesions. In three patients, the liver lesions were found intraoperatively to be unresectable, and resection of the primary tumor was performed. One patient had removal of the primary tumor after demonstrating a radiographic complete response in the liver. Four patients underwent resection of all liver metastases and the IPT, and for three this was performed as a combined surgery. In two of the three combined resections, the patients died of postoperative complications. One patient underwent a successful staged resection. In summary, three patients underwent successful resections of both the primary colon tumor and liver metastases without postoperative mortality.
SAEs Related to Chemotherapy
There were four deaths on this study possibly related to chemotherapy; they include chemotherapy-induced diarrhea, reversible posterior leukoencephalopathy syndrome, sudden death, and non-neutropenic sepsis. Four additional deaths met reporting requirements for SAEs (death within 3 months of study therapy) but were determined to be unrelated to protocol therapy by central review (Table 3). Six patients had grade 4 SAEs, which included infection with or without neutropenia, thromboembolic events, neuropathy, diarrhea, or dehydration. The median number of cycles of mFOLFOX6 plus bevacizumab for patients not having a morbidity event was 11.
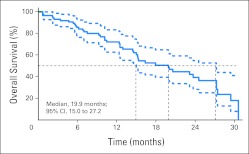
Median OS
After a median follow-up of 20.4 months at the time of analysis, the median OS was 19.9 months (95% CI, 15.0 to 27.2 months). The Kaplan-Meier estimates of survival are given in Figure 2.
Fig 2.
Kaplan-Meier estimates of overall survival with 95% CIs: National Surgical Adjuvant Breast and Bowel Project C-10 trial.
DISCUSSION
The challenges of managing unresectable metastatic colon cancer in the setting of an asymptomatic IPT have been an area of intense debate for the last decade.17,18 Clinicians and patients are faced with a decision requiring a careful balance of the following key issue: Is initiating systemic treatment for metastatic disease of greater importance than removal of the primary colon cancer? For patients presenting with symptoms related to the IPT, the decision to resect the IPT is straightforward. For the majority of patients, however, the IPT is asymptomatic, and a careful evaluation of the goal of noncurative colon resection is necessary. Arguments by medical oncologists and surgeons for initial removal of the IPT have been if the tumor obstructs, bleeds, or perforates, then urgent surgery is required while the patient is being given systemic chemotherapy.2–4 In the last decade, two more extremely important issues have surfaced that also need to be considered. The first is the increasing evidence that colon resection in this clinical setting carries a 10% 30-day mortality rate, significantly higher than colon resection in the nonmetastatic setting.11,19–21 In this study, the comparable mortality of the initial nonoperative approach is three (3.5%) of 86, which includes the two primary end point deaths as well as the single postoperative death following surgery for a primary that became symptomatic. Second, increasingly effective systemic chemotherapy has improved response rates both at the metastatic sites and at the IPT. One recent small series22 of patients with metastatic CRC and an IPT documented endoscopic tumor response of the colon tumor in essentially all patients treated with modern three-agent chemotherapy. Another series23 evaluating tumor responses of the metastatic lesions and the IPT noted a 65% response rate of the IPT. We did not measure primary tumor response in our study, so we cannot comment on how our series compares with these prior reports.
NSABP C-10 was designed to evaluate the safety of systemic chemotherapy (mFOLFOX6) combined with bevacizumab for patients with an asymptomatic IPT and unresectable distant metastases. To the best of our knowledge, this is the only prospective multicenter study to address this clinical dilemma. We identified that 86% of patients on this study did not develop symptoms from the IPT that required surgery or problems related to the IPT resulting in death. Of the 10 surgeries performed for symptoms related to the IPT, only four (4.7%) were urgent among all 86 patients, and only three patients (3.5%) required permanent ostomies. Three other colon resections were performed for enrolled patients. One patient had an ileostomy for a small bowel obstruction secondary to diffuse omental and peritoneal disease who, after a near-complete peritoneal response to treatment, had the ileostomy reversed and IPT removed electively. A second patient underwent resection of an asymptomatic IPT when CT imaging demonstrated disease progression in the liver and peritoneum. The third patient had disease progression in the liver and underwent resection of an asymptomatic IPT at the time of hepatic pump placement.
In our analysis of competing events, we accounted for primary tumors removed for both curative intent and for other reasons. At 24 months, the probability of major morbidity related to the IPT was 16.3% (95% CI, 7.6% to 25.1%). We prospectively defined a major complication rate of 25% as acceptable on the basis of prior retrospective reports advocating for an initial nonoperative approach. Those studies6,8,10 reported events related to IPT ranging from 9% to 29%, with obstruction at 10% to 20%, and bleeding and perforation at less than 5%. Therefore, 75% of patients with advanced stage IV colon cancer would avoid a major noncurative resection of the IPT. In the setting of unresectable metastatic disease, maintaining quality of life and extending OS are of greatest importance. We considered the alternative approach of initial major surgery for resection of the colon primary with attendant discomfort, recovery time delaying systemic therapy, postoperative morbidity reported in the 30% to 50% range, ostomy rates as high as 24%, and surgical mortality rates of 10% as an important opportunity to identify a better standard of care.2,4,11,20
Importantly, we identified perforation of the IPT as a relatively rare event that should dispel a widespread and persistent reluctance to use bevacizumab in the setting of an IPT. We identified only two cases of perforation. One patient with extensive metastatic disease had a suspected perforation and died without surgery. In the second patient, a chronic perforation with a contained abscess permitted nonurgent surgery after holding bevacizumab for 4 weeks before surgery. Although neither a defined primary nor a secondary objective of the study, 9.3% of patients had sufficient response of their metastatic disease to attempt curative resection, although successful curative resection of both the IPT and all metastases was rare (3.5%). Interestingly, this matches the rate of subsequent metastectomy (3%) identified by Temple et al11 in their SEER-Medicare–linked database study. The low conversion rate to resectability in our series likely reflects our entry criteria of the metastases being initially unresectable for cure as well as a considerable number of patients with multisite metastases. We believe this further supports initial systemic chemotherapy as the best option for patients with unresectable metastatic disease and an asymptomatic IPT.
Our study, like others, identified bowel obstruction as the most common clinical event related to the IPT. In total, obstruction occurred in 11 patients (eight required surgery, one contributed to patient death, one required stent placement, and one hospitalization was managed without intervention). Overall, this study identified that an initial nonoperative approach, using mFOLFOX6 combined with bevacizumab is a viable and safe option for patients faced with the dilemma of advanced, likely incurable colon cancer. The majority of patients (84%) were able to receive initial systemic therapy to better control distant disease and to avoid potential delays and complications or death related to initial surgical resection without compromising OS. This treatment approach allows patients to receive systemic therapy for widely metastatic disease earlier in their treatment. It does not entirely preclude future surgical resection, but it avoids initial surgery for the vast majority. A randomized controlled trial to further explore this controversy would be challenged by the difficulty of randomly assigning patients with unresectable metastases to noncurative major colon surgery. We believe this approach of primary systemic treatment with expectant observation of the IPT should define a new standard of care.
Supplementary Material
Acknowledgment
Supported by Public Health Service Grants No. U10CA-12027, U10CA-69974, U10CA-37377, U10CA-69651, and U24-CA-114732 from the National Cancer Institute, Department of Health and Human Services to the National Surgical Adjuvant Breast and Bowel Project.
Footnotes
See accompanying editorial on page 3165
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00321828.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Greg Yothers, Genentech (C); Norman Wolmark, Roche (U) Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Laurence E. McCahill, Greg Yothers, Saima Sharif, Nicholas J. Petrelli, Lawrence D. Wagman, Michael J. O'Connell, Norman Wolmark
Administrative support: Greg Yothers
Provision of study materials or patients: Laurence E. McCahill, Naftali Bechar, Jeffrey K. Giguere, Shaker R. Dakhil, Louis Fehrenbacher
Collection and assembly of data: Laurence E. McCahill, Greg Yothers, Saima Sharif, Lily Lau Lai, Naftali Bechar, Jeffrey K. Giguere, Shaker R. Dakhil, Louis Fehrenbacher
Data analysis and interpretation: Laurence E. McCahill, Greg Yothers, Saima Sharif, Louis Fehrenbacher, Samia H. Lopa, Lawrence D. Wagman, Michael J. O'Connell
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.American Cancer Society: Cancer facts & figures. 2010. http://www.cancer.org/Research/CancerFacts Figures/CancerFactsFigures/most-requested-tables-figures-2010.
- 2.Isbister WH. Audit of definitive colorectal surgery in patients with early and advanced colorectal cancer. ANZ J Surg. 2002;72:271–274. [PubMed] [Google Scholar]
- 3.Costi R, Mazzeo A, Di Mauro D, et al. Palliative resection of colorectal cancer: Does it prolong survival? Ann Surg Oncol. 2007;14:2567–2576. doi: 10.1245/s10434-007-9444-2. [DOI] [PubMed] [Google Scholar]
- 4.Rosen SA, Buell JF, Yoshida A, et al. Initial presentation with stage IV colorectal cancer: How aggressive should we be? Arch Surg. 2000;135:530–534. doi: 10.1001/archsurg.135.5.530. discussion 534-535. [DOI] [PubMed] [Google Scholar]
- 5.Galizia G, Lieto E, Orditura M, et al. First-line chemotherapy vs bowel tumor resection plus chemotherapy for patients with unresectable synchronous colorectal hepatic metastases. Arch Surg. 2008;143:352–358. doi: 10.1001/archsurg.143.4.352. [DOI] [PubMed] [Google Scholar]
- 6.Ruo L, Gougoutas C, Paty PB, et al. Elective bowel resection for incurable stage IV colorectal cancer: Prognostic variables for asymptomatic patients. J Am Coll Surg. 2003;196:722–728. doi: 10.1016/S1072-7515(03)00136-4. [DOI] [PubMed] [Google Scholar]
- 7.Poultsides GA, Servais EL, Saltz LB, et al. Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J Clin Oncol. 2009;27:3379–3384. doi: 10.1200/JCO.2008.20.9817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tebbutt NC, Norman AR, Cunningham D, et al. Intestinal complications after chemotherapy for patients with unresected primary colorectal cancer and synchronous metastases. Gut. 2003;52:568–573. doi: 10.1136/gut.52.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scoggins CR, Meszoely IM, Blanke CD, et al. Nonoperative management of primary colorectal cancer in patients with stage IV disease. Ann Surg Oncol. 1999;6:651–657. doi: 10.1007/s10434-999-0651-x. [DOI] [PubMed] [Google Scholar]
- 10.Sarela AI, Guthrie JA, Seymour MT, et al. Non-operative management of the primary tumour in patients with incurable stage IV colorectal cancer. Br J Surg. 2001;88:1352–1356. doi: 10.1046/j.0007-1323.2001.01915.x. [DOI] [PubMed] [Google Scholar]
- 11.Temple LK, Hsieh L, Wong WD, et al. Use of surgery among elderly patients with stage IV colorectal cancer. J Clin Oncol. 2004;22:3475–3484. doi: 10.1200/JCO.2004.10.218. [DOI] [PubMed] [Google Scholar]
- 12.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 13.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 14.Korn EL, Dorey FJ. Applications of crude incidence curves. Stat Med. 1992;11:813–829. doi: 10.1002/sim.4780110611. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 16.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 17.Cohen AM. What is the best treatment for stage IV colorectal cancer? Ann Surg Oncol. 2005;12:581–582. doi: 10.1245/ASO.2005.03.901. [DOI] [PubMed] [Google Scholar]
- 18.Petrelli NJ. Systemic chemotherapy should be the primary treatment of synchronous colorectal metastases in the asymptomatic patient [corrected] Ann Surg Oncol. 2006;13:137–139. doi: 10.1245/ASO.2006.05.059. [Erratum: Ann Surg Oncol 13:753, 2006] [DOI] [PubMed] [Google Scholar]
- 19.Cook AD, Single R, McCahill LE. Surgical resection of primary tumors in patients who present with stage IV colorectal cancer: An analysis of Surveillance, Epidemiology, and End Results data, 1988 to 2000. Ann Surg Oncol. 2005;12:637–645. doi: 10.1245/ASO.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Stillwell AP, Buettner PG, Siu SK, et al. Predictors of postoperative mortality, morbidity, and long-term survival after palliative resection in patients with colorectal cancer. Dis Colon Rectum. 2011;54:535–544. doi: 10.1007/DCR.0b013e3182083d9d. [DOI] [PubMed] [Google Scholar]
- 21.Longo WE, Virgo KS, Johnson FE, et al. Risk factors for morbidity and mortality after colectomy for colon cancer. Dis Colon Rectum. 2000;43:83–91. doi: 10.1007/BF02237249. [DOI] [PubMed] [Google Scholar]
- 22.Cameron S, Hünerbein D, Mansuroglu T, et al. Response of the primary tumor in symptomatic and asymptomatic stage IV colorectal cancer tocombined interventional endoscopy and palliative chemotherapy. BMC Cancer. 2009;9:218. doi: 10.1186/1471-2407-9-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gervaz P, Rubbia-Brandt L, Andres A, et al. Neoadjuvant chemotherapy in patients with stage IV colorectal cancer: A comparison of histological response in liver metastases, primary tumors, and regional lymph nodes. Ann Surg Oncol. 2010;17:2714–2719. doi: 10.1245/s10434-010-1056-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.