Abstract
The hepatocyte growth factor (HGF) and its receptor, the transmembrane tyrosine kinase cMET, promote cell proliferation, survival, motility, and invasion as well as morphogenic changes that stimulate tissue repair and regeneration in normal cells but can be co-opted during tumor growth. MET overexpression, with or without gene amplification, has been reported in a variety of human cancers, including breast, lung, and GI malignancies. Furthermore, high levels of HGF and/or cMET correlate with poor prognosis in several tumor types, including breast, ovarian, cervical, gastric, head and neck, and non–small-cell lung cancers. Gene amplification and protein overexpression of cMET drive resistance to epidermal growth factor receptor family inhibitors, both in preclinical models and in patients. It is increasingly apparent that the HGF-cMET axis signaling network is complex, and rational combinatorial therapy is needed for optimal clinical efficacy. Better understanding of HGF-cMET axis signaling and the mechanism of action of HGF-cMET inhibitors, along with the identification of biomarkers of response and resistance, will lead to more effective targeting of this pathway for cancer therapy.
INTRODUCTION
The cMET oncogene was isolated from a human osteosarcoma–derived cell line driven by a DNA rearrangement TPR-MET, where the translocated promoter region (TPR) locus on chromosome 1 fuses to the MET sequence on chromosome 71 and encodes for a prototype of the cMET receptor tyrosine kinase (RTK) subfamily. Shortly afterward, the ligand hepatocyte growth factor (HGF) or scatter factor was identified and shown to be a platelet-derived mitogen for hepatocytes and fibroblast-derived factor capable of inducing epithelial cell scattering.2
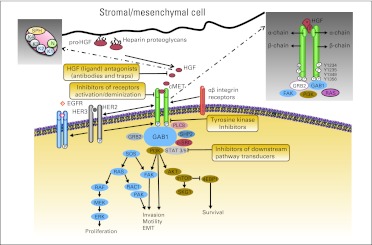
The cMET RTK subfamily is structurally distinct from most RTK subfamilies. The established form of the cMET receptor is a disulfide-linked heterodimer composed of an extracellular α-chain and transmembrane β-chain (Fig 1), resulting from the proteolytic cleavage of a precursor protein. The β-chain has an extracellular domain, transmembrane domain, and cytoplasmic portion. The cytoplasmic portion contains juxtamembrane and TK domains and a carboxy-terminal tail essential for substrate docking and downstream signaling.3 Like the cMET receptor, HGF is synthesized as an inactive precursor and is later converted into a two-chain, active heterodimer through proteolysis. The active form of HGF comprises an amino-terminal domain (N), four Kringle domains (K1 to K4), and a serine protease homology domain (SPH),4 where the N-K1 portion mediates receptor binding by engaging two cMET molecules, leading to receptor dimerization.5 Residues within the SPH domain may provide additional contacts with cMET.4 The binding of active HGF to functionally established cMET leads to receptor dimerization/multimerization, multiple tyrosine residue phosphorylation in the intracellular region, catalytic activation, and downstream signaling through docking of substrates, transducing multiple biologic activities such as motility, proliferation, survival, and morphogenesis (Fig 1).6,7
Fig 1.
The hepatocyte growth factor (HGF)–cMET axis signaling network and ongoing targeted therapy strategies. The pathway, which transduces invasive growth signals from mesenchymal to epithelial cells (secreted by mesenchymal cells), is activated by HGFA and binds to the cMET receptor on epithelial cells. cMET kinase activation results in trans-autophosphorylation and binding of adaptor proteins, forming scaffolds for recruitment and activation of signaling proteins. Signals generated from these structures lead to activation of signaling pathways related to increased proliferation, survival, motility, invasiveness, and stimulation of angiogenesis. EGFR, epidermal growth factor receptor; FAK, focal adhesion kinase; GRB2, growth factor receptor–bound protein 2; HER, human epidermal growth factor receptor; mTOR, mammalian target of rapamycin; PI3K, phosphatidylinositol 3-kinase; RAS, renin–angiotensin system; STAT, signal transducer and activator of transcription.
HGF binding induces cMET autophosphorylation on the tyrosine residues Y1234 and Y1235 at the TK domain, which regulates kinase activity. Phosphorylation on the Y1349 and Y1356 tyrosine residues near the COOH terminus forms a multifunctional docking site that recruits intracellular adapters through Src homology-2 domains and other motifs and activates downstream signaling.6,8 The main substrates and adapter proteins in this axis are signal transducer and activator of transcription 3 (STAT3), growth factor receptor–bound protein 2 (Grb2), Gab1, phosphatidylinositol 3-kinase (PI3K), phospholipase C-γ, Shc, Src, Shp2, and Ship1. Gab1 and Grb2 are critical effectors that interact directly with the receptor. They recruit a network of adaptor proteins that are involved in signaling and multiple biologic effects induced by the activated axis. Integrity of the entire signal transduction machinery is necessary for cMET to achieve its maximal activity in promoting invasive cell growth (Fig 1).6,8 One effect of HGF-mediated activation of cMET is the activation of downstream effectors involved in epithelial-mesenchymal transition through the renin–angiotensin system (RAS)/mitogen-activated protein kinase (MAPK) signaling pathway or through recruitment of the focal adhesion kinase (FAK)/paxillin complex.9,10
The HGF-cMET pathway is modulated by other proteins, including α6β4-integrin, which works as a signaling platform that potentiates HGF-triggered activation of RAS and PI3K11; plexin B1, which transactivates cMET in response to semaphorin stimulation12; and the death receptor Fas, which can associate with cMET, preventing Fas-ligand binding and inhibiting Fas-induced apoptosis.13 In addition, the activation of other RTKs may potentiate HGF-cMET effects. The epidermal growth factor receptor (EGFR) plays an important role in enhancing HGF-cMET–mediated proliferation and invasion of epithelial cells,14 and cMET can synergize with human epidermal growth factor receptor 2 to promote a malignant phenotype.15 cMET works together with the insulin-like growth factor 1 receptor to induce migration and invasion of pancreatic cancer cells.16 Other regulators include activated RAS protein, which induces cMET expression through a positive feedback loop,17 and hypoxia, which may regulate cMET activity through tumor angiogenesis.18 In summary, a complex system of interactions modulates and governs the magnitude and duration of cMET signaling in the cell.
HGF-CMET AXIS AND CANCER
Under normal conditions, HGF-induced cMET-TK activation is tightly regulated by ligand activation at the cell surface, ligand-activated receptor internalization/degradation, and paracrine ligand delivery. Despite these controls, pathway deregulation occurs in multiple neoplasms. HGF upregulates various genes, including cMET and those encoding proteases required for HGF and cMET metabolism, creating the potential for protein overexpression through persistent ligand stimulation.6 Other mechanisms of oncogenic pathway activation include aberrant paracrine or autocrine ligand production, constitutive kinase activation in the presence or absence of cMET gene amplification, and cMET gene mutations.19,20
Extensive work in preclinical models has been done to characterize the effects of sustained cMET activation. In vivo studies have shown that activation of HGF-cMET signaling promotes cell invasiveness and triggers metastases through direct involvement of angiogenic pathways.21 The oncogenic TPR-MET fusion protein is constitutively active, and in animal models, its transgenic expression leads to the development of malignancies.1 This rearrangement has been detected in human gastric cancer, in both precursor lesions and the adjacent normal mucosa, indicating predisposition to develop gastric cancer.22 A variety of cancer cell lines that exhibit cMET gene amplification are dependent on cMET for growth and survival, and cMET inhibition results in both decreased proliferation and cell death. This cMET-addicted phenotype has been described in cultured cells from non–small-cell lung carcinomas (NSCLCs) and in gastric carcinomas.19,23
The most frequent cause of constitutive cMET activation in human cancers is protein overexpression resulting from transcriptional upregulation in the absence of gene aberrations. High levels of cMET expression have been found in a variety of epithelial tumors.24 Multiple studies have been conducted to examine expression/overexpression of cMET in primary cancers. cMET has been shown to be overexpressed in neoplastic tissue compared with normal surrounding tissue, and the extent of expression has correlated with disease extension and outcome in several tumor types.25–27 Studies in NSCLC have shown strong cMET expression in up to 60% of cases,28 and phospho-cMET (p-cMET) in 40% to 100% of cases, depending on the specific lung cancer tissue assessed.25,28–30 Rates of over 80% of cMET overexpression have been reported in malignant renal cell carcinoma and pleural mesothelioma.31 cMET overexpression has been reported in breast27 and ovarian cancers32 and seems to be associated with advanced disease stage and poor outcome in NSCLC as well as colon, squamous cell carcinoma of the head and neck (SCCHN), breast, and ovarian cancers.27,30,33,34
cMET gene amplification causes protein overexpression and constitutive activation of the kinase domain19 and has been observed both in primary tumors or as secondary events affecting therapy sensitivity in cancer cells.23,35 cMET amplification has been reported in different human cancers including gastroesophageal carcinomas,36 colorectal cancers,37 NSCLC,38 NSCLC with acquired resistance to EGFR inhibitors,38 medulloblastomas,39 and glioblastomas.40 Additionally, several studies have shown that increased cMET copy number is an independent negative prognostic factor in surgically resected NSCLC38 or is associated with advanced stage and liver metastases in colorectal cancer.33
An additional mechanism, although rare, that causes cMET activation is the presence of activating mutations. Missense germ-line mutations in the TK domain have been described in patients with hereditary papillary renal carcinoma.41 Sporadic mutations are more prevalent and can involve the TK, juxtamembrane, or sema domain. Sporadic mutations have been detected in papillary renal carcinoma (RCC),41 gastric carcinoma,42 SCCHN,43 small-cell lung carcinoma (SCLC),44 NSCLC,28 mesothelioma,31 melanoma,45 and childhood hepatocellular carcinoma (HCC).46 However, only some of these mutant alleles have been proven to cause malignant transformation as a result of constitutive receptor activation posing the potential for therapeutic target.28 Oncogenic mutations have been found to be predominantly located in the nonkinase domain, mainly in regions encoding the extracellular semaphorin (E168D, L229F, S323G, and N375S) and intracellular juxtamembrane domains (R988C, T1010I, S1058P, and exon 14 deletions) of NSCLC cell lines, in 12.5% of patient cases of SCLC as well as in 8% of samples of lung human adenocarcinomas.28,44,47 The juxtamembrane domain regulates ligand-dependent cMET internalization by Y1003 phosphorylation in response to HGF binding, leading to cMET ubiquitination and degradation1; when an exon 14 deletion occurs, the loss of Y1003 results in cMET accumulation at the cell surface and persistent HGF stimulation, leading to tumorigenesis.1 Overall, cMET mutations occur at a lower frequency than other mechanisms of pathway activation; however, they provide strong evidence of the oncogenic potential of the axis and may identify patients that can either benefit from cMET-directed therapies or those in whom some of these therapies may not work.
A strong response to therapeutic inhibition with cMET small-molecule inhibitors has been demonstrated in cell line models harboring cMET oncogenic mutations when these cause increased cMET phosphorylation and downstream signaling.28,48 The presence of cMET mutations in lymph nodes and metastatic sites could suggest the selection of these mutated cells during metastatic progression.49 Little is known about the presence of cMET activation mutations and prognosis. Studies in SCCHN show that cMET mutations could be associated with resistance to radiotherapy and decrease progression-free survival (PFS).50
Although cMET receptor overexpression may lead to ligand-independent kinase activation, cMET activation in cancer occurs mostly via ligand-dependent mechanism. HGF itself is able to activate cMET transcription.51 HGF is particularly active in the reactive stroma of tumors and is expressed throughout the body,52 suggesting that it allows paracrine-positive feedback loops supporting the dissemination of cancer cells. cMET-activating mutations require HGF to boost their catalytic efficiency,53 and HGF can also aberrantly activate cMET in an autocrine manner in human cancers, including breast cancer,54 glioblastomas,55 and sarcomas.56
INCORPORATION OF ANTI–HGF-cMET–TARGETED THERAPY INTO CLINICAL PRACTICE
The prevalence of HGF-cMET pathway activation in human cancer has affected drug development. Currently, multiple agents are under study, and some are in phase III trials. These targeted therapies can be biologic antagonists, low molecular weight synthetic compounds, or small molecule inhibitors57,58 directed to target either ligand binding or receptor activation (Fig 1). Table 1 shows the HGF-cMET axis inhibitors in active clinical trials. Biologic antagonists are protein-based agents that can act through different mechanisms and have target selectivity and predictable pharmacokinetics. However, their molecular size restricts their action to extracellular events, and their complexity can affect drug manufacturing, administration routes, and shelf life.57 Synthetic small-molecule TK inhibitors (TKIs) outnumber other class compounds. Small-molecule downstream pathway inhibitors directed to STAT3 are just entering clinical trials.
Table 1.
HGF-cMET Axis Inhibitors in Active Clinical Trials
| Agent | Target | Type | Company | Development Phase |
|---|---|---|---|---|
| Ligand antagonists | ||||
| Ficlatuzumab (AV-299) | HGF | Monoclonal antibody | AVEO | I and II |
| Rilotumumab (AMG-102) | HGF | Monoclonal antibody | Amgen | II |
| TAK-701 | HGF | Monoclonal antibody | Millennium Pharmaceuticals | I |
| Receptor inhibitors | ||||
| Onartuzumab (OA5D5) | Human cMET | Monoclonal antibody | Genentech | II and III |
| LY-2875358 | cMET | Monoclonal antibody | Eli Lilly | II |
| Receptor TKIs | ||||
| Tivantinib (ARQ-197) | cMET | Non–ATP-competitive TKI | Daiichi Sankyo | II and III |
| INC-280 | cMET | ATP-competitive TKI | Novartis | I |
| Cabozantinib (XL-184) | cMET, RET, VEGFR1-3, KIT, FLT3, TIE2 | ATP-competitive TKI | Exelixis | II |
| Foretinib (XL-880) | cMET, RON, VEGFR1-3, PDGFR, KIT, FLT3, TIE2 | ATP-competitive TKI | Exelixis | II |
| EMD-1214063 | cMET | ATP-competitive TKI | EMD Serono | I |
| MGCD-265 | cMET, RON, VEGFR1-2, PDGFR, KIT, FLT3, TIE2 | ATP-competitive TKI | MethylGene | I to II |
| AMG 208 | cMET, VEGFR1-3, RON, TIE2 | ATP-competitive TKI | Amgen | I |
| AMG-337 | cMET | ATP-competitive TKI | Amgen | I |
| E-7050 | cMET | ATP-competitive TKI | Eisai | I and II |
| LY-2801653 | cMET, VEGFR2 | ATP-competitive TKI | Eli Lilly | I |
| Crizotinib (PF-02341066) | cMET | ATP-competitive TKI | Pfizer | II and III |
| PF-04217903 | cMET, ALK | ATP-competitive TKI | Pfizer | I |
| Downstream pathway inhibitors | ||||
| OPB-31121 | STAT3 | IL6-induced STAT3 phosphorylation inhibitors | Otsuka | I |
| OPB-51602 | STAT3 | IL6-induced STAT3 phosphorylation inhibitors | Otsuka | I |
Abbreviations: ALK, anaplastic lymphoma kinase; ATP, adenosine triphosphate; HGF, hepatocyte growth factor; IL6, interleukin-6; PDGFR, platelet-derived growth factor receptor; STAT, signal transducer and activator of transcription; TKI, tyrosine kinase inhibitor; VEGFR, vascular endothelial growth factor receptor.
HGF and cMET Biologic Antagonists
These molecules prevent interaction between the ligand and receptor or related cell-surface events such as receptor clustering, but they are unable to activate downstream signaling. HGF has two cMET binding sites: a high- affinity site that recognizes cMET independently of HGF status (pro HGF or HGF), and a low-affinity site accessible only to HGF and essential for cMET dimerization and activation.58 Some of these agents are in various stages of development and have completed clinical trials as single agents or in combination with other targeted therapies (Table 2).
Table 2.
Efficacy of Single-Agent and Combination Therapies With HGF-cMET Axis Inhibitors in Selected Phases Ib and II Clinical Trials
| Author | Study Treatment | Phase | Disease | No. of Patients | End Point |
|---|---|---|---|---|---|
| Single-agent therapy | |||||
| Schoffski et al59 | Rilotumumab | II | RCC | 61 | ORR: 2% |
| Wen et al60 | Rilotumumab | II | Glioblastoma multiforme | 60 | ORR: 0% |
| Kurzrock et al61 | Cabozantinib | Ib | Medullary thyroid cancer | 35 | ORR: 29% |
| Choueiri et al62 | Cabozantinib | II | RCC | 25 | ORR: 24% |
| Seiwert et al43 | Foretinib | II | SCCHN | 14 | ORR: 0% |
| Santoro et al63 | Tivantinib | Ib | Hepatocellular carcinoma | 10 | ORR: 0% |
| Combination therapy | |||||
| Mok et al64 | Gefitinib | II | NSCLC | 170 | Ongoing |
| Gefitinib/ficlatuzumab | |||||
| Malka et al64 | FOLFOX | II | Gastroesphageal adenocarcinoma | 165 | Ongoing |
| FOLFOX/panitumumab | |||||
| FOLFOX/rilotumumab | |||||
| Eng et al65 | Panitumumab | Ib/II | Colorectal cancer (KRAS wild type) | 48 | RR: 21% |
| Panitumuab/rilotumumab | 48 | RR: 31% | |||
| Panitumumab/ganitumab | 46 | RR: 22% | |||
| Ryan et al66 | Mitoxatrone/prednisone | II | Castrate-resistant prostate cancer | 45 | PFS: 13.4 months |
| Mitoxatrone/prednisone/rilotumumab | 48 | PFS: 11.6 months | |||
| Mitoxatrone/prednisone/rilotumumab | 49 | PFS: 12.2 months | |||
| Spigel et al67 | Erlotinib | II | NSCLC | 68 | ITT/PFS: 2.6 months |
| Erlotinib/onartuzumab | 31 | cMET+/PFS: 1.5 months | |||
| 69 | ITT/PFS: 2.2 months | ||||
| 35 | cMET+/PFS: 3 months | ||||
| Sequist et al68 | Erlotinib | II | NSCLC | 83 | PFS: 2.3 months |
| Erlotinib/tivantinib | 84 | PFS: 3.8 months | |||
| Wakelee et al69 | Erlotinib/cabozantinib | Ib/II | NSCLC | 54 | RR: 8% |
Abbreviations: FOLFOX, infusional fluorouracil, leucovorin, and oxaliplatin; HGF, hepatocyte growth factor; ITT, intention to treat; NSCLC, non–small-cell lung cancer; ORR, objective response rate; PFS, progression-free survival; RCC, renal cell carcinoma; RR, response rate; SCCHN, squamous cell carcinoma of the head and neck.
HGF-competitive analogs.
These compete with the ligand for receptor binding but do not induce cMET signaling, because they cannot cause cMET dimerization. NK2 is a truncated protein product of a naturally occurring alternative HGF mRNA transcript that competitively antagonizes growth stimulated by full-length HGF.70 However, its potential antioncogenic efficacy is compromised by its intrinsic mitogenic activity, which has enhanced HGF-driven metastasis in murine models.71 NK4 is a longer truncated isoform of full-length HGF proven to be a complete competitive antagonist of HGF-cMET signaling in preclinical models; it has been tested as administration of the purified protein or as gene therapy.72,73 Some of these compounds have entered human clinical trials,57 but there are no final reports available of further drug development, activity, or safety. Uncleavable HGF is a form of HGF locked in its inactive conformation that competes with active HGF for binding to cMET and with pro-HGF convertases for HGF activation, blocking cMET catalytic activation and HGF proteolytic development.74 No human studies have been reported.
cMET competitive variants.
These can competitively displace HGF and impair dimerization of the endogenous receptor, but they are not yet in the clinic. Decoy cMET is a recombinant, enzymatically inactive molecule that matches the whole cMET extracellular domain, interacting with both HGF and full-length cMET, sequestering the ligand, and impairing dimerization of the native receptor.75 Another compound in this class is an isolated sema domain that retains the ability to competitively inhibit ligand binding and receptor dimerization, impairing cMET-dependent transduction pathways and reducing HGF-triggered cell migration, tumor growth, and metastasis in mice.75,76
Antibodies against HGF.
Several monoclonal antibodies against HGF have been developed and have shown activity in preclinical models.77 Three compounds are being explored in clinical trials. AMG-102 (rilotumumab) binds to the HGF light chain, blocking HGF-cMET binding.78 It completed phase I in solid tumors with a maximum-tolerated dose of 20 mg/kg every 2 weeks and a mean half-life of 15.4 hours. Adverse events of fatigue, constipation, anorexia, and nausea/vomiting were low grade.79 Trials have evaluated the activity of rilotumumab as a single agent and in combination with chemotherapy, antiangiogenic therapy, and anti-EGFR inhibitors in various tumor types.64 No significant antitumor activity was reported from two single-agent phase II trials in patients with RCC and recurrent glioblastomas.59,60 However, in a randomized phase Ib/II trial in patients with KRAS wild-type colorectal cancer, the combination of panitumumab plus rilotumumab was superior in terms of response rate to panitumumab alone (31% v 21%).65 Rilotumumab is being combined with chemotherapy in advanced gastric cancer after promising data in patients with cMET-positive disease. AV-299 (ficlatuzumab) has completed phase I trials. This antibody was well tolerated in patients at doses up to 20 mg/kg every 2 weeks and had a similar 15-hour half-life.80 A phase Ib study evaluating gefitinib plus ficlatuzumab in patients with NSCLC demonstrated safety, with five responses seen in 15 patients.81 A randomized phase II trial in NSCLC comparing gefitinib with gefitinib plus ficlatuzumab is ongoing.64 TAK-701 is being explored in advanced nonhematologic malignancies in the phase I setting.82
Antibodies against cMET.
These monoclonal antibodies bind the cMET extracellular domain; however, one issue in their development has been the agonist activity of the dual-arm compounds. OA5D5 (onartuzumab [MetMAb; Genentech, San Francisco, CA]) is an engineered monovalent Fab fragment antibody with murine-variable domains that is extremely well tolerated.23 A phase II trial comparing single-agent erlotinib with erlotinib plus onartuzumab at 15 mg/kg once every 3 weeks in patients with refractory NSCLC demonstrated a significant improvement in PFS and overall survival (OS) in those patients whose tumors overexpressed cMET by immunohistochemistry (IHC).67 These promising results led to the development of a phase III trial.64 Additionally, onartuzumab has been successfully combined with bevacizumab in a phase Ib trial, with both drugs administered at full doses. In this study, a patient with gastric cancer had prolonged disease control.84 LY-2875358, a humanized immunoglobulin G4 antibody that binds to cMET and prevents HGF binding, is undergoing phase I testing.64 DN30 induces a proteolytic cleavage of the cMET extracellular domain, decreasing the number of receptor molecules on the cell surface and inhibiting HGF binding and cMET dimerization. It has been shown to reduce anchorage-independent growth and xenograft development in cMET-amplified gastric carcinoma cells and melanoma metastatic models.85 h224G11A is a humanized, bivalent monoclonal antibody that inhibits cMET phosphorylation and dimerization and blocks proliferation, migration, invasion, morphogenesis, and angiogenesis in in vitro studies.57
Synthetic Small-Molecule TKIs
Synthetic small-molecule TKIs are low molecular weight molecules. Most of them compete for the adenosine triphosphate (ATP) binding site in the TK domain of cMET, preventing receptor transactivation and recruitment of downstream effectors. In contrast, others can bind to a region of cMET outside of the ATP binding site, impairing kinase activation allosterically. There are several ongoing developmental paths for TKIs. Some of them are being developed as cMET receptor specific; others are more promiscuous and target other cytokine-directed pathways, including the vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), RON, TIE2, and EML4–anaplastic lymphoma kinase (ALK). Preclinical studies have shown that cMET TKIs potentially and selectively suppress growth, migration, and/or survival in a variety of models. These agents are in various stages of development. Table 2 highlights selected clinical trials of these as single agents or in combination with other targeted therapies.
Unselective cMET TKIs.
Crizotinib (PF-02341066) is an orally available 2-amino-3-benzyloxy-5-arylpyridine compound developed to target cMET; it has also been found to target ALK. This compound has shown antitumor activity and antiangiogenic activity in several models with constitutively activated forms of cMET or ALK.86 In clinic, it has shown efficacy at well-tolerated doses. It is currently in phase I/II/III clinical trials and approved for EML4-ALK–positive NSCLC. Foretinib (XL-880), also orally available, inhibits several kinases, including cMET, VEGFR2, PDGFR, RON, KIT, and TIE2.87 Phase II trials are ongoing in patients with poorly differentiated diffuse gastric cancer and papillary renal cell carcinoma. A phase II trial in refractory SCCHN failed to meet a prespecified end point for activity.43 Cabozantinib (XL-184) is an orally administrated TKI targeting cMET, RET, VEGFR1, VEGFR2, VEGFR3, KIT, FLT-3, and TIE-2 that exhibits significant oral bioavailability and blood-brain barrier penetration as well as significant activity in blastic oseous metastasis.88 It has also demonstrated activity in RCC in a phase II trial, with response rates of 24%.62 It is being developed for of medullary thyroid cancer, glioblastoma multiforme, prostate cancer, breast cancer, and NSCLC. A phase III trial investigating XL-184 as first-line treatment, compared with placebo, in patients with medullary thyroid cancer has completed accrual. MGCD-265 is an oral compound that targets cMET, VEGFR1, VEGFR2, VEGFR3, RON, and TIE2 receptor TK.89 It is currently in phase I single-agent clinical trials for solid tumors and in phase I/II trials for NSCLC in combination with docetaxel and erlotinib. E-7050 targets both cMET and VEGFR2; it has completed phase I testing and is being explored in combination with other targeted therapies.64
Selective cMET TKIs.
Tivantinib (ARQ-197) is a non–ATP-competitive drug. It was well tolerated in a single-agent phase Ib trial in cirrhotic patients with HCC.63 A phase II trial comparing single-agent erlotinib with erlotinib plus tivantinib in patients with refractory NSCLC failed to meet its primary end point (PFS) in the intent-to-treat population, although the combination demonstrated a trend toward improved survival outcomes in a planned subset analysis in nonsquamous NSCLC.68 A confirmatory phase III clinical trial in patients with nonsquamous NSCLC and other phase II trials in a variety of solid tumors are accruing patients.64 JNJ-38877605 has greater than 1,000-fold selectivity for the cMET kinase; however, a phase I study was terminated because of renal toxicities. PF-04217903 has completed a phase I trial with pending results. A number of other highly selective cMET TKIs, including EMD-1214063, LY-2801653, AMG-337, AMG 208, and INC-280, are undergoing evaluation in phase I studies.64
Downstream Pathway Inhibitors
OPB-31121 and OPB-51602 inhibit the interleukin-6 (IL6)– induced phosphorylation of STAT3. OPB-31121 was well tolerated in a phase I trial in patients with solid tumors, and a stable disease rate of 47% was reported.90
PATIENT SELECTION FOR TREATMENT WITH HGF-CMET AXIS INHIBITORS
One of the most important challenges in effectively using targeted therapeutics is identifying those tumors that are sensitive as well as the patients likely to benefit from them. Preclinical studies have been performed using some of these compounds in in vitro and in vivo models harboring aberrations in components of the HGF-cMET axis. Early clinical trials completed preplanned or retrospective tumor tissue and serum analyses to explore pharmacodynamic markers of target inhibition and outcomes. New studies are being designed to preselect patients for trial participation based on tumor biomarkers, including cMET protein overexpression by IHC, cMET amplification by copy number arrays or fluorescent in situ hybridization, trisomy of chromosome 7, and cMET somatic mutations.57,64 Figures 2 and 3, along with Appendix Figure A1 (online only), illustrate examples of molecular aberrations in the cMET receptor evaluated to select patients for anti–HGF-cMET axis–targeted therapies.
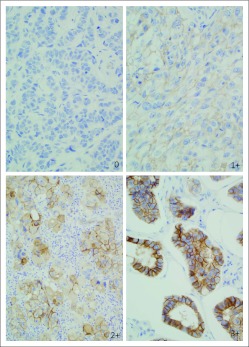
Fig 2.
Protein overexpression by immunohistochemistry, a molecular aberration in the cMET receptor evaluated to select patients for anti–hepatocyte growth factor–cMET axis–targeted therapies.
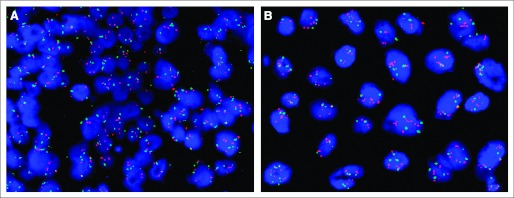
Fig 3.
Gene amplification by fluorescent in situ hybridization, a molecular aberration in the cMET receptor evaluated to select patients for anti–hepatocyte growth factor–cMET axis–targeted therapies. (A) Nonamplified; (B) amplified.
Pharmacodynamic Markers of Outcomes
Preclinical studies of anti-cMET agents have included evaluating activity against known cMET aberrations. Completed (Table 3) and ongoing trials have compared efficacy of these agents between patients with tumors that harbor these aberrations versus those with histologically similar tumors that do not. In a phase II randomized study in patients with KRAS wild-type advanced colorectal cancer, tumors that overexpressed cMET were more likely to respond to the combination of rilotumumab and panitumumab.65 However, in a study of rilotumumab for advanced RCC, neither baseline plasma HGF, soluble c-MET, nor cMET tumor expression correlated with outcome.59 cMET overexpression was a predictor of PFS and OS when erlotinib was combined with onartuzumab in advanced NSCLC.67 In the same study, baseline HGF levels and more than five copies of cMET were associated with OS.91 Similar studies have been completed with TKIs. The combination of tivatinib and erlotinib in advanced NSCLC was more effective in patients with tumors that either had a nonsquamous cell carcinoma histology, harbored KRAS mutations, were EGFR wild type, or had increased cMET copy number.68 In a phase II trial of foretinib in advanced gastric cancer, tumors with cMET amplification were more likely to respond to therapy.92 These findings are being used as the basis for patient selection in follow-up studies with these and other compounds.
Table 3.
Potential Predictors and Pharmacodynamic Markers of Response to HGF-cMET Axis Inhibitors
| Author | Marker | Disease | Treatment | End Point |
|---|---|---|---|---|
| Eng et al65 | cMET overexpression | Colorectal cancer (KRASwild type) | Panitumuab/rilotumumab | RR |
| Spigel et al,67 Yu et al91 | cMET overexpression | NSCLC | Erlotinib/onartuzumab | PFS and OS |
| cMET amplification | OS (trend) | |||
| Low HGF levels | OS | |||
| Sequist et al68 | Nonsquamous histology | NSCLC | Erlotinib/tivantinib | PFS and OS |
| KRAS mutations | ||||
| EGFR wild type | ||||
| cMET amplification | ||||
| Jhawer et al92 | cMET amplification | Gastric cancer | Foretinib | RR |
Abbreviations: HGF, hepatocyte growth factor; NSCLC, non–small-cell lung cancer; OS, overall survival; PFS, progression-free survival; RR, response rate.
Pharmacodynamic Markers of Target Inhibition
Pharmacokinetic-pharmacodynamic modeling is increasingly being applied in drug discovery and drug development with the aim of optimizing the design of early clinical trials and streamlining drug development. It is used to select drug candidates with favorable properties and to assist with prediction of exposure and clinical benefit. Comprehensive pharmacokinetic and pharmacodynamic studies were completed for crizotinib in animal models to characterize the relationship of drug plasma concentrations with p-cMET in tumor and the relationship of p-cMET with antitumor efficacy. Near-complete inhibition of cMET phosphorylation (> 90%) significantly inhibited tumor growth (> 50%).93 To identify a preclinical algorithm of soluble surrogate biomarkers indicative of response to cMET inhibition, investigators surveyed candidate molecules based on antibody proteomics and gene expression profiling. After enzyme-linked immunosorbent assay validation and analytic quantification, they identified four biomarkers that were strongly and consistently modulated by cMET inhibition in a panel of cMET-addicted gastric cancer cell lines but not in cMET-independent lines. Pharmacologic cMET inhibition was correlated with reduced secretion of IL8, growth regulated oncogene–α, and uPAR and with increased production of IL6 both in vitro (supernatants) and in vivo (plasma).94 Clinical trials have shown similar results of biomarker modulation after exposure to anti-cMET therapies. Treatment with tivantinib in patients with advanced solid tumors showed decreased tumor levels of total cMET, p-cMET, and FAK as well as increased apoptosis by terminal deoxynucleotidil transferase dUTP nick-end labeling assay.95 In a similar study of foretinib, post-treatment tissues showed decreased levels of p-cMET, p-RON, p-ERK, and p-AKT as well as an increase in apoptosis markers.96 When looking at soluble pharmacodynamic markers, the use of cabozantinib in patients with medullary thyroid cancer was associated with a significant decrease in serum calcitonin, placental growth factor, VEGFA, soluble VEGFR2, erythropoietin, and soluble cMET.61 In a phase II study of advanced RCC, therapy with foretinib decreased plasma levels of placental growth factor, VEGFA, soluble VEGFR2, erythropoietin, and soluble cMET.97 Plasma levels of HGF decreased after exposure to MGDC-265.98
Mechanisms of Resistance to HGF-cMET Axis Inhibitors
The use of new targeted agents is occurring along with the emergence of primary and acquired resistance, which should be considered in clinical trial design. Multiple mutations and bypass mechanisms can contribute to this problem. In preclinical in vitro and in vivo models using gastric carcinoma cell lines, investigators observed the simultaneous development of two mechanisms of resistance that resulted in maintenance of downstream PI3K and MAPK signaling in the presence of two TKIs: acquisition of a mutation in the cMET activation loop (Y1230), destabilizing the autoinhibitory conformation of cMET and abolishing an aromatic stacking interaction with the inhibitor; and activation of the EGFR by increased expression of transforming growth factor α, bypassing the need for cMET signaling to activate downstream signaling.99 A second study using in vitro and in vivo gastric cancer and NSCLC models showed that prolonged exposure to TKIs drove amplification, overexpression, and constitutive activation of cMET. The investigators also observed progressive amplification of KRAS, resulting in increased expression and activation of wild-type KRAS and in activation of the MAPK pathway.100 Strategies to overcome resistance include: therapy selection based on the presence of known susceptibility factors such as oncogene addiction, use of inhibitors at different levels of the pathway (ligand, receptor, and TK), and therapy combinations against multiple pathways to overcome bypass mechanisms. These strategies are being applied and tested in ongoing clinical trials.
DISCUSSION
The extensive basic knowledge of HGF-cMET biology has allowed a comprehensive assessment of the oncogenic potential of the axis and provided insights needed to develop selective and potent inhibitors now in clinic. Improvement on biomarker development for patient selection and evaluation of therapeutic activity are advancing as efforts to improve technologies progress. The issue of resistance needs to be considered in clinical trial design to enable mechanistic-driven combinations and careful patient selection.
Supplementary Material
Appendix
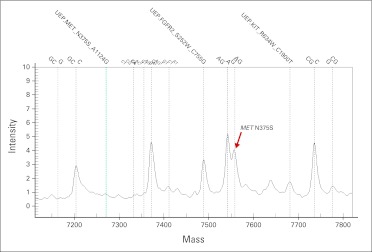
Fig A1.
Activating mutations by Sequenom (San Diego, CA), molecular aberrations in the cMET receptor evaluated to select patients for anti–hepatocyte growth factor–cMET axis–targeted therapies.
Footnotes
Supported in part by the Kleberg Center for Molecular Markers at the MD Anderson Cancer Center; by Grant No. P50-CA116199 from the National Cancer Institute (NCI) Breast Specialized Program for Research Excellence (G.R.B., G.B.M., A.M.G.-A.); by Support Grant No. P30 CA016672 to MD Anderson Cancer Center from the NCI; and by Grant No. SAC100004 from the Susan G. Komen Foundation (G.R.B., A.M.G.-A.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: George R. Blumenschein, Genentech (C), Bristol-Myers Squibb (C), EMD Serono (C), sanofi-aventis (C); Gordon B. Mills, Asuragen (U), Aushon (U), Catena (C), Daiichi Pharmaceutical (C), Foundation Medicine (U), Arcxix Biotechnologies (C), Targeted Molecular Diagnostics (C), Han AlBio Korea (C), Novartis (C), Tau Therapeutics (C); Ann M. Gonzalez-Angulo, Genentech (C) Stock Ownership: Gordon B. Mills, Catena, PVT Ventures, Spindle Top Ventures Honoraria: None Research Funding: George R. Blumenschein Jr, Genentech, GlaxoSmithKline, Bristol-Myers Squibb, Celgene, Abraxis, Novartis, AVEO, Exelixis, Merck; Gordon B. Mills, AstraZeneca, Celgene, CeMines, Exelixis/sanofi-aventis, GlaxoSmithKline, LPATH, Roche, SDI, Wyeth/Pfizer/Puma; Ana M. Gonzalez-Angulo, Genentech, GlaxoSmithKline, Bristol-Myers Squibb, Celgene, Abraxis, Novartis, AVEO, Merck Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Financial support: George R. Blumenschein Jr, Ana M. Gonzalez-Angulo
Administrative support: All authors
Provision of study materials or patients: Gordon B. Mills
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Peschard P, Park M. From Tpr-Met to Met, tumorigenesis and tubes. Oncogene. 2007;26:1276–1285. doi: 10.1038/sj.onc.1210201. [DOI] [PubMed] [Google Scholar]
- 2.Bottaro DP, Rubin JS, Faletto DL, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 3.Gherardi E, Youles ME, Miguel RN, et al. Functional map and domain structure of MET, the product of the c-met protooncogene and receptor for hepatocyte growth factor/scatter factor. Proc Natl Acad Sci U S A. 2003;100:12039–12044. doi: 10.1073/pnas.2034936100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lokker NA, Mark MR, Luis EA, et al. Structure-function analysis of hepatocyte growth factor: Identification of variants that lack mitogenic activity yet retain high affinity receptor binding. Embo J. 1992;11:2503–2510. doi: 10.1002/j.1460-2075.1992.tb05315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gherardi E, Sandin S, Petoukhov MV, et al. Structural basis of hepatocyte growth factor/scatter factor and MET signalling. Proc Natl Acad Sci U S A. 2006;103:4046–4051. doi: 10.1073/pnas.0509040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang YW, Vande Woude GF. HGF/SF-met signaling in the control of branching morphogenesis and invasion. J Cell Biochem. 2003;88:408–417. doi: 10.1002/jcb.10358. [DOI] [PubMed] [Google Scholar]
- 7.Rosario M, Birchmeier W. How to make tubes: Signaling by the Met receptor tyrosine kinase. Trends Cell Biol. 2003;13:328–335. doi: 10.1016/s0962-8924(03)00104-1. [DOI] [PubMed] [Google Scholar]
- 8.Corso S, Comoglio PM, Giordano S. Cancer therapy: Can the challenge be MET? Trends Mol Med. 2005;11:284–292. doi: 10.1016/j.molmed.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Stamos J, Lazarus RA, Yao X, et al. Crystal structure of the HGF beta-chain in complex with the sema domain of the Met receptor. Embo J. 2004;23:2325–2335. doi: 10.1038/sj.emboj.7600243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boccaccio C, Comoglio PM. Invasive growth: A MET-driven genetic programme for cancer and stem cells. Nat Rev Cancer. 2006;6:637–645. doi: 10.1038/nrc1912. [DOI] [PubMed] [Google Scholar]
- 11.Trusolino L, Bertotti A, Comoglio PM. A signaling adapter function for alpha6beta4 integrin in the control of HGF-dependent invasive growth. Cell. 2001;107:643–654. doi: 10.1016/s0092-8674(01)00567-0. [DOI] [PubMed] [Google Scholar]
- 12.Basile JR, Afkhami T, Gutkind JS. Semaphorin 4D/plexin-B1 induces endothelial cell migration through the activation of PYK2, Src, and the phosphatidylinositol 3-kinase-Akt pathway. Mol Cell Biol. 2005;25:6889–6898. doi: 10.1128/MCB.25.16.6889-6898.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gómez-Quiroz LE, Factor VM, Kaposi-Novak P, et al. Hepatocyte-specific c-Met deletion disrupts redox homeostasis and sensitizes to Fas-mediated apoptosis. J Biol Chem. 2008;283:14581–14589. doi: 10.1074/jbc.M707733200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puri N, Salgia R. Synergism of EGFR and c-Met pathways, cross-talk and inhibition, in non-small cell lung cancer. J Carcinog. 2008;7:9. doi: 10.4103/1477-3163.44372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shattuck DL, Miller JK, Carraway KL, 3rd, et al. Met receptor contributes to trastuzumab resistance of Her2-overexpressing breast cancer cells. Cancer Res. 2008;68:1471–1477. doi: 10.1158/0008-5472.CAN-07-5962. [DOI] [PubMed] [Google Scholar]
- 16.Bauer TW, Somcio RJ, Fan F, et al. Regulatory role of c-Met in insulin-like growth factor-I receptor-mediated migration and invasion of human pancreatic carcinoma cells. Mol Cancer Ther. 2006;5:1676–1682. doi: 10.1158/1535-7163.MCT-05-0175. [DOI] [PubMed] [Google Scholar]
- 17.Fan S, Meng Q, Laterra JJ, et al. Ras effector pathways modulate scatter factor-stimulated NF-kappaB signaling and protection against DNA damage. Oncogene. 2007;26:4774–4796. doi: 10.1038/sj.onc.1210271. [DOI] [PubMed] [Google Scholar]
- 18.Cooke VG, LeBleu VS, Keskin D, et al. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by Met signaling pathway. Cancer Cell. 2012;21:66–81. doi: 10.1016/j.ccr.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smolen GA, Sordella R, Muir B, et al. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc Natl Acad Sci U S A. 2006;103:2316–2321. doi: 10.1073/pnas.0508776103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lengyel E, Prechtel D, Resau JH, et al. C-Met overexpression in node-positive breast cancer identifies patients with poor clinical outcome independent of Her2/neu. Int J Cancer. 2005;113:678–682. doi: 10.1002/ijc.20598. [DOI] [PubMed] [Google Scholar]
- 21.Christensen JG, Burrows J, Salgia R. C-Met as a target for human cancer and characterization of inhibitors for therapeutic intervention. Cancer Lett. 2005;225:1–26. doi: 10.1016/j.canlet.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 22.Soman NR, Correa P, Ruiz BA, et al. The TPR-MET oncogenic rearrangement is present and expressed in human gastric carcinoma and precursor lesions. Proc Natl Acad Sci U S A. 1991;88:4892–4896. doi: 10.1073/pnas.88.11.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 24.Danilkovitch-Miagkova A, Zbar B. Dysregulation of Met receptor tyrosine kinase activity in invasive tumors. J Clin Invest. 2002;109:863–867. doi: 10.1172/JCI15418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ichimura E, Maeshima A, Nakajima T, et al. Expression of c-met/HGF receptor in human non-small cell lung carcinomas in vitro and in vivo and its prognostic significance. Jpn J Cancer Res. 1996;87:1063–1069. doi: 10.1111/j.1349-7006.1996.tb03111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cipriani NA, Abidoye OO, Vokes E, et al. MET as a target for treatment of chest tumors. Lung Cancer. 2009;63:169–179. doi: 10.1016/j.lungcan.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia S, Dalès JP, Charafe-Jauffret E, et al. Poor prognosis in breast carcinomas correlates with increased expression of targetable CD146 and c-Met and with proteomic basal-like phenotype. Hum Pathol. 2007;38:830–841. doi: 10.1016/j.humpath.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Ma PC, Jagadeeswaran R, Jagadeesh S, et al. Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res. 2005;65:1479–1488. doi: 10.1158/0008-5472.CAN-04-2650. [DOI] [PubMed] [Google Scholar]
- 29.Olivero M, Rizzo M, Madeddu R, et al. Overexpression and activation of hepatocyte growth factor/scatter factor in human non-small-cell lung carcinomas. Br J Cancer 74:1862- 1868;1996 doi: 10.1038/bjc.1996.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takanami I, Tanana F, Hashizume T, et al. Hepatocyte growth factor and c-Met/hepatocyte growth factor receptor in pulmonary adenocarcinomas: An evaluation of their expression as prognostic markers. Oncology. 1996;53:392–397. doi: 10.1159/000227594. [DOI] [PubMed] [Google Scholar]
- 31.Jagadeeswaran R, Ma PC, Seiwert TY, et al. Functional analysis of c-Met/hepatocyte growth factor pathway in malignant pleural mesothelioma. Cancer Res. 2006;66:352–361. doi: 10.1158/0008-5472.CAN-04-4567. [DOI] [PubMed] [Google Scholar]
- 32.Wong AS, Pelech SL, Woo MM, et al. Coexpression of hepatocyte growth factor-Met: An early step in ovarian carcinogenesis? Oncogene. 2001;20:1318–1328. doi: 10.1038/sj.onc.1204253. [DOI] [PubMed] [Google Scholar]
- 33.Zeng ZS, Weiser MR, Kuntz E, et al. C-Met gene amplification is associated with advanced stage colorectal cancer and liver metastases. Cancer Lett. 2008;265:258–269. doi: 10.1016/j.canlet.2008.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tolgay Ocal I, Dolled-Filhart M, D'Aquila TG, et al. Tissue microarray-based studies of patients with lymph node negative breast carcinoma show that met expression is associated with worse outcome but is not correlated with epidermal growth factor family receptors. Cancer. 2003;97:1841–1848. doi: 10.1002/cncr.11335. [DOI] [PubMed] [Google Scholar]
- 35.Carracedo A, Egervari K, Salido M, et al. FISH and immunohistochemical status of the hepatocyte growth factor receptor (c-Met) in 184 invasive breast tumors. Breast Cancer Res. 2009;11:402. doi: 10.1186/bcr2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Houldsworth J, Cordon-Cardo C, Ladanyi M, et al. Gene amplification in gastric and esophageal adenocarcinomas. Cancer Res. 1990;50:6417–6422. [PubMed] [Google Scholar]
- 37.Umeki K, Shiota G, Kawasaki H. Clinical significance of c-met oncogene alterations in human colorectal cancer. Oncology. 1999;56:314–321. doi: 10.1159/000011985. [DOI] [PubMed] [Google Scholar]
- 38.Cappuzzo F, Ciuleanu T, Stelmakh L, et al. SATURN: A double-blind, randomized, phase III study of maintenance erlotinib versus placebo following nonprogression with first-line platinum-based chemotherapy in patients with advanced NSCLC. J Clin Oncol. 2009;27:407s. (suppl 15; abstr 8001) [Google Scholar]
- 39.Tong CY, Hui AB, Yin XL, et al. Detection of oncogene amplifications in medulloblastomas by comparative genomic hybridization and array-based comparative genomic hybridization. J Neurosurg. 2004;100:187–193. doi: 10.3171/ped.2004.100.2.0187. [DOI] [PubMed] [Google Scholar]
- 40.Beroukhim R, Getz G, Nghiemphu L, et al. Assessing the significance of chromosomal aberrations in cancer: Methodology and application to glioma. Proc Natl Acad Sci U S A. 2007;104:20007–20012. doi: 10.1073/pnas.0710052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt L, Duh FM, Chen F, et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet. 1997;16:68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- 42.Lee JH, Han SU, Cho H, et al. A novel germ line juxtamembrane Met mutation in human gastric cancer. Oncogene. 2000;19:4947–4953. doi: 10.1038/sj.onc.1203874. [DOI] [PubMed] [Google Scholar]
- 43.Seiwert TY, Jagadeeswaran R, Faoro L, et al. The MET receptor tyrosine kinase is a potential novel therapeutic target for head and neck squamous cell carcinoma. Cancer Res. 2009;69:3021–3031. doi: 10.1158/0008-5472.CAN-08-2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma PC, Kijima T, Maulik G, et al. C-MET mutational analysis in small cell lung cancer: Novel juxtamembrane domain mutations regulating cytoskeletal functions. Cancer Res. 2003;63:6272–6281. [PubMed] [Google Scholar]
- 45.Puri N, Ahmed S, Janamanchi V, et al. C-Met is a potentially new therapeutic target for treatment of human melanoma. Clin Cancer Res. 2007;13:2246–2253. doi: 10.1158/1078-0432.CCR-06-0776. [DOI] [PubMed] [Google Scholar]
- 46.Park WS, Oh RR, Park JY, et al. Frequent somatic mutations of the beta-catenin gene in intestinal-type gastric cancer. Cancer Res. 1999;59:4257–4260. [PubMed] [Google Scholar]
- 47.Kong-Beltran M, Seshagiri S, Zha J, et al. Somatic mutations lead to an oncogenic deletion of met in lung cancer. Cancer Res. 2006;66:283–289. doi: 10.1158/0008-5472.CAN-05-2749. [DOI] [PubMed] [Google Scholar]
- 48.Graveel C, Su Y, Koeman J, et al. Activating Met mutations produce unique tumor profiles in mice with selective duplication of the mutant allele. Proc Natl Acad Sci U S A. 2004;101:17198–17203. doi: 10.1073/pnas.0407651101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Renzo MF, Olivero M, Martone T, et al. Somatic mutations of the MET oncogene are selected during metastatic spread of human HNSC carcinomas. Oncogene. 2000;19:1547–1555. doi: 10.1038/sj.onc.1203455. [DOI] [PubMed] [Google Scholar]
- 50.Lorenzato A, Olivero M, Patane S, et al. Novel somatic mutations of the MET oncogene in human carcinoma metastases activating cell motility and invasion. Cancer Res. 2002;62:7025–7030. [PubMed] [Google Scholar]
- 51.Boccaccio C, Gaudino G, Gambarotta G, et al. Hepatocyte growth factor (HGF) receptor expression is inducible and is part of the delayed-early response to HGF. J Biol Chem. 1994;269:12846–12851. [PubMed] [Google Scholar]
- 52.Aguirre Ghiso JA, Alonso DF, Farías EF, et al. Deregulation of the signaling pathways controlling urokinase production: Its relationship with the invasive phenotype. Eur J Biochem. 1999;263:295–304. doi: 10.1046/j.1432-1327.1999.00507.x. [DOI] [PubMed] [Google Scholar]
- 53.Michieli P, Basilico C, Pennacchietti S, et al. Mutant Met-mediated transformation is ligand-dependent and can be inhibited by HGF antagonists. Oncogene. 1999;18:5221–5231. doi: 10.1038/sj.onc.1202899. [DOI] [PubMed] [Google Scholar]
- 54.Tuck AB, Park M, Sterns EE, et al. Coexpression of hepatocyte growth factor and receptor (Met) in human breast carcinoma. Am J Pathol. 1996;148:225–232. [PMC free article] [PubMed] [Google Scholar]
- 55.Koochekpour S, Jeffers M, Rulong S, et al. Met and hepatocyte growth factor/scatter factor expression in human gliomas. Cancer Res. 1997;57:5391–5398. [PubMed] [Google Scholar]
- 56.Ferracini R, Olivero M, Di Renzo MF, et al. Retrogenic expression of the MET proto-oncogene correlates with the invasive phenotype of human rhabdomyosarcomas. Oncogene. 1996;12:1697–1705. [PubMed] [Google Scholar]
- 57.Cecchi F, Rabe DC, Bottaro DP. Targeting the HGF/Met signalling pathway in cancer. Eur J Cancer. 2010;46:1260–1270. doi: 10.1016/j.ejca.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cañadas I, Rojo F, Arumí-Uría M, et al. C-MET as a new therapeutic target for the development of novel anticancer drugs. Clin Transl Oncol. 2010;12:253–260. doi: 10.1007/s12094-010-0501-0. [DOI] [PubMed] [Google Scholar]
- 59.Schoffski P, Garcia JA, Stadler WM, et al. A phase II study of the efficacy and safety of AMG 102 in patients with metastatic renal cell carcinoma. BJU Int. 2011;108:679–686. doi: 10.1111/j.1464-410X.2010.09947.x. [DOI] [PubMed] [Google Scholar]
- 60.Wen PY, Schiff D, Cloughesy TF, et al. A phase II study evaluating the efficacy and safety of AMG 102 (rilotumumab) in patients with recurrent glioblastoma. Neuro Oncol. 2011;13:437–446. doi: 10.1093/neuonc/noq198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kurzrock R, Sherman SI, Ball DW, et al. Activity of XL184 (cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol. 2011;29:2660–2666. doi: 10.1200/JCO.2010.32.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choueiri TK, Pal SK, McDermott DF, et al. Activity of cabozantinib (XL184) in patients (pts) with metastatic, refractory renal cell carcinoma (RCC) J Clin Oncol. 2012;30 (suppl; abstr 364) [Google Scholar]
- 63.Zucali P, Santoro A, Rodriguez-Lope C, et al. Final results from ARQ 197-114: A phase Ib safety trial evaluating ARQ 197 in cirrhotic patients (pts) with hepatocellular carcinoma (HCC) J Clin Oncol. 2010;28:334s. (suppl 15; abstr 4137) [Google Scholar]
- 64.A phase 1/2b study in Asian subjects with non-small cell lung cancer. ClinicalTrials.gov NCT01039948.
- 65.Eng C, Van Cutsem E, Nowara E, et al. A randomized, phase Ib/II trial of rilotumumab (AMG 102; ril) or ganitumab (AMG 479; gan) with panitumumab (pmab) versus pmab alone in patients (pts) with wild-type (WT) KRAS metastatic colorectal cancer (mCRC): Primary and biomarker analyses. J Clin Oncol. 2011;29:221s. (suppl 15; abstr 3500) [Google Scholar]
- 66.Ryan CJ, Rosenthal M, Ng S, et al. A multicenter, randomized phase II study of rilotumumab (R) (AMG 102) or placebo (Pbo) plus mitoxantrone (M) and prednisone (P) in patients (pts) with previously treated castrate-resistant prostate cancer (CRPC) J Clin Oncol. 2012;30 (suppl; abstr 115) [Google Scholar]
- 67.Spigel DR, Ervin TJ, Ramlau R, et al. Final efficacy results from OAM4558g, a randomized phase II study evaluating MetMAb or placebo in combination with erlotinib in advanced NSCLC. J Clin Oncol. 2011;29:477s. (suppl 15; abstr 7505) [Google Scholar]
- 68.Sequist LV, von Pawel J, Garmey EG, et al. Randomized phase II study of erlotinib plus tivantinib versus erlotinib plus placebo in previously treated non–small-cell lung cancer. J Clin Oncol. 2011;29:3307–3315. doi: 10.1200/JCO.2010.34.0570. [DOI] [PubMed] [Google Scholar]
- 69.Wakelee HA, Gettinger SN, Engelman JA, et al. A phase Ib/II study of XL184 (BMS 907351) with and without erlotinib (E) in patients (pts) with non-small cell lung cancer (NSCLC) J Clin Oncol. 2010;28:237s. (suppl 15; abstr 3017) [Google Scholar]
- 70.Chan AM, Rubin JS, Bottaro DP, et al. Identification of a competitive HGF antagonist encoded by an alternative transcript. Science. 1991;254:1382–1385. doi: 10.1126/science.1720571. [DOI] [PubMed] [Google Scholar]
- 71.Yu Y, Ross SA, Halseth AE, et al. Role of PYK2 in the development of obesity and insulin resistance. Biochem Biophys Res Commun. 2005;334:1085–1091. doi: 10.1016/j.bbrc.2005.06.198. [DOI] [PubMed] [Google Scholar]
- 72.Matsumoto K, Nakamura T. NK4 (HGF-antagonist/angiogenesis inhibitor) in cancer biology and therapeutics. Cancer Sci. 2003;94:321–327. doi: 10.1111/j.1349-7006.2003.tb01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matsumoto K, Nakamura T. NK4 gene therapy targeting HGF-Met and angiogenesis. Front Biosci. 2008;13:1943–1951. doi: 10.2741/2813. [DOI] [PubMed] [Google Scholar]
- 74.Mazzone M, Basilico C, Cavassa S, et al. An uncleavable form of pro-scatter factor suppresses tumor growth and dissemination in mice. J Clin Invest. 2004;114:1418–1432. doi: 10.1172/JCI22235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Michieli P, Mazzone M, Basilico C, et al. Targeting the tumor and its microenvironment by a dual-function decoy Met receptor. Cancer Cell. 2004;6:61–73. doi: 10.1016/j.ccr.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 76.Kong-Beltran M, Stamos J, Wickramasinghe D. The sema domain of Met is necessary for receptor dimerization and activation. Cancer Cell. 2004;6:75–84. doi: 10.1016/j.ccr.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 77.Kim KJ, Wang L, Su YC, et al. Systemic anti-hepatocyte growth factor monoclonal antibody therapy induces the regression of intracranial glioma xenografts. Clin Cancer Res. 2006;12:1292–1298. doi: 10.1158/1078-0432.CCR-05-1793. [DOI] [PubMed] [Google Scholar]
- 78.Burgess TL, Sun J, Meyer S, et al. Biochemical characterization of AMG 102: A neutralizing, fully human monoclonal antibody to human and nonhuman primate hepatocyte growth factor. Mol Cancer Ther. 2010;9:400–409. doi: 10.1158/1535-7163.MCT-09-0824. [DOI] [PubMed] [Google Scholar]
- 79.Gordon MS, Sweeney CS, Mendelson DS, et al. Safety, pharmacokinetics, and pharmacodynamics of AMG 102, a fully human hepatocyte growth factor-neutralizing monoclonal antibody, in a first-in-human study of patients with advanced solid tumors. Clin Cancer Res. 2010;16:699–710. doi: 10.1158/1078-0432.CCR-09-1365. [DOI] [PubMed] [Google Scholar]
- 80.Ramanathan R, Payumo F, Papadopoulos K, et al. A phase 1, first in human, study of SCH 900105, an antihepatocyte growth factor (HGF) monoclonal antibody, in subjects with advanced solid tumors. Presented at the 21st Annual American Association for Cancer Research–National Cancer Institute–European Organisation for Research and Treatment of Cancer International Conference on Molecular Targets and Cancer Therapeutics; November 15-19, 2009; Boston, MA. abstr A100. [Google Scholar]
- 81.Tan E, Park K, Lim WT, et al. Phase Ib study of ficlatuzumab (formerly AV-299), an anti-hepatocyte growth factor (HGF) monoclonal antibody (MAb) in combination with gefitinib (G) in Asian patients (pts) with NSCLC. J Clin Oncol. 2011;29:493s. (suppl 15; abstr 7571) [Google Scholar]
- 82.Jones SF, Cohen RB, Bendell JC, et al. Safety, tolerability, and pharmacokinetics of TAK-701, a humanized anti-hepatocyte growth factor (HGF) monoclonal antibody, in patients with advanced nonhematologic malignancies: First-in-human phase I dose-escalation study. J Clin Oncol. 2011;28:253s. (suppl 15; abstr 3081) [Google Scholar]
- 83.Salgia R, Peterson A, Eppler SA. Phase I, open-label, dose escalation study of the safety and pharmacology of MetMAb, a monovalent antagonist antibody to the recetor c-Met, administered IV in patients with locally advanced or metastatic solid tumors. Presented at the 20th Annual American Association for Cancer Research–National Cancer Institute–European Organisation for Research and Treatment of Cancer International Conference on Molecular Targets and Cancer Therapeutics; October 21-24, 2008; Geneva, Switzerland. (abstr 411) [Google Scholar]
- 84.Moss RA, Bothos JG, Filvaroff E, et al. Phase Ib dose-escalation study of MetMAb, a monovalent antagonist antibody to the receptor MET, in combination with bevacizumab in patients with locally advanced or metastatic solid tumors. J Clin Oncol. 2010;28 (suppl; abstr e13050) [Google Scholar]
- 85.Petrelli A, Circosta P, Granziero L, et al. Ab-induced ectodomain shedding mediates hepatocyte growth factor receptor down-regulation and hampers biological activity. Proc Natl Acad Sci U S A. 2006;103:5090–5095. doi: 10.1073/pnas.0508156103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zou HY, Li Q, Lee JH, et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007;67:4408–4417. doi: 10.1158/0008-5472.CAN-06-4443. [DOI] [PubMed] [Google Scholar]
- 87.Eder JP, Vande Woude GF, Boerner SA, et al. Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin Cancer Res. 2009;15:2207–2214. doi: 10.1158/1078-0432.CCR-08-1306. [DOI] [PubMed] [Google Scholar]
- 88.Hussain M, Smith MR, Sweeney C, et al. Cabozantinib (XL184) in metastatic castration-resistant prostate cancer (mCRPC): Results from a phase II randomized discontinuation trial. J Clin Oncol. 2011;29:293s. (suppl 15; abstr 4516) [Google Scholar]
- 89.Kollmannsberger CK, Hurwitz H, Vlahovic G, et al. Phase I study of daily administration of MGCD265 to patients with advanced malignancies (Study 265-101) J Clin Oncol. 2009;27 (suppl; abstr e14525) [Google Scholar]
- 90.Oh D, Han S, Kim TM, et al. A phase I, open-label, nonrandomized trial of OPB-31121, a STAT3 inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2010;28 (suppl; abstr e13056) [Google Scholar]
- 91.Yu W, Pandita A, Penuel E, et al. Exploratory biomarker analyses from OAM4558g: A placebo-controlled phase II study of erlotinib with or without MetMAb in patients with advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2011;29:483s. (suppl 15; abstr 7529) [Google Scholar]
- 92.Jhawer MP, Kindler HL, Wainberg ZA, et al. Preliminary activity of XL880, a dual MET/VEGFR2 inhibitor, in MET amplified poorly differentiated gastric cancer (PDGC): Interim results of a multicenter phase II study. J Clin Oncol. 2008;26:231s. (suppl 15; abstr 4572) [Google Scholar]
- 93.Yamazaki S, Skaptason J, Romero D, et al. Pharmacokinetic-pharmacodynamic modeling of biomarker response and tumor growth inhibition to an orally available cMet kinase inhibitor in human tumor xenograft mouse models. Drug Metab Dispos. 2008;36:1267–1274. doi: 10.1124/dmd.107.019711. [DOI] [PubMed] [Google Scholar]
- 94.Torti D, Sassi F, Galimi F, et al. A preclinical algorithm of soluble surrogate biomarkers that correlate with therapeutic inhibition of the MET oncogene in gastric tumors. Int J Cancer. 2012;130:1357–1366. doi: 10.1002/ijc.26137. [DOI] [PubMed] [Google Scholar]
- 95.Yap TA, Olmos D, Brunetto AT, et al. Phase I trial of a selective c-MET inhibitor ARQ 197 incorporating proof of mechanism pharmacodynamic studies. J Clin Oncol. 2011;29:1271–1279. doi: 10.1200/JCO.2010.31.0367. [DOI] [PubMed] [Google Scholar]
- 96.Eder JP, Shapiro GI, Appleman LJ, et al. A phase I study of foretinib, a multi-targeted inhibitor of c-Met and vascular endothelial growth factor receptor 2. Clin Cancer Res. 2010;16:3507–3516. doi: 10.1158/1078-0432.CCR-10-0574. [DOI] [PubMed] [Google Scholar]
- 97.Srinivasan R, Choueiri TK, Vaishampayan U, et al. A phase II study of the dual MET/VEGFR2 inhibitor XL880 in patients (pts) with papillary renal carcinoma (PRC) J Clin Oncol. 2008;26:275s. (suppl 15; abstr 5103) [Google Scholar]
- 98.O'Dwyer PJ, Papadopoulos KP, Tolcher AW, et al. MGCD265, an oral Met/VEGFR multitargeted receptor tyrosine kinase inhibitor, in combination with erlotinib: Phase I clinical experience. J Clin Oncol. 2011;29:214s. (suppl 15; abstr 3083) [Google Scholar]
- 99.Qi J, McTigue MA, Rogers A, et al. Multiple mutations and bypass mechanisms can contribute to development of acquired resistance to MET inhibitors. Cancer Res. 2011;71:1081–1091. doi: 10.1158/0008-5472.CAN-10-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cepero V, Sierra JR, Corso S, et al. MET and KRAS gene amplification mediates acquired resistance to MET tyrosine kinase inhibitors. Cancer Res. 2010;70:7580–7590. doi: 10.1158/0008-5472.CAN-10-0436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.