Abstract
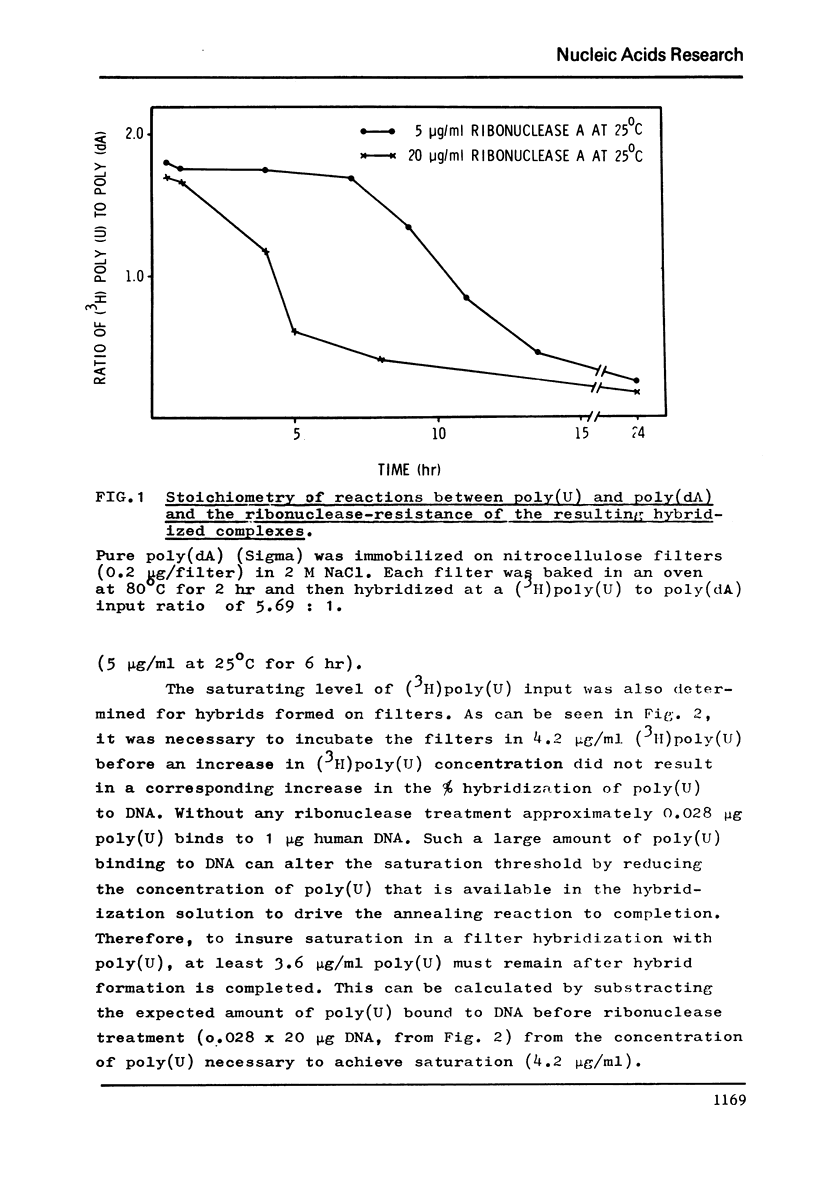
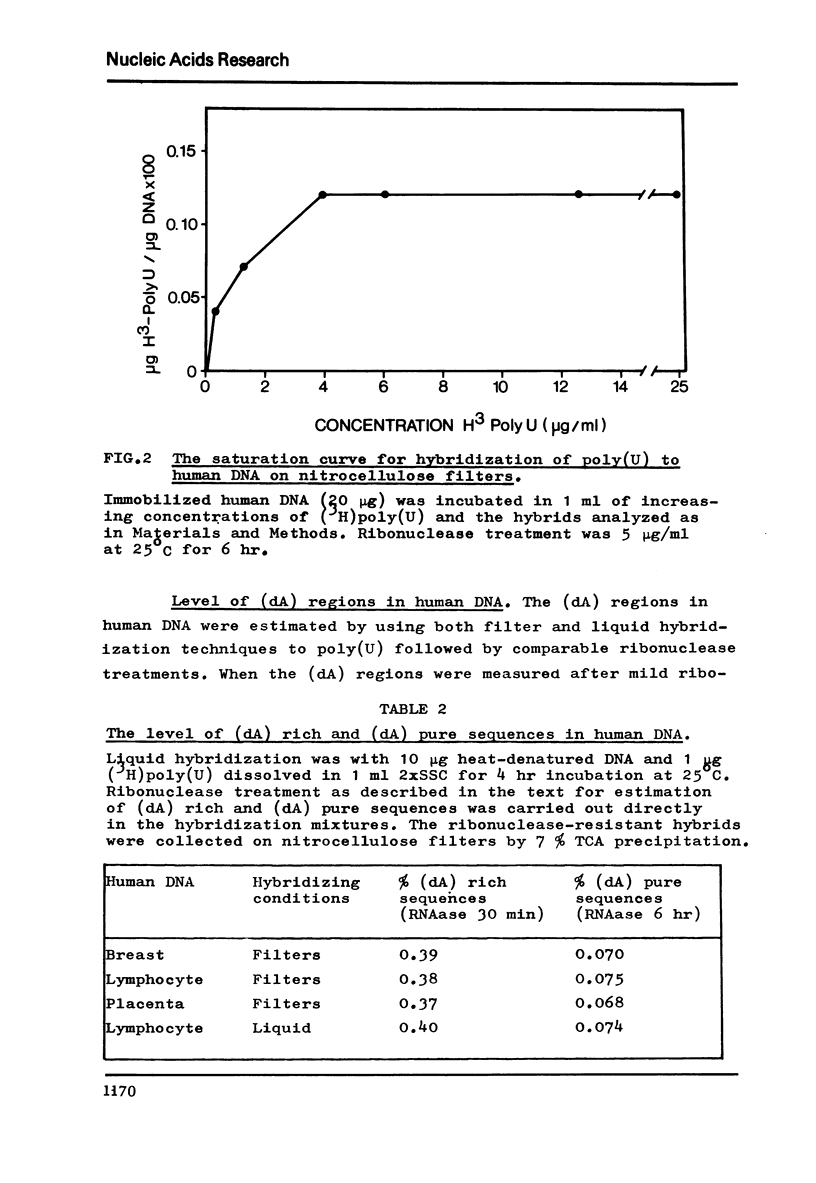
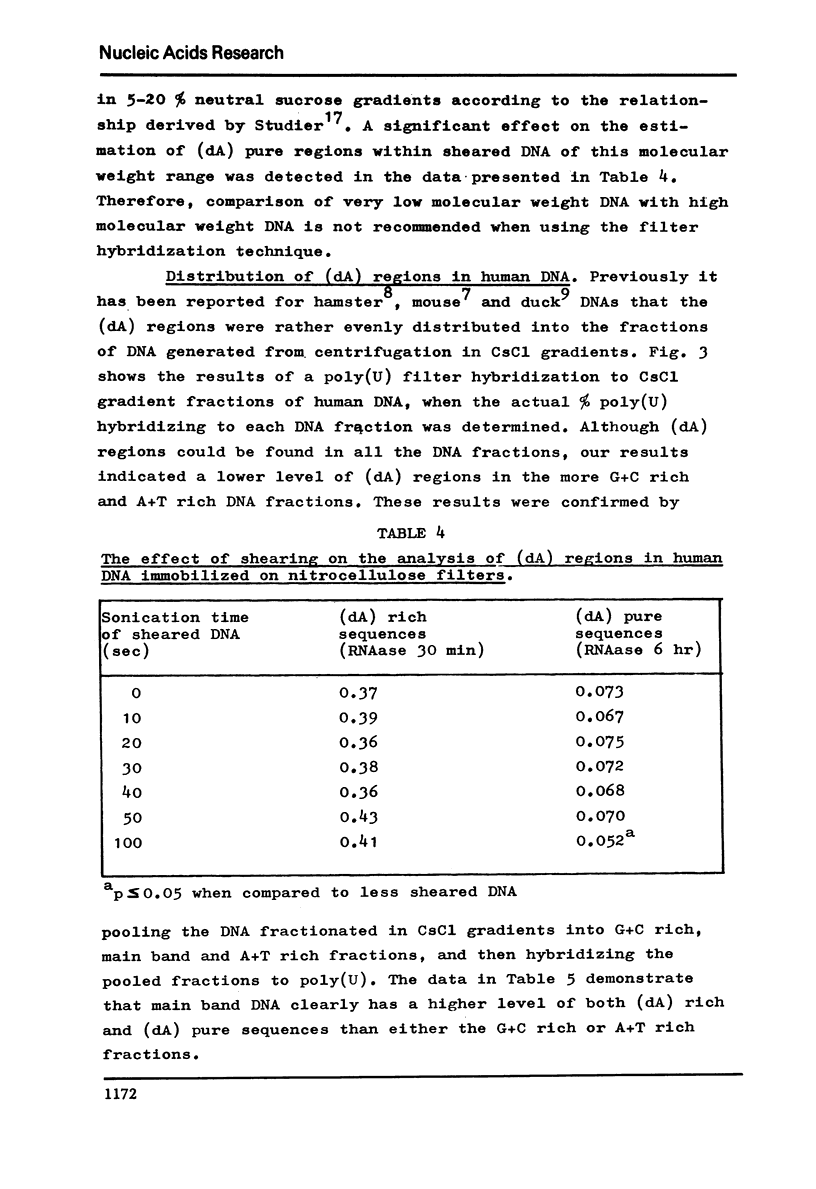
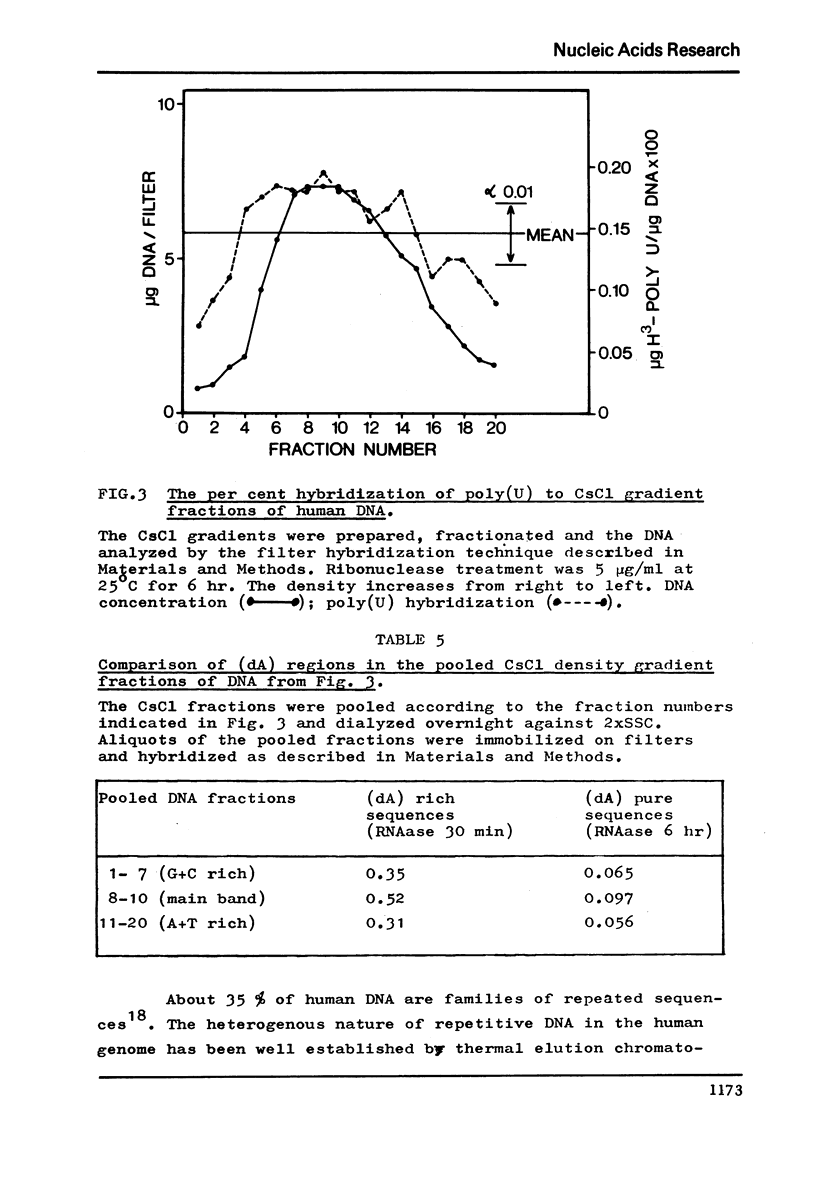
The level of deoxyadenylate (da) regions in human DNA was estimated from formation of poly(U)-poly(da) triplexes on nitrocellulose filters that were RNAase resistant. The (dA) rich sequences were determined following mild ribonuclease treatment of the poly(U)-DNA hybrids (5 mug/ml at 25 degreesC for 30 min), where as exhaustive ribonuclease treatment (5 mug/ml at 25 degrees C for 6 hr) estimated the more (dA) pure sequences. The level of (dA) rich regions was 0.39% of the DNA and for the more (dA) pure regions it was 0.07%. The (dA) regions were widely distributed throughout human DNA regardless of base composition or sequence repetition. However, a concentration of (dA) regions into main band CsC1 gradient fractions of DNA and into repeated DNA was observed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Mitchel R. E., Straus N. A. Analysis of long pyrimidine polynucleotides in HeLa cell nuclear DNA: absence of polydeoxythymidylate. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2189–2192. doi: 10.1073/pnas.70.8.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. O., Rosbash M. Polynucleotide sequences in eukaryotic DNA and RNA that form ribonuclease-resistant complexes with polyuridylic acid. J Mol Biol. 1974 May 5;85(1):75–86. doi: 10.1016/0022-2836(74)90130-2. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Rosbash M. Reiteration frequency of duck haemoglobin genes. Nat New Biol. 1973 Feb 14;241(111):204–207. doi: 10.1038/newbio241204a0. [DOI] [PubMed] [Google Scholar]
- Burdon R. H., Shenkin A. Uridylate-rich sequences in rapidly labelled RNA of mammalian cells. FEBS Lett. 1972 Jul 15;24(1):11–14. doi: 10.1016/0014-5793(72)80813-5. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Wall R., Tushinski R. J. An adenylic acid-rich sequence in messenger RNA of HeLa cells and its possible relationship to reiterated sites in DNA. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1321–1325. doi: 10.1073/pnas.68.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firtel R. A., Jacobson A., Lodish H. F. Isolation and hybridization kinetics of messenger RNA from Dictyostelium discoideum. Nat New Biol. 1972 Oct 25;239(95):225–228. doi: 10.1038/newbio239225a0. [DOI] [PubMed] [Google Scholar]
- Goldberg R. B., Galau G. A., Britten R. J., Davidson E. H. Nonrepetitive DNA sequence representation in sea urchin embryo messenger RNA. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3516–3520. doi: 10.1073/pnas.70.12.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRBY K. S. ISOLATION AND CHARACTERIZATION OF RIBOSOMAL RIBONUCLEIC ACID. Biochem J. 1965 Jul;96:266–269. doi: 10.1042/bj0960266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby K. S., Cook E. A. Isolation of deoxyribonucleic acid from mammalian tissues. Biochem J. 1967 Jul;104(1):254–257. doi: 10.1042/bj1040254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein W. H., Murphy W., Attardi G., Britten R. J., Davidson E. H. Distribution of repetitive and nonrepetivite sequence transcripts in HeLa mRNA. Proc Natl Acad Sci U S A. 1974 May;71(5):1785–1789. doi: 10.1073/pnas.71.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall S., Gillespie D. Poly U tracts absent from viral RNA. Nat New Biol. 1972 Nov 8;240(97):43–45. doi: 10.1038/newbio240043a0. [DOI] [PubMed] [Google Scholar]
- Molloy G. R., Thomas W. L., Darnell J. E. Occurrence of uridylate-rich oligonucleotide regions in heterogeneous nuclear RNA of HeLa cells. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3684–3688. doi: 10.1073/pnas.69.12.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato H., Edmonds M., Kopp D. W. Differential metabolism of large and small poly(A) sequences in the heterogeneous nuclear RNA of HeLa cells. Proc Natl Acad Sci U S A. 1974 Jan;71(1):200–204. doi: 10.1073/pnas.71.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards G. M. Modifications of the diphenylamine reaction giving increased sensitivity and simplicity in the estimation of DNA. Anal Biochem. 1974 Feb;57(2):369–376. doi: 10.1016/0003-2697(74)90091-8. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Saunders G. F., Shirakawa S., Saunders P. P., Arrighi F. E., Hsu T. C. Populations of repeated DNA sequences in the human genome. J Mol Biol. 1972 Feb 14;63(3):323–334. doi: 10.1016/0022-2836(72)90430-5. [DOI] [PubMed] [Google Scholar]
- Shenkin A., Burdon R. H. Deoxyadenylate-rich and deoxyguanylate-rich regions in mammalian DNA. J Mol Biol. 1974 May 5;85(1):19–39. doi: 10.1016/0022-2836(74)90126-0. [DOI] [PubMed] [Google Scholar]
- Shenkin A., Burdon R. H. Deoxyadenylate-rich sequences in mammalian DNA. FEBS Lett. 1972 May 1;22(2):157–160. doi: 10.1016/0014-5793(72)80033-4. [DOI] [PubMed] [Google Scholar]
- Sullivan D., Palacios R., Stavnezer J., Taylor J. M., Faras A. J., Kiely M. L., Summers N. M., Bishop J. M., Schimke R. T. Synthesis of a deoxyribonucleic acid sequence complementary to ovalbumin messenger ribonucleic acid and quantification of ovalbumin genes. J Biol Chem. 1973 Nov 10;248(21):7530–7539. [PubMed] [Google Scholar]
- Suzuki Y., Gage L. P., Brown D. D. The genes for silk fibroin in Bombyx mori. J Mol Biol. 1972 Oct 14;70(3):637–649. doi: 10.1016/0022-2836(72)90563-3. [DOI] [PubMed] [Google Scholar]
- Tapiero H., Monier M. N., Shaool D., Harel J. Distribution of repetitious sequences in chick nuclear DNA. Nucleic Acids Res. 1974 Feb;1(2):309–322. doi: 10.1093/nar/1.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]


