Abstract
The authors investigated variations in cognitive ability by gestational age among 13,824 children at age 6.5 years who were born at term with normal weight, using data from a prospective cohort recruited in 1996–1997 in Belarus. The mean differences in the Wechsler Abbreviated Scales of Intelligence were examined by gestational age in completed weeks and by fetal growth after controlling for maternal and family characteristics. Compared with the score for those born at 39–41 weeks, the full-scale intelligence quotient (IQ) score was 1.7 points (95% confidence interval (CI): −2.7, −0.7) lower in children born at 37 weeks and 0.4 points (95% CI: −1.1, 0.02) lower at 38 weeks after controlling for confounders. There was also a graded relation in postterm children: a 0.5-points (95% CI: −2.6, 1.6) lower score at 42 weeks and 6.0 points (95% CI: −15.1, 3.1) lower at 43 weeks. Compared with children born large for gestational age (>90th percentile), children born small for gestational age (<10th percentile) had the lowest IQ, followed by those at the 10th–50th percentile and those at the >50th–90th percentile. These findings suggest that, even among healthy children born at term, cognitive ability at age 6.5 years is lower in those born at 37 or 38 weeks and those with suboptimal fetal growth.
Keywords: birth weight, cognition, gestational age, term birth
Recent studies have shown that pregnancy outcomes such as perinatal death, low 5-minute Apgar score, and maternal hemorrhage vary by gestational age, even at term (1, 2). Zhang and Kramer (3) have found that infant mortality (both neonatal and postneonatal) and neonatal morbidity vary by week of gestation among term births. These findings suggest considerable heterogeneity in outcome among births at or beyond 37 completed weeks.
It is well established that children born small for gestational age or preterm (<37 weeks) have lower cognitive ability than their appropriate-for-gestational-age or term counterparts (4–13). It is therefore often assumed that children born at term are homogeneous with respect to long-term cognitive development. Recently, studies have reported a positive association between birth weight and cognitive ability even among children with birth weight within the normal range (14–16), although residual confounding by unmeasured family characteristics may have biased the results (17, 18). Although there are many studies on cognitive ability variations by birth weight, studies to examine the variation by gestational age are very limited. To our knowledge, there are only 2 such studies, but 1 did not adjust for important confounders including socioeconomic position (19) and the other was based on men only (20). Moreover, neither of these studies focuses on variations in cognitive ability by each week of gestation among children born at or beyond 37 completed weeks of gestation.
In this study, we took advantage of a 6.5-year follow-up of Belarusian children participating in a large, randomized trial known as the Promotion of Breastfeeding Intervention Trial (PROBIT) to examine whether child cognitive ability is associated with gestational duration and birth weight for gestational age among healthy, normally grown term births.
MATERIALS AND METHODS
Participants
A full description of the original trial has been published elsewhere (21). In brief, PROBIT is a cluster-randomized controlled trial in the Republic of Belarus of a breastfeeding promotion intervention modeled on the World Health Organization/United Nations Children's Fund (formerly the United Nations International Children's Emergency Fund) Baby-Friendly Hospital Initiative. A total of 17,046 mothers and their healthy, full-term infants whose birth weight was at least 2,500 g were recruited from 31 maternity hospitals and affiliated polyclinics during their postpartum stay between June 1996 and December 1997. After frequent follow-up visits during the first year of life, 13,889 of the children were interviewed and examined at age 6.5 years. The study received approval from the institutional review board of the Montreal Children's Hospital, and signed consent in Russian was obtained from all participating mothers.
Measures
At the 6.5-year follow-up, cognitive ability was measured by the Wechsler Abbreviated Scales of Intelligence (WASI) (22). The WASI consists of 4 subtests of vocabulary, similarities, block designs, and matrices. The WASI was translated from English to Russian and back-translated to ensure comparability of the Russian version. It was administered by the polyclinic pediatricians after extensive training and follow-up monitoring by child psychologists and psychiatrists in Minsk, Belarus. Interpediatrician agreement was high, with Pearson correlation coefficients of 0.80 (95% confidence interval (CI): 0.67, 0.89) for vocabulary, 0.72 (95% CI: 0.54, 0.83) for similarities, 0.80 (95% CI: 0.67, 0.89) for block designs, and 0.79 (95% CI: 0.66, 0.88) for matrices in a convenience sample of 45 children during a 1-week training workshop (23). The present study used the total score of the WASI, the full-scale intelligence quotient (IQ), for a measure of general cognitive ability of children. We also, however, assessed the associations with verbal and performance IQ.
Of children followed up at 6.5 years of age, those who had begun school were also evaluated by their teachers in mathematics, reading, writing, and other subjects according to a 5-point Likert scale as far below (1), somewhat below, at, somewhat above, or far above (5) his or her grade level based on items in the Teacher Report Form of the Child Behavior Checklist (24). Associations with gestational age and birth weight for gestational age were also examined for these academic performance measures.
Gestational age in completed weeks and birth weight were obtained from hospital records during the maternity stay. Gestational age was confirmed by ultrasound dating for 93.9% of the children. In only 3.8% was the gestational age estimate based solely on maternal report of the last menstrual period and in 2.3% by obstetric and/or pediatric clinical estimates. Fetal growth was based on the Canadian sex-specific reference for birth weight for gestational age (25) (Canadian standards were used because no Belarusian reference is available). Birth weight for gestational age was categorized as <10th percentile (small for gestational age), 10th–50th percentile, >50th–90th percentile, and >90th percentile (large for gestational age).
Potentially confounding maternal and family characteristics were obtained by maternal report at enrollment. They include maternal age at the birth of the index child, height, smoking and drinking during pregnancy, marital status, number of children in the household at the time of birth, and parental education and occupation.
Statistical analysis
As PROBIT is a cluster-randomized trial, children were nested within polyclinics where all child outcomes were measured by pediatricians. Clustering introduces similarities of study variables among children within the clusters and violates the requirement for independence among the units of statistical analysis (children). We previously reported an intraclass (within-cluster) correlation coefficient for full-scale IQ of 0.31 (23). The intraclass correlation coefficient for academic performance assessed by teachers was 0.09. To account for the clustering, we used a linear generalized estimating equations regression analysis. This approach allows the intracluster correlation to be estimated and taken into account to generate the regression coefficients and their correct standard errors. Even if the chosen correlation structure is incorrect or if the strength of the correlation varies among clusters, estimation of the coefficients is not affected (26). A generalized estimation equation approach was thus used to estimate population-average effects of gestational age across clusters while controlling for the potential effects of the clustering on cognitive ability variations. The mean differences in cognitive ability by each week of gestation (adjusted for clustering) were estimated compared with those born at 39–41 weeks of gestation as reference.
RESULTS
The 13,889 children followed up at age 6.5 years represented 81.5% of the original sample of 17,046 children, of whom 13,824 received the WASI tests and are included in the present study. Children who were lost to follow-up were not different from those followed up with respect to mean gestational age and birth weight, but the proportion of children born at 41 weeks was slightly higher in those lost to follow-up (8% vs. 6%). Those lost to follow-up also included more first-born children (63% vs. 56%), children from cohabiting or unmarried couples (15% vs. 11%), children whose father was a university graduate (18% vs. 12%), and children whose mother smoked during pregnancy (3% vs. 2%).
Table 1 presents the characteristics of children by gestational weeks. Of the total sample of 13,824 children, 3.4% were born at 37 weeks, 15.2% at 38 weeks, 30.3% at 39 weeks, 43.1% at 40 weeks, 6.7% at 41 weeks, 1.2% at 42 weeks, and 0.1% at 43 weeks.
Table 1.
Characteristics (% or Mean (SD)) of 13,824 PROBIT Children Recruited in 1996–1997 and Followed Up at Age 6.5 Years by Gestational Age
| 37 Weeks (n = 469) | 38 Weeks (n = 2,100) | 39 Weeks (n = 4,194) | 40 Weeks (n = 5,956) | 41 Weeks (n = 924) | 42 Weeks (n = 171) | 43 Weeks (n = 10) | All (n = 13,824) | |
| Birth weight, kg | 3.08 (0.37) | 3.27 (0.39) | 3.41 (0.39) | 3.51 (0.41) | 3.64 (0.43) | 3.71 (0.49) | 3.82 (0.60) | 3.44 (0.42) |
| Boys, % | 54.4 | 53.8 | 50.8 | 51.7 | 49.5 | 52.0 | 60.0 | 51.7 |
| Maternal age, % | ||||||||
| <20 years | 16.8 | 15.1 | 13.4 | 13.5 | 13.3 | 13.5 | 20.0 | 13.8 |
| 20–34 years | 77.2 | 80.2 | 82.2 | 82.4 | 84.1 | 83.0 | 80.0 | 82.0 |
| ≥35 years | 6.0 | 4.7 | 4.4 | 4.1 | 2.6 | 3.5 | 0.0 | 4.2 |
| Maternal height, cm | 164.3 (5.3) | 164.0 (5.6) | 164.4 (5.6) | 164.4 (5.6) | 164.7 (6.0) | 164.3 (5.6) | 166.9 (7.9) | 164.4 (5.6) |
| Missing, % | 5.2 | 15.5 | 25.8 | 46.4 | 5.8 | 1.3 | 0.0 | 0.1 |
| Smoking during pregnancy, % | 3.2 | 2.1 | 1.7 | 2.1 | 3.1 | 2.3 | 0.0 | 2.1 |
| Drinking during pregnancy, % | 2.8 | 2.1 | 2.3 | 2.1 | 2.6 | 2.3 | 0.0 | 2.2 |
| Marital status, % | ||||||||
| Registered marriage | 85.5 | 87.2 | 88.8 | 89.9 | 90.4 | 91.8 | 70.0 | 89.1 |
| Unregistered marriage | 9.0 | 7.8 | 6.9 | 6.7 | 6.5 | 6.4 | 20.0 | 7.0 |
| Unmarried | 5.5 | 5.0 | 4.2 | 3.3 | 3.1 | 1.7 | 10.0 | 3.9 |
| No. of children at home, % | ||||||||
| 1 child | 59.5 | 59.0 | 56.5 | 55.4 | 59.1 | 58.5 | 70.0 | 56.7 |
| 2 children | 28.8 | 33.6 | 35.9 | 35.3 | 32.5 | 26.9 | 20.0 | 34.7 |
| 3 children | 11.7 | 7.4 | 7.6 | 9.3 | 8.4 | 14.6 | 10.0 | 8.6 |
| Maternal education, % | ||||||||
| Less than secondary | 4.5 | 3.8 | 3.5 | 3.7 | 3.4 | 4.7 | 0.0 | 3.7 |
| Secondary | 36.0 | 31.8 | 31.1 | 32.6 | 30.0 | 36.3 | 50.0 | 32.0 |
| Partial university | 47.1 | 51.6 | 52.2 | 50.1 | 53.7 | 49.7 | 20.0 | 51.1 |
| University | 12.4 | 12.8 | 13.2 | 13.6 | 12.9 | 9.3 | 30.0 | 13.2 |
| Maternal occupation, % | ||||||||
| Manual | 29.0 | 22.9 | 26.8 | 29.4 | 31.1 | 36.8 | 30.0 | 27.8 |
| Service | 36.9 | 46.7 | 44.8 | 42.8 | 41.3 | 33.3 | 50.0 | 43.6 |
| Farmer | 7.5 | 5.9 | 5.8 | 6.0 | 4.3 | 8.2 | 10.0 | 5.9 |
| Pupil | 2.3 | 1.2 | 1.1 | 1.1 | 0.6 | 0.0 | 0.0 | 1.1 |
| Student | 1.9 | 2.2 | 2.0 | 2.0 | 1.8 | 2.9 | 0.0 | 2.0 |
| Housewife | 13.4 | 12.3 | 11.4 | 11.0 | 12.3 | 7.0 | 0.0 | 11.4 |
| Unemployed | 9.0 | 8.8 | 8.0 | 7.7 | 8.4 | 11.7 | 10.0 | 8.1 |
| Paternal education, % | ||||||||
| Less than secondary | 3.4 | 2.1 | 2.3 | 2.2 | 2.5 | 1.7 | 10.0 | 2.4 |
| Secondary | 40.7 | 32.2 | 34.1 | 38.3 | 35.7 | 39.8 | 40.0 | 37.2 |
| Partial university | 44.7 | 50.2 | 47.1 | 43.6 | 47.6 | 45.0 | 30.0 | 47.5 |
| University | 11.2 | 11.0 | 13.2 | 12.9 | 12.3 | 12.3 | 10.0 | 12.9 |
| Missing | 0.0 | 4.4 | 3.8 | 2.9 | 1.8 | 1.2 | 10.0 | 3.4 |
| Paternal occupation, % | ||||||||
| Manual | 48.6 | 40.9 | 44.2 | 45.9 | 48.0 | 49.1 | 50.0 | 44.9 |
| Service | 20.0 | 30.7 | 29.5 | 27.2 | 26.1 | 24.6 | 20.0 | 28.0 |
| Farmer | 11.5 | 9.0 | 8.6 | 10.1 | 8.2 | 12.9 | 0.0 | 9.4 |
| Pupil | 0.2 | 0.2 | 0.2 | 0.2 | 0.3 | 0.0 | 0.0 | 0.2 |
| Student | 0.6 | 0.9 | 0.9 | 1.0 | 0.8 | 0.0 | 0.0 | 1.0 |
| Unemployed | 13.6 | 13.2 | 12.0 | 12.2 | 13.2 | 11.1 | 20.0 | 12.4 |
| Unknown | 5.3 | 5.0 | 4.5 | 3.5 | 3.4 | 2.3 | 10.0 | 4.1 |
Abbreviations: PROBIT, Promotion of Breastfeeding Intervention Trial; SD, standard deviation.
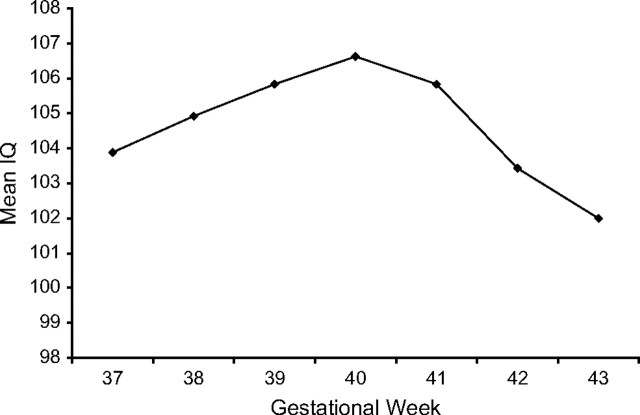
Figure 1 shows the mean full-scale IQ scores by gestational age. The mean IQ increased with each completed week of gestation from 37 to 40 weeks and decreased among those born postterm. As no statistically significant differences in full-scale IQ were observed among children born at 39, 40, and 41 weeks, we combined these 3 gestational ages as a reference group. As no differences were observed in the magnitude of associations by sex (all Pinteraction > 0.3), we present sex-adjusted results.
Figure 1.
Mean full-scale IQ score by gestational age in completed weeks among the PROBIT children recruited in 1996–1997 and followed up at age 6.5 years. Mean full-scale IQ scores were based on 469 children at 37 weeks of gestation, 2,100 children at 38 weeks, 4,194 children at 39 weeks, 5,956 children at 40 weeks, 924 children at 41 weeks, 171 children at 42 weeks, and 10 children at 43 weeks. IQ, intelligence quotient; PROBIT, Promotion of Breastfeeding Intervention Trial.
Table 2 presents the crude and adjusted mean IQ differences by gestational week. Compared with the score for children born at 39–41 weeks, the full-scale IQ score was 2.6 points (95% CI: −3.7, −1.4) lower in those born at 37 weeks and 0.5 points (95% CI: −1.1, −0.01) lower at 38 weeks. A graded relation was also found among children born postterm compared with those born at 39–41 weeks: 1.4 points (95% CI: −3.5, 0.6) lower at 42 weeks and 5.8 points (95% CI: −14.0, 2.5) lower at 43 weeks. These associations were attenuated with adjustment for potential confounding factors, mainly owing to differences in maternal age and family socioeconomic position. However, the overall pattern remained unchanged. After controlling for all potential confounding factors, we found that the full-scale IQ was lower by 1.7 points (95% CI: −2.7, −0.7) in children born at 37 weeks and by 0.4 points (95% CI: −1.2, 0.2) at 38 weeks compared with those born at 39–41 weeks. In the fully adjusted model, the IQ was lower by 0.5 points (95% CI: −2.6, 1.6) in children born at 42 weeks and by 6.0 points (95% CI: −15.1, 3.1) in those born at 43 weeks.
Table 2.
Crude and Adjusted Associations Between Cluster-adjusted Mean Full-Scale IQ Score and Gestational Age in Completed Weeks Among PROBIT Children Recruited in 1996–1997 and Followed Up at Age 6.5 Years
| Gestational Week | Mean Difference in Full-Scale IQ, Comparing Children Born at 39–41 Weeks and at Other Weeks of Gestation |
|||||||
| Crude |
Model 1a |
Model 2b |
Model 3c |
|||||
| Mean Difference | 95% Confidence Interval | Mean Difference | 95% Confidence Interval | Mean Difference | 95% Confidence Interval | Mean Difference | 95% Confidence Interval | |
| 37 (n = 469) | −2.6 | −3.7, −1.4 | −2.7 | −3.8, −1.5 | −2.4 | −3.5, −1.3 | −1.7 | −2.7, −0.7 |
| 38 (n = 2,100) | −0.6 | −1.1, −0.01 | −0.6 | −1.2, −0.1 | −0.5 | −1.0, 0.1 | −0.4 | −1.1, 0.2 |
| 39–41 (n = 11,074) | 0.0 | 0.0 | 0.0 | 0.0 | ||||
| 42 (n = 171) | −1.4 | −3.5, 0.6 | −1.4 | −3.4, 0.6 | −1.4 | −3.4, 0.6 | −0.5 | −2.6, 1.6 |
| 43 (n = 10) | −5.8 | −14.0, 2.5 | −5.9 | −14.2, 2.4 | −6.2 | −14.4, 1.4 | −6.0 | −15.1, 3.1 |
Abbreviations: IQ, intelligence quotient; PROBIT, Promotion of Breastfeeding Intervention Trial.
Model 1: adjusted for birth weight for gestational age and sex.
Model 2: model 1 + maternal height and age at birth, smoking, and drinking during pregnancy.
Model 3: model 2 + parental marital status, number of children in household, parental education, and occupation.
For fetal growth (birth weight for gestational age), 9.1% of children were born small for gestational age, and 7.6% were born large for gestational age. The full-scale IQ increased steadily with fetal growth. The cluster-adjusted mean full-scale IQ was 105.0 among children born small for gestational age, 106.4 among those in the 10th–50th percentile, 107.4 at the >50th–90th percentile, and 107.8 among those born large for gestational age. Compared with children who were born large for gestational age, those born small for gestational age had the lowest score of −2.7 (95% CI: −3.5, −1.9), followed by −1.6 (95% CI: −2.5, −0.7) for those at the 10th–50th percentile and −0.8 (95% CI: −1.7, 0.1) for those at the >50th–90th percentile after controlling for gestational age and maternal and family characteristics (Table 3).
Table 3.
Adjusteda Mean Difference and 95% Confidence Interval in Full-Scale IQ Score by Baseline Characteristics Among 13,824 PROBIT Children Recruited in 1996–1997 and Followed Up at Age 6.5 Years
| Mean Difference | 95% Confidence Interval | |
| Gestational ageb | ||
| 37 weeks | −1.7 | −2.7, −0.7 |
| 38 weeks | −0.4 | −1.1, 0.2 |
| 39–41 weeks (reference) | 0.0 | |
| 42 weeks | −0.4 | −2.5, 1.7 |
| 43 weeks | −5.9 | −15.0, 3.3 |
| Birth weight for gestational age | ||
| <10th percentile | −2.7 | −3.5, −1.9 |
| 10th–50th percentile | −1.6 | −2.5, −0.7 |
| >50th–90th percentile | −0.8 | −1.7, 0.1 |
| >90th percentile (reference) | 0.0 | |
| Sex, male | 0.2 | −0.3, 0.6 |
| Maternal age at birth | ||
| <20 years | −1.4 | −2.1, −0.7 |
| 20–34 years (reference) | 0.0 | |
| ≥35 years | −0.1 | −1.2, 1.1 |
| Maternal height, cm | 0.1 | 0.0, 0.1 |
| Pregnancy behavior | ||
| Smoking (yes) | −0.3 | −2.2, 1.5 |
| Drinking (yes) | 0.0 | −1.4, 1.5 |
| Parents’ marital status | ||
| Married (reference) | 0.0 | |
| Cohabitating | −0.9 | −1.9, −0.0 |
| Unmarried | −0.8 | −3.1, 1.6 |
| No. of children in household | −2.6 | −3.0, −2.2 |
| Parental education | ||
| Mother | ||
| University degree (reference) | 0.0 | |
| Partial university/special secondary | −3.8 | −4.9, −2.8 |
| Common secondary | −6.4 | −7.5, −5.3 |
| < Secondary | −8.4 | −10.5, −6.3 |
| Father | ||
| University degree (reference) | 0.0 | |
| Partial university/special secondary | −3.0 | −3.8, −2.2 |
| Common secondary | −4.1 | −5.1, −3.0 |
| < Secondary | −6.0 | −8.2, −3.7 |
| Parental occupation | ||
| Father | ||
| Manual | −1.1 | −1.9, −0.4 |
| Service (reference) | 0.0 | |
| Farmer | −5.0 | −6.7, −3.2 |
| Pupil | −1.5 | −6.0, 3.0 |
| Student | 1.4 | −0.8, 3.6 |
| Unemployed | −1.3 | −2.1, −0.6 |
| Unknown | −2.5 | −3.7, −1.3 |
| Mother | ||
| Manual | −2.0 | −2.8, −1.2 |
| Service (reference) | 0.0 | |
| Farmer | −5.4 | −6.9, −4.0 |
| Pupil | −0.4 | −2.8, 2.1 |
| Student | 1.9 | 0.4, 3.3 |
| Housewife | −1.7 | −3.1, −0.3 |
| Unemployed | −1.2 | −2.2, −0.1 |
Abbreviations: IQ, intelligence quotient; PROBIT, Promotion of Breastfeeding Intervention Trial.
Adjusted for cluster and all other variables included in the table.
The interaction between gestational age and fetal growth was not statistically significant (P = 0.13). In addition, the mean differences in full-scale IQ associated with gestational age and fetal growth were essentially unchanged with further adjustment for breastfeeding status.
We also examined whether length and head circumference at birth (standardized for sex and gestational age), as other measures of fetal growth, were associated with full-scale IQ. Although both measures were positively associated with full-scale IQ, the associations were small and disappeared after adjustment for birth weight. The mean differences in full-scale IQ per standard deviation were 0.34 (95% CI: −0.05, 0.72) for birth length and 0.35 (95% CI: −0.06, 0.76) for birth head circumference, but associations with birth weight for gestational age were unchanged after adjustment for birth length or head circumference.
The patterns of association found for verbal and performance IQ were consistent with that observed for full-scale IQ. For verbal IQ, compared with children born at 39–41 weeks of gestation, children born at 37 weeks scored 1.2 points (95% CI: −2.1, 0.3) lower, 0.5 points (95% CI: −1.0, 0.1) lower at 38 weeks, 1.0 points (95% CI: −2.7, 0.7) lower at 42 weeks, and 4.5 points (95% CI: −19.2, 10.2) lower at 43 weeks after adjustment for potential confounders. For performance IQ, the adjusted mean differences were −1.8 (95% CI: −3.1, −0.5) points for 37 weeks, −0.3 (95% CI: −1.0, 0.4) points at 38 weeks, 0.4 (95% CI: −2.1, 2.9) points at 42 weeks, and −6.0 (95% CI: −10.2, −1.7) points at 43 weeks. The adjusted mean differences in verbal IQ from children born large for gestational age were −2.4 (95% CI: −3.5, −1.2) among those born small for gestational age, −1.7 (95% CI: −2.6, −0.8) among the 10th–50th percentile, and −0.8 (95% CI: −1.7, 0.1) among the >50th–90th percentile. For performance IQ, the corresponding values were −2.4 (95% CI: −3.5, −1.3), −1.1 (95% CI: −2.0, −0.2), and −0.6 (95% CI: −1.5, 0.3), respectively.
Although academic performance ratings tended to be lower among children born at early term (37–38 weeks) and postterm (42–43 weeks) compared with those born at 39–41 weeks, the differences were small and statistically nonsignificant. For example, the adjusted mean rating differences in math were −0.03 (95% CI: −0.11, 0.05) at 37 weeks, −0.02 (95% CI: −0.06, 0.02) at 38 weeks, 0 (95% CI: −0.13, 0.12) at 42 weeks, and 0.06 (95% CI: −0.53, 0.65) at 43 weeks. The adjusted mean differences in writing were −0.01 (95% CI: −0.09, 0.06) at 37 weeks, −0.05 (95% CI: −0.09, −0.01) at 38 weeks, −0.01 (95% CI: −0.11, 0.13) at 42 weeks, and −0.23 (95% CI: −0.80, 0.34) at 43 weeks. The adjusted mean differences in math by fetal growth were −0.14 (95% CI: −0.21, −0.07) among children born small for gestational age, −0.08 (95% CI: −0.14, −0.02) among the 10th–50th percentile, and −0.02 (95% CI: −0.07, 0.04) among those in the >50th–90th percentile. The corresponding values for writing were −0.20 (95% CI: −0.27, −0.13), −0.10 (95% CI: −0.15, −0.03), and −0.03 (95% CI: −0.09, 0.03), respectively.
Sensitivity analyses
As pregnancy and birth complication rates are more frequent among early term and postterm births, we assessed the association after excluding children with any delivery, postpartum maternal, or infant complications such as maternal hemorrhage, cephalhematoma, and postpartum infection (n = 2,729). Results remained unchanged from those of the main analysis. Compared with values for children born at 39–41 weeks, the adjusted mean differences in full-scale IQ were −1.9 (95% CI: −3.0, −0.8) points at 37 weeks, −0.5 (95% CI: −1.2, 0.2) points at 38 weeks, −1.1 (95% CI: −3.3, 1.1) points at 42 weeks, and −2.0 (95% CI: −12.8, 8.8) points at 43 weeks. For fetal growth, compared with values for children born large for gestational age, the adjusted mean differences were −2.9 (95% CI: −4.0, −1.7) among those small for gestational age, −1.8 (95% CI: −2.8, −0.9) among the 10th–50th percentile, and −1.0 (95% CI: −1.9, −0.1) among the >50th–90th percentile.
In order to avoid potential bias from misclassification of gestational age, we reanalyzed our data after restriction to children whose gestational age was based on ultrasound (n = 12,985). The adjusted mean full-scale IQ scores were lower by 1.8 (95% CI: −2.9, −0.7) points at 37 weeks and by 0.5 (95% CI: −1.1, 0.1) points at 38 weeks. The mean IQ score of 0.1 (95% CI: −2.2, 2.4) was not different in children born at 42 weeks but, at −5.8 (95% CI: −14.9, 3.2), it was nonsignificantly lower at 43 weeks compared with those born at 39–41 weeks. The full-scale IQ was also lower by 2.4 (95% CI: −3.5, −1.3) among children born small for gestational age, by 1.4 (95% CI: −2.3, −0.5) among the 10th–50th percentile, and by 0.6 (95% CI: −1.5, 0.3) among the >50th–90th percentile compared with those born large for gestational age.
After restriction to spontaneous vaginal births (n = 12,220), the adjusted mean IQ scores were 1.8 (95% CI: −3.1, −0.5) points lower among children born at 37 weeks, 0.5 (95% CI: −1.2, 0.1) points lower at 38 weeks, 0.3 (95% CI: −3.0, 2.5) points lower at 42 weeks, and 8.3 (95% CI: −16.2, −0.5) points lower at 43 weeks. The adjusted mean differences from those large for gestational age were −2.8 (95% CI: −4.0, −1.7) among children small for gestational age, −1.8 (95% CI: −2.8, −0.9) among the 10th–50th percentile, and −1.0 (95% CI: −1.9, −0.1) among the 50th–90th percentile.
DISCUSSION
We found that IQ scores varied by gestational age, even among healthy children born at ≥37 completed weeks of gestation. We observed a gradual increase in IQ from 37 to 40 weeks of gestation and a gradual decrease thereafter for postterm. However, the mean differences compared with 39–41 weeks were small and statistically significant only at 37 completed weeks of gestation after controlling for maternal and family characteristics. Our results also confirm previous reports that fetal growth is positively associated with cognitive ability in children with normal birth weight (14–16). Differences in academic performances by gestational age and fetal growth were small, but the patterns were consistent with the results of IQ—lower scores in early term and postterm and monotonically higher scores with increases in fetal growth.
Our results are based on a large sample of healthy term births, permitting us to estimate gestational week-specific associations with cognitive ability. The effects estimated in our study are probably generalizable to other developed country settings, since Belarus resembles Western developed countries with respect to basic health services, sanitary conditions, and readily accessible health-care facilities, even in rural areas. Belarus has the lowest infant and child mortality rates among the Commonwealth of Independent States, with an infant mortality rate of 7 per 1,000 livebirths in 2002.
Although we adjusted for a wide range of important maternal and family characteristics, residual confounding by unmeasured family characteristics cannot be excluded. For example, child cognitive development is strongly determined by maternal cognitive ability (27), which was not measured in our study. Some studies using family-based analyses comparing siblings within and between families suggest residual confounding in the well-known positive association between birth weight and cognitive ability in children (17, 18), although results are not consistent across such studies (16). As family socioeconomic position partly confounded the association observed in our and other studies (15, 17, 18), differences in unmeasured indicators of family socioeconomic position may further confound the observed association. However, Belarus is one of the countries with the lowest degree of income inequality, as indicated by their Gini index of 29.7 compared with 40.8 in the United States (one of the highest inequality countries) and 25.0 in Sweden (one of the lowest inequality countries). Thus, the potential residual confounding by socioeconomic factors may not be as important as in other settings.
It should also be noted that the association between gestational age and IQ observed in the present study could be confounded by the underlying causes of earlier birth or suboptimal fetal growth, causes that might themselves lead to suboptimal brain development. Nonetheless, after restricting our analysis to children without delivery or postpartum maternal or neonatal complications identified by maternity hospital records, we found nearly identical results as in our main analysis, although pregnancy complications such as gestational diabetes were not recorded.
It is worth noting the clustering of mean IQ scores across polyclinics, as denoted by the high intraclass correlation coefficient of 0.31 (28). Since IQ was measured by a single pediatrician for all children at 24 of the 31 polyclinics (2 pediatricians shared the work in 7 of the busiest polyclinics), the high intraclass correlation coefficient suggests that, despite our efforts to standardize measurements across pediatricians, IQ assessment varied across pediatricians, probably due to differences in strictness or leniency in scoring or timing of responses, rather than true geographic differences in IQ (29). However, neither gestational age nor birth weight was measured by the pediatricians, neither substantially differed by polyclinic, and neither covaried with IQ across polyclinics. Thus, the issue here is not confounding but loss of statistical power and consequently wide confidence intervals (29). We also note that the mean IQ in our study is higher than the English/American norms for the test (i.e., mean of 100). This probably reflects our inclusion criteria, which restricted the study sample to healthy infants born at term with birth weights of ≥2,500 g. The high mean IQ scores might also reflect the study pediatricians’ permissive scoring of children's responses or time allotted to complete tasks or answer questions. High literacy rates in Belarusian parents, however, might result in truly higher IQ in their children. Finally, the Russian version of the WASI used in our study has not been formally validated by comparison with the Russian version of the full Wechsler scales or other instruments. Despite the lack of such validation, however, the WASI scores we obtained were strongly associated with parental socioeconomic factors and by parental education in particular. The scores were also positively correlated with children's academic performances (correlation coefficients ∼ 0.3). As shown in Table 3, other factors considered in our study were also associated with IQ scores in the expected directions.
The positive association that we observed between fetal growth and cognitive ability in children born at term is consistent with results from other studies (14–16). However, studies among full-term births (30, 31) are less common. The present study adds to the existing literature suggesting a positive association between fetal growth and IQ across the entire distribution of fetal growth, even among children born at term and after controlling for important confounding factors.
We have recently examined variations in cognitive ability by fetal growth comparing siblings with nonsiblings and found that the positive association disappeared in within-sibling analyses (18). As noted earlier, the positive association observed in the present study could thus be due to residual confounding by unmeasured family characteristics. Alternatively, the disappearing effect of fetal growth in our sibling-based study could be explained by the potential biases due to relatively poorly measured birth weight and gestational age, which were based solely on maternal recall within a 2-year time window. Variations in measurement of cognitive ability might also explain inconsistent results.
Consistent with our findings, lower cognitive ability among children born postterm (≥42 weeks of gestation) has been reported in a few previous studies (19, 20). Our study further demonstrates that cognitive ability increases with each additional week of gestation between 37 and 41 weeks and in both sexes. This pattern of association across gestational weeks among term births is consistent with recent reports of increased risks of infant mortality and morbidity in late preterm (34–36 weeks) births (32, 33) and even at 37 and 38 weeks of gestation (3). Chyi et al. (34) have recently shown that healthy, late-preterm children without significant neonatal complications have poorer reading and math scores compared with those born at term. Our results suggest that mild cognitive deficit can occur even among infants born at early term compared with those born at 39–41 weeks. The gradient observed suggests that each additional week of gestation is beneficial for brain development, at least up to 39 weeks (35).
In conclusion, recent trends toward greater fetal surveillance and more frequent and earlier labor induction (36, 37) are shifting the gestational age distribution to earlier weeks, which in turn increases both late-preterm and early term births. Although the effects that we observed are not large from the standpoint of the individual child, they should be considered by pregnant women and their caregivers when making decisions about elective induction and cesarean delivery despite some potential benefits of earlier birth (38, 39).
Acknowledgments
Author affiliations: Department of Epidemiology, Biostatistics, and Occupational Health, McGill University, Montreal, Quebec, Canada (Seungmi Yang, Robert W. Platt, Michael S. Kramer); The Research Institute of McGill University Health Centre, Montreal, Quebec, Canada (Seungmi Yang, Robert W. Platt, Michael S. Kramer); and Department of Pediatrics, McGill University, Montreal, Quebec, Canada (Robert W. Platt, Michael S. Kramer).
This research was supported by the Canadian Institute of Health Research (grant MOP-53155).
The authors thank Dr. Frances Aboud for help developing the IQ measure in this research and comments on previous versions of the manuscript.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- IQ
intelligence quotient
- PROBIT
Promotion of Breastfeeding Intervention Trial
- WASI
Wechsler Abbreviated Scales of Intelligence
References
- 1.Heimstad R, Romundstad PR, Eik-Nes SH, et al. Outcomes of pregnancy beyond 37 weeks of gestation. Obstet Gynecol. 2006;108(3 pt 1):500–508. doi: 10.1097/01.AOG.0000227783.65800.0f. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson JM, Kellar LC, Kellar GM. The impact of the interaction between increasing gestational age and obstetrical risk on birth outcomes: evidence of a varying optimal time of delivery. J Perinatol. 2006;26(7):392–402. doi: 10.1038/sj.jp.7211528. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, Kramer MS. Variations in mortality and morbidity by gestational age among infants born at term. J Pediatr. 2009;154(3):358–362. doi: 10.1016/j.jpeds.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Fitzhardinge PM, Steven EM. The small-for-date infant. II. Neurological and intellectual sequelae. Pediatrics. 1972;50(1):50–57. [PubMed] [Google Scholar]
- 5.Calame A, Fawer CL, Claeys V, et al. Neurodevelopmental outcome and school performance of very-low-birth-weight infants at 8 years of age. Eur J Pediatr. 1986;145(6):461–466. doi: 10.1007/BF02429043. [DOI] [PubMed] [Google Scholar]
- 6.Harvey D, Prince J, Bunton J, et al. Abilities of children who were small-for-gestational-age babies. Pediatrics. 1982;69(3):296–300. [PubMed] [Google Scholar]
- 7.McCormick MC, Brooks-Gunn J, Workman-Daniels K, et al. The health and developmental status of very low-birth-weight children at school age. JAMA. 1992;267(16):2204–2208. [PubMed] [Google Scholar]
- 8.Breslau N. Psychiatric sequelae of low birth weight. Epidemiol Rev. 1995;17(1):96–106. doi: 10.1093/oxfordjournals.epirev.a036191. [DOI] [PubMed] [Google Scholar]
- 9.Goldenberg RL, DuBard MB, Cliver SP, et al. Pregnancy outcome and intelligence at age five years. Am J Obstet Gynecol. 1996;175(6):1511–1515. doi: 10.1016/s0002-9378(96)70099-6. [DOI] [PubMed] [Google Scholar]
- 10.Hutton JL, Pharoah PO, Cooke RW, et al. Differential effects of preterm birth and small gestational age on cognitive and motor development. Arch Dis Child Fetal Neonatal Ed. 1997;76(2):F75–F81. doi: 10.1136/fn.76.2.f75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strauss RS. Adult functional outcome of those born small for gestational age: twenty-six-year follow-up of the 1970 British Birth Cohort. JAMA. 2000;283(5):625–632. doi: 10.1001/jama.283.5.625. [DOI] [PubMed] [Google Scholar]
- 12.Lundgren EM, Cnattingius S, Jonsson B, et al. Birth characteristics and different dimensions of intellectual performance in young males: a nationwide population-based study. Acta Paediatr. 2003;92(10):1138–1143. [PubMed] [Google Scholar]
- 13.Shenkin SD, Starr JM, Deary IJ. Birth weight and cognitive ability in childhood: a systematic review. Psychol Bull. 2004;130(6):989–1013. doi: 10.1037/0033-2909.130.6.989. [DOI] [PubMed] [Google Scholar]
- 14.Sørensen HT, Sabroe S, Olsen J, et al. Birth weight and cognitive function in young adult life: historical cohort study. BMJ. 1997;315(7105):401–403. doi: 10.1136/bmj.315.7105.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richards M, Hardy R, Kuh D, et al. Birth weight and cognitive function in the British 1946 birth cohort: longitudinal population based study. BMJ. 2001;322(7280):199–203. doi: 10.1136/bmj.322.7280.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matte TD, Bresnahan M, Begg MD, et al. Influence of variation in birth weight within normal range and within sibships on IQ at age 7 years: cohort study. BMJ. 2001;323(7308):310–314. doi: 10.1136/bmj.323.7308.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawlor DA, Clark H, Smith GD, et al. Intrauterine growth and intelligence within sibling pairs: findings from the Aberdeen children of the 1950s cohort. Pediatrics. 2006;117(5):e894–e902. doi: 10.1542/peds.2005-2412. [DOI] [PubMed] [Google Scholar]
- 18.Yang S, Lynch J, Susser ES, et al. Birth weight and cognitive ability in childhood among siblings and nonsiblings. Pediatrics. 2008;122(2):e350–e358. doi: 10.1542/peds.2007-3851. [DOI] [PubMed] [Google Scholar]
- 19.Record RG, McKeown T, Edwards JH. The relation of measured intelligence to birth weight and duration of gestation. Ann Hum Genet. 1969;33(1):71–79. doi: 10.1111/j.1469-1809.1969.tb01631.x. [DOI] [PubMed] [Google Scholar]
- 20.Eide MG, Oyen N, Skjaerven R, et al. Associations of birth size, gestational age, and adult size with intellectual performance: evidence from a cohort of Norwegian men. Pediatr Res. 2007;62(5):636–642. doi: 10.1203/PDR.0b013e31815586e9. [DOI] [PubMed] [Google Scholar]
- 21.Kramer MS, Chalmers B, Hodnett ED, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285(4):413–420. doi: 10.1001/jama.285.4.413. [DOI] [PubMed] [Google Scholar]
- 22.Wechsler D. Wechsler Abbreviated Scales of Intelligence. San Antonio, TX: Psychological Corp; 1999. [Google Scholar]
- 23.Kramer MS, Aboud F, Mironova E, et al. Breastfeeding and child cognitive development: new evidence from a large randomized trial. Arch Gen Psychiatry. 2008;65(5):578–584. doi: 10.1001/archpsyc.65.5.578. [DOI] [PubMed] [Google Scholar]
- 24.Achenback TM, Rescola LA. Manual for the ASEBA School-Age Forms and Profiles. Burlington, VT: 2001. University of Vermont Research Center for Children, Youth, and Families; [Google Scholar]
- 25.Kramer MS, Platt RW, Wen SW, et al. A new and improved population-based Canadian reference for birth weight for gestational age [electronic article] Pediatrics. 2001;108(2):E35. doi: 10.1542/peds.108.2.e35. [DOI] [PubMed] [Google Scholar]
- 26.Diggle PJ, Heagerty P, Liang KY, et al. Analysis of Longitudinal Data. 2nd ed. Oxford, United Kingdom: Oxford University Press; 2002. [Google Scholar]
- 27.Deary IJ, Der G, Shenkin SD. Does mother's IQ explain the association between birth weight and cognitive ability in childhood? Intelligence. 2005;33(5):445–454. [Google Scholar]
- 28.Taljaard M, Donner A, Villar J, et al. Intracluster correlation coefficients from the 2005 WHO Global Survey on Maternal and Perinatal Health: implications for implementation research. Paediatr Perinat Epidemiol. 2008;22(2):117–125. doi: 10.1111/j.1365-3016.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- 29.Kramer MS, Martin RM, Sterne JAC, et al. The “double jeopardy” of clustered measurement and cluster randomization. BMJ. 2009;339(7719):501–503. doi: 10.1136/bmj.b2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jelliffe-Pawlowski LL, Hansen RL. Neurodevelopmental outcome at 8 months and 4 years among infants born full-term small-for-gestational-age. J Perinatol. 2004;24(8):505–514. doi: 10.1038/sj.jp.7211111. [DOI] [PubMed] [Google Scholar]
- 31.Heinonen K, Räikkönen K, Pesonen AK, et al. Prenatal and postnatal growth and cognitive abilities at 56 months of age: a longitudinal study of infants born at term. Pediatrics. 2008;121(5):e1325–e1333. doi: 10.1542/peds.2007-1172. [DOI] [PubMed] [Google Scholar]
- 32.Raju TNK, Higgins RD, Stark AR, et al. Optimizing care and outcome for late-preterm (near-term) infants: a summary of the workshop sponsored by the National Institute of Child Health and Human Development. Pediatrics. 2006;118(3):1207–1214. doi: 10.1542/peds.2006-0018. [DOI] [PubMed] [Google Scholar]
- 33.Engle WA, Tomashek KM, Wallman C, et al. “Late-preterm” infants: a population at risk. Pediatrics. 2007;120(6):1390–1401. doi: 10.1542/peds.2007-2952. [DOI] [PubMed] [Google Scholar]
- 34.Chyi LJ, Lee HC, Hintz SR, et al. School outcomes of late preterm infants: special needs and challenges for infants born at 32 to 36 weeks gestation. J Pediatr. 2008;153(1):25–31. doi: 10.1016/j.jpeds.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 35.Kinney HC. The near-term (late preterm) human brain and risk for periventricular leukomalacia: a review. Semin Perinatol. 2006;30(2):81–88. doi: 10.1053/j.semperi.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Kirby RS. Trends in labor induction in the United States: is it true that what goes up must come down? Birth. 2004;31(2):148–151. doi: 10.1111/j.0730-7659.2004.00294.x. [DOI] [PubMed] [Google Scholar]
- 37.Yuan H, Platt RW, Morin L, et al. Fetal deaths in the United States, 1997 vs 1991. Am J Obstet Gynecol. 2005;193(2):489–495. doi: 10.1016/j.ajog.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Caughey AB, Musci TJ. Complications of term pregnancies beyond 37 weeks of gestation. Obstet Gynecol. 2004;103(1):57–62. doi: 10.1097/01.AOG.0000109216.24211.D4. [DOI] [PubMed] [Google Scholar]
- 39.Caughey AB, Stotland NE, Washington AE, et al. Maternal and obstetric complications of pregnancy are associated with increasing gestational age at term. Am J Obstet Gynecol. 2007;196(2):155. doi: 10.1016/j.ajog.2006.08.040. e1–155.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]