Abstract
Handgrip strength is a strong indicator of total body muscle strength and is a predictor of poor outcomes in older adults. The aging suppressor gene klotho encodes a single-pass transmembrane protein that is secreted as a circulating hormone. In mice, disruption of klotho expression results in a syndrome that includes sarcopenia, atherosclerosis, osteoporosis, and shortened lifespan, and conversely, overexpression of klotho leads to a greater longevity. The objective was to determine whether plasma klotho levels are related to skeletal muscle strength in humans. We measured plasma klotho in 804 adults, ≥65 years, in the InCHIANTI study, a longitudinal population-based study of aging in Tuscany, Italy. Grip strength was positively correlated with plasma klotho at threshold <681 pg/mL. After adjusting for age, sex, education, smoking, physical activity, cognition, and chronic diseases, plasma klotho (per 1 standard deviation increase) was associated with grip strength (beta = 1.20, standard error = 0.35, P = 0.0009) in adults with plasma klotho <681 pg/mL. These results suggest that older adults with lower plasma klotho have poor skeletal muscle strength.
Keywords: aging, klotho, muscle strength, sarcopenia
Aging is accompanied by sarcopenia, defined as the loss of skeletal muscle mass and muscle strength (25). Humans lose ~20-40% of both skeletal muscle mass and strength from 20 to 80 years of age (2, 5). Poor skeletal muscle strength is predictive of disability (10, 27) and mortality (28). Handgrip strength is strongly correlated with other measures of muscle strength and is often considered representative of total body muscle strength (28). Among the many underlying causes of sarcopenia are increased oxidative stress and inflammation, hormonal changes, anorexia of aging, and lack of physical activity (5).
Klotho, a recently discovered hormone, may play a potential role in the pathogenesis of sarcopenia. The aging suppressor gene klotho encodes a single-pass transmembrane protein that is predominantly expressed in the distal tubule cells of the kidney, parathyroid glands, and choroid plexus of the brain. The klotho gene was named after one of the three Fates in Greek mythology, the goddess who spins the thread of life. Klotho was originally identified in a mutant mouse strain that could not express klotho, developed multiple disorders resembling human aging, and had a shortened life span (17). The aging phenotypes included atherosclerosis, endothelial dysfunction, decreased bone mineral density, sarcopenia, skin atrophy, and impaired cognition (13, 18, 30).
In vivo gene delivery of klotho has been shown to protect against endothelial dysfunction in an atherosclerotic mouse model (29) and hypertension and renal damage in a hypertensive rat model (33). Overexpression of klotho in transgenic mice resulted in a significant extension of life span compared with wild-type mice (19). These findings suggest that some phenotypes of aging may be ameliorated and lifespan increased by increased expression of klotho.
There are two forms of klotho, membrane and secreted, and each has different functions. Membrane klotho acts as an obligate co-receptor for fibroblast growth factor (FGF)-23, a bone-derived hormone that induces phosphate excretion into urine (32). Secreted klotho is involved in regulation of nitric oxide production in the endothelium (29, 30), calcium homeostasis in the kidney (3, 14), inhibition of intracellular insulin and insulin-like growth factor-1 signaling (19), and inhibition of transforming growth factor-1 signaling (6). Klotho gene transcripts for a putative secreted form of klotho protein were described in 1998 (22). In 2004, Imura and colleagues demonstrated that klotho protein was present in both human sera and cerebrospinal fluid (14). The relationship of circulating klotho with clinical phenotypes in human aging has not been studied because of the lack of a sensitive and reliable assay for measurement of secreted klotho protein in the blood. For example, it is not known whether low plasma klotho levels are associated with poor muscle strength in humans. Recently, a sensitive and specific assay was developed for the measurement of soluble klotho in humans (34).
We hypothesized that low plasma klotho concentrations were independently associated with skeletal muscle strength. To address this hypothesis, we measured plasma klotho levels in a large, population-based study of aging.
MATERIALS AND METHODS
The study participants consisted of men and women, aged 65 and older, who participated in the Invecchiare in Chianti, “Aging in the Chianti Area” (InCHIANTI) study, conducted in two small towns in Tuscany, Italy. The rationale, design, and data collection have been described elsewhere, and the main outcome of this longitudinal study is mobility disability (7). Briefly, in August 1998, 1270 people aged 65 years and older were randomly selected from the population registry of Greve in Chianti (pop. 11,709) and Bagno a Ripoli (pop. 4,704), and of 1,256 eligible subjects, 1,155 (90.1%) agreed to participate. Participants received an extensive description of the study and participated after written, informed consent. The study protocol complied with the Declaration of Helsinki and was approved by the Italian National Institute of Research and Care on Aging Ethical Committee and by the Institutional Review Board of the Johns Hopkins University School of Medicine.
Participants were evaluated again for a three-year follow-up visit from 2001-2003 (n = 926) and six-year follow-up visit from 2004-2006 (n = 844), and nine-year follow-up visit from 2007-2009 (n = 768). Of the 926 participants seen at the three-year follow-up visit, 804 (86.8%) had blood drawn and plasma available for analysis. There were no significant differences in age, sex, other demographic factors, or subsequent mortality between those who did or did not participate in the blood drawing. Plasma klotho was measured at the three-year follow-up visit and not the enrollment visit because of the greater availability of archived plasma samples from the three-year visit. The three year visit will be referred to as the baseline visit for the present study of klotho and grip strength. There were 775 participants who had measurements of both plasma klotho and grip strength at baseline, and of these, grip strength measurements were available on 608 participants at the six-year visit and 472 participants at the nine-year visit.
Demographic information and information on smoking and medication use were collected using standardized questionnaires. Smoking history was determined from self-report. Daily alcohol intake, expressed in gm/day, was determined based upon the European Prospective Investigation into Cancer and Nutrition food frequency questionnaire that had been validated in the Italian population. Education was recorded as years of school.
All participants were examined by a trained geriatrician. Diseases were ascertained according to standard, pre-established criteria and algorithms based upon those used in the Women's Health and Aging Study for diabetes mellitus, coronary heart disease, chronic heart failure, stroke, and cancer (12). Those who did not have diabetes by the algorithm but had a fasting plasma glucose >125 mg/dL (1) were also considered to have diabetes.
Systolic and diastolic blood pressures were calculated from the mean of three measures taken with a standard mercury sphygmomanometer during the physical examination. Weight was measured using a high-precision mechanical scale. Standing height was measured to the nearest 0.1 cm. Body mass index (BMI) was calculated as weight/height2 (kg/m2). Mini-Mental State Examination (MMSE) was administered at enrollment, and an MMSE score <24 was considered consistent with cognitive impairment (8). Chronic kidney disease was defined as estimated glomerular filtration rate of <60 mL/min/1.73 m2 using the four-variable Modification of Diet in Renal Disease Study equation of Levey and colleagues (20).
Grip strength was measured with a Jamar dynamometer (Irvington, NY). Before testing, the examiner asked the subject if he/she had pain in one hand or in both (for example, a new acute phase of an arthrosis process or a recent attack of gout), or if he had undergone any surgery to the hand. If the response was affirmative, the affected limb was not tested; if both hands were affected, the test was deferred. The subject was seated in front of a bench with the arm of the test hand supported on the bench with the elbow flexed to 45 degrees. The examiner adjusted the handle of the dynamometer such that the proximal interphalangeal joints of the hand were bent to 90 degrees. The examiner asked the subject to perform a practice test and said: ‘Try once to use the device to understand how it works. Squeeze the handle until your hand is closed. It does not matter whether you use your maximum strength. Instead, tell me whether you feel you are in a comfortable position to carry out the task.’ The examiner then adjusted the device, if necessary, and started the test saying: ‘Now begin the test. When I say go, begin to squeeze with all your might. You are ready. Squeeze. Fine, just relax.’ The test lasted about 5 seconds and two sessions were performed. Both hands were tested, unless contraindicated as above.
Grip strength is a strong predictor of mortality (27), thus, the competing risk of mortality was also taken into account in this analysis. At the end of the field data collection, mortality data of the original InCHIANTI cohort were collected using data from the Mortality General Registry maintained by the Tuscany Region. Analyses include those who refused to participate in the follow-up after baseline or those who moved away but were known to be alive at the time of censoring of this analysis.
Blood samples were collected in the morning after a 12-h fast. Aliquots of serum and plasma were immediately obtained and stored at -80° C. Soluble α-klotho was measured in EDTA-plasma using a solid phase sandwich enzyme-linked immunosorbent assay (ELISA) (Immuno-Biological Laboratories, Takasaki, Japan) (34). The minimum level of detectability of the assay is 6.15 pg/mL. The minimum level is below the plasma concentrations that were found in our study. The intra-assay and inter-assay coefficients of variation were 4.1% and 8.9% for klotho measurements in the investigator's (R.D.S.) laboratory. The designation α-klotho is used to describe the original klotho gene and its product (15) and to distinguish it from a homolog which was named β-klotho (16). Throughout this paper, the term klotho refers to α-klotho. Commercial enzymatic tests (Roche Diagnostics) were used for measuring serum total cholesterol, triglycerides, and high-density lipoprotein (HDL) cholesterol concentrations. Low-density lipoprotein (LDL) cholesterol was calculated by the Friedewald formula (9).
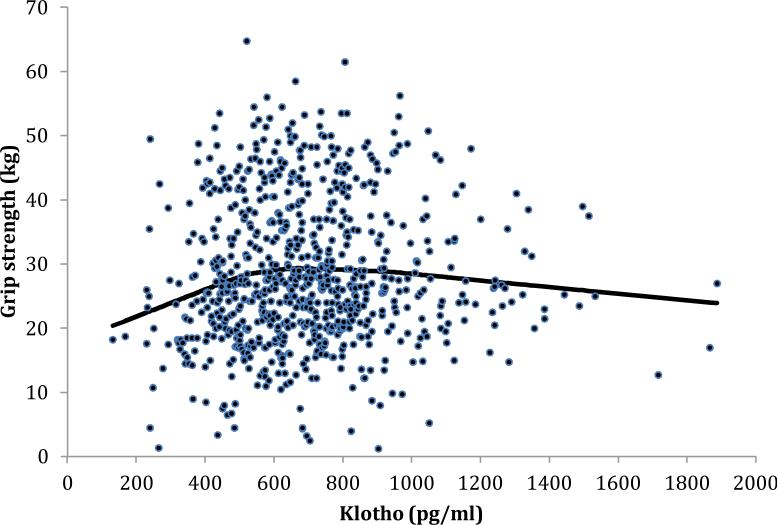
Variables are reported as medians (25th, 75th percentiles) or as percentages. An exploratory analysis using scatterplots and lowess smoothing curves suggested a threshold between plasma klotho and grip strength. Nonparametric deviance reduction (26) was used to identify the threshold of 681 pg/mL of plasma klotho in relation to grip strength. Given the threshold of plasma klotho, we used piecewise regression (23) to examine the relationship between plasma klotho and grip strength at the 3-year (baseline), 6-year, and 9-year follow-up visits. In multivariable linear mixed models, no significant plasma klotho and time interactions were found in the individual models. All analyses were performed using SAS (v. 9.1.3, SAS Institute, Inc., Cary, NC) with a type I error of 0.05.
RESULTS
The baseline characteristics of the participants are shown in Table 1. The relationship between plasma klotho and grip strength is shown in Figure 1. As noted in the Methods, nonparametric deviance reduction was used to identify a threshold relationship between plasma klotho and grip strength at baseline of 681 pg/mL. The proportion of participants who were above and below the threshold was 46.8% and 53.2%, respectively. Univariate linear regression models were used to examine the relationship between participant characteristics and grip strength at baseline (Table 2). Piecewise regression was used to examine the relationship between plasma klotho, above and below 681 pg/mL, with grip strength at baseline. Older age, physical inactivity, cognitive impairment, congestive heart failure, stroke, chronic obstructive pulmonary disease, chronic kidney disease, depression, cancer, and plasma klotho, per 1 SD increase, for participants ≥681 pg/mL had significant negative association with grip strength. Current smoking and plasma klotho, per 1 SD increase, for participants <681 pg/mL, were significantly and positively associated with grip strength.
Table 1.
Baseline characteristics of adults, ≥65 years, in the InCHIANTI study1
| Characteristic | Median (25th, 75th Percentile) or % | |
|---|---|---|
| Age, y | 75.0 (71.0, 80.0) | |
| Sex (%) | Male | 44.2 |
| Female | 55.8 | |
| Education, y | 5.0 (4.0, 6.0) | |
| Current smoker (%) | 11.0 | |
| Body mass index (kg/m2) | 26.4 (23.7, 28.7) | |
| Physical activity (%) | Inactive | 26.1 |
| Low | 48.6 | |
| Moderate-High | 25.3 | |
| Grip strength | 26.5 (20.3, 36.5) | |
| Plasma klotho (pg/mL) | 664 (521, 811) | |
| Mini-Mental State Exam Score <24 (%) | 29.0 | |
| Hypertension (%) | 72.3 | |
| Coronary artery disease (%) | 6.1 | |
| Heart failure (%) | 7.7 | |
| Peripheral artery disease (%) | 13.7 | |
| Stroke (%) | 7.6 | |
| Diabetes mellitus (%) | 14.3 | |
| Cancer (%) | 8.7 | |
| Chronic kidney disease (%) | 28.6 | |
| Depression (%) | 28.5 | |
Baseline for this study is the three-year follow-up visit of this prospective study cohort.
Figure 1.
Scatterplot of plasma klotho and grip strength at baseline, with lowess smoothing curve.
Table 2.
Univariate linear regression models of plasma klotho and other variables with grip strength at baseline
| Characteristic | Beta | SE | P | |
|---|---|---|---|---|
| Age, y | -0.78 | 0.05 | <0.0001 | |
| Male sex | 15.2 | 0.60 | <0.0001 | |
| Education, y | 0.88 | 0.11 | <0.0001 | |
| Current smoking | 4.23 | 1.26 | 0.0008 | |
| Body mass index, kg/m2 | -0.01 | 0.10 | 0.91 | |
| Physical activity | Inactive | -14.95 | 1.00 | <0.0001 |
| Low | -7.80 | 0.86 | <0.0001 | |
| MMSE score <24 | -8.65 | 0.85 | <0.0001 | |
| Hypertension | -1.01 | 0.90 | 0.26 | |
| Coronary heart disease | 0.13 | 1.69 | 0.94 | |
| Congestive heart failure | -7.13 | 1.52 | <0.0001 | |
| Peripheral artery disease | -1.80 | 1.17 | 0.12 | |
| Stroke | -6.90 | 1.58 | <0.0001 | |
| Diabetes mellitus | -1.99 | 1.15 | 0.08 | |
| Chronic obstructive pulmonary disease | -3.68 | 0.88 | <0.0001 | |
| Chronic kidney disease | -3.68 | 0.89 | <0.0001 | |
| Depression | -7.28 | 0.85 | <0.0001 | |
| Cancer | -3.36 | 1.42 | 0.02 | |
| Plasma klotho (per 1 SD increase) | <681 pg/mL | 2.69 | 0.58 | <0.0001 |
| ≥681 pg/mL | -1.14 | 0.39 | 0.004 | |
Multivariable linear mixed models using piecewise regression were used to examine the relationship between plasma klotho and other covariates with grip strength at baseline and at 3 and 6 years following the baseline visit (Table 3). In models adjusting for age and sex (model 1), additionally for education, smoking, and physical activity (model 2), and finally adding MMSE score, stroke, heart failure, chronic kidney disease, cancer, and depression, in participants with plasma klotho <681 pg/mL, an increase in plasma klotho was significantly associated with increased grip strength (per 1 SD increase, beta = 1.20, standard error = 0.35, P = 0.0009). Plasma klotho ≥681 pg/mL had no significant relationship with grip strength once adjusted for other covariates in the three models above.
Table 3.
Multivariable linear mixed models for plasma klotho and grip strength
| Model 1 Adjusted for age, sex | Model 2 Adjusted for age, sex, education, smoking, physical activity | Model 3 Adjusted for age, sex, education, smoking, physical activity, MMSE score, stroke, heart failure, chronic kidney disease, cancer, and depression | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Beta | SE | P | Beta | SE | P | Beta | SE | P | ||
| Plasma klotho (per 1 SD increase) | <681 pg/mL | 1.27 | 0.37 | 0.0007 | 1.24 | 0.36 | 0.0008 | 1.20 | 0.35 | 0.0009 |
| ≥681 pg/mL | -0.11 | 0.25 | 0.65 | -0.14 | 0.25 | 0.58 | -0.19 | 0.24 | 0.44 | |
We examined the relationship between tertiles of plasma klotho (<575, 575-763, >763 pg/mL) and decline in grip strength over six years of follow-up. Mean (standard deviation) decline in grip strength from the lowest to the highest tertile of plasma klotho was 8.27 (4.83), 7.54 (5.04), and 7.25 (4.70) kg, respectively. In multivariable linear mixed models adjusting for age, sex, baseline grip strength, and other covariates above, tertile of plasma klotho at baseline was not significantly associated with decline in grip strength (data not shown). The proportion of participants who died during six years of follow-up from lowest to the highest tertile of plasma klotho was 31.1%, 24.2%, and 17.1% (P <0.0001). The mortality results from this cohort are being reported in detail in a separate paper.
DISCUSSION
The present study shows that older community-dwelling adults with low plasma klotho concentrations have poor grip strength. To our knowledge, this is the first study to describe the relationship between circulating klotho hormone and skeletal muscle strength in humans. The findings are consistent with sarcopenia that has been described in the klotho mouse model of aging (13, 18). Plasma klotho concentrations at baseline were associated with grip strength measurements measured during a six year study period. Although plasma klotho was not an independent predictor of decline in grip strength over six years of follow-up, there was a strong competing risk of mortality. Participants with low plasma klotho concentrations were at a higher risk of death, as described in greater detail in a separate paper (31).
Sarcopenia is characterized by loss of skeletal muscle strength and muscle mass due to enhanced catabolic activity in muscle. Two major pathways are involved in the catabolism of protein in muscle cells. In the autophagic-lysosomal pathway, macromolecules and organelles are absorbed into autophagosomes and degraded after fusion with lysosomes and subsequent lysosomal degradation (24). In the ubiquitin-proteasomal pathway, proteins are conjugated to multiple ubiquitins and then degraded within the proteasome complex (4). Insulin/insulin-like growth factor-1 (IGF-1) signaling regulates both pathways through Akt and FoxO3 in skeletal muscle (11, 21). Circulating klotho is believed to regulate insulin/IGF-1 signaling, but the biological mechanism has yet to be identified (18). A recent study suggests that the autophagiclysosomal pathway is activated in mutant klotho mice, but there was no evidence for activation of the ubiquitin-proteasomal pathway or insulin/IGF-1 signaling in klotho mice (13).
The strengths of the present study are that the participants were drawn from a population-based sample of older adults living in the community. Grip strength was carefully assessed using standardized methodology. Findings were based upon baseline and six years of follow-up data. Although the analyses were adjusted for potential confounders such as age and chronic diseases, as with any epidemiological study, there may be unmeasured confounding factors that could influence the relationship between plasma klotho and muscle strength. The study populations consisted of white adults from the rural Chianti region of Tuscany, Italy, and whether these findings can be extrapolated to populations in other parts of the world and to minorities is not known.
Future studies are needed to examine the relationship between circulating klotho and skeletal muscle mass and changes in skeletal muscle mass over time.
Acknowledgments
GRANTS
This work was supported by National Institute on Aging (NIA) Grant R01 AG027012, R01 HL094507, the Italian Ministry of Health (ICS110.1/RF97.71), NIA contracts 263 MD 9164, 263 MD 821336, N.1-AG-1-1, N.1-AG-1-2111, and N01-AG-5-0002, the Intramural Research Program of NIA, National Institutes of Health, Baltimore, Maryland.
REFERENCES
- 1.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29(suppl 1):S43–S48. [PubMed] [Google Scholar]
- 2.Carmeli E, Coleman R, Reznick AZ. The biochemistry of aging muscle. Exp Gerontol. 2002;37:477–489. doi: 10.1016/s0531-5565(01)00220-0. [DOI] [PubMed] [Google Scholar]
- 3.Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoederop JG. The β-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–493. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- 4.Clague MJ, Urbé S. Ubiquitin: same molecule, different degradation pathways. Cell. 2010;143:682–685. doi: 10.1016/j.cell.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Doherty TJ. Aging and sarcopenia. J Appl Physiol. 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 6.Doi S, Zou Y, Togao O, Pastor JV, Johns GB, Wang L, Shiizaki K, Gotschall R, Schiavi S, Yorioka N, Takahashi M, Boothman DA, Kuro-o M. Klotho inhibits transforming growth actor-β-1 (TGF-β1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. 2011 Jan 5; doi: 10.1074/jbc.M110.174037. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 8.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 9.Friedewald WT, Levy RI, Frederickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparation ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 10.Giampaoli S, Ferrucci L, Cecchi F, Lo Noce C, Poce A, Dima F, Santaquilani A, Vescio MF, Menotti A. Hand-grip strength predicts incident disability in non-disabled older men. Age Ageing. 1999;28:283–288. doi: 10.1093/ageing/28.3.283. [DOI] [PubMed] [Google Scholar]
- 11.Glass DJ. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nature Cell Biol. 2003;5:87–90. doi: 10.1038/ncb0203-87. [DOI] [PubMed] [Google Scholar]
- 12.Guralnik JM, Fried LP, Simonsick EM, et al. The Women's Health and Aging Study: Health and Social Characteristics of Older Women with Disability. National Institute on Aging; Bethesda, MD: 1995. NIH Publication No. 95-4009. [Google Scholar]
- 13.Iida R, Kanko S, Suga T, Morito M, Yamane A. Autophagic-lysosomal pathway functions in the masset and tongue muscles in the klotho mouse, a mouse model for aging. Mol Cell Biochem. 2011;348:89–98. doi: 10.1007/s11010-010-0642-z. [DOI] [PubMed] [Google Scholar]
- 14.Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. Secreted klothoprotein in sera and CSF: implications for post-translational cleavage in release of klotho protein from cell membrane. FEBS Lett. 2004;565:143–147. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 15.Imura A, Tsuji Y, Murata M, Maeda R, Kubota K, Iwano A, Obuse C, Togashi K, Tominaga M, Kita N, Tomiyama K, Iijima J, Nabeshima Y, Fujioka M, Asato R, Tanaka S, Kojima K, Ito J, Nozaki K, Hashimoto N, Ito T, Nishio T, Uchiyama T, Fujimori T, Nabeshima YI. α-klotho as a regulator of calcium homeostasis. Science. 2007;316:1615–1618. doi: 10.1126/science.1135901. [DOI] [PubMed] [Google Scholar]
- 16.Ito S, Kinoshita S, Shiraishi N, Nakagawa S, Sekine S, Fujimori T, Nabeshima YI. Molecular cloning and expression analyses of mouse betaklotho, which encodes a novel Klotho family protein. Mech Dev. 2000;98:115–119. doi: 10.1016/s0925-4773(00)00439-1. [DOI] [PubMed] [Google Scholar]
- 17.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima Y. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;1997:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 18.Kuro-o M. Klotho. Eur J Physiol. 2010;459:333–343. doi: 10.1007/s00424-009-0722-7. [DOI] [PubMed] [Google Scholar]
- 19.Kurosu H, Yamamoto M, Clark JE, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone klotho. Science. 2005;308:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 21.Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458–471. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Comm. 1998;242:626–630. doi: 10.1006/bbrc.1997.8019. [DOI] [PubMed] [Google Scholar]
- 23.McGee VE, Willard TC. Piecewise regression. J Am Stat Assoc. 1970;65:1109–1124. [Google Scholar]
- 24.Mehrpour M, Esclatine A, Beau I, Codogno P. Autophagy in health and disease. I. Regulation and significance of autophagy: an overview. Am J Physiol Cell Physiol. 2010;298:C776–C785. doi: 10.1152/ajpcell.00507.2009. [DOI] [PubMed] [Google Scholar]
- 25.Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001;137:231–243. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- 26.Qian SS, King RS, Richardson CJ. Two statistical methods for the detection of environmental thresholds. Ecological Modelling. 2003;166:87–97. [Google Scholar]
- 27.Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, White L. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–560. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- 28.Rantanen T, Volpato S, Ferrucci L, Heikkinen E, Fried LP, Guralnik JM. Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J Am Geriatr Soc. 2003;51:636–641. doi: 10.1034/j.1600-0579.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- 29.Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, Kuro-o M, Nabeshima Y, Kurabayashi M, Nagai R. In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Comm. 2000;276:767–772. doi: 10.1006/bbrc.2000.3470. [DOI] [PubMed] [Google Scholar]
- 30.Saito Y, Yamagishi T, Nakamura T, Ohyama Y, Aizawa H, Suga T, Matsumura Y, Masuda H, Kurabayashi M, Kuro-o M, Nabeshima Y, Nagai R. Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Comm. 1998;248:324–329. doi: 10.1006/bbrc.1998.8943. [DOI] [PubMed] [Google Scholar]
- 31.Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, Guralnik JM, Ferrucci L. Plasma klotho and mortality risk in older community-dwelling adults. J Gerontol A Biol Sci Med Sci. doi: 10.1093/gerona/glr058. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444 doi: 10.1038/nature05315. doi:10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Sun Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension. 2009;54:810–817. doi: 10.1161/HYPERTENSIONAHA.109.134320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, Hasegawa H, Yamashita T, Nakatani K, Saito Y, Okamoto N, Kurumatani N, Namba N, Kitaoka T, Ozono K, Sakai T, Hataya H, Ichikawa S, Imel EA, Econs MJ, Nabeshima YI. Establishment of a sandwich ELISA for soluble alpha-klotho measurements: age-dependent change of soluble alpha-klotho levels in healthy subjects. Biochem Biophys Res Commun. 2010;398:513–518. doi: 10.1016/j.bbrc.2010.06.110. [DOI] [PMC free article] [PubMed] [Google Scholar]