Abstract
The link between Attention-deficit/Hyperactivity Disorder (ADHD) and substance use disorders (SUD) continues to be an area of great interest. In this report we discuss more recent work exploring the developmental relationship between ADHD and SUD and associated concurrent disorders. Recent work highlights the role of treatment of ADHD in children on subsequent cigarette smoking and SUD in adolescence and adulthood. Recent diagnostic issues examining ADHD in SUD populations are highlighted. Recent studies in patients with ADHD and SUD suggest that SUD treatment needs to be sequenced initially with ADHD treatment quickly thereafter.
Keywords: ADHD, SUD, Treatment, Prevention treatment
Introduction
The intersection of Attention deficit/Hyperactivity Disorder (ADHD) and alcohol or drug abuse or dependence (referred to here as substance use disorders [SUD]) in adolescents and adults has been an area of increasing awareness. ADHD affects from 6 to 9% of children and adolescents and up to 5% of adults worldwide [1*, 2**]. Longitudinal data suggest that childhood ADHD persists in 75% of cases into adolescence and in approximately one-half of cases into adulthood (for review see [1*]). SUD usually onset in adolescence or early adulthood and affect between 10 to 30% of U.S. adults, and approximately 8.9% of adolescents manifest a drug use disorder and 6.4% an alcohol use disorder [2**]. The study of comorbidity between SUD and ADHD is relevant to both research and clinical practice in developmental pediatrics, psychology, and psychiatry with implications for diagnosis, prognosis, treatment, and health care delivery.
ADHD in SUD
Higher than expected rates of ADHD are found in populations with SUD. Studies incorporating structured psychiatric diagnostic interviews assessing ADHD and other disorders in substance abusing groups have indicated that from one quarter of adults with SUD and one-half of adolescents with SUD have ADHD. Data ascertained from adult groups with SUD also show a higher risk for ADHD, as well as earlier onset and more severe SUD associated with ADHD (for review see [1*, 3*, 4**]).
ADHD as a Risk Factor for SUD
Since ADHD manifests earlier than SUD, the risk for SUD resulting in ADHD is unlikely (see Figure 1). Thus, it is important to evaluate to what extent ADHD is a precursor of SUD. Prospective studies of ADHD children have provided evidence that ADHD itself is a risk for later cigarette smoking and SUD [4**]. For instance, a recent meta-analysis of 13 studies showed that ADHD was associated with alcohol and drug use disorders in adulthood and nicotine use in adolescence. Children with ADHD who also have conduct and/or bipolar disorders co-occurring with ADHD seem to have the poorest outcome with respect to developing SUD and major morbidity. For instance, in a longitudinal study of adolescents with and without ADHD, conduct disorder, and SUD, Brook et al. found that ADHD is related to SUD, but that the main effect was related to conduct disorder [5*]. Also, as part of an ongoing prospective study of ADHD we found differences in the risk for SUD in ADHD adolescents and young adults (mean age 21 years) compared to non-ADHD controls which were highest in those with comorbid conduct disorder at baseline [6*]. Interestingly, as part of a 10-year follow-up through adolescence, we did not find that any features within ADHD such as family history of SUD, cognitive impairment, socialization, or family environment predicted later SUD [6*]. Similarly, we did not find that operationally defined neuropsychological abnormalities indicative of executive function deficits predicted SUD in our ADHD or control youth in older adolescence and young adulthood [7*].
Figure 1.
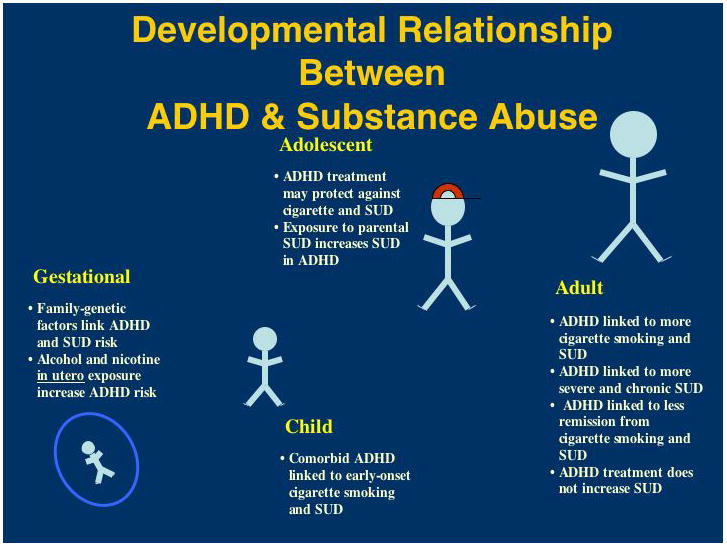
Developmental relationship between ADHD and substance abuse across the lifespan.
ADHD Treatment and SUD
Controversy continues in the lay and scientific literature about the effects of ADHD treatment and later SUD. Since prospective studies in ADHD youth are naturalistic, and hence not randomized for treatment, attempts to unravel positive or deleterious effects of treatment from the severity of the underlying condition(s) creates serious confounds. Although concerns of the potential kindling of specific types of abuse (i.e. stimulants) secondary to early stimulant exposure in ADHD children have been raised, the preponderance of clinical data do not support such a contention.
Recent work has shed more light on this issue. Biederman et al. [8] and Mannuzza et al. [9] simultaneously reported that early stimulant treatment did not increase nor decrease the risk for subsequent SUD in young adulthood. We reported in a sample of adolescent girls with ADHD that stimulant treatment resulted in an almost two-fold reduction in cigarette smoking and SUD [10]. Of interest, we did not find that the onset or duration of stimulant treatment was related to the risk for SUD in distinction to Mannuzza et al. [9]. Interestingly, in reviewing neuroimaging findings, some have conjectured that ADHD and SUD-related craving share neurobiological similarities, and that treatment of ADHD may reduce craving for substances and subsequently reduce the risk for relapse to substance use [3*].
An aggregate of the literature seems to suggest that early stimulant treatment reduces or delays the onset of SUD and perhaps cigarette smoking into adolescence; however, the protective effect is lost in adulthood. The loss of the protective effect in adults may be a reflection of findings in adolescents not spanning through the full age of risk of SUD or that most adolescents have stopped their ADHD treatment during later adolescence and young adulthood, thus losing the protective effect of stimulants [11*].
SUD Pathways Associated with ADHD
An increasing body of literature shows an intriguing association between ADHD and cigarette smoking [12*, 13]. ADHD is a significant predictor of early initiation cigarette smoking (before age 15), and conduct and mood disorders coupled with ADHD put youth at particularly high risk for early onset smoking. Data also suggest that smoking kindles youth with ADHD for an even higher risk for SUD. This is not surprising given that smoking leads to peer group pressures and availability of illicit substances and that biologically, nicotine exposure may make the brain more susceptible to later behavioral problems and SUD. Furthermore, nicotinic modulating agents are being evaluated for the treatment of ADHD.
The precise mechanism(s) mediating the expression of SUD in ADHD populations remains to be seen. The self-medication hypothesis is compelling in ADHD considering that the disorder is chronic and often associated with demoralization and failure, factors frequently associated with SUD in adolescents. Among substance abusing adolescents with and without ADHD, ADHD adolescents have reported using substances more frequently to attenuate their moods and to help them sleep. However, no evidence of differences in types of substances has emerged between groups [14]. Other important links include family/genetic contributions [15] and exposure to parental SUD during vulnerable developmental phases [16].
Diagnosis and Treatment of ADHD and SUD
There has been much debate on the age of onset criteria currently used for diagnosing older adolescents and adults with ADHD. Barkley has proposed that requiring child-based symptoms for adults to meet the diagnosis of ADHD is too stringent [17]. Studies have shed important light on establishing the diagnosis of ADHD in adults with ADHD and SUD who fail to have clear recollection of symptom onset in childhood. Faraone et al. [18] have examined differences between groups of adults with ADHD based on age of onset of symptoms of ADHD. In this study, researchers investigated whether differences existed between 79 adults who met full current criteria for ADHD but did not have a clear track of symptoms prior to age 7 years and 127 adults who had their ADHD onset prior to age 7 years [18]. Interestingly, no differences in rates of psychiatric comorbidity, SUD, family history of ADHD, or impairment were found between groups of adults with full criteria ADHD and those who had a longitudinal track of ADHD without clear onset in youth, supporting the growing contention of problems with using the current onset of symptoms prior to age 7 years stringently, particularly in adults with SUD. In a study examining symptom prevalence of ADHD in a residential substance abuse treatment program using contemporary methods, McAweeney et al. [19**] found that the prevalence rate of ADHD from the program clinical records was 3%, while the prevalence rate from the study assessments was 44% suggesting that ADHD may be under diagnosed in addiction treatment programs.
The treatment needs of individuals with SUD and ADHD need to be considered simultaneously; however, if possible, the SUD should be addressed initially. If the SUD is active, immediate attention needs to be paid to stabilization of the addiction(s). Depending on the severity and duration of the SUD, individuals may require inpatient treatment. Self help groups offer a helpful treatment modality for many with SUD. In tandem with addiction treatment, SUD individuals with ADHD require intervention(s) for ADHD (and if applicable, comorbid psychiatric disorders).
The efficacy of various psychotherapeutic interventions for populations with ADHD and SUD remains to be established. However, data suggests efficacy of cognitive behavioral therapies for adults with ADHD in both individual [20*] and group settings [21*]—as well for SUD. It appears that effective psychotherapy for this comorbid group combines the following elements: structured and goal-directed sessions, proactive therapist involvement, and knowledge of SUD and ADHD.
Medication serves an important role in reducing the symptoms of ADHD and other concurrent psychiatric disorders. Effective agents for ADHD include the stimulants, noradrenergic agents, and catecholaminergic antidepressants. In general, while open studies are more encouraging, results from controlled trials with stimulants and/or bupropion suggest that ADHD pharmacotherapy used in adults with ADHD plus SUD has meager effects on the ADHD and substance use or cravings. Multiple recent studies shed light on this issue. A well conducted, pilot, placebo-controlled 12-week study of osmotic-release oral system methylphenidate (OROS MPH) (72 mg) in 24 adults with amphetamine abuse and ADHD indicated no significant differences in outcome for neither ADHD nor SUD [22*]. A multisite, NIH funded placebo-controlled study of stimulants in adult smokers with ADHD was recently published [23**]. In this 11-week study, OROS MPH/placebo was dosed to 72 mg/day in 255 adults with ADHD who were also treated with the nicotine patch to examine the effects on cigarette cessation and ADHD. The results of this trial showed improved ADHD but no effects on rates of cigarette cessation [23**]. Of interest, there was no increased cigarette smoking in the medicated group and side effects in these adults were similar to those noted in previous stimulant trials. Data from another NIH multisite study of treatment of adolescents with ADHD and SUD was also recently reported [24]. In this 16-week placebo-controlled study, 300 adolescents with mixed SUD received OROS MPH/placebo to 72 mg/day along with weekly individual cognitive behavioral therapy. Both treatment arms resulted in significant improvement compared to baseline; however, there was no significant improvement in ADHD (investigator/parent) or SUD (adolescent self report) between treatment groups. Side effects were reminiscent of adolescent studies and the medication was reported to be of low abuse liability.
In ADHD adults with recent SUD, the nonstimulant agents (atomoxetine), antidepressants (bupropion), and extended-release or longer acting stimulants with lower abuse liability and diversion potential are preferable [3*]. The broad spectrum of activity in ADHD, lack of abuse liability, and recent work showing relative safety in the context of active SUD generate particular interest in studying atomoxetine in adults with alcohol use disorders [25]. In a 12-week multisite study in recently abstinent alcoholics, atomoxetine (compared to placebo) was effective in treating ADHD, and reducing recurrent episodes of heavy drinking, but not relapse [25]. In a separate analysis, atomoxetine administration in heavy relative to light or nondrinkers was associated with more side effects; yet, there were no serious adverse events nor evidence of impaired liver functioning in the heavy drinkers in these relatively short terms trials [26]. In a similar 10-week, open-label study, atomoxetine was effective in treating ADHD symptoms as well as intensity, frequency, and length of cravings in recently abstinent adults with SUD and comorbid ADHD, and no serious adverse events were reported [27*]. However, these promising data in abstinent alcoholics need to be tempered against recent work in currently using adolescents with SUD. Thurstone et al. [28*] studied 70 adolescents with ADHD and at least one active non-nicotine SUD who received 12 weeks of atomoxetine or placebo in addition to motivational interviewing/cognitive behavioral therapy. There were no differences between ADHD scores or in use of substances between treatment groups that emerged during the study. The authors speculated that the therapy may have contributed to a larger than expected placebo response. The above findings linked with an older literature suggest that medications used in adolescents and adults with ADHD plus active SUD have only a meager effect on ADHD, and an even smaller effect on cigarette or substance use. It may be that some abstinence may result in improved outcomes for both ADHD and SUD. Regardless of the pharmacotherapy being administered, individuals with ADHD and SUD should be monitored frequently. Evaluation of compliance with treatment, questionnaires, random toxicology screens as indicated, and coordination of care with addiction counselors and other caregivers are integral components of effective treatment.
Issues of Misuse and Diversion
There has been substantial interest in misuse and diversion of stimulants prescribed for ADHD (for review see [29]). The majority of individuals treated for their ADHD use their medications appropriately, however survey studies have indicated that approximately 5% of college students have misused stimulants [29]. This practice is more common in competitive colleges and the stimulants are more often misused for their pro-cognitive effects than euphoria. In a secondary analysis of an internet-based survey, young adults with higher Adult ADHD Self-Report Scale (ASRS) scores were significantly more likely to engage in non-medical use of ADHD medications, while young adults with lower scores were significantly less likely to engage in non-medical use [30*]. In a longitudinal study utilizing the ASRS, significantly higher symptoms of ADHD were observed in college students who had an ongoing pattern of persistent non-medical use of prescription stimulants [31*]. These studies suggest that young adult non-medical users are self-medicating undiagnosed or untreated ADHD. The majority of high school and college students who misuse stimulants obtain them from friends, with only a minority reporting “scamming” local practitioners to obtain stimulants. For instance, of high school students who were receiving prescribed stimulants, 15% had given and 7% had sold their medications to other students [29]. Not surprisingly, a recent study reported that students with ADHD are often pressured into selling or letting others have their own stimulants [32*]. Similar to other studies [29], those to whom the stimulants were diverted misused the stimulants in context with other substances of abuse and other psychopathology such as depression and conduct disorder.
Basic science and behavioral studies suggest that the preparations of stimulants may have an impact on misuse and diversion [33]. For instance, less misuse of extended- compared to immediate-release stimulants has been reported. Likewise, using simultaneous neuroimaging and self-report queries, less likeability has been reported with extended-release methylphenidate compared to equipotent doses of immediate-release methylphenidate [34]. Recent work further separates abuse liability among the various extended-release formulations of methylphenidate [35*]. Work with lisdexamfetamine, a prodrug stimulant that is metabolized in vivo to d-amphetamine, indicates less likelihood for intravenous or intranasal abuse compared to equipotent d-amphetamine [36*]. Hence, a growing literature suggests the consideration of extended-release stimulant preparations in populations at higher risk to misuse or divert their stimulants.
Conclusion
While the existing literature has provided important information on the relationship of ADHD and SUD, it also points to a number of areas in need of further study. The mechanism by which untreated ADHD leads to SUD, as well as the specificity of risk reduction of ADHD treatment on cigarette smoking and SUD at apparent developmental points needs to be better understood. Given the prevalence and major morbidity and impairment caused by ADHD and SUD, prevention and treatment strategies for these adolescents and adults need be further developed and evaluated.
Acknowledgments
This research was supported by NIH R01 DA14419 and K24 DA016264 to TW. Dr. Wilens is receiving or has received grant support from Abbott, McNeil, Lilly, NIH (NIDA), Merck, and Shire; has been a speaker for Eli Lilly, McNeil, Novartis, and Shire, and is or has been a consultant for Abbott, AstraZeneca, McNeil, NIH, Lilly, Novartis, Merck, and Shire.
References
Note: Papers of particular interest, published within the annual period of review, have been highlighted as:
*of special interest
**of outstanding interest
- *1.Wilens TE, Spencer TJ. Understanding attention-deficit/hyperactivity disorder from childhood to adulthood. Postgrad Med. 2010 Sep;122(5):97–109. doi: 10.3810/pgm.2010.09.2206. This review provides a comprehensive discussion pertaining to the co-occurring disorders, diagnoses, and treatment modalities of ADHD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **2.Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010 Oct;49(10):980–9. doi: 10.1016/j.jaac.2010.05.017. This article’s findings provide contemporary prevalence data on a broad range of mental disorders in a nationally representative sample of U.S. adolescents and suggest that approximately one in every four to five youth in the U.S. meets criteria for a mental disorder with severe impairment across their lifetime. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *3.Frodl T. Comorbidity of ADHD and substance use disorder (SUD): a neuroimaging perspective. J Atten Disord. 2010 Sep;14(2):109–20. doi: 10.1177/1087054710365054. This review of the literature identifies neurobiological similarities between ADHD and SUD. [DOI] [PubMed] [Google Scholar]
- **4.Charach A, Yeung E, Climans T, Lillie E. Childhood attention-deficit/hyperactivity disorder and future substance use disorders: comparative meta-analyses. J Am Acad Child Adolesc Psychiatry. 2011 Jan;50(1):9–21. doi: 10.1016/j.jaac.2010.09.019. This meta-analysis points to the risk of developing SUD and nicotine use in children diagnosed with ADHD. The findings suggest that childhood ADHD is associated with alcohol and drug use disorders in adulthood and with nicotine use in adolescence. [DOI] [PubMed] [Google Scholar]
- *5.Brook DW, Brook JS, Zhang C, Koppel J. Association between attention-deficit/hyperactivity disorder in adolescence and substance use disorders in adulthood. Arch Pediatr Adolesc Med. 2010 Oct;164(10):930–4. doi: 10.1001/archpediatrics.2010.180. This is a longitudinal study that examines the role of conduct disorder in context of the relationship between ADHD and SUD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *6.Wilens T. Does ADHD predict substance use disorders? A 10-year follow-up study of young adults with ADHD. J Am Acad Child Adolesc Psychiatry. doi: 10.1016/j.jaac.2011.01.021. In press. This 10-year longitudinal follow-up study examines the risk of SUD in ADHD populations and evaluates the role of risk factors including comorbid disorders and cognitive dysfunction within ADHD that predicts later SUD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *7.Wilens T, Martelon M, Fried R, et al. Do executive function deficits predict later substance use disorders among adolescents and young adults? J Am Acad Child Adolesc Psychiatry. In press. This is among the largest longitudinal studies examining cognitive dysfunction and later SUD. The results of this study do not support the hypothesis that neuropsychologically defined executive function deficits predict later stable cigarette smoking or SUD in children with or without ADHD growing up, however stable cigarette smoking is associated with subsequent executive function deficits. [Google Scholar]
- 8.Biederman J, Monuteaux MC, Spencer T, et al. Stimulant therapy and risk for subsequent substance use disorders in male adults with ADHD: a naturalistic controlled 10-year follow-up study. Am J Psychiatry. 2008 May;165(5):597–603. doi: 10.1176/appi.ajp.2007.07091486. [DOI] [PubMed] [Google Scholar]
- 9.Mannuzza S, Klein RG, Truong NL, et al. Age of methylphenidate treatment initiation in children with ADHD and later substance abuse: prospective follow-up into adulthood. Am J Psychiatry. 2008 May;165(5):604–9. doi: 10.1176/appi.ajp.2008.07091465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilens TE, Adamson J, Monuteaux MC, et al. Effect of prior stimulant treatment for attention-deficit/hyperactivity disorder on subsequent risk for cigarette smoking and alcohol and drug use disorders in adolescents. Arch Pediatr Adolesc Med. 2008 Oct;162(10):916–21. doi: 10.1001/archpedi.162.10.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *11.Bejerot S, Ryden EM, Arlinde CM. Two-year outcome of treatment with central stimulant medication in adult attention-deficit/hyperactivity disorder: a prospective study. J Clin Psychiatry. 2010 Dec;71(12):1590–7. doi: 10.4088/JCP.09m05168pur. Bejerot et al.’s prospective study of stimulant treatment in ADHD provides further evidence of the longer term effectiveness and general tolerability of stimulants in adults with ADHD. [DOI] [PubMed] [Google Scholar]
- *12.Kollins SH, McClernon FJ, Van Voorhees EE. Monetary incentives promote smoking abstinence in adults with attention deficit hyperactivity disorder (ADHD) Exp Clin Psychopharmacol. 2010 Jun;18(3):221–8. doi: 10.1037/a0019565. Kollins et al. investigate the use of monetary incentives as an evidence-based approach to reducing cigarette smoking in ADHD populations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willoughby MT, Kollins SH, McClernon FJ. Association between smoking and retrospectively reported attention-deficit/hyperactivity disorder symptoms in a large sample of new mothers. Nicotine Tob Res. 2009 Mar;11(3):313–22. doi: 10.1093/ntr/ntp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biederman J, Wilens T, Mick E, et al. Is ADHD a risk for psychoactive substance use disorder? Findings from a four year follow-up study. J Am Acad Child Adolesc Psychiatry. 1997;36:21–9. doi: 10.1097/00004583-199701000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Biederman J, Petty CR, Wilens TE, et al. Familial risk analyses of attention deficit hyperactivity disorder and substance use disorders. Am J Psychiatry. 2008 Jan;165(1):107–15. doi: 10.1176/appi.ajp.2007.07030419. [DOI] [PubMed] [Google Scholar]
- 16.Yule AM, Wilens TE, Martelon M, et al. Impact of exposure to parental substance use disorders (SUD) on SUD risk in girls and their siblings. American Academy of Child and Adolescent Psychiatry; 2010 October; New York, NY. [Google Scholar]
- 17.Barkley RA. ADHD in Adults. Sudbury, MA: Jones and Bartlett Publishers; 2010. [Google Scholar]
- 18.Faraone SV, Biederman J, Spencer TJ, et al. Diagnosing adult attention deficit hyperactivity disorder: Are late onset and subthreshold diagnoses valid? Am J Psychiatry. 2006 Oct;163(10):1720–9. doi: 10.1176/ajp.2006.163.10.1720. [DOI] [PubMed] [Google Scholar]
- **19.McAweeney M, Rogers NL, Huddleston C, et al. Symptom prevalence of ADHD in a community residential substance abuse treatment program. J Atten Disord. 2010 May;13(6):601–8. doi: 10.1177/1087054708329973. This is a systematic examination of chart-report vs assessment based prevalence of ADHD in a SUD treatment center. The ADHD Self-Report Scale (ASRS) is discussed and recommended in the context of substance abuse treatment programs. The authors assert that ADHD may be under diagnosed in these treatment programs. [DOI] [PubMed] [Google Scholar]
- *20.Safren SA, Sprich S, Mimiaga MJ, et al. Cognitive behavioral therapy vs relaxation with educational support for medication-treated adults with ADHD and persistent symptoms: a randomized controlled trial. JAMA. 2010 Aug 25;304(8):875–80. doi: 10.1001/jama.2010.1192. This randomized controlled trial suggests that individually administered cognitive behavioral therapy can be used in conjunction with medication to reduce severe ADHD symptoms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *21.Solanto MV, Marks DJ, Wasserstein J, et al. Efficacy of meta-cognitive therapy for adult ADHD. Am J Psychiatry. 2010 Aug;167(8):958–68. doi: 10.1176/appi.ajp.2009.09081123. This controlled study highlights the efficacy of a form of meta-cognitive therapy for ADHD in a group modality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *22.Konstenius M, Jayaram-Lindstrom N, Beck O, Franck J. Sustained release methylphenidate for the treatment of ADHD in amphetamine abusers: a pilot study. Drug Alcohol Depend. 2010 Apr 1;108(1–2):130–3. doi: 10.1016/j.drugalcdep.2009.11.006. Other studies have examined the efficacy of stimulant treatment on cocaine and alcohol dependent individuals with ADHD, but this pilot study sought to test the feasibility of treating amphetamine dependent patients. [DOI] [PubMed] [Google Scholar]
- **23.Winhusen TM, Somoza EC, Brigham GS, et al. Impact of attention-deficit/hyperactivity disorder (ADHD) treatment on smoking cessation intervention in ADHD smokers: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2010 Dec;71(12):1680–8. doi: 10.4088/JCP.09m05089gry. This multisite controlled study shows the efficacy of osmotic-release oral system methylphenidate (OROS MPH) in terms of ADHD treatment but maintains that OROS MPH does not improve smoking cessation success in adult smokers with ADHD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riggs P. Multi-site of OROS-MPH for ADHD in substance abusing adolescents. Scientific proceedings of the 56th annual meeting for the American Academy of Child and Adolescent Psychiatry AACAP; 2009; Hawaii. [Google Scholar]
- 25.Wilens TE, Adler LA, Weiss MD, et al. Atomoxetine treatment of adults with ADHD and comorbid alcohol use disorders. Drug Alcohol Depend. 2008 Jul 1;96(1–2):145–54. doi: 10.1016/j.drugalcdep.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Adler L, Wilens T, Zhang S, et al. Retrospective safety analysis of atomoxetine in adult ADHD patients with or without comorbid alcohol abuse and dependence. Am J Addict. 2009 Sep-Oct;18(5):393–401. doi: 10.3109/10550490903077663. [DOI] [PubMed] [Google Scholar]
- *27.Adler L, Guida F, Irons S, Shaw D. Open label pilot study of atomoxetine in adults with ADHD and substance use disorder. J Dual Diagnosis. 2010;6:196–207. In this open-label atomoxetine trial, investigator-rated and participant-rated ADHD symptoms in abstinent alcohol/drug abusers improved, suggesting the potential efficacy of atomoxetine in ADHD plus SUD populations. [Google Scholar]
- *28.Thurstone C, Riggs PD, Salomonsen-Sautel S, Mikulich-Gilbertson SK. Randomized, controlled trial of atomoxetine for attention-deficit/hyperactivity disorder in adolescents with substance use disorder. J Am Acad Child Adolesc Psychiatry. 2010 Jun;49(6):573–82. doi: 10.1016/j.jaac.2010.02.013. This randomized and controlled trial of atomoxetine for adolescents with ADHD and SUD failed to show separation between groups; however, the authors speculated that therapy may have contributed to a large treatment response in the placebo group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilens TE, Adler LA, Adamson J, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008 Jan;47(1):21–31. doi: 10.1097/chi.0b013e31815a56f1. [DOI] [PubMed] [Google Scholar]
- *30.Upadhyaya HP, Kroutil LA, Deas D, et al. Stimulant formulation and motivation for nonmedical use of prescription attention-deficit/hyperactivity disorder medications in a college-aged population. Am J Addict. 2010 Nov;19(6):569–77. doi: 10.1111/j.1521-0391.2010.00078.x. This secondary analysis of a survey study of college students investigates the motives behind non-medical use of ADHD medications. The authors suggest that a substantial portion of non-medical use of ADHD medications may be related to self-medication of ADHD symptoms. [DOI] [PubMed] [Google Scholar]
- *31.Amelia AM, Garnier-Dykstra LM, Caldeira KM, et al. Persistent nonmedical use of prescription stimulants among college students: possible association with ADHD symptoms. J Atten Disord. 2010;XX(X):1–10. doi: 10.1177/1087054710367621. This longitudinal study examines the association between untreated ADHD symptoms and persistent non-medical use of prescription stimulants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *32.Janusis GM, Weyandt LL. An exploratory study of substance use and misuse among college students with and without ADHD and other disabilities. J Atten Disord. 2010 Nov;14(3):205–15. doi: 10.1177/1087054710367600. The results of this study suggest that students with ADHD are more likely to use or misuse prescription stimulant medication than students without ADHD. [DOI] [PubMed] [Google Scholar]
- 33.Kollins SH. ADHD, substance use disorders, and psychostimulant treatment: current literature and treatment guidelines. J Atten Disord. 2008 Sep;12(2):115–25. doi: 10.1177/1087054707311654. [DOI] [PubMed] [Google Scholar]
- 34.Spencer TJ, Biederman J, Ciccone PE, et al. PET study examining pharmacokinetics, detection and likeability, and dopamine transporter receptor occupancy of short- and long-acting oral methylphenidate. Am J Psychiatry. 2006 Mar;163(3):387–95. doi: 10.1176/appi.ajp.163.3.387. [DOI] [PubMed] [Google Scholar]
- *35.Spencer TJ, Bonab AA, Dougherty DD, et al. A PET study examining pharmacokinetics and dopamine transporter occupancy of two long-acting formulations of methylphenidate in adults. Int J Mol Med. 2010 Feb;25(2):261–5. This study examines differences between peripheral pharmacokinetics and central nervous system dopamine transporter (DAT) occupancies of osmotically controlled-release oral delivery system (OROS) and diffucaps bead-delivery system (DBDS) methylphenidate - demonstrating likeability differences based on preparation of the extended release stimulant. [PubMed] [Google Scholar]
- 36.Jasinski DR, Krishnan S. Abuse liability and safety of oral lisdexamfetamine dimesylate in individuals with a history of stimulant abuse. J Psychopharmacol. 2009 Jun;23(4):419–27. doi: 10.1177/0269881109103113. [DOI] [PubMed] [Google Scholar]


