Abstract
The auditory system of monkeys includes a large number of interconnected subcortical nuclei and cortical areas. At subcortical levels, the structural components of the auditory system of monkeys resemble those of nonprimates, but the organization at cortical levels is different. In monkeys, the ventral nucleus of the medial geniculate complex projects in parallel to a core of three primary-like auditory areas, AI, R, and RT, constituting the first stage of cortical processing. These areas interconnect and project to the homotopic and other locations in the opposite cerebral hemisphere and to a surrounding array of eight proposed belt areas as a second stage of cortical processing. The belt areas in turn project in overlapping patterns to a lateral parabelt region with at least rostral and caudal subdivisions as a third stage of cortical processing. The divisions of the parabelt distribute to adjoining auditory and multimodal regions of the temporal lobe and to four functionally distinct regions of the frontal lobe. Histochemically, chimpanzees and humans have an auditory core that closely resembles that of monkeys. The challenge for future researchers is to understand how this complex system in monkeys analyzes and utilizes auditory information.
The auditory system of mammals includes a large number of interconnected nuclei and cortical areas (Fig. 1). The complexity of this array of interacting structures presents a challenge to researchers to determine how this system analyzes and uses auditory information. Although most of the component subcortical nuclei can be identified in a range of studied mammals, considerable variability in at least cortical organization appears to exist in different lines of evolution, and this variability amplifies the task from understanding the auditory system to understanding auditory systems and their variability.
Figure 1.
Cortical and subcortical connections of the primate auditory system. Major cortical and subcortical regions are color coded. Subdivisions within a region have the same color. Solid black lines denote established connections. Dashed lines indicate proposed connections based on findings in other mammals. Joined lines ending in brackets denotes connections with all fields in that region. The belt region may include an additional field, MM (see Fig. 5). Abbreviations of subcortical nuclei: AVCN, anteroventral cochlear nucleus; PVCN, posteroventral cochlear nucleus; DCN, dorsal cochlear nucleus; LSO, lateral superior olivary nucleus; MSO, medial superior olivary nucleus; MNTB, medial nucleus of the trapezoid body; DNLL, dorsal nucleus of the lateral lemniscus; VNLL, ventral nucleus of the lateral lemniscus; ICc, central nucleus of the inferior colliculus; ICp, pericentral nucleus of the inferior colliculus; ICdc, dorsal cortex of the inferior colliculus; ICx, external nucleus of the inferior colliculus; MGv, ventral nucleus of the medial geniculate complex; MGd, dorsal nucleus of the medial geniculate complex; MGm, medial/magnocellular nucleus of the medial geniculate complex; Sg, suprageniculate nucleus; Lim, limitans nucleus; PM, medial pulvinar nucleus. Abbreviations of cortical areas: AI, auditory area I; R, rostral area; RT; rostrotemporal area; CL, caudolateral area; CM, caudomedial area; ML, middle lateral area; RM, rostromedial area; AL, anterolateral area; RTL, lateral rostrotemporal area; RTM, medial rostrotemporal area; CPB, caudal parabelt; RPB, rostral parabelt; Tpt, temporoparietal area; TS1,2, superior temporal areas 1 and 2. Frontal lobe areas numbered after the tradition of Brodmann and based on the parcellation of Preuss and Goldmann-Rakic (1): 8a, periarcuate; 46d, dorsal principal sulcus; 12vl, ventrolateral area; 10, frontal pole; orb, orbitofrontal areas.
We have been studying the auditory system of primates, partly because we are interested in how our auditory system works, but also because primates vary in size, auditory behavior, and brain complexity. Thus, we wonder what components of the auditory system are basic and similar across the major taxonomic groups of primates, and what features vary. In particular, we are concerned about auditory cortex, because different lines of evolution vary considerably in cortical organization. For example, whereas both cats and monkeys have quite a number of visual areas, perhaps 30 or more in monkeys, they share only a few (see ref. 2). Most visual areas have emerged independently as both carnivores and primates evolved more complex visual systems. Although the organization of visual cortex in primates has been intensively studied in many laboratories over the last 30 years, efforts to determine the subdivisions and interconnections of auditory cortex have been much more limited. Yet considerable progress has been made, especially recently. Here we review theories of the organization of auditory cortex in primates. These theories have been based largely on studies of the tonotopy, connections, and architecture of auditory cortex in monkeys, but additional relevant information is starting to emerge from investigations of cortical organization in prosimian galagos, histochemical studies in chimpanzees and humans, and noninvasive functional studies in humans.
The Core Areas of Auditory Cortex
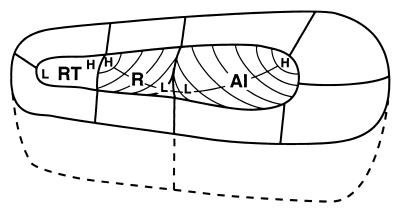
Originally, auditory cortex of monkeys was thought to be organized much as in cats, with a single primary area, AI, in the cortex of the lower bank of the lateral sulcus and a second area, AII, deeper in the sulcus (e.g., ref. 3). This concept fits well with the early view that auditory, somatosensory, and visual systems all have two fields. However, we now know that primates have a number of sensory representations for each modality, and several somatosensory and auditory fields can be considered primary or primary like in character. In the auditory system, three fields have similar primary-like features. These fields constitute the auditory core, which is surrounded by a belt of secondary fields, and a more lateral parabelt of fields at a third level of processing (Fig. 2). The core includes a most caudal AI, a more rostral field R, and an even more rostral temporal field, RT (Fig. 3). These fields are distinguished from each other by having different systematic representations of the cochlea (6–12). In caudal AI, neurons are best activated by high-frequency tones, whereas neurons in rostral AI are best activated by low-frequency tones. The lines of isorepresentation along rows of neurons across AI having similar best frequencies are curved so that neurons deeper in the fissure have higher best frequencies. The tonotopic organization of R is reversed from that in AI, so that low frequencies are represented caudally in R, and higher frequencies are represented rostrally. Again, the lines of isorepresentation are curved so that neurons deeper in the lateral sulcus have higher best frequencies. The tonotopic organization of RT has not been studied adequately, but it appears that high frequencies are represented caudally and low frequencies, rostrally.
Figure 2.
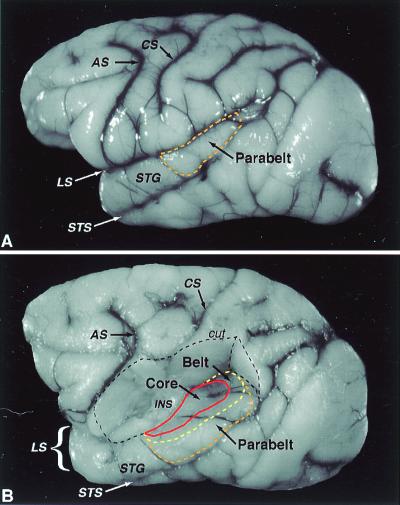
Lateral view of the macaque cerebral cortex. (A) The approximate location of the parabelt region on the superior temporal gyrus (dashed orange line). (B) Dorsolateral view of the same brain as in A after removal of the overlying parietal cortex, exposing the ventral bank of the lateral sulcus and insula. The approximate locations of the core region (solid red line), caudal and lateral portions of the belt region (dashed yellow line), and the parabelt region (dashed orange line) are shown. The medial portion of the belt region and the rostral pole of the core in the ventral circular sulcus are not visible. Dashed black line defines portion of cortex cut away. AS, arcuate sulcus; CS central sulcus; INS, insula; LS, lateral sulcus; STG superior temporal gyrus; STS, superior temporal sulcus. Adapted from ref. 4.
Figure 3.
Tonotopic organization in the core. Auditory core fields (AI, R, RT) are surrounded by belt fields (not labeled). Curved lines within each field depict isofrequency contours. High- (H) frequency acoustic stimuli are represented caudomedially in AI, rostromedially in R. Low- (L) frequency stimuli are represented rostrolaterally in AI, caudolaterally in R. Tonotopic organization in RT is not as certain but may mirror that found in R. See Fig. 1 for abbreviations.
Each of the core areas has features that are characteristic of primary sensory cortex. First, neurons in all three areas respond well and with short latencies to pure tones. The neurons respond best to specific (best) frequencies, and they have narrow frequency-response curves. Second, all three areas receive dense thalamic inputs from the principal or ventral nucleus of the medial geniculate complex (e.g., refs. 9–11, 13–19). These inputs appear to be in parallel so that the ablation of one of the fields does not deactivate the others (20). Third, all three fields have the architectonic characteristics of primary sensory cortex (5, 11, 21, 22). Thus, the fields have a well-developed layer 4 of granule cells and a dense band of myelinated fibers in the middle layers of cortex. In addition, the middle layers of the core densely express the metabolic enzyme, cytochrome oxidase (CO), the enzyme for deactivating the neurotransmitter/neuromodulator acetylcholine (acetylcholinesterase, AChE), and the calcium-binding protein, parvalbumin (Pb) (Fig. 4). Primary sensory areas characteristically express large amounts of CO, AChE, and Pb (e.g., refs. 5, 23–25), although AChE may be more obvious in developing primary areas in some mammals (26, 27). The persistence of large amounts of AChE in the auditory core of adult monkeys is interesting in that acetylcholine is a neurotransmitter associated with developmental plasticity (e.g., ref. 28). Possibly, primary auditory cortex of adult monkeys is especially plastic, so that neurons are capable of changing their response characteristics (29–31). Although the three core areas are similar in architecture and responsiveness to tones, they are unlikely to be identical in how they process auditory information. Indeed, a number of differences in response characteristics between neurons in AI and R have already been reported (32). In addition, the architectonic features of AI and R are quite similar, but they are somewhat muted in RT. Thus, RT is the least certain member of the core.
Figure 4.
Architectonic fields in auditory cortex. Macaque brain section flattened and cut parallel to pial surface at 40 μm. Parvalbumin immunohistochemistry. The core fields are the most darkly stained. The caudal belt fields (ML, CL, CM) are moderately dark. Scale bar = 5 mm. Adapted from ref. 5. See Fig. 1 for abbreviations.
The cortical connections of the core areas (see refs. 10, 11, 21, 22, 33) are somewhat unusual for primary sensory cortex. Each core area densely interconnects with its neighbor, and even AI and RT have some interconnections (Fig. 5). Thus, core areas must influence each other strongly. In addition, each core area projects to a collection of adjacent belt areas, and the core areas appear to be responsible for activating those belt areas (20, 32). The ipsilateral cortical connections of the core are exclusively, or nearly so, with the narrow surrounding belt of cortex. There are few or no long projections to more distant auditory fields. This means that the belt is an obligatory second stage of cortical processing. The belt is not bypassed, and more distant fields (e.g., parabelt) do not have direct access to core information. In contrast, primary visual cortex, V1, projects both to a surrounding belt, V2, and to a number of more distant visual areas, especially quite distant middle temporal visual area (see ref. 34). Area 3b of somatosensory cortex projects to bordering areas 3a and 1, but also more distantly to areas 2, S2, PV, and even motor cortex (e.g., refs. 35, 36). Thus, primary sensory information is more widely distributed in the somatosensory and visual systems, whereas the auditory system clearly separates three levels of cortical processing.
Figure 5.
Auditory cortical connections of AI. Area AI, as well as other core areas, has dense reciprocal connections with adjacent areas of the core and belt (solid lines with arrows). Connections with nonadjacent fields are less dense (dashed lines with arrows). The core has few, if any, connections with the parabelt or more distant cortex. See Fig. 1 for abbreviations.
The connection pattern of the auditory core is unusual in another way. The core areas also project callosally to the core of the other cerebral hemisphere, where the most dense terminations appear to be in tonotopically matched locations of the same area, and to the adjacent belt. Unlike primary visual cortex (V1) and primary area 3b of somatosensory cortex of monkeys, which have large regions of few or no callosal connections, the auditory core has major interhemispheric connections.
The clear manner in which chemoarchitectonic preparations distinguish the core from the belt in monkeys allowed us to examine other primates for the existence of a core. The core in brain sections from temporal cortex of humans and chimpanzees is quite distinct (unpublished observations; refs. 37, 38) and can be identified and delineated with great precision (Fig. 6). In both chimpanzees and humans, the areal extent of the core is greater than in macaque monkeys, but the core in all three primates has the same elongated shape. This suggests that chimpanzees and humans also have three areas within the core (AI, R, RT), but direct evidence is lacking. We do know from studies in humans that a tonotopic pattern of organization can be revealed crudely in the region of the core. A variety of studies using evoked potentials (39, 40), magnetoencephalography (41–52), positron emission tomography (53–56), and functional magnetic resonance imaging (57–60) have produced convincing evidence of tonotopic organization in the human transverse temporal gyrus of Heschl (TTG). As observed in monkeys, high frequencies are represented in the posteromedial TTG of humans, whereas lower frequencies generate activity in anterolateral TTG. Further, apparent reversals in the tonotopic gradient suggest that more than one cochleotopic field may exist in the TTG (45), consistent with evidence for multiple tonotopic fields in the core region of primates.
Figure 6.
Architectonic identification of core and belt regions. Coronal sections showing the border between the core and belt regions of auditory cortex (arrowheads). Acetylcholinesterase histochemistry. (A) Macaque monkey; (B) chimpanzee; (C) human. Compared with the belt region, the density of acetylcholinesterase in the middle cortical layers (IIIc and IV) is particularly high in the core.
The Auditory Belt Cortex
The auditory belt is the narrow 2- to 3-mm fringe of cortex immediately surrounding the core with dense interconnections with the core. The belt appears to receive only sparse inputs from the ventral nucleus of the medial geniculate complex, with most of its thalamic inputs coming from the dorsal and medial (magnocellular) divisions of the complex. Despite these thalamic inputs, the belt seems to depend on core inputs for activation. However, this dependence has been demonstrated directly only in one subdivision of the belt, the caudomedial area (CM). Ablation of the core abolishes responses to auditory stimuli in CM (20).
The belt appears to consist of about eight auditory areas, each with a distinct representation of the cochlea. The evidence for these separate areas is largely of two types. First, the core areas connect most densely with adjacent portions of the belt (Fig. 7). This means that the most dense projections to the caudal fields of the belt are from AI, whereas more rostral belt fields get their densest inputs from R or RT. Furthermore, portions of the belt get their densest inputs from immediately adjoining portions of the core, suggesting input patterns that would support multiple, but crude, representations of the cochlea. More specifically, the connection patterns support the possibility of three to four medial belt fields and four lateral belt fields with different sequences of tonotopic organization. These suppositions are supported by the results of microelectrode recordings from the belt. Although neurons in the belt respond much less vigorously to tones than neurons in the core, they respond well enough to indicate that tonotopic gradients in belt areas parallel those of adjacent core areas (12, 61). Neurons in the lateral belt respond better to narrow bands of noise than pure tones, and the center frequency of the band can be varied to indicate best frequencies for neurons (61). These studies provide evidence for three tonotopic representations lateral to the core, the caudolateral area (CL), mediolateral area (ML), and the anterior lateral area (AL). However, microelectrode recording studies have provided only limited evidence for a tonotopic representation in CM (12, 20, 32), and other medial belt areas have not been studied adequately. Thus, these divisions of the belt are more tentatively proposed and are largely based on connection patterns (see ref. 5). Although the belt is architecturally quite distant from the core, differences between proposed belt areas are not so obvious. CL and CM are somewhat darker than other belt areas in brain sections processed for parvalbumin.
Figure 7.
Auditory cortical connections of ML. Area ML, and other belt areas, have dense connections with adjacent areas of the core, belt, and parabelt (solid lines with arrows). Connections with nonadjacent fields tend to be less dense (dashed lines with arrows). The belt fields also have topographically organized connections with functionally distinct fields in prefrontal cortex. Abbreviations defined in Fig. 1.
Besides connections with the adjoining core, belt areas connect with adjoining belt areas, more distant belt areas, the parabelt region, and to the frontal lobe (Fig. 7). Only the connections of the more accessible lateral belt areas have been studied directly, so all of the targets of the medial belt are not known. Nevertheless, it is clear that belt areas are widely interconnected with each other, and they distribute principally to the parabelt, a third distinct level of cortical processing.
The Lateral Parabelt of Auditory Cortex
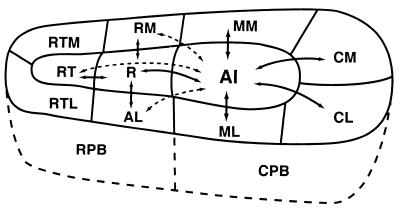
The parabelt of auditory cortex, just lateral to the lateral belt (Fig. 2), is defined as that region of the temporal lobe where injections of tracers label large numbers of neurons in the belt but few or no neurons in the core (5). The full extent of the parabelt has not been determined accurately, but injections rostral to the parabelt in the TS1,2 region and caudal to the parabelt in the Tpt region label the parabelt and other regions of cortex but not the belt. The parabelt may have functionally distinct subdivisions, but little is now known about how to divide the parabelt. Subdivisions are not obvious in the architecture, and systematic recordings with microelectrodes have not yet been attempted. We have tentatively divided the parabelt into rostral and caudal halves on the basis of differences in connections (Fig. 8). The rostral parabelt (RPB) connects largely with the rostral belt areas, whereas the caudal parabelt (CPB) largely connects with the caudal belt areas. However, both divisions connect with the rostromedial area (RM) of the belt. Callosal connections are with largely homotopic portions of the contralateral parabelt and roughly matching levels of the medial and lateral belt. Although the parabelt gets some inputs from the dorsal and medial divisions of the medial geniculate complex, it gets other thalamic inputs from the suprageniculate nucleus, nucleus limitans, and medial pulvinar. The parabelt neurons likely depend on belt inputs rather than thalamic inputs for suprathreshold auditory activation.
Figure 8.
Auditory cortical connections of CPB. Parabelt area CPB, as well as RPB, has dense connections with adjacent areas of the belt and RM in the medial belt (solid lines with arrows). Connections with nonadjacent fields of the belt tend to be less dense (dashed lines with arrows). The parabelt fields have few, if any, connections with the core areas. The parabelt also has connections with the polysensory areas in the superior temporal sulcus (STS) and with functionally distinct fields in prefrontal cortex. Abbreviations defined in Fig. 1.
Targets of the Parabelt: Additional Levels of Auditory Processing
The parabelt is interconnected with adjacent portions of the temporal and parietal lobe and with several regions of the frontal lobe. These target regions can be considered components of an additional fourth level of auditory processing, with thalamic inputs from the suprageniculate, limitans, and medial pulvinar nuclei (Fig. 1; refs. 62–64).
Much of the parabelt connects with nearby cortex of the upper and lower banks of the superior temporal sulcus. The caudal end of this sulcus is occupied by visual areas, such as the middle temporal visual area, and there is no evidence for direct parabelt connections with these visual areas. However, more rostral cortex in the superior temporal sulcus has been referred to as the superior temporal polysensory cortex (STP; see ref. 65 for review) where neurons respond to auditory, visual, and even somatosensory stimulation. The functions of this polysensory cortex are unknown, but visual stimuli are known to influence strongly the perceived locations of sound sources, and bimodal auditory and visual neurons in STP would be well suited for this interaction. The rostral parabelt connects with more rostral portions of the superior temporal gyrus. On the basis of connections (5), the latter is likely to be largely auditory in function. The caudal parabelt connects with cortex of the caudal end of the superior temporal gyrus, which also may be largely auditory in function, although a nearby region of posterior parietal cortex, 7a, has somatosensory and multimodal functions and neurons involved in reaching for objects (66, 67). Area 7a projects to premotor areas of the frontal lobe that are also involved in guiding reach.
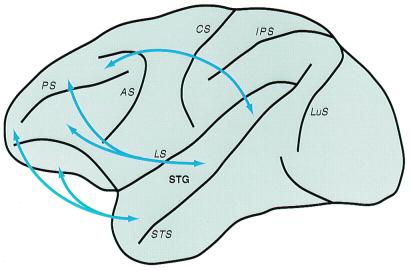
The parabelt also projects to four major regions of the frontal lobe (Fig. 9; refs. 4, 69, 70). These include cortex near or within the frontal eye field. This region of cortex (area 8a) is important for directing gaze toward objects of interest. Clearly, sounds from locations in space often would be of visual interest. Other projections are to dorsolateral prefrontal cortex of the principal sulcus in monkeys (area 46d). This cortex is thought to be important in the short-term temporary storage of information, sometimes called working memory (71, 72). Neurons in some locations in this prefrontal region respond to auditory stimuli (73–76). The third projection zone in the frontal lobe is more rostral and ventral (area 12v1). This region of cortex may be involved in the multimodal characterization of objects. In the ventrolateral prefrontal cortex, a number of single-unit studies indicate that this region subserves working memory for nonspatial tasks, such as stimulus recognition (see ref. 4). The fourth target of the parabelt is in orbital–frontal cortex, which is a multimodal region with a role in assigning value to stimuli. The region is associated with the reward system, and it is considered emotive or motivational in function (77–81). Many neurons in orbital–frontal cortex respond to auditory stimuli (82).
Figure 9.
Prefrontal connections of auditory cortex in macaque monkeys. Arrows summarize the topographic connections of the lateral belt and parabelt auditory regions with functionally distinct areas of prefrontal cortex. The targets of caudal auditory fields favor spatial domains of prefrontal cortex (e.g., 8a, caudal 46d), whereas more rostral fields exhibit stronger connections with nonspatial regions (e.g., areas 12vl, 10, mediofrontal, orbitofrontal). Connections with intermediate temporal fields tend to overlap more. See Fig. 1 and ref. 83 for more detailed connections. Adapted from ref. 68, with permission from S. Karger A, Basel.
On the basis of specific patterns of connections between temporal and frontal cortex, some investigators have distinguished separate pathways for processing spatial and nonspatial auditory information (e.g., refs. 69, 70). These data suggest that spatial (i.e., areas 8a, caudal 46d) and nonspatial (i.e., areas 10, 12vl, rostral 46d) domains in prefrontal cortex are the targets of separate processing streams originating in caudal and rostral fields of nonprimary auditory cortex, respectively. The connection patterns support this distinction, in general, but raise the possibilities of additional streams and significant interactions between streams (83).
Evidence for Levels of Cortical Processing in Humans
In addition to evidence for a core of distinct tonotopic fields in humans, there is also evidence in this literature for hierarchical processing in auditory cortex such that auditory-related activity in cortical fields outside of the core region can be dissociated from activity within by using various techniques (). Howard et al. (104) recorded evoked potentials from auditory cortex of patients undergoing surgery for intractable epilepsy. They were able to dissociate auditory responsive fields in Heschl's gyrus (HG) and the posterolateral superior temporal gyrus (PLST) on the basis of differences in responses to auditory stimuli and sensitivity to anesthesia. In addition, short-latency potentials evoked by electrical stimulation of HG were recorded from PLST, suggesting activation via direct or indirect connections between these areas. Scheich et al. (96) used a foreground–background decomposition task to dissociate three distinct auditory cortical regions with functional MRI (fMRI). The activated regions on the superior temporal gyrus (T1, T2, and T3) corresponded to distinct cytoarchitectonic fields (KA, PaAi, and PaAe, respectively) of human auditory cortex (37), which are comparable in relative position to core, lateral belt, and parabelt fields in monkeys. These results compare well with those of Binder et al. (105), in which distinct foci of peak activation were resolved in fMRI studies with speech and nonspeech stimuli. All auditory stimuli produced equivalent activations in the medial half of HG, but activation in the surrounding fields varied. White noise activation was centered on the HG with some spread into the surrounding fields, whereas activation produced by frequency-modulated tones was more expansive laterally than for noise. The extent of activation was greatest for speech stimuli, irrespective of linguistic significance, spreading ventrolaterally into cortex of the superior temporal sulcus. The authors concluded that such contrasts provided support for a hierarchical model of auditory word processing in the human temporal lobe based on increasing complexity and integration of temporal and spectral features. Overall, the results of functional studies of auditory cortex in humans indicate the presence of multiple hierarchically arranged fields, consistent with key features of the primate model.
Conclusions
The auditory systems of all mammals include a large number of complexly interconnected auditory nuclei, and at least several areas of auditory cortex. Although the subcortical auditory system of primates and other animals may have similar component nuclei, further study will likely reveal specializations in types and numbers of neurons and connections. Thus, we do not yet know how similar or different the subcortical auditory systems of various animals might be. At the cortical level, primates appear to have evolved an elaborate network of areas that is quite different from the networks in other mammals. Although most of our present understanding of auditory cortex in primates depends on studies in monkeys, there is some evidence that early levels of cortical processing are similar in monkeys, chimpanzees, and humans, although the human auditory system undoubtedly includes specializations for language. Our growing understanding of the complexity of auditory cortex in monkeys opens the door for many productive studies of the nature of processing within the network.
The auditory cortex of monkeys includes a network of at least 20 interconnected auditory areas or multimodal regions. The cortical system is hierarchical with at least four distinct levels of processing, with several areas at each level. Information is widely distributed within levels, between areas of the same level of opposite cerebral hemispheres, and between areas of one level and the next. Yet, there is enough separation in distributions of connections to suggest the existence of at least distinct rostral and caudal processing streams. The existence of multiple areas at even the primary level of cortical processing has obvious implications. Because areas of the same level have similar connections, they may have overlapping functions. Thus, the system should be able to compensate largely for losses produced by lesions that leave some of the areas of any level intact, but the highly serial nature of processing from level to level indicates that extensive damage to any level would be devastating.
Footnotes
This paper was presented at the National Academy of Sciences colloquium “Auditory Neuroscience: Development, Transduction, and Integration,” held May 19–21, 2000, at the Arnold and Mabel Beckman Center in Irvine, CA.
References
- 1.Preuss T M, Goldman-Rakic P S. J Comp Neurol. 1991;310:429–474. doi: 10.1002/cne.903100402. [DOI] [PubMed] [Google Scholar]
- 2.Kaas J H, Krubitzer L A. In: Neuroanatomy of Visual Pathways and Their Retinotopic Organization. Dreher B, Robinson S R, editors. London: MacMillan; 1991. pp. 302–359. [Google Scholar]
- 3.Walzl E M. Laryngoscope. 1947;57:778–787. doi: 10.1288/00005537-194712000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Hackett T A, Stepniewska I, Kaas J H. Brain Res. 1999;817:45–58. doi: 10.1016/s0006-8993(98)01182-2. [DOI] [PubMed] [Google Scholar]
- 5.Hackett T A, Stepniewska I, Kaas J H. J Comp Neurol. 1998;394:475–495. doi: 10.1002/(sici)1096-9861(19980518)394:4<475::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 6.Merzenich M M, Brugge J F. Brain Res. 1973;50:275–296. doi: 10.1016/0006-8993(73)90731-2. [DOI] [PubMed] [Google Scholar]
- 7.Imig T J, Ruggero M A, Kitzes L F, Javel E, Brugge J F. J Comp Neurol. 1977;171:111–128. doi: 10.1002/cne.901710108. [DOI] [PubMed] [Google Scholar]
- 8.Aitkin L M, Merzenich M M, Irvine D R F, Clarey J C, Nelson J E. J Comp Neurol. 1986;252:175–185. doi: 10.1002/cne.902520204. [DOI] [PubMed] [Google Scholar]
- 9.Leuthke L E, Krubitzer L A, Kaas J H. J Comp Neurol. 1989;285:487–513. doi: 10.1002/cne.902850406. [DOI] [PubMed] [Google Scholar]
- 10.Morel A, Kaas J H. J Comp Neurol. 1992;318:27–63. doi: 10.1002/cne.903180104. [DOI] [PubMed] [Google Scholar]
- 11.Morel A, Garraghty P E, Kaas J H. J Comp Neurol. 1993;335:437–459. doi: 10.1002/cne.903350312. [DOI] [PubMed] [Google Scholar]
- 12.Kosaki H, Hashikawa T, He J, Jones E G. J Comp Neurol. 1997;386:304–316. [PubMed] [Google Scholar]
- 13.Mesulam M M, Pandya D N. Brain Res. 1973;60:315–333. doi: 10.1016/0006-8993(73)90793-2. [DOI] [PubMed] [Google Scholar]
- 14.Burton H, Jones E G. J Comp Neurol. 1976;168:249–302. doi: 10.1002/cne.901680204. [DOI] [PubMed] [Google Scholar]
- 15.Fitzpatrick K A, Imig T J. J Comp Neurol. 1978;177:537–556. doi: 10.1002/cne.901770402. [DOI] [PubMed] [Google Scholar]
- 16.Aitkin L M, Kudo M, Irvine D R F. J Comp Neurol. 1988;269:235–248. doi: 10.1002/cne.902690208. [DOI] [PubMed] [Google Scholar]
- 17.Pandya D N, Rosene D L, Doolittle A M. J Comp Neurol. 1994;345:447–471. doi: 10.1002/cne.903450311. [DOI] [PubMed] [Google Scholar]
- 18.Hashikawa T, Rausell E, Molinari M, Jones E G. Brain Res. 1995;544:335–341. doi: 10.1016/0006-8993(91)90076-8. [DOI] [PubMed] [Google Scholar]
- 19.Molinari M, Dell'Anna M E, Rausell E, Leggio M G, Hashikawa T, Jones E G. J Comp Neurol. 1995;362:171–194. doi: 10.1002/cne.903620203. [DOI] [PubMed] [Google Scholar]
- 20.Rauschecker J P, Tian B, Pons T, Mishkin M. J Comp Neurol. 1997;382:89–103. [PubMed] [Google Scholar]
- 21.Galaburda A M, Pandya D N. J Comp Neurol. 1983;221:169–184. doi: 10.1002/cne.902210206. [DOI] [PubMed] [Google Scholar]
- 22.Jones E G, Dell'Anna M E, Molinari M, Rausell E, Hashikawa T. J Comp Neurol. 1995;362:153–170. doi: 10.1002/cne.903620202. [DOI] [PubMed] [Google Scholar]
- 23.Tootell R B H, Hamilton S L, Silverman M S. J Neurosci. 1985;5:2786–2800. doi: 10.1523/JNEUROSCI.05-10-02786.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitzpatrick D, Diamond I T. J Comp Neurol. 1980;194:703–719. doi: 10.1002/cne.901940402. [DOI] [PubMed] [Google Scholar]
- 25.Blumcke I, Hof P R, Morrison J H, Celio M R. J Comp Neurol. 1990;301:417–432. doi: 10.1002/cne.903010307. [DOI] [PubMed] [Google Scholar]
- 26.Krmpotic-Nemanic J, Kostovic I, Kelovic Z, Nemanic D. Acta Otolaryngol. 1980;89:388–392. doi: 10.3109/00016488009127153. [DOI] [PubMed] [Google Scholar]
- 27.Robertson R T, Mostamand F, Kageyama G H, Gallardo K A, Yu J. Dev Brain Res. 1991;58:81–95. doi: 10.1016/0165-3806(91)90240-j. [DOI] [PubMed] [Google Scholar]
- 28.Bear M F, Winger W. Nature (London) 1986;320:172–176. doi: 10.1038/320172a0. [DOI] [PubMed] [Google Scholar]
- 29.Weinberger N M. Annu Rev Neurosci. 1995;18:129–158. doi: 10.1146/annurev.ne.18.030195.001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinberger N M. Neurobiol Learn Mem. 1998;70:226–251. doi: 10.1006/nlme.1998.3850. [DOI] [PubMed] [Google Scholar]
- 31.Bjordahl T S, Dimyan M A, Weinberger N M. Behav Neurosci. 1998;112:467–479. doi: 10.1037//0735-7044.112.3.467. [DOI] [PubMed] [Google Scholar]
- 32.Recanzone G H, Guard D C, Phan M L. J Neurophysiol. 2000;83:2315–2331. doi: 10.1152/jn.2000.83.4.2315. [DOI] [PubMed] [Google Scholar]
- 33.Fitzpatrick K A, Imig T J. J Comp Neurol. 1980;192:589–610. doi: 10.1002/cne.901920314. [DOI] [PubMed] [Google Scholar]
- 34.Casagrande V A, Kaas J H. In: Cerebral Cortex. Peters A, Rockland K, editors. Vol. 10. New York: Plenum; 1994. pp. 201–259. [Google Scholar]
- 35.Krubitzer L A, Kaas J H. J Neurosci. 1990;10:952–974. doi: 10.1523/JNEUROSCI.10-03-00952.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stepniewska I, Preuss T M, Kaas J H. J Comp Neurol. 1993;330:238–271. doi: 10.1002/cne.903300207. [DOI] [PubMed] [Google Scholar]
- 37.Galaburda A M, Sanides F. J Comp Neurol. 1980;190:597–610. doi: 10.1002/cne.901900312. [DOI] [PubMed] [Google Scholar]
- 38.Rivier F, Clarke S. Neuroimage. 1997;6:288–304. doi: 10.1006/nimg.1997.0304. [DOI] [PubMed] [Google Scholar]
- 39.Verkindt C, Bertrand O, Perrin F, Echallier J F, Pernier J. Electroencephalogr Clin Neurophysiol. 1995;96:143–156. doi: 10.1016/0168-5597(94)00242-7. [DOI] [PubMed] [Google Scholar]
- 40.Howard M A, Volkov I O, Abbas P J, Damasio H, Ollendieck M C, Granner M A. Brain Res. 1996;724:260–264. doi: 10.1016/0006-8993(96)00315-0. [DOI] [PubMed] [Google Scholar]
- 41.Elberling C, Bak C, Kofoed B, Lebeck J, Saermark K. Scand Audiol. 1982;11:61–65. doi: 10.3109/01050398209076201. [DOI] [PubMed] [Google Scholar]
- 42.Romani G L, Williamson S J, Kaufman L. Science. 1982;216:1339–1340. doi: 10.1126/science.7079770. [DOI] [PubMed] [Google Scholar]
- 43.Hoke E S, Ross B, Hoke M. NeuroReport. 1998;9:3065–3068. doi: 10.1097/00001756-199809140-00027. [DOI] [PubMed] [Google Scholar]
- 44.Pantev C, Hoke M, Lehnertz K, Lutkenhoner B, Anogianakis G, Wittkowski W. Electroencephalogr Clin Neurophysiol. 1988;69:160–170. doi: 10.1016/0013-4694(88)90211-8. [DOI] [PubMed] [Google Scholar]
- 45.Pantev C, Bertrand O, Eulitz C, Verkindt C, Hampson S, Schuierer G, Elbert T. Electroencephalogr Clin Neurophysiol. 1995;94:26–40. doi: 10.1016/0013-4694(94)00209-4. [DOI] [PubMed] [Google Scholar]
- 46.Bertrand O, Perrin F, Pernier J. Acta Otolaryngol. 1991;S491:116–123. doi: 10.3109/00016489109136788. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto T, Uemura Y, Llinas R. Acta Otolaryngol. 1992;112:201–204. doi: 10.1080/00016489.1992.11665404. [DOI] [PubMed] [Google Scholar]
- 48.Tiitinen H, Alho K, Huotilainen R, Ilmoniemi R J, Simola J, Naatanen R. Psychophysiology. 1993;30:537–540. doi: 10.1111/j.1469-8986.1993.tb02078.x. [DOI] [PubMed] [Google Scholar]
- 49.Huotilainen M, Tiitinen H, Lavikainen J, Ilmoniemi R J, Pekkonen E, Sinkkonen J, Laine P, Naatanen R. NeuroReport. 1995;6:841–844. doi: 10.1097/00001756-199504190-00004. [DOI] [PubMed] [Google Scholar]
- 50.Langner G, Sams M, Heil P, Schultze H. J Comp Physiol A. 1997;181:665–676. doi: 10.1007/s003590050148. [DOI] [PubMed] [Google Scholar]
- 51.Lutkenhoner B, Steinstrater O. Audiol Neurootol. 1998;3:191–213. doi: 10.1159/000013790. [DOI] [PubMed] [Google Scholar]
- 52.Rosburg T, Kreitschmann-Andermahr I, Emmerich E, Nowak H, Sauer H. Neurosci Lett. 1998;258:105–108. doi: 10.1016/s0304-3940(98)00865-9. [DOI] [PubMed] [Google Scholar]
- 53.Lauter J L, Herscovitch P, Formby C, Raichle M E. Hear Res. 1985;2:199–205. doi: 10.1016/0378-5955(85)90024-3. [DOI] [PubMed] [Google Scholar]
- 54.de Rossi G, Paludetti G, di Nardo W, Calcagni M L, di Guida D, Almadori G, Galli J. Nuklearmedizin. 1996;35:112–115. [PubMed] [Google Scholar]
- 55.Ottaviani F, di Girolamo S, Briglia G, de Rossi G, di Giuda D, di Nardo W. Audiology. 1997;36:241–248. doi: 10.3109/00206099709071977. [DOI] [PubMed] [Google Scholar]
- 56.Lockwood A H, Salvi R J, Coad M L, Arnold S A, Wack D S, Murphy B W, Burkard R F. Cereb Cortex. 1999;9:65–76. doi: 10.1093/cercor/9.1.65. [DOI] [PubMed] [Google Scholar]
- 57.Strainer J C, Ulmer J L, Yetkin F Z, Haughton V M, Daniels D L, Millen S J. Am J Neuroradiol. 1997;18:601–610. [PMC free article] [PubMed] [Google Scholar]
- 58.Wessinger C M, Buonocore M H, Kussmaul C L, Mangun G R. Hum Brain Mapp. 1997;5:18–25. doi: 10.1002/(SICI)1097-0193(1997)5:1<18::AID-HBM3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 59.Bilecen D, Scheffler K, Schmid N, Tschopp K, Seelig J. Hear Res. 1998;126:19–27. doi: 10.1016/s0378-5955(98)00139-7. [DOI] [PubMed] [Google Scholar]
- 60.Yang Y, Engelien A, Engelien W, Xu S, Stern E, Silbersweig D A. Magn Reson Med. 2000;43:185–190. doi: 10.1002/(sici)1522-2594(200002)43:2<185::aid-mrm4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 61.Rauschecker J P, Tian B, Hauser M. Science. 1995;268:111–114. doi: 10.1126/science.7701330. [DOI] [PubMed] [Google Scholar]
- 62.Yeterian E H, Pandya D N. J Comp Neurol. 1989;282:80–97. doi: 10.1002/cne.902820107. [DOI] [PubMed] [Google Scholar]
- 63.Kosmal A, Malinowska M, Kowalska D M. Acta Neurobiol Exp (Warsz) 1997;57:165–188. doi: 10.55782/ane-1997-1224. [DOI] [PubMed] [Google Scholar]
- 64.Romanski L M, Giguere M, Bates J F, Goldman-Rakic P S. J Comp Neurol. 1997;379:313–332. [PubMed] [Google Scholar]
- 65.Cusick C G. In: Cerebral Cortex, vol. 12. Rockland K, Kaas J H, Peters A, editors. New York: Plenum; 1997. pp. 435–468. [Google Scholar]
- 66.Ghosh S, Gattera R. Somatosens Mot Res. 1995;12:359–378. doi: 10.3109/08990229509093668. [DOI] [PubMed] [Google Scholar]
- 67.Wise S P, Boussaoud D, Johnson P B, Caminiti R. Annu Rev Neurosci. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]
- 68.Kaas J H, Hackett T A. Audiol Neurootol. 1998;3:73–85. doi: 10.1159/000013783. [DOI] [PubMed] [Google Scholar]
- 69.Romanski L M, Bates J F, Goldman-Rakic P S. J Comp Neurol. 1999a;403:141–157. doi: 10.1002/(sici)1096-9861(19990111)403:2<141::aid-cne1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 70.Romanski L M, Tian B, Fritz J, Mishkin M, Goldman-Rakic P S, Rauschecker J P. Nat Neurosci. 1999b;2:1131–1136. doi: 10.1038/16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fuster J M. In: Epilepsy and the Functional Anatomy of the Frontal Lobe. Jasper H H, Riggio S, Goldman-Rakic P S, editors. New York: Raven; 1995. pp. 9–20. [Google Scholar]
- 72.Schall J D. In: Cerebral Cortex. Rockland K, Kaas J H, Peters A, editors. Vol. 12. New York: Plenum; 1997. pp. 527–637. [Google Scholar]
- 73.Ito S-I. Brain Res. 1982;247:39–47. doi: 10.1016/0006-8993(82)91025-3. [DOI] [PubMed] [Google Scholar]
- 74.Niki H, Sugita S, Watanabe M. In: Vision, Memory, and the Temporal Lobe. Iwai E, Mishkin M, editors. Amsterdam: Elsevier; 1990. pp. 295–304. [Google Scholar]
- 75.Watanabe M. Exp Brain Res. 1992;89:233–247. doi: 10.1007/BF00228241. [DOI] [PubMed] [Google Scholar]
- 76.Bodner M, Kroger J, Fuster J M. NeuroReport. 1996;7:1905–1908. doi: 10.1097/00001756-199608120-00006. [DOI] [PubMed] [Google Scholar]
- 77.Mishkin M, Manning F J. Brain Res. 1978;143:313–324. doi: 10.1016/0006-8993(78)90571-1. [DOI] [PubMed] [Google Scholar]
- 78.Thorpe S J, Rolls E T, Maddison S. Exp Brain Res. 1983;49:93–115. doi: 10.1007/BF00235545. [DOI] [PubMed] [Google Scholar]
- 79.Gaffan D, Murray E A. J Neurosci. 1990;10:3479–3493. doi: 10.1523/JNEUROSCI.10-11-03479.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schoenbaum G, Chiba A A, Gallagher M. Nat Neurosci. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- 81.Tremblay L, Schultz W. Nature (London) 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- 82.Benevento L A, Fallon J H, Davis B J, Rezak M. Exp Neurol. 1977;57:849–872. doi: 10.1016/0014-4886(77)90112-1. [DOI] [PubMed] [Google Scholar]
- 83.Kaas J H, Hackett T A. Nat Neurosci. 1999;2:1045–1047. doi: 10.1038/15967. [DOI] [PubMed] [Google Scholar]
- 84.Petersen S E, Fox P T, Posner M I, Mintum M, Raichle M E. Nature (London) 1988;231:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- 85.Liegeois-Chauvel C, Peretz I, Babai M, Laguitton V, Chauvel P. Brain. 1998;121:1853–1867. doi: 10.1093/brain/121.10.1853. [DOI] [PubMed] [Google Scholar]
- 86.Liegeois-Chauvel C, Musolino A, Badier J, Marquis P, Chauvel P. Electroencephalogr Clin Neurophysiol. 1994;92:204–214. doi: 10.1016/0168-5597(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 87.Price C, Wise R, Ramsay S, Friston K, Howard D, Patterson K, Frackoiak R. Neurosci Lett. 1992;146:179–182. doi: 10.1016/0304-3940(92)90072-f. [DOI] [PubMed] [Google Scholar]
- 88.Demonet J F, Chollet F, Ramsay S, Cardebat D, Nespoulous J L, Wise R, Rascol A, Frackowiak R. Brain. 1992;115:1753–1768. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
- 89.Zatorre R J, Evans A C, Meyer E, Gjedde A. Science. 1992;256:846–849. doi: 10.1126/science.1589767. [DOI] [PubMed] [Google Scholar]
- 90.Binder J R, Rao S M, Hammeke T A, Yetkin F Z, Jewmanowicz A, Bandettini P A, Wong E C, Estkowski L D, Goldstein M D, Haughton V M, Hyde J S. Ann Neurol. 1994;35:662–672. doi: 10.1002/ana.410350606. [DOI] [PubMed] [Google Scholar]
- 91.Berry I, Demonet J-F, Warach S, Viallard G, Boulanouar K, Franconi J M, Marc-Vergnes J-P, Edelman R, Manelfe C. Neuroimage. 1995;2:215–219. doi: 10.1006/nimg.1995.1028. [DOI] [PubMed] [Google Scholar]
- 92.Hickock G, Love T, Swinney D, Wong E C, Buxton R B. Brain Lang. 1997;58:197–201. doi: 10.1006/brln.1997.1868. [DOI] [PubMed] [Google Scholar]
- 93.Gaschler-Markefski B, Baumgart F, Tempelmann C, Woldorff M G, Scheich H. Neural Plast. 1998;6:69–75. doi: 10.1155/NP.1998.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Griffiths T D, Rees G, Rees A, Green G G, Witton C, Rowe D, Buchel C, Turner R, Frackowiak R S. Nat Neurosci. 1998a;1:74–79. doi: 10.1038/276. [DOI] [PubMed] [Google Scholar]
- 95.Griffiths T D, Buchel C, Frackowiak R S, Patterson R D. Nat Neurosci. 1998b;1:422–427. doi: 10.1038/1637. [DOI] [PubMed] [Google Scholar]
- 96.Scheich H, Baumgart F, Gaschler-Markefski B, Tegeler C, Tempelmann C, Heinze H J, Schindler F, Stiller D. Eur J Neurosci. 1998;10:803–809. doi: 10.1046/j.1460-9568.1998.00086.x. [DOI] [PubMed] [Google Scholar]
- 97.Baumgart F, Gaschler-Markefski B, Woldorff M G, Heinze H J, Scheich H. Nature (London) 1999;400:724–726. doi: 10.1038/23390. [DOI] [PubMed] [Google Scholar]
- 98.Nishimura H, Hashikawa K, Doi K, Iwaki T, Watanabe Y, Kusuoka H, Nishimura T, Kubo T. Nature (London) 1999;397:116. doi: 10.1038/16376. [DOI] [PubMed] [Google Scholar]
- 99.Belin P, Zatorre R J, Hoge R, Evans A C, Pike B. Neuroimage. 1999;10:417–429. doi: 10.1006/nimg.1999.0480. [DOI] [PubMed] [Google Scholar]
- 100.Belin P, Zatorre R J, Lafaille P, Ahad P, Pike B. Nature (London) 2000;403:309–312. doi: 10.1038/35002078. [DOI] [PubMed] [Google Scholar]
- 101.Celsis P, Boulanouar K, Doyon B, Ranjeva J P, Berry I, Nespoulous J L, Chollet F. Neuroimage. 1999;9:135–144. doi: 10.1006/nimg.1998.0389. [DOI] [PubMed] [Google Scholar]
- 102.Jancke L, Mirzazade S, Shah N J. Neurosci Lett. 1999;266:125–128. doi: 10.1016/s0304-3940(99)00288-8. [DOI] [PubMed] [Google Scholar]
- 103.Mummery C J, Ashburner J, Scott S K, Wise R J. J Acoust Soc Am. 1999;106:449–457. doi: 10.1121/1.427068. [DOI] [PubMed] [Google Scholar]
- 104.Howard M A, Volkov I O, Mirsky R, Garell P C, Noh M D, Granner M, Damasio H, Steinschneider M, Reale R A, Hind J E, Brugge J F. J Comp Neurol. 2000;416:79–92. doi: 10.1002/(sici)1096-9861(20000103)416:1<79::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 105.Binder J R, Frost J A, Hammeke T A, Bellgowan P S F, Springer J A, Kaufman J N, Possing E T. Cereb Cortex. 2000;10:512–528. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]