Abstract
Background
Arterial stiffening is one of the hallmarks of vascular aging, and is an important risk factor for cardiovascular morbidity and mortality. Aging is also associated with bone demineralization. Accumulating evidence indicate that arterial stiffness and bone demineralization might share common pathways. The aims of this study were to evaluate whether the association between arterial stiffness and bone demineralization is independent of age, and to explore putative mechanisms that may mediate their relationship.
Methods
A cross-sectional analysis was performed using data from 321 men (68 ± 12 years) and 312 women (65 ± 13 years) of the Baltimore Longitudinal Study of Aging. Arterial stiffness was assessed by carotid-femoral pulse wave velocity (PWV) and cross-sectional cortical bone area (cCSA) was assessed at the level of the mid-tibia with CT imaging.
Results
Age was significantly correlated with PWV in men (r=0.38, p<0.0001) and women (r=0.35, p<0.0001). Age was associated with cCSA in women (r=−0.14, p=0.0008), but not in men. Age-adjusted linear regression analysis showed a significant inverse association between PWV and cCSA, in women but not in men. The association between PWV and cCSA remained significant in women after adjusting for age, mean arterial pressure, obesity, menopause, drugs, alcohol intake, physical activity, renal function, serum calcium, and total estradiol concentration.
Conclusions
Independent of age and other shared risk factors, arterial stiffness is inversely related to cortical bone area in women. The sex-specific signaling and molecular pathways that putatively underlie the cross-talk between central arteries and bone are not completely understood.
Keywords: Arterial Stiffness, Bone Demineralization, Pulse Wave Velocity, Cortical Bone Area, Aging, Baltimore Longitudinal Study of Aging
INTRODUCTION
Aging is associated with structural changes of the arterial wall, which result in decreased distensibility and increased arterial stiffness (1). Arterial stiffness can be evaluated by measuring pulse wave velocity (PWV) between two sites in the arterial tree, with higher PWV indicating greater stiffness (2). Arterial stiffness increases with advancing age even in relatively healthy people who are at low risk for cardiovascular disease (3). In addition, arterial stiffness is a predictor of mortality and morbidity (4,5), hypertension (6), and decline in cognitive function (7).
Aging is also associated with bone demineralization, a process that is accelerated in women compared to men (8). Several lines of evidence suggest that age-associated bone demineralization and arterial calcification share common mechanisms and signaling pathways (9).
We therefore sought to test the hypothesis that arterial stiffness (measured by carotid-femoral PWV) is associated with bone demineralization (assessed by middle tibia cortical cross-sectional area, cCSA) independent of age; and to explore putative mechanisms that might mediate this relationship in a normative aging study population.
METHODS
Study design and patients
The Baltimore Longitudinal Study of Aging (BLSA) is a prospective study of normative aging conducted by the National Institute on Aging, Intramural Research Program since 1958. BLSA participants are healthy volunteers 21–96 years of age who undergo standardized testing across multiple body systems over 2 to 3 days at regular intervals (10). The study cohort for this cross-sectional analysis included all participants with pulse wave velocity (PWV) and tibial CT scan data obtained during their regularly scheduled BLSA study visit.
Pulse wave velocity (PWV)
PWV was measured with the participant supine in a quiet room. After simultaneous acquisition of pressure waveforms in the right common carotid artery and right femoral artery, pulse transit time between these two sites were automatically measured by the Complior SP device ® (Artech Medical, Pantin, France), as previously described and validated (11). The average of 3 measurements was used in the analyses.
Computed Tomography (CT) scan
A 10-mm axial slice of the tibia at 38% of the tibial length, renamed middle tibia, was obtained using a Siemens Somatom Sensation 10 CT scanner. Each image was assessed for quality and accurate positioning using ImageJ (NIH, Bethesda, MD). Middle tibia cortical cross-sectional area, (cCSA) of the cortical bone was determined using Geanie software (Bonalyse Oy, Jyvaskyla, Finland) and indexed to body mass index (BMI).
Anthropometrics, smoking status assessment and physical activity level
Height (m) and weight (kg) were measured for all participants. BMI was calculated as body weight in kilograms (kg) divided by their height in meters (m) squared (kg/m2). Obesity was defined as a BMI of 30 and above. Systolic (SBP) and diastolic (DBP) blood pressure, mean arterial pressure (MAP) and heart rate (HR) determinations were performed at the time of PWV analysis with an oscillometric device (Dash 4000 ® Monitor, General Electric), following a five-minute quiet resting period. Smoking status was ascertained by a questionnaire that classified each subject as ever or never smoker. Alcohol intake data were ascertained by a questionnaire that classified each subject as ever or never had alcohol consumption in the last year before the visit. Medication use (antihypertensives, loop and thiazide diuretics, and hypoglycemic agents), hormone replacement therapy (testosterone in men, estrogens/progesterone in women) and calcium and/or vitamin D supplementation were also verified at the time of the BLSA visit. Participants were classified with respect to their levels of weekly physical activity and exercise program (metabolic equivalents, METs per week) using validated standard questionnaires (12).
Laboratory Testing
Blood samples were drawn, at rest, from the antecubital vein after an overnight fast between 7 and 8 AM. Serum albumin was measured with a commercial enzymatic test (Roche Diagnostics GmbH, Mannheim, Germany). Automated chemical analysis was used for analysis of calcium and creatinine. Corrected calcium (Cac) was computed as Cac= (4.0 g per dL − (plasma albumin)) × 0.8 + (serum calcium) (13). Estimated glomerular filtration rate (eGFR) was computed according the Modification of Diet in Renal Disease Study (MDRD) equation (14). Concentrations of plasma triglycerides and total cholesterol were determined by enzymatic method (Abbott Laboratories ABA-200 ATC Biochromatic Analyzer, Irving, Texas). The concentration of high-density lipoprotein (HDL) cholesterol was determined by a dexstran sulfate-magnesium precipitation procedure. Low-density lipoprotein (LDL) cholesterol concentrations were estimated by using the Friedewald formula. The fasting plasma glucose concentration was measured by the glucose oxidase method (Beckman Instruments, Inc., Fullerton, California). Total estradiol testing was performed by Esoterix, Inc (Calabasas Hills, CA). Total estradiol was measured by 2-dimensional liquid chromatography (HPLC) with mass spectrometry detection after liquid-liquid extraction, yielding a lower limit of detection of 0.1 ng/dL, and between assay coefficients of variation (CV) < 4%. Total testosterone (available in 317 out of 321 men and in all women) was assayed using commercial radioimmunologic kits (Diagnostic Systems Laboratories, Webster, TX). The minimum detectable concentration was 0.03 nmol/L; intra-assay and inter-assay CV were 8.1% and 8.4%, respectively.
Statistical Analysis
For continuous variables, unpaired t-test was used to compare means. Correlations between variables were assessed with Pearson’s correlation coefficient (r). The associations between PWV and cCSA were evaluated with hierarchical multiple linear regression analysis. The interaction term between cCSA and sex was highly significant (p=0.0001), thus analyses were performed separately in men and women. In the initial model, age, MAP, obesity, menopause status (in women) and cCSA were included as independent variables (Model 1). In Model 2, eGFR and serum calcium levels were added as covariates to Model 1. In Model 3, the effects of medications were evaluated by adding hormone replacement therapy, antihypertensive medications, thiazide-type diuretics, and calcium/vitamin D supplementation to Model 2. Subsequently, in Model 4, alcohol intake and physical activity level were added to Model 3. Finally, in Model 5, total estradiol was added to Model 4. Multi-collinearity among the covariates was tested using the variance inflation factor. Total estradiol and total testosterone levels were log-transformed because of the skewed distribution and expressed as median and interquartile range. Data are expressed as means ± standard deviation (SD) or proportions. All analyses were performed using SAS (Version 9.1, Cary, NC) with significance set at P<0.05.
RESULTS
The study cohort consisted of 321 men aged 68 ± 12 years and 312 women aged 65 ± 13 years. Descriptive and clinical characteristics for these men and women are summarized in Table 1. Sixty-three percent of men and 53% of women were Caucasian.
Table 1.
Descriptive and clinical characteristics of the study cohort
| Men (n = 321) | Women (n = 312) | |
|---|---|---|
| Demographics | ||
| Age (years) | 68 ± 12 | 65 ± 13 |
| BMI (kg/m2) | 27 ± 4 | 26 ± 5 |
| Caucasian (n, %) | 204 (63) | 184 (53) |
| Smoking (n, %) | 146 (45) | 127 (41) |
| Physical activity level (METs/week) | 107 ± 74 | 106 ± 65 |
| Hypertension (n, %) | 171 (53) | 104 (33) |
| Diabetes (n, %) | 33 (10) | 8 (2) |
| Post menopause status (n, %) | --- | 241 (77) |
| Age at menopause (years) | --- | 49 ± 6 |
| Cardiovascular parameters | ||
| SBP (mmHg) | 128 ± 17 | 123 ± 20 |
| DBP (mmHg) | 70 ± 9 | 64 ± 9 |
| MAP (mmHg) | 89 ± 10 | 83 ± 11 |
| HR (beats/min) | 63 ± 9 | 66 ± 9 |
| PWV (m/s) | 7.1 ± 1.9 | 6.5 ± 1.8 |
| Medications | ||
| Antihypertensive drugs (n, %) | 171 (53) | 104 (33) |
| Loop diuretics | 12 (4) | 4 (1) |
| Thiazide diuretics | 54 (16) | 54 (17) |
| Hypoglycemic agents (n, %) | 33 (10) | 8 (2) |
| Hormone replacement therapy (n, %) | 20 (6) | 80 (25) |
| Calcium supplements (n,%) | 19 (6) | 40 (13) |
| Vitamin D supplements (n,%) | 15 (5) | 22 (7) |
| Calcium and Vitamin D supplements (n,%) | 43 (13) | 76 (24) |
| Laboratory | ||
| Fasting glucose (mg/dl) | 96 ± 15 | 90 ± 13 |
| Creatinine (mg/dl) | 1.1 ± 0.2 | 0.9 ± 0.2 |
| Albumin (g/dl) | 4.1 ± 0.3 | 4.1 ± 0.3 |
| eGFR (ml/min/1.73m2) | 74 ± 15 | 74 ± 17 |
| Cac (mg/dl) | 9.2 ± 0.3 | 9.2 ± 0.4 |
| Total cholesterol (mg/dl) | 182 ± 37 | 199 ± 38 |
| LDL- cholesterol (mg/dl) | 108 ± 32 | 115 ± 34 |
| HDL- cholesterol (mg/dl) | 54 ± 14 | 65 ± 17 |
| Triglycerides (mg/dl) | 100 ± 62 | 98 ± 48 |
| Total Estradiol (ng/dl) | 2.2 (0.9)* | 0.5 (0.8)* |
| Total Testosterone (ng/ml) | 453 (211)* | 23.7 (13.5)* |
| Computed tomography scan | ||
| Cortical CSA (mm2) | 241 ± 29 | 180 ± 22 |
median, interquartile range.
Abbreviations: BMI, body mass index; Cac, corrected calcium; CSA, cross-sectional area; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; HR, heart rate; LDL, low-density lipoprotein; MAP, mean arterial pressure; PWV, pulse wave velocity; SBP, systolic blood pressure.
PWV was 7.1 ± 1.9 m/s (range 3.0 – 15.5) in men, and was 6.5 ± 1.8 m/s (range 2.8 – 15.4) in women. Men with hypertension (n = 171) had higher PWV compared to non-hypertensive men (n=150) (7.3 ± 1.8 vs. 6.9 ± 1.7, p=0.04, respectively). Women with hypertension (n=104) had higher PWV compared to non-hypertensive women (n=208) (7.1 ± 1.9 vs. 6.2 ± 1.7, p<0.0001, respectively). Men with diabetes (n = 33) had higher PWV compared to non diabetic men (n=288) (7.8 ± 2.0 vs. 7.0 ± 1.9, p=0.02, respectively). Women with diabetes (n=8) had higher PWV compared to non diabetic women (n=304) (8.5 ± 2.8 vs. 6.4 ± 1.8, p=0.002, respectively).
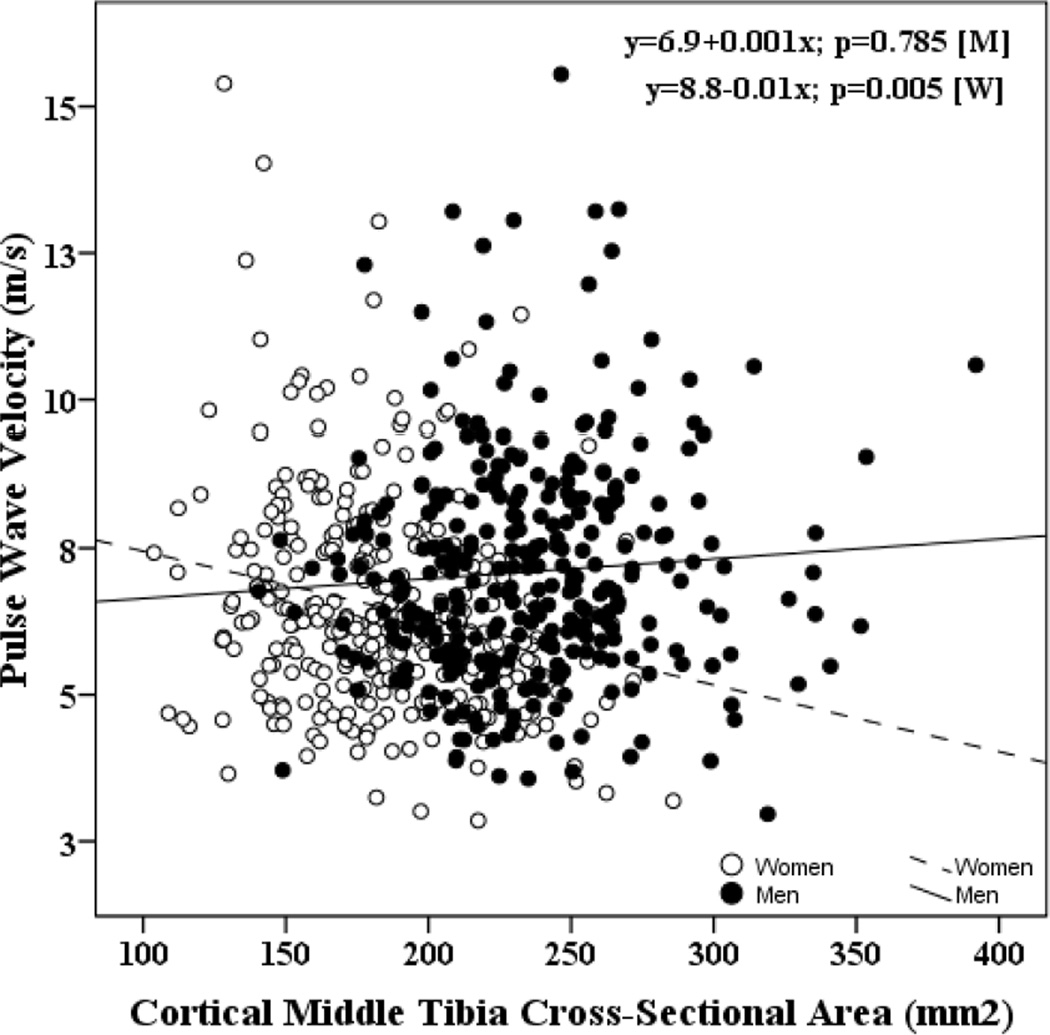
Bivariate correlates of PWV are shown in Table 2. In men, PWV was directly correlated with age (r=0.38, p<0.001) and inversely correlated with eGFR (r=−0.11, p<0.05). No significant correlation between PWV and cCSA was observed in men with or without hypertension or diabetes. In women, PWV was directly correlated with age (r=0.37, p<0.0001) and MAP (r=0.25, p<0.0001); and inversely correlated with eGFR (r=−0.20, p=0.0003). An inverse correlation between PWV and cCSA was observed in women (r=−0.22, p<0.0001), but not in men (r=0.07, p=0.21). Regression lines depicting the relationship between carotid-femoral pulse wave velocity and cCSA are shown in Figure 1.
Table 2.
Bivariate correlates of PWV
| Pulse Wave Velocity | ||||
|---|---|---|---|---|
| Men | Women | |||
| r | P-value | r | P-value | |
| Age | 0.38 | <0.0001 | 0.36 | <0.0001 |
| Mean Arterial Pressure | 0.10 | 0.06 | 0.25 | <0.0001 |
| Heart Rate | −0.01 | 0.99 | 0.09 | 0.16 |
| Smoking Status | 0.03 | 0.55 | −0.03 | 0.51 |
| Menopausal Status | --- | --- | 0.23 | <0.0001 |
| eGFR | −0.11 | 0.03 | −0.20 | <0.001 |
| Serum Calcium | 0.03 | 0.54 | 0.01 | 0.57 |
| Hormone Replacement Therapy | 0.01 | 0.89 | −0.08 | 0.14 |
| Antihypertensives | 0.11 | 0.043 | 0.24 | <0.0001 |
| Physical Activity Level | −0.21 | <0.001 | −0.22 | <0.0001 |
| Total Estradiol | −0.10 | 0.06 | −0.26 | <0.0001 |
| Cortical CSA | 0.07 | 0.21 | −0.22 | <0.0001 |
Abbreviations: CSA, cross-sectional area; eGFR, estimated glomerular filtration rate.
Figure 1.
Regression lines (best fit lines) depicting the relationship between carotid-femoral pulse wave velocity and cortical middle tibial cross-sectional area (cCSA).
After adjusting for age, we found a significant inverse correlation between PWV and cCSA in women (Standardized [Std] β = −0.17, p=0.001), but not in men (Std β = 0.05, p=0.33). Next, we attempted to identify potential mediators of the association between PWV and cCSA by successively adding variables known to influence PWV or bone demineralization (Table 3). After adjusting for MAP, obesity and menopause status, the association between PWV and cCSA remained highly statistically significant in women (Model 1) but not in men. The association between PWV and cCSA remained unchanged after eGFR and serum calcium levels were added to the model (Model 2). Similarly, this association was not influenced by the addition of medications that affect PWV or bone demineralization (Model 3), or alcohol intake and physical activity levels (Model 4). Finally, the association between PWV and cCSA still remained significant after adjusting for total estradiol levels (Model 5). The fully-adjusted model (Table 3, Model 5) showed that, in women, for every SD decrease in cCSA, PWV increased by 0.14 SD. Notably, only three other variables remained independently associated with PWV, with age providing the greatest contribution (Std β = 0.24, p=0.002), followed by menopause (Std β = 0.16, p=0.002), and hormone replacement therapy (Std β = −0.13 p=0.01).
Table 3.
Hierarchical Multivariate Models Examining the Relationship between Pulse Wave Velocity and Middle Tibia Cortical Cross-Sectional Area in Women
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Covariates | Std B | P | Std B | P | Std B | P | Std B | P | B | Std B | P |
| Age | 0.29 | <0.0001 | 0.28 | <0.0001 | 0.29 | <0.0001 | 0.27 | <0.001 | 0.03 | 0.24 | 0.002 |
| MAP | 0.11 | 0.04 | 0.11 | 0.04 | 0.08 | 0.13 | 0.08 | 0.13 | 0.01 | 0.08 | 0.13 |
| Obesity | −0.03 | 0.61 | −0.03 | 0.64 | −0.04 | 0.57 | −0.03 | 0.54 | −0.14 | −0.03 | 0.60 |
| Menopause status | 0.16 | 0.001 | 0.16 | 0.002 | 0.17 | 0.002 | 0.17 | 0.001 | 0.13 | 0.16 | 0.002 |
| eGFR | … | … | −0.04 | 0.43 | −0.04 | 0.44 | −0.04 | 0.46 | −0.004 | −0.04 | 0.45 |
| cCa | … | … | −0.03 | 0.46 | −0.03 | 0.52 | −0.03 | 0.51 | −0.18 | −0.03 | 0.47 |
| Thiazide-type diuretics use | … | … | … | … | 0.0006 | 0.99 | 0.002 | 0.97 | 0.01 | 0.002 | 0.97 |
| Antihypertensives | … | … | … | … | 0.08 | 0.25 | 0.08 | 0.26 | 0.31 | 0.08 | 0.28 |
| Hormone Replacement Therapy | … | … | … | … | −0.14 | 0.006 | −0.14 | 0.007 | −0.57 | −0.13 | 0.01 |
| Vitamin D | … | … | … | … | 0.07 | 0.19 | 0.07 | 0.19 | 0.49 | 0.07 | 0.19 |
| Calcium | … | … | … | … | −0.007 | 0.88 | −0.007 | 0.89 | −0.04 | −0.007 | 0.89 |
| Vitamin D plus Calcium | … | … | … | … | −0.08 | 0.10 | −0.09 | 0.10 | −0.39 | −0.09 | 0.09 |
| Alcohol intake | … | … | … | … | … | −0.02 | 0.72 | −0.09 | −0.02 | 0.68 | |
| Physical activity level | … | … | … | … | … | −0.04 | 0.45 | −0.001 | −0.05 | 0.41 | |
| Total estradiol | … | … | … | … | … | … | … | … | −0.05 | −0.04 | 0.52 |
| cCSA | −0.15 | 0.02 | −0.15 | 0.02 | −0.14 | 0.03 | −0.14 | 0.05 | −0.007 | −0.14 | 0.04 |
For each Model, standardized β-coefficient (Std B) and P values are given. For Model 5, unstandardized β-coefficient (B), standardized β-coefficient (Std B) and P values are given.
Our findings were not changed when the analyses were repeated with cCSA not indexed to BMI or indexed to height.
DISCUSSION
In the present study we found an inverse association between arterial stiffness and bone demineralization in women but not in men, independent of age and several confounders.
Arterial stiffness and bone demineralization in women
Four previous studies that explored the association between arterial stiffness and bone demineralization in women (15–18) also found an inverse relationship between these two variables. It should be noted that these 4 studies focused on postmenopausal women, measured bone mineral density with dual energy x-ray absorptiometry (DEXA), and all but one (17) assessed PWV at the brachial and ankle sites. In our study, we examined community-dwelling women with a wide range of bone mineral density (BMD); we assessed PWV at the carotid and femoral sites, which is the gold standard for the non invasive assessment of arterial stiffness (11); and we measured BMD with computed tomography, which, unlike DEXA, can distinguish between cortical and trabecular bone. Using peripheral quantitative CT, Tanaka and colleagues also found a significant association between arterial stiffness and para-articular trabecular bone loss at the distal radius, in patients with rheumatoid arthritis (19).
We found that every SD decrease in cCSA is associated with a 0.14 SD increase in PWV. Although this effect size is quite modest, it is not very surprising as age is the main determinant of PWV. Previous studies that explored the relationship between PWV and BMD reported somewhat stronger effect sizes. Sumino et al. (15) found that in postmenopausal women (n=95), after adjusting for age, years since menopause, systolic and diastolic blood pressure, every SD decrease in lumbar spine bone mineral density (BMD) is associated with a 0.26 SD increase in PWV. Similarly, Frost et al. (17) found that in postmenopausal women (n=54), after adjusting for age, mean arterial pressure, total cholesterol, LDL, HDL, triglycerides, every SD decrease in total hip BMD is associated with a 0.25 SD increase in PWV; and every SD decrease in lumbar spine BMD is associated with a 0.17 SD increase in PWV.
Arterial stiffness and bone demineralization in men
In our study, we did not find an association between PWV and cCSA in men. Similarly, Benetos and colleagues (20) did not observe an association between osteoporosis and aortic stiffness in seemingly healthy French men over the age of 60. On the other hand, a clinical study in Japanese men spanning a very large age range (21–80 years), found a correlation between brachial ankle PWV and the osteo-sono index, an ultrasound-based method for detecting osteoporosis (21). However, the osteo-sono index represents a surrogate marker of BMD and reflects elastic properties that may be relatively independent of bone mineralization.
Putative mediators of the relationship between arterial stiffness and bone demineralization
Both arterial stiffening and bone demineralization are highly regulated processes. A number of mechanisms may explain the association between arterial stiffness and bone demineralization (9). For example, elastin breakdown may stimulate calcium deposition (22). Elastin degradation products produce cathepsin S, a proteinase that is regulated by inflammation; is overexpressed in diseased arteries and can trigger osteogenesis (22).
Moreover, experimental studies have shown that under appropriate conditions, cells either residing in the vascular wall (smooth muscle cells, SMC) or precursor cells with the potential for mesenchymal differentiation acquire osteogenic properties, a process that appears to be regulated by bone morphogenetic protein (BMP), Smad6 and core-binding factor a-1 (cbfa-1) signaling pathways. These osteoblast-like cells deposit bone matrix proteins that can subsequently become mineralized. In addition, matrix vesicles and apoptotic bodies from vascular SMC can also become calcified, a process that appears to be regulated by fetuin-A and matrix Gla protein (MGP) (9). Recently, Pirro et al. (23) found that postmenopausal osteoporotic women have both high PWV and circulating immature osteoprogenitor cells (OPCs) compared to non-osteoporotic subjects, and that the number of circulating OPCs was directly associated with PWV. Hence, although circulating OPCs are believed to play a beneficial influence on bone by actively contributing to mineralization (24), it is also possible that circulating cells expressing both stem cell and osteogenic markers (i.e. CD34, alkaline phosphatase and osteocalcin), may have a role in the process of arterial stiffening (23).
In our study, we evaluated several clinically available markers that are plausible mediators of the age-independent and inverse relationship between arterial stiffness and bone demineralization (Table 3), but none of them significantly altered the association between PWV and cCSA. However, an important finding in our study is that the relationship between arterial stiffness and bone demineralization is sex-specific, suggesting that mediators of this association are probably differentially regulated between men and women. Inorganic phosphorus, which was not assessed in our study, is a putative candidate mediator: Serum phosphorus levels differ between men and women (25). Estradiol influences phosphorus levels (26). In women, serum phosphorus levels are increased and urine phosphorus levels are decreased after menopause (27). In community-living older men, higher serum estradiol and testosterone levels are each independently associated with lower serum phosphate levels (28). Importantly, phosphorus can induce vascular SMC to calcify in vitro (29), and phosphorus levels are associated with arterial calcification (30) and vascular stiffness (31,32) as well as cardiovascular disease and all-cause mortality (33, 34).
Interestingly, menopause and hormone replacement therapy were the only two variables beyond age and cCSA that remained independently associated with PWV. Their relationship with PWV was in opposite direction to each other. This suggests that the previously reported sex differences in arterial stiffness (35) may be related to differential effects of sex hormones on arterial structure and function (36).
Study limitations
Our results suggest that pathways differentially regulated by sex hormones, particularly those involving mineral metabolism, may play a pivotal role in mediating the association between arterial stiffness and bone mineral density. A major limitation of our study is that we did not measure any sex hormone beyond total estradiol and total testosterone, nor did we measure any marker of mineral metabolism beyond serum calcium (e.g. intact parathyroid hormone, 25-hydroxy-vitamin D, inorganic phosphorus).
This study is based on previously collected data. A pre-hoc analysis was not performed in order to ensure the absence of type II error in the lack of significance regarding men. The cross-sectional nature of this study does not allow us to ascertain whether arterial stiffness precedes or follows bone demineralization. Since eGFR is not accurate at a true GFR level >60, we cannot exclude residual confounding by subtle differences in kidney dysfunction.
Despite the aforementioned limitations, this study has several unique strengths. First, this study measured carotid-femoral PWV, which is the gold standard for the non-invasive assessment of arterial stiffness (2), and used a state of the art method to assess bone density. Second, this study was performed in the context of a normative aging study that included both sexes with careful assessment of several potential confounders of the arterial stiffness/bone demineralization relationship. Finally, all analyses were performed separately by sex, given important sex differences in age-associated bone demineralization (accelerated in women) and arterial stiffening (accelerated in older women) (3).
Conclusions
The present study provides evidence for an inverse association between arterial stiffness and bone demineralization in women but not in men, independent of age and several confounders. The sex-specific signaling and molecular pathways that putatively underlie the cross-talk between central arteries and bone are not completely understood and warrant further investigation.
Acknowledgements
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging; and in part by the “Programma di Scambi Internazionali con Universitá ed Istituti di Ricerca Stranieri per la Mobiltá di Breve Durata di Docenti, Ricercatori e Studiosi” of the University of Naples “Federico II”.
Footnotes
Disclosure
The authors have no financial or personal conflicts of interest. None of the sponsoring institutions interfered with the design, methods, subject recruitment, data collections, analysis and preparation of the manuscript.
References
- 1.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 2.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 4.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A Health ABC Study. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 5.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 6.Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, Spurgeon HP, Ferrucci L, Lakatta EG. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2008;51:1377–1383. doi: 10.1016/j.jacc.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51:99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- 8.Lauretani F, Bandinelli S, Griswold ME, Maggio M, Semba R, Guralnik JM, Ferrucci Longitudinal changes in BMD and bone geometry in a population-based study. J Bone Miner Res. 2008;23:400–408. doi: 10.1359/JBMR.071103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofbauer LC, Brueck CC, Shanahan CM, Schoppet M, Dobnig H. Vascular calcification and osteoporosis--from clinical observation towards molecular understanding. Osteoporos Int. 2007;18:251–259. doi: 10.1007/s00198-006-0282-z. [DOI] [PubMed] [Google Scholar]
- 10.Shock NW, Greulich RC, Andres RA, Arenberg D, Costa PT, Jr, Lakatta EG, Tobin JD. Normal human aging: the Baltimore Longitudinal Study of Aging. Washington, DC: U.S. Government Printing Office; 1984. p. 45. NIH publication no. 84–2450. [Google Scholar]
- 11.Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac AM, Target R, Levy BI. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26:485–490. doi: 10.1161/01.hyp.26.3.485. [DOI] [PubMed] [Google Scholar]
- 12.Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, Lakatta EG. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 13.Bringhurst FR, Demay MB, Kronenberg HM. Hormones and disorders of mineral metabolism. Philadelphia: Saunders; 1998. pp. 1155–1209. [Google Scholar]
- 14.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 15.Sumino H, Ichikawa S, Kasama S, Takahashi T, Kumakura H, Takayama Y, Kanda T, Sakamaki T, Kurabayashi M. Elevated arterial stiffness in postmenopausal women with osteoporosis. Maturitas. 2006;55:212–218. doi: 10.1016/j.maturitas.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Mikumo M, Okano H, Yoshikata R, Ishitani K, Ohta H. Association between lumbar bone mineral density and vascular stiffness as assessed by pulse wave velocity in postmenopausal women. J Bone Miner Metab. 2009;27:89–94. doi: 10.1007/s00774-008-0014-x. [DOI] [PubMed] [Google Scholar]
- 17.Frost ML, Grella R, Millasseau SC, Jiang BY, Hampson G, Fogelman I, Chowienczyk PJ. Relationship of calcification of atherosclerotic plaque and arterial stiffness to bone mineral density and osteoprotegerin in postmenopausal women referred for osteoporosis screening. Calcif Tissue Int. 2008;83:112–120. doi: 10.1007/s00223-008-9153-2. [DOI] [PubMed] [Google Scholar]
- 18.Seo SK, Cho S, Kim HY, Choi YS, Park KH, Cho DJ, Lee BS. Bone mineral density, arterial stiffness, and coronary atherosclerosis in healthy postmenopausal women. Menopause. 2009;16:937–943. doi: 10.1097/gme.0b013e3181a15552. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka K, Inaba M, Goto H, Nagata-Sakurai M, Sakai S, Yamada S, Ueda M, Ishimura E, Nishizawa Y. Paraarticular trabecular bone loss at the ultradistal radius and increased arterial stiffening in postmenopausal patients with rheumatoid arthritis. J Rheumatol. 2006;33:652–658. [PubMed] [Google Scholar]
- 20.Benetos A, Zervoudaki A, Kearney-Schwartz A, Perret-Guillaume C, Pascal-Vigneron V, Lacolley P, Labat C, Weryha G. Effects of lean and fat mass on bone mineral density and arterial stiffness in elderly men. Osteoporos Int. 2009;20:1385–1391. doi: 10.1007/s00198-008-0807-8. [DOI] [PubMed] [Google Scholar]
- 21.Hirose K, Tomiyama H, Okazaki R, Arai T, Koji Y, Zaydun G, Hori S, Yamashina A. Increased pulse wave velocity associated with reduced calcaneal quantitative osteo-sono index: possible relationship between atherosclerosis and osteopenia. J Clin Endocrinol Metab. 2003;88:2573–2578. doi: 10.1210/jc.2002-021511. [DOI] [PubMed] [Google Scholar]
- 22.Aikawa E, Aikawa M, Libby P, Figueiredo JL, Rusanescu G, Iwamoto Y, Fukuda D, Kohler RH, Shi GP, Jaffer FA, Weissleder R. Arterial and aortic valve calcification abolished by elastolytic cathepsin S deficiency in chronic renal disease. Circulation. 2009;119:1785–1794. doi: 10.1161/CIRCULATIONAHA.108.827972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pirro M, Schillaci G, Mannarino MR, Scarponi AM, Manfredelli MR, Callarelli L, Leli C, Fabbriciani G, Helou RS, Bagaglia F, Mannarino E. Circulating immature osteoprogenitor cells and arterial stiffening in postmenopausal osteoporosis. Nutr Metab Cardiovasc Dis. 2010 May 27; doi: 10.1016/j.numecd.2010.01.015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Eghbali-Fatourechi GZ, Lamsam J, Fraser D, Nagel D, Riggs BL, Khosla S. Circulating osteoblast-lineage cells in humans. N Engl J Med. 2005;352 doi: 10.1056/NEJMoa044264. 1959e66. [DOI] [PubMed] [Google Scholar]
- 25.Cirillo M, Ciacci C, De Santo NG. Age, renal tubular phosphate reabsorption, and serum phosphate levels in adults. N Engl J Med. 2008;359:864–866. doi: 10.1056/NEJMc0800696. [DOI] [PubMed] [Google Scholar]
- 26.Faroqui S, Levi M, Soleimani M, Amlal H. Estrogen downregulates the proximal tubule type IIa sodium phosphate cotransporter causing phosphate wasting and hypophosphatemia. Kidney Int. 2008;73:1141–1150. doi: 10.1038/ki.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carrillo-López N, Román-García P, Rodríguez-Rebollar A, Fernández-Martín JL, Naves-Díaz M, Cannata-Andía JB. Indirect regulation of PTH by estrogens may require FGF23. J Am Soc Nephrol. 2009;20:2009–2017. doi: 10.1681/ASN.2008121258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meng J, Ohlsson C, Laughlin GA, Chonchol M, Wassel CL, Ljunggren O, Karlsson MK, Mellstrom D, Orwoll ES, Barrett-Connor E, Ix JH Osteoporotic Fractures in Men (MrOs) Study Group. Associations of estradiol and testosterone with serum phosphorus in older men: the Osteoporotic Fractures in Men study. Kidney Int. 2010;78:415–422. doi: 10.1038/ki.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10–E17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 30.Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA. Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol. 2009;20:397–404. doi: 10.1681/ASN.2008020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ix JH, De Boer IH, Peralta CA, Adeney KL, Duprez DA, Jenny NS, Siscovick DS, Kestenbaum BR. Serum phosphorus concentrations and arterial stiffness among individuals with normal kidney function to moderate kidney disease in MESA. Clin J Am Soc Nephrol. 2009;4:609–615. doi: 10.2215/CJN.04100808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meng J, Wassel CL, Kestenbaum BR, Collins TC, Criqui MH, Lewis CE, Cummings SR, Ix JH Osteoporotic Fractures in Men (MrOS) Study Group. Serum phosphorus levels and the spectrum of ankle-brachial index in older men: the Osteoporotic Fractures in Men (MrOS) study. Am J Epidemiol. 2010;171:909–916. doi: 10.1093/aje/kwq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D'Agostino RB, Sr, Gaziano JM, Vasan RS. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879–885. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 34.Foley RN, Collins AJ, Ishani A, Kalra PA. Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2008;156:556–563. doi: 10.1016/j.ahj.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 35.Vermeersch SJ, Rietzschel ER, De Buyzere ML, De Bacquer D, De Backer G, Van Bortel LM, Gillebert TC, Verdonck PR, Segers P. Age and gender related patterns in carotid-femoral PWV and carotid and femoral stiffness in a large healthy, middle-aged population. J Hypertens. 2008;26:1411–1419. doi: 10.1097/HJH.0b013e3282ffac00. [DOI] [PubMed] [Google Scholar]
- 36.Miller VM. Sex-based differences in vascular function. Women’s Health (Lond Engl) 2010;6:737–752. doi: 10.2217/whe.10.53. [DOI] [PubMed] [Google Scholar]