Abstract
The induction of the heat shock response is accepted to be a protective response, reducing injury and improving cell survival. However, when inflammation precedes heat shock there is an unexpected increase in injury, known as the heat shock paradox, which is hypothesized to be a mechanism underlying multi-organ dysfunction. We hypothesized that the heat shock paradox would occur in adult cardiac myocytes and that heat shock factor (HSF)1 would contribute to injury. Heat shock (HS) at 42°C and TNF (10 ng/ml) were used as the HS and the inflammatory insult, respectively. The combination of TNF followed by HS (TNF/HS) caused the greatest amount of apoptosis in adult rat cardiac myocytes. TNF/HS resulted in an increase in heat shock protein (HSP) 60, compared to untreated cells, those receiving HS/TNF, or TNF alone. There was no increase in heme oxygenase 1 in any of the groups. HSP72 increased in all the groups, with the greatest levels with TNF/HS. NFκB activation was greatest with TNF/HS. Pretreatment with a DNA binding decoy for HSF1 prevented the increase in HSPs and decreased apoptosis in all groups. However, the increase in iNOS, seen in all treatment groups, was unaffected by the HSF1 binding decoy. We conclude that the heat shock paradox occurs in adult cardiac myocytes, that HSP60 is increased as part of the heat shock paradox, and that HSF1 activation contributes to injury.
Keywords: HSF1, NFkappaB, HSP60, apoptosis, MODS, iNOS
Introduction
Induction of the heat shock response with increased expression of the heat shock proteins is well-established as a protective response.(1–3) However, when an inflammatory stimulus precedes heat shock there is an unexpected increase in injury, known as the heat shock paradox.(4;5) The heat shock paradox has been reported in several different settings including endothelial cells and a mouse model of sepsis, and may be a mechanism for multi-organ dysfunction.(4;5) The mechanism of this deleterious response remains elusive. The heat shock paradox is of great interest because of its potential role in the multi-organ dysfunction syndrome (MODS) seen in critically ill patients in the intensive care unit. We investigated the heat shock paradox using isolated rat adult cardiac myocytes. We hypothesized that activation of heat shock factor (HSF) 1, the heat shock protein transcription factor activated in response to stress, was a critical element for injury, despite the overall protective effects of the expression of heat shock proteins (HSPs).
Using TNFα (TNF) as the inflammatory stimulus, we found that the addition of heat shock (HS) markedly decreased I6B, drove activation of NFκB and increased expression of iNOS, along with the expected increase in HSPs. HS or TNF alone or HS followed by TNF did not decrease I6B. Using DNA binding decoys for HSF, we dissected the role of HSF activation in these changes. The results of these experiments are reported here. To our knowledge, this is the first report of the heat shock paradox in cardiac myocytes.
Materials and Methods
Isolated Adult Rat Cardiac Myocytes
The animal protocol was approved by the University of California, Davis Animal Research committee in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Cardiac myocytes were isolated from adult male Sprague Dawley rats as previously described.(6)
Experimental Protocol
Experimental groups consisted of: 1. Control, untreated cells; 2. HS at 42°C for 1 h followed by 18 h of recovery at 37°C; 3. TNF (10 ng/ml, R&D Systems) for 19 h.; 4. TNF with 1 h of HS at 42°C at 6 h and then 12 h of recovery at 37°C; 5. HS at 42°C for 1 h followed by TNF (10 ng/ml) for 18h at 37EC. Heat shock was delivered in a special CO2 incubator set to 42°C (Brunswick). All protein samples were collected at the end of the experiment, 19 h, for analysis. NFκB activation was measured 1 h. after the last treatment (HS or TNF).
Western Blotting
was performed as previously described.(7) Antibodies were used to HSP72 (StressGen, 1:1000) HSP60 (SPA806, 1:1000, StressGen), heme oxygenase 1 (HO-1, also known as HSP32, 1:1000, StressGen), IκB (1:1000, Cell Signaling), and iNOS (1:1000, BD Transduction). Antibody to GAPDH (1:40,000, Fitzgerald Industries) was used as a loading control. Nitrocellulose membranes after blocking and antibody incubations were developed with a chemiluminescent system (Pierce). Densitometry was done on scanned images using U-Scan It software.
NFκB Activation Assay
This assay measures binding of nuclear extract to a microtiter plate coated with the DNA binding element for NFκB (Pierce). The plate is washed and developed with antibody for p65 following the instructions of the manufacturer. Previously, we have found this assay to correlate well with the results of EMSA, but to provide more precise quantification and to allow for the comparison of multiple samples.(8)
LDH Release
was measured as an index of necrosis as previously described.(7)
Apoptosis
was assessed by measuring caspase 3 activity using an assay that detects cleavage of AC-DEVD-AMC (Calbiochem, San Diego, CA). DNA fragmentation was used as a second index of apoptosis and was measured by the Cell Death ELISA (Roche) following instructions of the manufacturer. This assay measures oligosome formation, an index of DNA fragmentation. The results were normalized to control, untreated cells to correct for any variation amongst cardiac myocyte isolations.
Cell Viability
was determined with the Live/Dead Assay (Invitrogen) as previously described.(7)
HSF Decoy
DNA binding decoys act as a cytosolic sponge, binding activated transcription factor.(9;10) A scrambled nonspecific sequence was used as a control as previously described.(11) The phosphorothioate DNA was synthesized and purified by Trilink (San Diego). The small DNA dimers consist of oligonucleotides encoding a consensus DNA binding domain for HSF as previously detailed.(11) Cells were pretreated with 2 uM HSF1 decoy or a scrambled control sequence for 6h, as reported previously, prior to the start of the experiment.(8;11)
Statistics
Data was analyzed by an ANOVA followed by a Student Neuman Keuls test. Normalized data was analyzed by an ANOVA on RANKS followed by an appropriate secondary test. A p < 0.05 was considered significant. Results are expressed as mean α the standard error of the mean (SEM).
Results
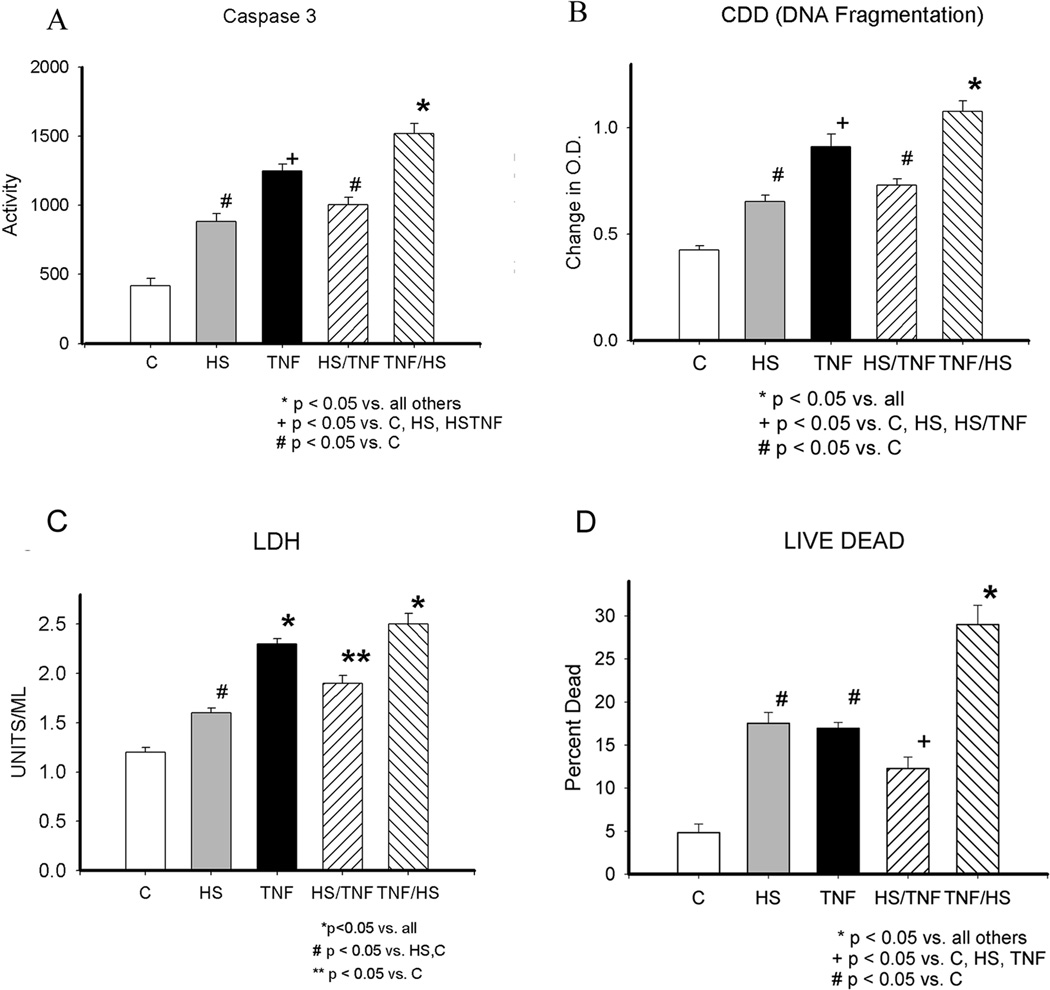
The effect of heat shock (HS), TNF, HS/TNF and TNF/HS on cardiac myocyte injury were compared. Figure 1 summarizes the time line for the treatment protocols. As shown in figure 2A, caspase 3 activation occurred with all treatments, but the greatest activation was seen with TNF treatment, which was used as an inflammatory injury, followed by HS (TNF/HS). Similarly, DNA fragmentation, as measured by the CDD assay, was increased with all treatments, but greatest with TNF /HS (figure 2B). LDH release was mild to moderately increased by the treatments, but in contrast to the apoptosis indices, TNF and TNF plus heat shock resulted in similar amounts of LDH release (figure 2C). To assess the percent of cells undergoing apoptosis/cell death, the Live/Dead Assay was used, as shown in figure 2D. TNF/HS was associated with the highest cell death with almost 30% of the cells dead, compared to 12.3% with HS/TNF. Thus, HS prior to TNF treatment diminished the injury caused by TNF alone for both apoptosis and necrosis parameters (figure 2A–D).
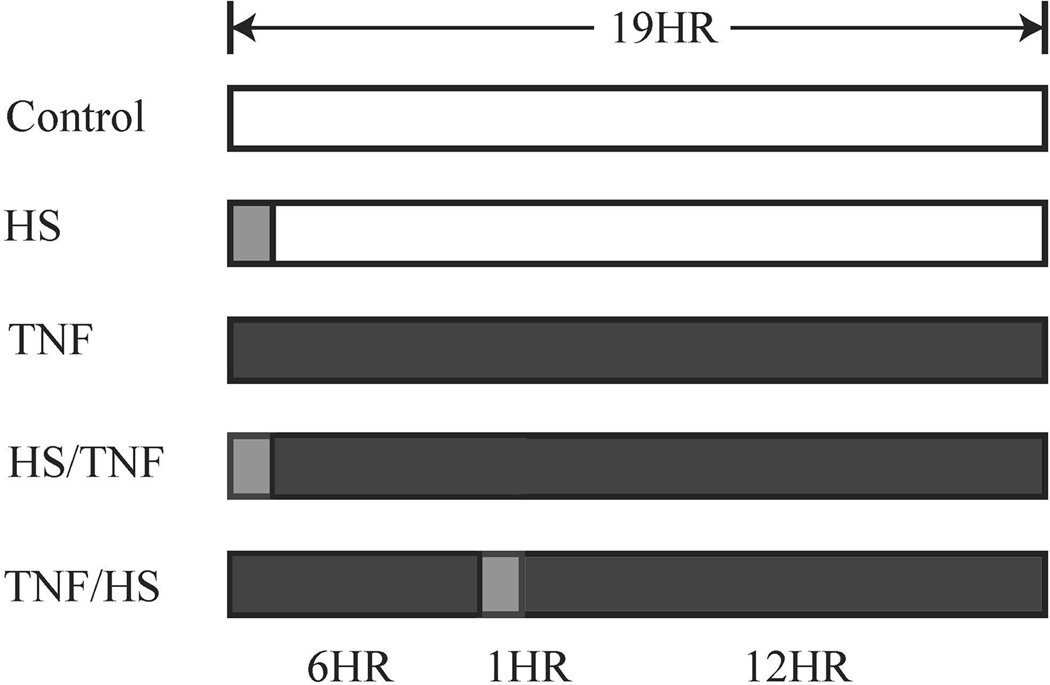
Figure 1.
Treatment protocols are summarized in diagram. For CDD assay, live dead assay and westerns, samples were studies at 19 hr., which is the completion of all protocols, on the far right. Caspase 3 activity, which increases before DNA fragmentation based on pilot studies, was measured at 16 hr. NFκB activity was postulated to peak early after treatments. Therefore, NFκB activity was measured at 7.5 h, which is 30 min. after completion of the last HS treatment. Dark grey bar represents TNF treatment. In the TNF groups, TNF was added at the beginning of experiment and not removed at any point, nor was new TNF added after HS. For the HS/TNF group, as HS was the first treatment, TNF added after completion of the 1h HS. Light grey bar represents 1 hr heat shock. HS - heat shock at 42°C for 1 h followed by 18 h at 37EC in a standard cell culture incubator; TNF - TNFα 10 ng/ml; HS/TNF - heat shock at 42°C for 1 h followed by the addition of TNF 10 ng/ml for 18 h; TNF/HS - TNFα 10 ng/ml for 6 h followed by heat shock at 42°C for 1 h, and then 12 h at 37EC in a standard cell culture incubator.
Figure 2.
A) Caspase 3 activity at 16h. B) DNA fragmentation by CDD or cell death assay (Roche) at 19h. C) LDH release at 19 h. D) Live Dead Assay. Percent of cells dead based on ethidium bromide uptake vs. metabolism of calcein in live cells at 19 h. C - control; HS - heat shock; HSTNF - 1 h hs followed by TNF treatment; TNFHS - 6 h TNF followed by 1 h hs. * p < 0.05 vs. all others, + p < 0.05 vs. C, HS, HSTNF, # p < 0.05 vs. C. Graphs summarize 3–6 experiments with 9–18/group.
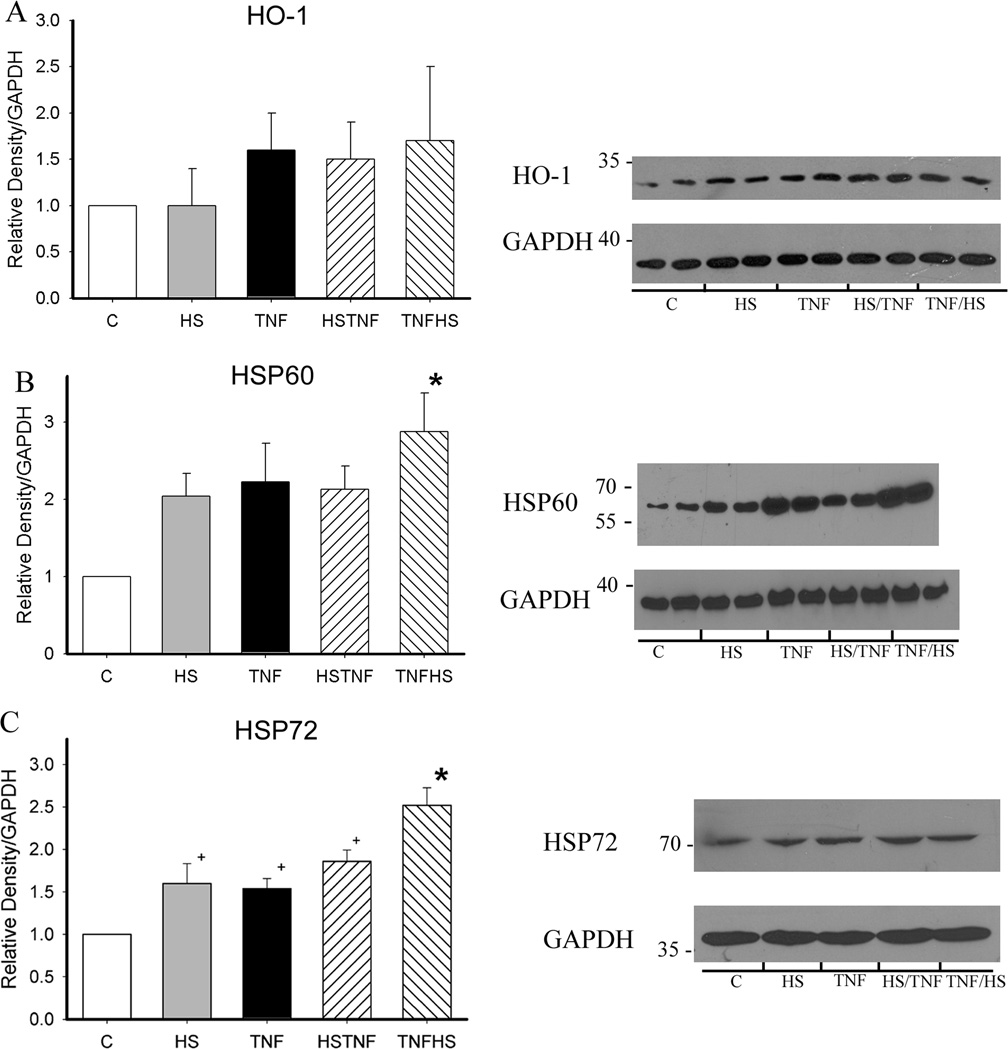
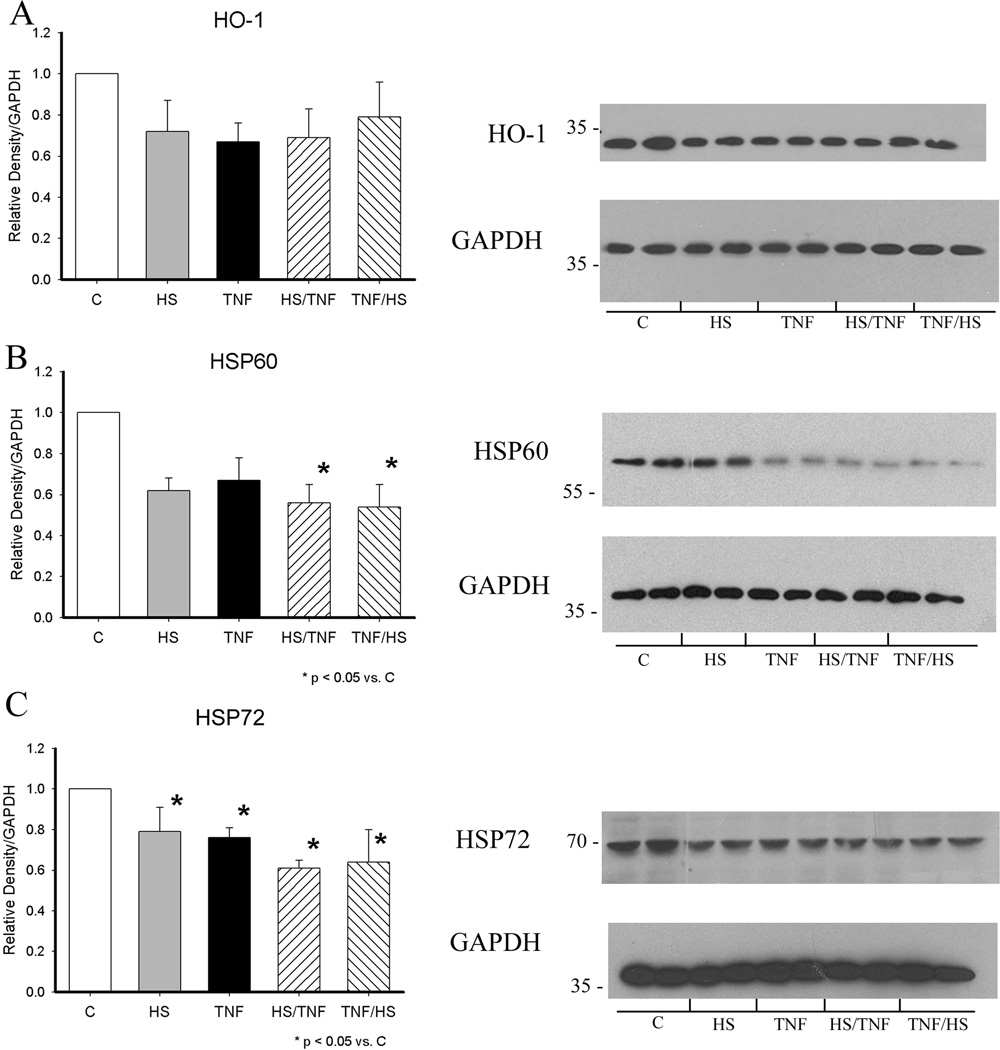
Both HS and TNF activate HSF-1 and this would be expected to increase HSP levels.(6) Expression of HSP72, HSP60 and HO-1 (HSP32) were examined by western blotting at 19h. HO-1 levels did not change significantly, but the combination of TNF/HS increased HSP60 levels by greater than 2.5 fold (figure 3A and B). HSP72, which is more readily induced by stresses such as heat, was significantly increased by all four treatments with TNF/HS having the greatest effect (figure 3C, p<0.05), and HS exceeding the effect of TNF or HS/TNF.
Figure 3.
Heat shock protein expression at 19 h. Expression was analyzed by western with GAPDH as a loading control. Graphs summarize results of 4–6 separate experiments. A) HO-1. B) HSP60. C) HSP72. C - control; HS - heat shock; HSTNF - 1 h hs followed by TNF treatment; TNFHS - 6 h TNF followed by 1 h hs. * p < 0.05 vs. all others. + p < 0.05 vs. control. N = 4–6/group.
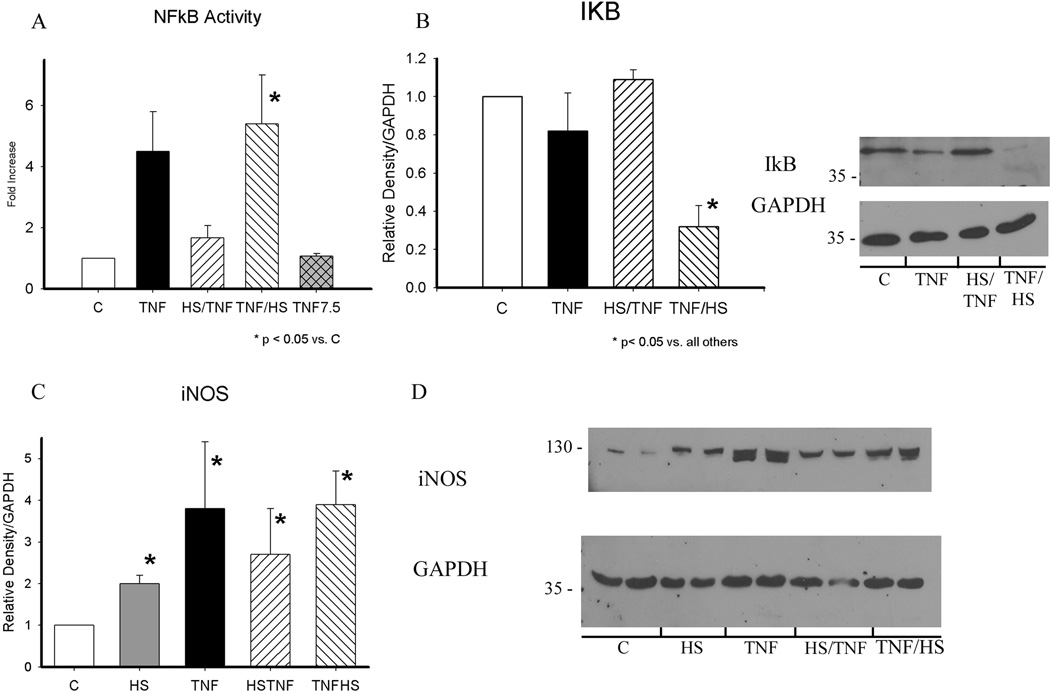
Activation of NFκB is thought to have a role in the heat shock paradox. We examined NFκB activation after treatment with TNF, HS/TNF and TNF/HS. For each treatment, NFκB activation was examined 30 min. after treatment. As the TNF/HS combination has the second treatment (HS) at 6 h, NFκB 7.5 h after TNF treatment alone was examined for comparison (figure 4A). TNF treatment increased NFκB activation, but the difference was not significant. There was no change in NFκB activation when TNF was given after HS. In contrast, NFκB activation was markedly increased when TNF was followed by HS (p<0.05). This was 7.5 h after initial TNF treatment. TNF treatment alone by 7.5h had no evidence of NFkB activation. To investigate the underlying mechanism, levels of IkB were examined. Only TNF followed by HS resulted in a decrease in IκB expression (figure 4B, p < 0.05). In addition, as shown in figure 4C/D, all four treatments increased expression of iNOS.
Figure 4.
A) NFκB activation was measured by assay 30 min. after completion of all treatments. TNF at 30 min. (black bar) and at 7.5 h (cross-hatched bar) are shown for comparison. * p < 0.05 vs. C (control). B) I6Bα expression was examined at 19h. Levels were decreased in TNF/HS group only. Graph summarizes results. Representative western with GAPDH on the same blot shown on right. * p <0.05 vs. all. C) iNOS levels were increased in all groups. * p < 0.05 vs. C. D) Representative western for iNOS. GAPDH for same blot shown below. All protein samples collected at 19 h. Graphs summarize results of 3–5 separate experiments. C - control; HS - heat shock; HS/TNF - 1 h hs followed by TNF treatment; TNF/HS - 6 h TNF followed by 1 h hs.
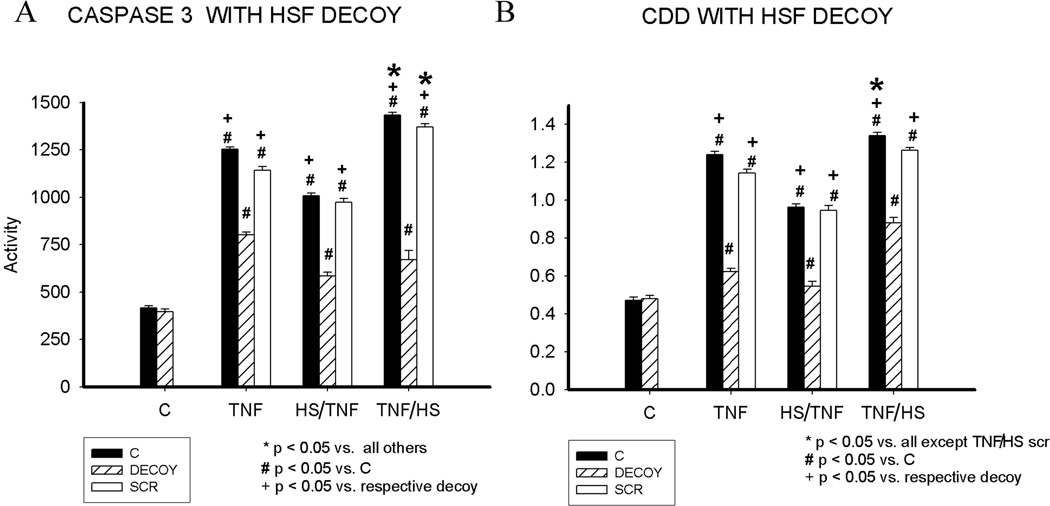
HS and TNF both activate HSF1.(6) HSF DNA binding decoys, as previously described, were used to inhibit the effects of HSF1 activation. The decoys are small phosphorothioate oligonucleotide dimers encoding the binding element (heat shock element, HSE) for HSF. The decoys act as a sponge for activated HSF1. For the decoy experiments, we focused on apoptosis. Cells were pretreated with HSF1 decoy or a scrambled control sequence for 6h, as reported previously.(8;11) The HSF1 decoy inhibited caspase 3 activation after TNF, HS/TNF or TNF/HS (figure 5A). Similarly, DNA fragmentation was greatly reduced by the HSF decoy (figure 5B). The scrambled control decoy had no effect.
Figure 5.
Effect of HSF binding decoy on apoptosis. A) Caspase 3 activity at 16h. B) DNA fragmentation at 19h. Graphs summarize 3 separate experiments with 9–15/group. C - control; HS - heat shock; HS/TNF - 1 h hs followed by TNF treatment; TNF/HS - 6 h TNF followed by 1 h hs. SCR - scrambled sequence decoy (i.e. nonfunctional). * p < 0.05 vs. all. # P < 0.05 vs. C. + p < 0.05 vs. respective decoy.
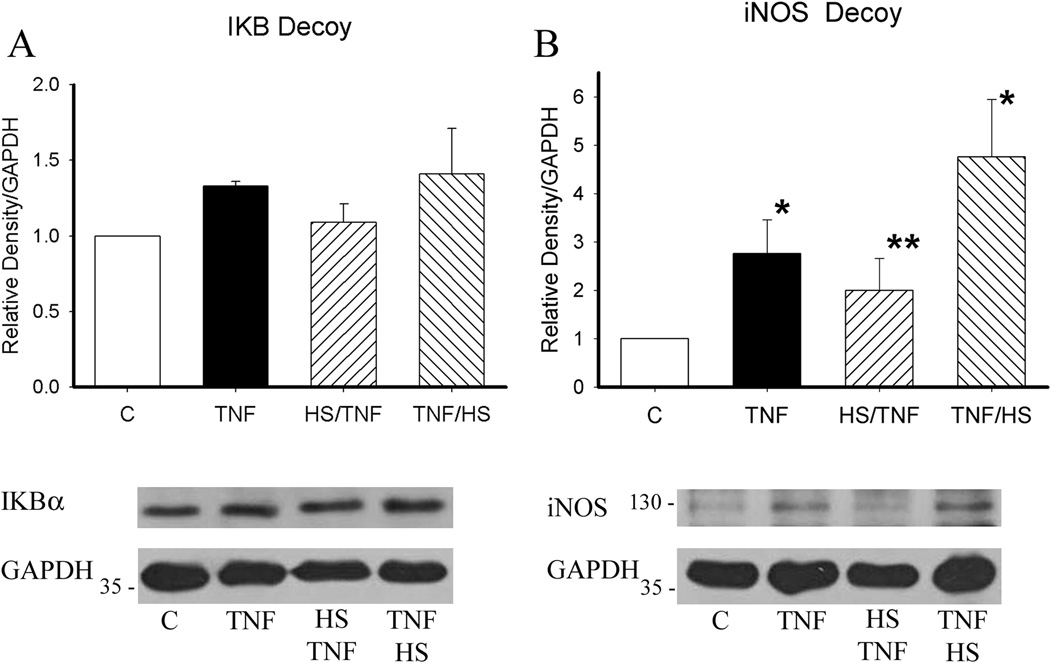
Western blotting was done to analyze the effect of the HSF decoy on the protein changes seen with the treatments. HO-1 expression was not significantly changed, similar to the baseline experiments. HSP60 expression was decreased in the HS/TNF and TNF/HS groups (figure 6A and B). This is in contrast to the increase in HSP60 expression seen with TNF/HS in the absence of the decoy. HSP72 expression was decreased in all groups (figure 6C). The HSF decoy blocked the decrease in IkB seen with TNF/HS (figure 7A). INOS levels were not affected by the HSF decoy (figure 7B).
Figure 6.
HSF DNA binding decoy and HSP expression at 19 h. A) HO-1 protein levels were unchanged. B) HSP60 protein expression was decreased in all groups compared to control. C) HSP72 expression was decreased in all groups compared to control. Graphs summarize results of multiple westerns. Data normalized to control group. Representative western for each protein and GAPDH on same blot shown. N = 3–4/group. * p < 0.05 vs. C.
Figure 7.
Effect of HSF binding decoy on IkB and iNOS expression at 19 h. A) HSF binding decoy blocked the decrease in IkB seen with TNF/HS. B) HSF binding decoy had no effect on iNOS expression. Graphs summarize results of multiple westerns. Data normalized to control group. Representative western for each protein and GAPDH on same blot shown. N = 4/group. * p < 0.05 vs. C.
Discussion
This study investigates the role of HSF in the heat shock paradox, where induction of the heat shock response after an inflammatory insult is unexpectedly more injurious than the inflammatory insult alone.(5) While most experimental studies focus on a single injury, in the intensive care setting it is common to have multiple sequential injuries, such as the trauma victim, who develops a fever, infection and other complications, or in the elderly, who at baseline have elevation of inflammatory cytokines such as IL-6.(12) Thus, the heat shock paradox may be quite important in the hospital course of severely ill patients. In the current study, we report for the first time to our knowledge that adult cardiac myocytes exhibit the heat shock paradox. TNF/HS, an inflammatory insult followed by heat shock, caused more apoptotic injury than any of the other treatments. Overall, HS prior to TNF was protective, as would be expected. Interestingly, only TNF/HS significantly increased HSP60 expression. In contrast, all groups had increased HSP72, but the greatest increase was seen with TNF/HS. TNF/HS caused a marked increase in NFkB activity. Only in this group was IkB expression significantly decreased. INOS was used as another index of an inflammatory response. All treatment groups had an increase in iNOS.
Inhibition of HSF and the Heat Shock Paradox
A DNA binding decoy was used to block the effect of any activated HSF. Others have investigated the role of NFκB activation in the deleterious effects of the heat shock paradox by inhibiting NFκB; however, many inhibitors of NFκB are actually inducers of the heat shock proteins, which are known to inhibit activation of NFκB.(13–15) Thus, it is difficult to separate the effects of NFκB inhibition from the induction of the heat shock response by the inhibitors. In the current study, we focused on the role of HSF activation. The DNA binding decoys are more selective than chemical inhibitors. HSF1 is the main mediator of the stress response, but HSF2 can be activated as well. HSFs are inactive in the cytosol of the normal, unstressed cell. Phosphorylation, trimerization and translocation to the nucleus are important steps in activation. Both activated HSF1 and HSF2 will be blocked by the DNA binding decoy, which acts as a cytosolic sponge for activated HSF.(9) Interestingly, the HSF DNA binding decoy significantly decreased apoptosis in all groups, but did not completely prevent apoptosis. Although brief heat shock is thought to induce protection, even the HS/TNF group had a marked decrease in apoptosis with the HSF DNA binding decoy. These results suggest that HSF activation can contribute to injury, as well as activating protective responses. The HSF binding decoy also blocked the increases in HSP60 and HSP72, and tended to decrease the expression of the three HSPs in all groups, likely reflecting inhibition of normal synthesis . The binding decoy had no effect on iNOS expression, indicating that the upregulation of iNOS was independent of HSF.
NFκB and the HSPs: A Complex Dance
There is an intertwining of the actions of the HSPs, HSF1, TNF and NFkB.(14;16) These interactions may help maintain the balance between health and inflammation. HSF1 inhibits both NFκB and the expression of TNF.(17) There is a chronic increase in TNF levels in HSF1 knockout mice, and this is associated with increased sensitivity to endotoxin.(18) The TNF promoter has an HSF1 binding site, and HSF1 functions as a repressor of TNF expression.(19;20) In contrast, TNF can inhibit the activation of HSF-1 via activation of phosphatases mediated by TNF-R1.(21) Low dose TNF increases HSP72 levels in cardiac myocytes, and we have found that TNF can activate HSF1 in cardiac myocytes (Chang and Knowlton, unpublished).(22) Others have reported that TNF increases resistance to heat injury in cancer through induction of MnSOD, increased HSF1 binding and greater HSP72 expression.(20;23) Activation of HSF1 is regulated by a complex mixture of phosphorylation and desphosphorylation, with phosphorylations at distinct sites promoting and inhibiting activity.(24–26) The heat shock paradox reflects a disassembly of the balance between HSPs and NFκB, viability and apoptosis, health and inflammation. The involved signaling pathways are complex. HSF1 is primarily known as the transcription factor for the HSPs. Slowly additional genes regulated by HSF1 are being identified including most recently, FasL, which has heat shock elements (HSE) in its promoter and is upregulated by HSF1. (20) HSF1 has also been found to block the activation and recruitment of SP1 and AP1.(20;27) In vascular smooth muscle cells knockdown of HSF1 with siRNA increased angiotensin II induced inflammation. Our understanding of the interaction of these fundamental pro- and anti-inflammatory proteins and pathways is incomplete, and much remains to be understood.
HSF, NFκB Activation and HSP60
In the current study, HS prior to TNF attenuated injury, but HS post-TNF increased injury. All the treatments increased expression of HSP72, although this increase was greatest with TNF/HS. HSP60 was only increased with the combination of TNF/HS, and this likely reflects the strong sequential activation NFκB occurring with first TNF and then HS combined with HSF1 activation. HS prior to TNF was associated with lower activation of NFκB. We have recently reported that the HSP60 gene contains two NFκB binding sites, and that these drive expression of HSP60 in cardiac myocytes.(28) Thus, TNF can increase the expression of HSP60. The HSF DNA binding decoy inhibited all of the changes in HSPs seen with the different treatments, and significantly decreased basal expression of both HSP60 and HSP72, likely through inhibition of underlying HSF1 mediated expression of these proteins.
These findings have important implications for our understanding of evolving illness in the critical care setting. In the absence of chest pain or pulmonary edema, most emphasis is placed on the function of organs other than the heart, assuming that without these signs and symptoms the heart is normal. However cardiac dysfunction is amongst the criteria for MODS, and rising TNF levels have been found in patients who go on to develop MODS.(29;30) MODS and other critical illness states, for which the criteria remain in flux, are associated with autonomic dysfunction with loss of the normal variability of heart rate and other parameters.(31;32) Increased troponin I leak, suggesting cardiac myocyte damage, in the absence of acute coronary syndrome or heart failure is associated with poorer outcomes.(33) However, cardiac myocyte apoptosis, as seen in the current study with TNF/HS, will not result in the release of troponin I, but has the potential to negatively impact cardiac function both acutely and in the long term. Our results support a more aggressive monitoring of cardiac function with cardiac echo, as well as invasive monitoring, such as cardiac output and pressure measurement, which is more routinely done in the setting of MODS.
The heat shock paradox, or two-hit hypothesis, has been studied primarily in cell culture. It has also been demonstrated that mice have the heat shock paradox when heat follows cecal ligation and puncture (CLP).(5) Repetitive injury is not only likely important in the clinic, it may very well be the norm. In the current study we demonstrate that adult cardiac myocytes manifest the heat shock paradox, and that this is accompanied by a significant increase in HSP60. HSP60 itself can cause inflammation and apoptosis, and further work will need to address the importance of the increase in this protein.(34) (14;35)
Acknowledgments
Supported by HL079071, HL077281 and a VA Merit Award, all to AAK. The authors thank Prof. Dr. H. Schimassek, Dr. G. Seitz and Dr. J. L. Kobba for their support of S. Kobba's stipend and travel to U.S. The authors thank S. Gupta for technical assistance.
Reference List
- 1.Currie RW, Karmazyn M. Improved post-ischemic ventricular recovery in the absence of changes in energy metabolism in working rat hearts following heat-shock. Journal of Molecular and Cellular Cardiology. 1990;22(6):631–636. doi: 10.1016/0022-2828(90)91006-s. [DOI] [PubMed] [Google Scholar]
- 2.Christians E, Yan L-J, Benjamin I. Heat shock factor 1 and heat shock proteins: Critical partners in protection against acute cell injury. Critical Care Medicine. 2002;30(1 Supp):S43–S50. [PubMed] [Google Scholar]
- 3.Gupta S, Knowlton AA. HSP60, Bax, apoptosis and the heart. Journal of Cellular and Molecular Medicine. 2005;9(1):51–58. doi: 10.1111/j.1582-4934.2005.tb00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchman TG, Abello PA, Smith EH, Bulkley GB. Induction of heat shock response leads to apoptosis in endothelial cells previously exposed to endotoxin. American Journal of Physiology. 1993;265(1 Pt 2):H165–H170. doi: 10.1152/ajpheart.1993.265.1.H165. [DOI] [PubMed] [Google Scholar]
- 5.Wizorek JJ, Coopersmith CM, Laramie JM, Tong A, Stromberg PE, Hotchkiss RS, Buchman TG, Cobb JP. Sequence makes a difference: Paradoxical effects of stress in vivo. Shock. 2004;22:229–233. doi: 10.1097/01.shk.0000133593.55400.ca. [DOI] [PubMed] [Google Scholar]
- 6.Sun L, Chang J, Kirchhoff SR, Knowlton AA. Activation of HSF and Selective Increase in Heat Shock Proteins by Acute Dexamethasone Treatment. American Journal of Physiology. 2000;278(4):H1091–H1096. doi: 10.1152/ajpheart.2000.278.4.H1091. [DOI] [PubMed] [Google Scholar]
- 7.Nakano M, Mann DL, Knowlton AA. Blocking the endogenous increase in HSP72 increases susceptibility to hypoxia and reoxygenation in isolated adult feline cardiocytes. Circulation. 1997;95(6):1523–1531. doi: 10.1161/01.cir.95.6.1523. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton KL, Mbai FN, Gupta S, Knowlton AA. Estrogen, Heat Shock Proteins, and NF{kappa}B in Human Vascular Endothelium. Arterioscler Thromb Vasc Biol. 2004;24(9):1628–1633. doi: 10.1161/01.ATV.0000137188.76195.fb. [DOI] [PubMed] [Google Scholar]
- 9.Bielinska A, Shivdasani RA, Zhang L, Nabel GJ. Regulation of gene expression with double-stranded phosphorothioate oligonucleotides. Science. 1990;250(4983):997–1000. doi: 10.1126/science.2237444. [DOI] [PubMed] [Google Scholar]
- 10.Morishita R, Gibbons GH, Horiuchi M, Ellison KE, Nakajima M, Zhang L, Kaneda Y, Ogihara T, Dzau VJ. A gene therapy strategy using a transcription factor decoy of the E2F binding site inhibits smooth muscle proliferation in vivo. Proceedings of the National Academy of Science, USA. 1995;92(13):5855–5859. doi: 10.1073/pnas.92.13.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton KL, Gupta S, Knowlton AA. Estrogen and regulation of heat shock protein expression in female cardiomyocytes: cross-talk with NFκB signaling. Journal of Molecular and Cellular Cardiology. 2004;36(4):577–584. doi: 10.1016/j.yjmcc.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo A, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Research Reviews. 2009;8(1):18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.May MJ, D'Acquisto F, Madge LA, Glockner J, Pober JS, Ghosh S. Selective inhibition of NFkB activation by a peptide that blocks the interaction of NEMO with the IkB kinase complex. Science. 2000;289(5484):1550–1554. doi: 10.1126/science.289.5484.1550. [DOI] [PubMed] [Google Scholar]
- 14.Knowlton AA. NF[kappa]B, heat shock proteins, HSF-1, and inflammation. Cardiovascular Research. 2006;69(1):7–8. doi: 10.1016/j.cardiores.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 15.DeMeester S, Buchman TG, Cobb JP. The heat shock paradox: does NF-{kappa}B determine cell fate? FASEB J. 2001;15(1):270–274. doi: 10.1096/fj.00-0170hyp. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Voegeli TS, Liu PP, Noble EG, Currie RW. Heat Shock Paradox and a new Role of Heat Shock Proteins and their Receptors as Anti-Inflammation Targets. Inflammation and Allergy - Drug Targets. 2007;6(2):91–100. doi: 10.2174/187152807780832274. [DOI] [PubMed] [Google Scholar]
- 17.Song M, Pinsky MR, Kellum JA. Heat shock factor 1 inhibits nuclear factor-κB nuclear binding activity during endotoxin tolerance and heat shock. Journal of Critical Care. 2008;23(3):406–415. doi: 10.1016/j.jcrc.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Xiao X, Zuo X, Davis AA, McMillan DR, Curry BB, Richardson JA, Benjamin IJ. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 1999;18(21):5943–5952. doi: 10.1093/emboj/18.21.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh IS, He JR, Calderwood S, Hasday JD. A High Affinity HSF-1 Binding Site in the 5'-Untranslated Region of the Murine Tumor Necrosis Factor-alpha Gene Is a Transcriptional Repressor. Journal of Biological Chemistry. 2002;277(7):4981–4988. doi: 10.1074/jbc.M108154200. [DOI] [PubMed] [Google Scholar]
- 20.Cooper ZA, Singh IS, Hasday JD. Febrile range temperature represses TNF-α gene expression in LPS-stimulated macrophages by selectively blocking recruitment of Sp1 to the TNF-α promoter. Cell Stress and Chaperones. 2010;15(5):665–673. doi: 10.1007/s12192-010-0179-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schett G, Steiner C, Xu Q, Smolen JS, Steiner G. TNFα mediates susceptibility to heat-induced apoptosis by protein phosphatase-mediated inhibition of the HSF1/hsp70 stress response. Cell Death and Differentiation. 2003;10(10):1126–1136. doi: 10.1038/sj.cdd.4401276. [DOI] [PubMed] [Google Scholar]
- 22.Nakano M, Knowlton AA, Yokoyama T, Lesslauer W, Mann DL. Tumor necrosis factor-α-induced expression of heat shock protein 72 in adult feline cardiac myocytes. American Journal of Physiology. 1996;270(4 part 2):H1231–H1239. doi: 10.1152/ajpheart.1996.270.4.H1231. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe N, Tsuji N, Kobayashi D, Yamauchi N, Akiyama S, Sasaki H, Sato T, Okamoto T, Niitsu Y. Endogenous tumor necrosis factor functions as a resistant factor against hyperthermic cytotoxicity in pancreatic carcinoma cells via enhancement of the heat shock element-binding activity of heat shock factor 1. Chemotherapy. 1997;43(6):406–414. doi: 10.1159/000239599. [DOI] [PubMed] [Google Scholar]
- 24.Chu B, Soncin F, Price BD, Stevenson MA, Calderwood SK. Sequential Phosphorylation by Mitogen-activated Protein Kinase and Glycogen Synthase Kinase 3 Represses Transcriptional Activation by Heat Shock Factor-1. Journal of Biological Chemistry. 1996;271(48):30847–30857. doi: 10.1074/jbc.271.48.30847. [DOI] [PubMed] [Google Scholar]
- 25.Guettouche T, Boellmann F, Lane W, Voellmy R. Analysis of phosphorylation of human heat shock factor 1 in cells experiencing a stress. BMC Biochemistry. 2005;6(4):1–14. doi: 10.1186/1471-2091-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossi A, Elia G, Santoro MG. Activation of the Heat Shock Factor 1by Serine Protease Inhibitors. An effect associated with nuclear factor-kappa b inhibition. Journal of Biological Chemistry. 1998;273(26):16446–16452. doi: 10.1074/jbc.273.26.16446. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Arrigo AP, Currie RW. Heat shock treatment suppresses angiotensin II-induced activation of NF-{kappa}B pathway and heart inflammation: a role for IKK depletion by heat shock? Am J Physiol Heart Circ Physiol. 2004;287(3):H1104–H1114. doi: 10.1152/ajpheart.00102.2004. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Chen L, Hagiwara N, Knowlton AA. Regulation of heat shock protein 60 and 72 expression in the failing heart. Journal of Molecular and Cellular Cardiology. 2010;48(2):360–366. doi: 10.1016/j.yjmcc.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baue AE. MOF, MODS, and SIRS: What is in a name or an acronym? Shock. 2006;26(5):438–449. doi: 10.1097/01.shk.0000228172.32587.7a. [DOI] [PubMed] [Google Scholar]
- 30.Frink M, Pape HC, van Griensven M, Krettek C, Chaudry IH, Hildebrand F. Influence of Sex and Age on Mods and Cytokines After Multiple Injuries. Shock. 2007;27(2):151–156. doi: 10.1097/01.shk.0000239767.64786.de. [DOI] [PubMed] [Google Scholar]
- 31.Werdan K, Schmidt H, Ebelt H, Zorn-Pauly K, Koidl B, Hoke RS, Heinroth K, Müller-Werdan U. Impaired regulation of cardiac function in sepsis, SIRS, and MODS. Canadian Journal of Physiology and Pharmacology. 2009;87(4):266–274. doi: 10.1139/Y09-012. [DOI] [PubMed] [Google Scholar]
- 32.Seiver AJ, Szaflarski NL. Report of a Case Series of Ultra Low-Frequency Oscillations in Cardiac Output in Critically Ill Adults with Sepsis, Systemic Inflammatory Response Syndrome, and Multiple Organ Dysfunction Syndrome. Shock. 2003;20(2):101–109. doi: 10.1097/01.shk.0000075570.93053.65. [DOI] [PubMed] [Google Scholar]
- 33.Kelly WE, Januzzi JL, Christenson RH. Increases of Cardiac Troponin in Conditions other than Acute Coronary Syndrome and Heart Failure. Clinical Chemistry. 2009;55(12):2098–2112. doi: 10.1373/clinchem.2009.130799. [DOI] [PubMed] [Google Scholar]
- 34.Kim SC, Stice JP, Chen L, Jung JS, Gupta S, Wang Y, Baumgarten G, Trial J, Knowlton AA. Extracellular Heat Shock Protein 60, Cardiac Myocytes and Apoptosis. Circ Res. 2009;105(12):1186–1195. doi: 10.1161/CIRCRESAHA.109.209643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stice JP, Knowlton AA. Estrogen, NFκB and the Heat Shock Response. Molecular Medicine. 2008;14(7–8):517–518. doi: 10.2119/2008-00026.Stice. [DOI] [PMC free article] [PubMed] [Google Scholar]