Abstract
Clinical and epidemiological studies provide strong data for a relationship between prenatal ethanol exposure and the risk for abuse in adolescent and young adult humans. However, drug-acceptance results in response to fetal exposure have differed by study, age at evaluation, and experimental animal. In the present study, the authors tested whether voluntary ethanol intake was enhanced in both the infantile and adult rat (15 and 90 days of age, respectively), as a consequence of chronic fetal drug experience. Experimental rats were exposed in utero by administering ethanol to a pregnant dam in a liquid diet during gestational Days 6–20. Compared with those for isocaloric pair-fed and ad lib chow control animals, the results for experimental animals demonstrated that fetal exposure significantly increased infantile affinity for ethanol ingestion without affecting intake patterns of an alternative fluid (water). Heightened affinity for ethanol was absent in adulthood. Moreover, the results argue against malnutrition as a principal factor underlying the infantile phenomenon. These data add to a growing literature indicative of heightened early postnatal acceptance patterns resulting from maternal use or abuse of ethanol during pregnancy.
Keywords: fetal ethanol exposure, postnatal ethanol affinity, ethanol abuse, ethanol drinking behavior, fetal learning
It has been well established that prenatal exposure to ethanol can exert profound detrimental effects in the developing fetus. The effects can be widespread, severe, and generally permanent. Depending on timing of exposure, and its duration and dose, these include stereotypical craniofacial malformations, poor suckling reflexes, decreased birth weight (Abel & Hannigan, 1995; Astley, Clarren, Little, Sampson, & Daling, 1992; Clareen & Smith, 1978; Jones, Smith, Ulleland, & Streissguth, 1973; Lemoine, Harousseau, Borteyru, & Meneut, 1968; Martin, Martin, Streissguth, & Lund, 1977; Osborn, Harris, & Weinberg, 1993; Oulette, Rosett, Rosman, & Weiner, 1977; Streissguth, Landesman-Dwyer, Martin, & Smith, 1980; Ulleland, Wennburg, Igo, & Smith, 1970; Van Dyke, MacKay, & Ziaylek, 1982), and other profound neurodevelopmental effects in the nervous system (Hannigan, Spear, Spear, & Goodlett, 1999; Miller, 1992b). Prenatal exposure to ethanol can cause behavioral changes, such as hyperactivity, and learning and memory deficits: Indeed, gestational exposure is a leading known cause of mental retardation (Abel & Hannigan, 1995; Abel & Sokol, 1992; Sampson et al., 1997; Stratton, Howe, & Battaglia, 1996).
The above notwithstanding, there are subtler yet potentially just as detrimental long-term consequences. Recent clinical and epidemiological studies indicate that prenatal exposure to ethanol is strongly associated with the risk for ethanol abuse in adolescent and young adult humans (Alati et al., 2006; Baer, Bar, Bookstein, Sampson, & Streissguth, 1998; Streissguth, 1998; Yates, Cadoret, Troughton, Steward, & Giunta, 1998). In effect, gestational exposure in humans is perhaps the best predictor of later ethanol abuse during adolescence (Streissguth, 1998; Yates et al., 1998). What then is the biological underpinning to these outcomes?
The chemical senses are among the earliest neural systems to develop (Gottlieb, 1971; Schaal & Orgeur, 1992; Smotherman & Robinson, 1990), and in the chemosensory world of the uterus, they may have a unique salience. A substantial literature has developed around the question of what the fetus “learns” behaviorally about ethanol as a consequence of ethanol contamination of the prenatal environment. A number of studies have demonstrated both the general capacity of the fetus to process salient chemosensory cues present in the fetal environment and for retaining this information over a significant time span (e.g., Schaal, Marlier, & Soussignan, 2000; Smotherman, 1982a, 1982b; Smotherman & Robinson, 1985, 1987, 1988, 1990; Stickrod, Kimble, & Smotherman, 1982). The response to fetal ethanol exposure appears to follow suit with these other chemosensory stimuli; however, the results have differed by experimental study, age of evaluation, and also the species of animal examined. It is clear that fetal experience with ethanol can indeed modulate subsequent responsiveness to certain attributes of the drug in the early postnatal animal (between P8 and P15). The data acquired using a rat model suggest that the fetus can both acquire information about ethanol’s sensory cues and display a memory of the prenatal experience with the drug in terms of (a) changes in orienting response to ethanol odor (as measured by an autonomic response; Chotro, Kraebel, McKinzie, Molina, & Spear, 1996; Chotro & Molina, 1992), (b) enhanced consumption of an ethanol solution (Chotro & Molina, 1990; Dominguez, Chotro, & Molina, 1993; Molina, Chotro, & Dominguez, 1995), (c) enhanced ethanol odor preference (Chotro et al., 1996), and (d) ethanol’s ability to act as an unconditioned stimulus mediating associative learning processes (Abate, Pepino, Dominguez, Spear, & Molina, 2000, Abate, Spear, & Molina, 2001).
In contrast with the studies that focused on early postnatal observations in rats, later acceptance and responsiveness to the chemosensory attributes of the drug are less clear. Relatively few studies have tested the effect of either acute or chronic prenatal ethanol exposure upon acceptance of the drug during different postnatal stages in development (Chotro, Arias, & Laviola, 2007; Spear & Molina, 2005). Further, the outcomes of these studies have been quite varied, particularly in the older literature involving the consequences of an extensive series of prenatal ethanol exposure. Bond and DiGiusto (1976) and Phillips and Stainbrook (1976) reported clear increases in adult preference or intake of ethanol among rat offspring exposed to ethanol relatively continuously throughout gestation. However, neither Abel and York (1979) nor McGivern, Clancy, Mousa, Couri, and Noble (1984) found an enhanced acceptance effect. Others have found qualified increases in later acceptance for ethanol after gestational exposure. For example, Randall, Hughs, Williams, and Anton (1983) found that mouse fetuses whose dams were exposed to the drug between G8 and birth consumed more ethanol during P25–P32 (i.e., the age range of juvenile and early adolescent development) than did controls, but this effect was not seen between P33 and P46. Reyes, Garcia, and Jones (1985) found that prenatal exposure from G1 until birth yielded greater preference for ethanol (in males only) for only the first half of a 30-day test in late adolescent/young adult rats (P45–P60). More recent studies with more selective prenatal exposure to ethanol (maternal intragastric administration of 1 or 2 g/kg ethanol during late gestation (G17–G20) have uniformly reported enhanced ethanol intake early in development, prior to weaning and in adolescence (Chotro et al., 2007; Molina, Spear, Spear, Mennella, & Lewis, 2007; Spear & Molina, 2005). But there remains the question of the inconsistent or negative results with other prenatal procedures, such as extensive exposure to ethanol, and older ages at the time of testing.
To further explore this question, in the present experiment, we focused on testing whether, and to what extent, ethanol intake is enhanced in both the early postnatal and adult rat, as a consequence of fetal experience with the drug. Our results provide additional data regarding the forms of experiential factors that may contribute to ethanol intake and acceptance patterns and the persistence of these effects.
Method
Treatment of Dams and Subject Selection
On gestational Day 5, pregnant Long–Evans female rats (Harlan Sprague–Dawley, Indianapolis, IN) were weighed and divided into weight-matched groups of 3 dams (a block for analytic purposes). Dams within a triad were then randomly assigned to one of three treatment groups (ethanol, pair-fed, and chow control). For the ethanol group (ET), ethanol administration was an ad lib liquid diet (ET: L10251, Research Diets, NJ) that was supplemented with 6.7% (v/v) ethanol (i.e., 35% daily calories derived from ethanol) from G11–G20 (Miller, 1992a). Animals were weaned onto this diet between G6 and G10 with diets containing increasing amounts of ethanol (namely, 2.2% v/v ethanol on G6–G8, 4.5% v/v ethanol on G9–G10). With this regimen of ethanol exposure, peak blood ethanol concentrations in pregnant females specifically assayed for this purpose on G17 were approximately equivalent to 150 mg/dl (Miller, 1992a).
As noted above, there were two control treatment groups. The first was a weight-matched group that was pair-fed (PF) to the ET group and received an isocaloric, isonutritive liquid diet (PF L100252, Research Diets, NJ). This PF group therefore received the same volume and caloric intake as the ET group. To accomplish this, on any given day, we gave the PF rat within a triad the same amount of diet consumed the day before by its respective weight-matched ET rat. By contrast, the chow control group (CH) had continuous access to standard lab chow (Labdiet, Richmond, IN) and water.
The timing of birth may vary between the ET, PF, and CH dams. Therefore, to minimize potential variability in the maturational state of the central nervous system among neonates, we based the age of the pups on a scale anchored by gestational age, rather than age postparturition. G22 was arbitrarily designated P0 regardless of when the actual birth occurred (i.e., whether a litter was born on G22 or G23, the birth was considered to be G22). All litters were culled to 10 and surrogate-fostered to nonexperimental dams that were fed chow and water during gestation.
In this study, a total of 10 triads of dams (ET, PF, and CH) were produced. No more than 2 randomly selected animals of each sex from any given litter were entered into an experimental condition and further randomly allocated to the P15 and P90 time points. Thus, for each age, a total of 10 male and 10 female animals from each treatment group participated in the study (60 animals at P15 and 60 animals at P90).
Ethanol Intake
Postnatal Day 15 animals
P15 animals do not readily ingest fluid from a drinking bottle. Therefore, we tested animals for ethanol intake by infusing a specified solution directly into the mouth via an intraoral cannula (Abate et al., 2000, 2001; Dominguez, Lopez, Chotro, & Molina, 1996; Dominguez, Lopez, & Molina, 1998; Hunt, Kraebel, Rabine, Spear, & Spear, 1993; Pepino, Kraebel, Lopez, Spear, & Molina, 1998, Pepino, Lopez, Spear, & Molina, 1999, Pepino, Spear, & Molina, 2001). Three hours prior to testing, pups were singly placed in a clean cage and deprived of food, fluid, and maternal/sibling presence. Temperature during this period was maintained with a heat lamp. Two hours prior to intake testing, an intraoral cannula (PE10 tubing; length 5 cm; Clay Adams, Parsippany, NJ) was implanted. The cannulation procedures have been extensively described (Abate et al., 2000, 2001; Dominguez et al., 1996, 1998; Hunt et al., 1993; Pepino et al., 1998, 1999, 2001). In brief, the flanged end of the cannula was positioned in the middle of the oral mucosa of the right cheek. The cannula was placed with a 30-gauge needle attached to the nonflanged end of the tubing by passing the needle through the cheek (from inside to outside) until the flange rested on the oral mucosa. The procedure took less than 20 s per pup. The intraoral position of the cannula permitted the pups to accept (i.e., swallow) or reject (i.e., active rejection through head shaking or by licking the floor and walls of the chamber or passive dripping) the solution.
Prior to testing, the anogenital region of each pup was gently stroked to stimulate it to void its bowel and bladder. The pups were then weighed (to the nearest 0.01 g) and placed in a plastic chamber (15 × 7 × 15 cm), and their cannula was attached to a peristaltic infusion pump that delivered a 5.0% v/v ethanol solution. Ethanol solution was infused for 15 min (following a 10-min habituation period) with a 3.0-s “on” and 10-s “off” duty cycle at a rate permitting delivery of 5.5% of the animal’s preinfusion body weight. Following testing, the pups were weighed again (to the nearest 0.01 g). For each animal, ethanol-intake score was calculated in terms of grams of absolute ethanol consumed per kilogram of body weight.
Postnatal Day 90 animals
Following the approach used by Molina and Spear (e.g., Pepino, Abate, Spear, & Molina, 2004; Ponce, Pautassi, Spear, & Molina, 2004), voluntary ethanol-drinking sessions were conducted in standard microisolator cages equipped with two graduated drinking tubes (volume: 30 ml with 0.1 ml graduation) mounted on one wall of the cage. Each voluntary intake session was preceded by 22 hr of water deprivation, and each intake session lasted for 2 hr. Rats were weighed just prior to each session.
During the first 4 days, both drinking tubes contained normal tap water. These water-only sessions served to adapt the animals to the testing cages and familiarize them with drinking from the tubes. Following the adaptation sessions, the animals were given simultaneous access to tap water and a given ethanol solution (on Days 5–10). Starting on Day 5, a 3% v/v ethanol solution was used, and the concentration of the solution was increased on subsequent testing days as follows: 4%, 5%, 6%, 7%, and 10% v/v by the 10th day. The position of the ethanol drinking-tube location (i.e., ethanol vs. tap water) was randomized and balanced across testing days, within and across treatment groups, within a block, and across blocks.
For the P90 animals, ethanol-drinking scores for each concentration were determined by calculating the grams of absolute ethanol consumed per kilogram body weight (g/kg). In addition, liquid ingestion from both the ethanol and water drinking tubes for each testing session was calculated in terms of milliliters consumed per 100 grams body weight.
Results
Postnatal Day 15 Intake Tests
Prenatal ethanol treatment appeared to have a profound consequence on ethanol acceptance. As a percentage of their initial body weight (ET group, M = 24.32 g, SEM = 0.423; PF group, M = 26.59 g, SEM = 0.423; CH group, M = 28.79 g, SEM = 0.423), animals receiving prenatal ethanol exposure gained, on average, 1.56× and 2.2× more weight than did control PF and CH animals, respectively, during testing. PF animals gained 1.33× more weight than did CH animals. This occurred despite the observation that, in rank order, ET animals were physically smaller (i.e., weighed less) than PF animals that, in turn, were smaller than CH controls. Indeed, a randomized-blocks analysis of variance (ANOVA) indicated that body weights significantly varied as a function of prenatal treatment, F(2, 45) = 7.28, p < .0001, however, there was no effect of sex, F(1, 45) = 0.67, p > .4, or Sex × Treatment interaction, F(2, 45) = 0.99, p > .3. Post hoc tests of differences between treatment groups with the Newman–Keuls criterion for multiple comparisons demonstrated significant effects for all pairwise comparisons (for ET vs. PF, ET vs. CH, and PF vs. CH, all ps < .0025).
Because the focus of the study was to test for a primary causal relationship between prenatal exposure and infantile ethanol acceptance, our formal analysis of the data proceeded in two steps. First, we evaluated the unconditional effect of maternal treatment on ethanol acceptance in the P15 animals (i.e., without adjusting for the potential intermediate response of animal weight, as a consequence of maternal treatment. Randomized-blocks ANOVA demonstrated a significant main effect of prenatal treatment, F(2, 45) = 6.29, p < .005, on ethanol acceptance. There was no evidence of a differential effect of sex, F(1, 45) = 0.40, p > .5, or Sex × Treatment interaction, F(2, 45) = 0.01, p > .9. Newman–Keuls post hoc tests of differences between treatment groups demonstrated that ET pups ingested significantly more ethanol relative to PF or CH controls (both ps < .01). However, PF and CH offspring exhibited similar levels of absolute ethanol intake. This latter point is noteworthy given the finding that PF and CH animals differed in terms of body weight at the time of testing.
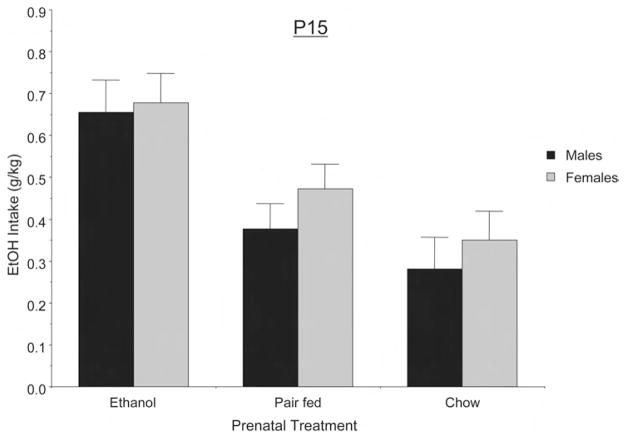
Second, we evaluated the conditional effect of prenatal treatment by performing a randomized-blocks analysis of covariance (ANCOVA) that analytically adjusted for the potential intermediate effect of maternal treatment on the animals’ weights. In this respect, the ANCOVA evaluated the following question: For animals of a given weight, does the ingestion of ethanol per unit weight differ for those with or without prenatal ethanol exposure? Randomized-blocks ANCOVA demonstrated a significant main effect of treatment, F(2, 44) = 6.54, p < .005, on ethanol acceptance. There was no evidence of a differential effect of sex, F(1, 44) = 1.60, p > .2, or Sex × Treatment interaction, F(2, 44) = 0.18, p = .832. Figure 1 illustrates P15 ethanol intake (expressed as grams of absolute ethanol consumed per kilogram of body weight) as a function of sex and maternal treatment. As can be seen in this figure, there was a clear effect of prenatal ethanol treatment on subsequent ethanol avidity.
Figure 1.
Grams of absolute ethanol consumed per kilogram of body weight corresponding to the 15-day-old (P15) rats as a function of prenatal treatment. Data are the means ± SEM.
In a follow-up experiment, we evaluated whether prenatal ethanol exposure was also likely to affect intake of a nonethanol solution. In other words, the explicit intention was to assess whether the increased intake observed for ethanol was a specific effect or a generalized response to any fluid that is intraorally delivered. To accomplish this, we randomly selected 1 animal of each gender from five additional triads of dams (ET, PF, and CH) for testing water consumption at P15. Thus, a total of 10 animals (5 male, 5 female) from each treatment group were evaluated for their intake of tap water. Note that, at P15, pups have no experience with water, because the dam provides all nutritional and fluid support. The procedures were identical to those used for the ethanol-acceptance study at this age. Expressed as a percentage of body-weight gain, a two-way randomized-blocks ANCOVA (Pre-natal Treatment × Sex) with the animals’ weights as a covariate provided no evidence of an altered intake pattern for the neutral fluid stimulus, tap water, F(2, 19) = 0.61, p > .5. The means ± SEM for the ET, PF, and CH animals were 1.03 ± 0.19%, 0.93 ± 0.14%, and 1.13 ± 0.15%, respectively.
Postnatal Day 90 Animals
Table 1 illustrates the body weights of adult animals during the course of the ethanol-drinking tests. A 3 × 2 × 6 mixed ANOVA (Prenatal Treatment × Sex × Days) indicated significant effects of the three main factors: prenatal treatment, F(2, 86) = 9.12; sex, F(1, 86) = 717.0; and days, F(5, 430) = 38.8; all ps < .001. Further, this analysis also indicated a significant interaction between prenatal treatment and days, F(10, 430) = 2.48, p < .01. As might be expected, females weighed significantly less than males. Newman–Keuls post hoc tests (p < .05) showed that ET animals exhibited significantly lower body weights relative to PF and CH rats. PF adults had lower weights than CH adults, but this difference failed to achieve significance (p > .07). Because there is no unambiguous choice of post hoc tests between and within factors, we further analyzed the interaction between prenatal treatment and days using an alternative ANOVA in which the dependent variable was the relative loss of body weight during the course of the testing sessions. As can be observed in Table 1, it appears that the regimen of fluid deprivation during the course of the testing procedure differentially affected body-weight changes across treatment groups. Overall percentage change in body weight for each treatment was calculated as follows: 100 × {(Day 6 body weight − Day 1 body weight)/Day 1 body weight}. ANOVA indicated a significant effect of prenatal treatment, F(2, 89) = 10.03, p < .01. Newman–Keuls post hoc tests further showed that ET rats lost significantly more weight than PF and CH adults during the course of the test. In turn, PF adults lost more weight than CH controls. Percentage body-weight changes for each group were as follows: ET, −3.34 ± 0.32%; PF, −2.38 ± 0.67%; and CH, −1.34 ± 0.27%.
Table 1.
Mean Body Weights (in Grams) During Adulthood ± SEM
| Prenatal treatment | Day 1, Water vs. 3% EtOH | Day 2, Water vs. 4% EtOH | Day 3, Water vs. 5% EtOH | Day 4, Water vs. 6% EtOH | Day 5, Water vs. 7% EtOH | Day 6, Water vs. 10% EtOH |
|---|---|---|---|---|---|---|
| Ethanol | ||||||
| Female | 224.1 ± 5.6 | 221.6 ± 5.3 | 219.7 ± 5.4 | 217.7 ± 5.4 | 216.9 ± 5.4 | 214.5 ± 5.4 |
| Male | 378.2 ± 11.8 | 374.3 ± 11.7 | 373.4 ± 12.0 | 371.6 ± 12.0 | 370.4 ± 11.8 | 368.6 ± 11.7 |
| Pair-fed | ||||||
| Female | 234.8 ± 5.8 | 232.1 ± 5.7 | 230.3 ± 5.9 | 229.8 ± 5.5 | 228.6 ± 5.8 | 227.9 ± 5.6 |
| Male | 403.9 ± 8.5 | 398.3 ± 7.3 | 400.3 ± 8.4 | 398.3 ± 8.4 | 397.8 ± 8.5 | 396.6 ± 8.6 |
| Chow | ||||||
| Female | 245.9 ± 2.7 | 244.7 ± 2.6 | 243.8 ± 2.9 | 242.8 ± 2.9 | 242.6 ± 2.7 | 241.5 ± 2.5 |
| Male | 414.9 ± 7.7 | 412.8 ± 7.5 | 413.0 ± 7.6 | 411.8 ± 7.4 | 412.2 ± 7.1 | 411.3 ±6.8 |
Overall fluid-consumption patterns (ml/100 gbw of water + ml/100 gbw of ethanol; see Table 2) were also subjected to an ANOVA with prenatal treatment and sex as between factors and days as repeated measures. The results of this analysis showed significant effects of the three main factors: prenatal treatment, F(2, 86) = 11.4; sex, F(1, 86) = 190.0; and days, F(5, 430) = 12.7; all ps < .001. Males drank significantly more fluid than did females. Overall fluid ingestion increased as a function of the progression of testing. Indeed, during the last 2 days (Days 5 and 6), fluid-intake scores were significantly higher than those registered during the first three testing sessions (Days 1, 2, and 3). In regard to the main effect of prenatal treatment, post hoc tests indicated that ET and PF adults drank significantly less fluid than did CH controls.
Table 2.
Mean (± SEM) Overall Fluid Ingestion (in Milliliters per 100 g Body Weight) During Adulthood
| Prenatal treatment | Day 1, Water vs. 3% EtOH | Day 2, Water vs. 4% EtOH | Day 3, Water vs. 5% EtOH | Day 4, Water vs. 6% EtOH | Day 5, Water vs. 7% EtOH | Day 6, Water vs. 10% EtOH |
|---|---|---|---|---|---|---|
| Ethanol | ||||||
| Female | 4.1 ± 0.3 | 4.8 ± 0.3 | 4.8 ± 0.3 | 5.0 ± 0.3 | 4.9 ± 0.2 | 5.1 ± 0.3 |
| Male | 3.8 ± 0.2 | 4.2 ± 0.2 | 4.3 ± 0.2 | 4.3 ± 0.2 | 4.1 ± 0.3 | 4.5 ± 0.3 |
| Pair-fed | ||||||
| Female | 3.7 ± 0.2 | 4.0 ± 0.2 | 4.4 ± 0.2 | 4.7 ± 0.4 | 5.1 ± 0.3 | 4.7 ± 0.3 |
| Male | 3.9 ± 0.2 | 4.2 ± 0.2 | 4.1 ± 0.2 | 4.4 ± 0.2 | 4.4 ± 0.2 | 4.4 ± 0.2 |
| Chow | ||||||
| Female | 4.6 ± 0.2 | 4.9 ± 0.2 | 4.8 ± 0.2 | 5.4 ± 0.2 | 5.4 ± 0.2 | 5.2 ± 0.3 |
| Male | 4.2 ± 0.2 | 4.5 ± 0.2 | 4.3 ± 0.3 | 4.7 ± 0.3 | 4.6 ± 0.3 | 5.0 ± 0.3 |
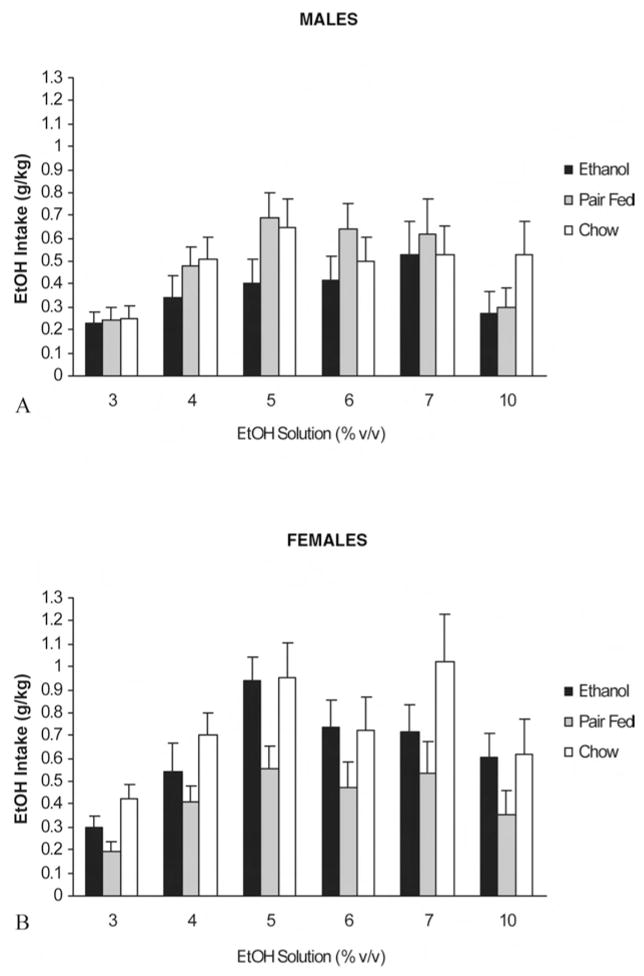
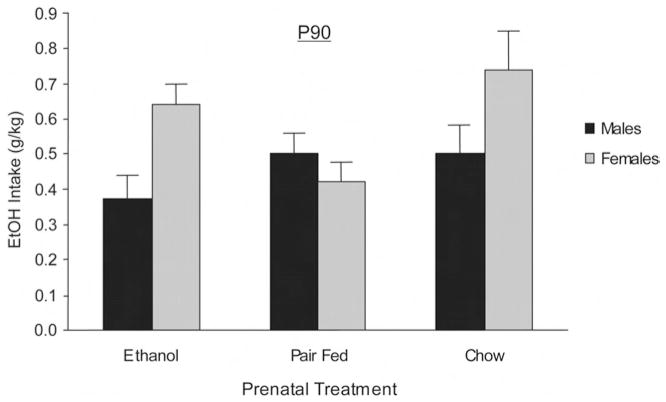
Figure 2 illustrates absolute ethanol-intake scores (g/kg) during adulthood testing. ANOVA demonstrated significant main effects of days, F(5, 430) = 17.2, p < .0001, and of sex, F(1, 86) = 5.55, p < .025. Moreover, the interaction between prenatal treatment and sex was also significant, F(2, 86) = 3.28, p < .05. Post hoc tests showed that ethanol-intake scores were higher when animals had access to the intermediate ethanol concentrations (4%, 5%, and 6% v/v ethanol on Days 3, 4, and 5, respectively) than when they had access to 3%, 7%, or 10% v/v ethanol solutions. Further, post hoc tests indicated that ET females drank significantly more ethanol than did their male counterparts. ET females were also found to ingest significantly more ethanol than were PF females. CH females also consumed significantly more ethanol than did CH males and PF females. The intake pattern of ET and CH females was not found to be significantly different. No treatment differences were observed when we focused on male consumption scores. The interaction between prenatal treatment and sex is depicted in Figure 3.
Figure 2.
Absolute ethanol intake (grams per kilogram) corresponding to adult rats as a function of prenatal treatment, sex, and ethanol concentration available during each testing session. Data are the means ± SEM.
Figure 3.
Absolute ethanol intake (grams per kilogram) during adulthood as a function of the interaction between prenatal treatment and sex. Data have been collapsed across test sessions of the 90-day-old (P90) animals and represent the means ± SEM.
In summary of the P90 data, the detrimental effects of the prenatal manipulations upon body weight were still observable during adulthood, but there was no evidence that prenatal exposure to ethanol resulted in heightened ethanol intake. Adults born to dams subjected to a restricted-food diet during pregnancy (PF group) still exhibited significant decrements in body weight compared with CH controls. This reduction in overall body weight was even more pronounced in males and females that were treated with ethanol during pregnancy, a result that parallels what was observed during infancy. In addition, ET and PF rats were observed to ingest somewhat less fluid during the drinking sessions than were CH rats. This effect was observed despite the fact that intake was adjusted in accordance with individual body weights (milliliter of fluid per 100 g of body weight, see Table 2). Nonetheless, despite this decrement in overall fluid ingestion, ET females were observed to ingest ethanol in quantities similar to those ingested by CH controls. Undernutrition during pregnancy, as operationalized through pair-feeding procedures, resulted in a reduction in female affinity for ethanol ingestion. Males in the different prenatal conditions were observed to ingest similar amounts of the drug.
Discussion
The present study was crafted from the perspective that a combined study designed to examine ethanol intake in both early postnatal and adult rats, as a consequence of the same prenatal exposure paradigm, would shed further light on the effects of fetal exposure to early ethanol-acceptance patterns and the persistence of these effects. More specifically, infantile rats and their adult littermates were evaluated for ethanol intake as a consequence of the presence or absence of in utero ethanol exposure throughout gestation (delivered in the dam’s diet). Consequently, at least for the current exposure paradigm, this fundamental approach provided the important unifying design element of the study. In this respect, recall that in prior studies, researchers have observed variable adult intake patterns (i.e., enhanced, marginal, and absent) in the face of a variable set of procedures without the benefit of providing contrasting infantile acceptance patterns in similarly manipulated littermates.
Our results demonstrated that fetal exposure throughout gestation (at a dose equivalent to moderate-to-high daily ethanol intake; e.g., Driscoll, Streissguth, & Riley, 1990; Vavrousek-Jakuba, Baker, & Shoemaker, 1991) significantly increased infantile affinity for ethanol ingestion. That is, although there were no differences between the male and female progeny within each of the three maternal treatment groups, ET animals drank significantly more ethanol than did the PF and CH animals, and these latter two treatment groups were not different from each other. Moreover, two additional key observations were noteworthy. First, intake patterns for tap water were unambiguously the same across the progeny of all three maternal treatment groups, a result that argues against a generalized enhancement or lack of motor control in terms of fluid ingestion as major factors underlying infantile ethanol-acceptance results. Second, the observation that there was no early predisposition to ingest ethanol in the offspring of dams subjected to dietary restrictions during gestation (i.e., the PF group) and that these animals were equivalent to CH animals strongly argues against fetal malnutrition as the sole or principal factor that underlies the enhanced ethanol-acceptance phenomenon here reported. As such, the infantile data add to the more general growing body of experimental and epidemiological literature indicative of heightened early postnatal ethanol-acceptance patterns in the preweanling and adolescent rat resulting from maternal use or abuse of ethanol during pregnancy (for recent reviews, see Chotro et al., 2007; Molina et al., 2007; Spear & Molina, 2005).
In contrast with the above, the adult picture was more complicated. Although the effects of chronic exposure to ethanol during pregnancy upon ethanol-intake affinity were notably absent in adulthood, the intake patterns were extensively affected by both a potential long-term nutritional effect on the PF females and an interaction between prenatal treatment and sex. During adulthood, there were marked differences between the progeny of ET and PF dams. Specifically, females prenatally exposed to the drug drank significantly more ethanol than did PF females. However, ET and CH females showed similar ethanol-intake levels. Regarding this observation, it has been previously reported that females suffering undernutrition during the period spanning from gestation through adolescence strongly reject ethanol solutions during adulthood (Cordoba, Molina, Basso, & Orsingher, 1990). According to these authors, early malnourished females appear to be highly sensitive to ethanol’s chemosensory cues. Their responses represented an exacerbated neophobia that failed to be attenuated even after numerous sensory-familiarization experiences with the drug’s odor. In the present study, we observed that the reluctance of adult PF females to accept ethanol was evident throughout the entire testing procedure. It is interesting that malnourishment is likely to be derived from the specific experimental manipulations applied to ET dams during gestation, as well. However, adult females representative of this treatment group drank as much ethanol as did CH control females. Thus, it could be suggested that alternative effects derived from prenatal ethanol exposure can ameliorate the effects of early undernutrition upon ethanol affinity but not sufficiently so as to result in an adult preference beyond that of CH counterparts.
ET female animals were also found to ingest more ethanol than their male counterparts. This gender difference was also observed in the case of control animals delivered by an untreated dam (CH group). That is, female CH animals drank more than their male counterparts. This sex difference was not encountered in PF adults. Exactly why these differences exist is a matter of speculation at this time.
In summary of the above, it is clear from our results that, at least for the chronic-exposure feeding paradigm and the testing methodology we employed, the infantile affinity for ethanol in the ET group was lost by the time of adult littermate testing. In considering this result, it is worth examining a possible underlying mechanism through which prenatal ethanol exposure resulted in a heightened avidity for ethanol that ameliorated with time. In this respect, ethanol’s flavor attributes (the integration of taste, smell, and somatosensory input) certainly contribute to the development of drug preference. Without a doubt, there is an extensive animal and human literature that addresses the role that fetal and postnatal learning about ethanol’s chemosensory attributes plays in later perception, preference, and consumption of ethanol (e.g., Abate et al., 2000, 2001; Arias & Chotro, 2005; Chotro & Arias, 2003; Mennella, 1999, 2001; Mennella & Beauchamp, 1998; Molina et al., 1995, Molina, Dominguez, Lopez, Pepino, & Faas, 1999; Spear & Molina, 2001, 2005). What is notably absent from this published literature are studies examining whether ethanol exposure functionally alters one or more of the neural systems (and, by extension, their contribution to the behavioral response) involved in the perception of ethanol’s flavor.
The olfactory system is highly plastic throughout the animal’s life (from the fetal period into and including adulthood; e.g., Coopersmith & Leon, 1984, 1986; Hudson & Distel, 1998; Johnson, Woo, Duong, Nguyen, & Leon, 1995; McCollum, Woo, & Leon, 1997; McLean & Harley, 2004; Rochefort, Gheusi, Vincent, & Lledo, 2002; Salcedo, Zhang, Kronberg, & Restrepo, 2005; Semke, Distel, & Hudson, 1995; Sullivan & Leon, 1986; Sullivan, McGaugh, & Leon, 1991; Sullivan, Wilson, & Leon, 1989; Woo, Coopersmith, & Leon, 1987; Woo, Oshita, & Leon, 1996; Wilson & Sullivan, 1990; Youngentob & Kent, 1995), and stimulus-induced plasticity has been hypothesized to be a mechanism for modifying olfactory function to emphasize the encoding of odorants that are significant for survival and reproductive fitness (Hudson, 1999). Recently, using an identical experimental fetal-exposure paradigm to the one employed in the current study, Youngentob et al. (in press) demonstrated that, compared with isocaloric PF and ad lib CH controls, ET rats showed that (a) a tuned neurophysiologic response of the olfactory epithelium to ethanol odor in the early postnatal animal (P15), and this effect was absent by adulthood (P90); (b) the neural effect observed in the infantile animal occurred in conjunction with an altered odorant-induced reflexive sniffing response to ethanol odor across a series of odorant concentrations; (c) the behavioral effect was specific to ethanol odor and was ameliorated by adulthood; and (d) a significant component of the infantile behavioral effect was attributable to ethanol’s effect on the olfactory neural modality.
The conjunction of the present results and those of Youngentob et al. (in press) suggests the intriguing possibility that in utero experience-induced olfactory plasticity, in response to ethanol, tuned olfactory system function (both behaviorally and neurophysiologically), thereby emphasizing the transduction of its odor-related attributes. Ethanol avidity was thus enhanced in the infantile animal. However, absent further experience with the drug, ethanol “lost” its biological significance (Hudson, 1999) with the passage of time, and by P90, the olfactory system function returned to an ethanol-neutral status (i.e., lack of biological significance). As a result, ethanol affinity in the ET animals was absent in adulthood. The exact mechanism by which the observed changes in olfactory system function and subsequent recovery occurred in the Youngentob et al. study are unknown at this time. Nevertheless, the remarkable parallel between the outcomes of the two data sets suggest they are intimately related.
Acknowledgments
This research was supported by National Institute on Alcohol Abuse and Alcoholism Grant AA014871 to Steven L. Youngentob.
Contributor Information
Steven L. Youngentob, Department of Neuroscience and Physiology, SUNY Upstate Medical University, and SUNY Developmental Ethanol Research Center;
Juan C. Molina, Department of Neuroscience and Physiology, SUNY Upstate Medical University, SUNY Developmental Ethanol Research Center, and Department of Psychology, Binghamton University
Norman E. Spear, SUNY Developmental Ethanol Research Center and Department of Psychology, Binghamton University
Lisa M. Youngentob, Department of Neuroscience and Physiology, SUNY Upstate Medical University, and SUNY Developmental Ethanol Research Center
References
- Abate P, Pepino MY, Dominguez HD, Spear NE, Molina JC. Fetal associative learning mediated through maternal alcohol intoxication. Alcoholism: Clinical and Experimental Research. 2000;24:39–47. [PubMed] [Google Scholar]
- Abate P, Spear NE, Molina JC. Fetal and infantile alcohol-mediated associative learning in the rat. Alcoholism: Clinical and Experimental Research. 2001;25:989–998. [PubMed] [Google Scholar]
- Abel EL, Hannigan JH. Maternal risk factors in fetal alcohol syndrome: Provocative and permissive influences. Neurotoxicology and Teratology. 1995;17:448–462. doi: 10.1016/0892-0362(95)98055-6. [DOI] [PubMed] [Google Scholar]
- Abel EL, Sokol RJ. A revised conservative estimate of the incidence of FAS and economic impact. Alcoholism: Clinical and Experimental Research. 1992;15:514–524. doi: 10.1111/j.1530-0277.1991.tb00553.x. [DOI] [PubMed] [Google Scholar]
- Abel EL, York JL. Absence of effect of prenatal ethanol on adult emotionality and ethanol consumption in rats. Journal of Studies on Alcohol. 1979;40:547–553. doi: 10.15288/jsa.1979.40.547. [DOI] [PubMed] [Google Scholar]
- Alati R, AlMamum A, Williams GM, O’Callagham M, Najman JM, Bor W. In utero alcohol exposure and prediction of alcohol disorders in early adulthood: A birth cohort study. Archive of General Psychiatry. 2006;63:1009–1016. doi: 10.1001/archpsyc.63.9.1009. [DOI] [PubMed] [Google Scholar]
- Arias C, Chotro MG. Increased preference for ethanol in the infant rat after prenatal ethanol exposure, expressed on intake and taste reactivity tests. Alcoholism: Clinical and Experimental Research. 2005;29:337–346. doi: 10.1097/01.alc.0000156115.35817.21. [DOI] [PubMed] [Google Scholar]
- Astley SJ, Clarren SK, Little RE, Sampson PD, Daling JR. Analysis of facial shape in children gestationally exposed to marijuana, alcohol, and/or cocaine. Pediatrics. 1992;89:67–77. [PubMed] [Google Scholar]
- Baer JS, Bar HM, Bookstein FL, Sampson PD, Streissguth AP. Prenatal alcohol exposure and family history of alcoholism in the etiology of adolescent alcohol problems. Journal of Studies on Alcohol. 1998;59:533–543. doi: 10.15288/jsa.1998.59.533. [DOI] [PubMed] [Google Scholar]
- Bond NW, DiGiusto EL. Effects of prenatal alcohol consumption on open-field behaviour and alcohol preference in rats. Psychopharmacology. 1976;46:163–165. doi: 10.1007/BF00421386. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Arias C. Prenatal exposure to ethanol increases ethanol consumption: A conditioned response? Alcohol. 2003;30:19–28. doi: 10.1016/s0741-8329(03)00037-5. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Arias C, Laviola G. Increased ethanol intake after prenatal exposure: Studies with animals. Neuroscience and Biobehavioral Reviews. 2007;31:181–191. doi: 10.1016/j.neubiorev.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Kraebel KS, McKinzie DL, Molina JC, Spear N. Prenatal and postnatal ethanol exposure influences preweanling rats’ behavioral and autonomic responding to ethanol odor. Alcohol. 1996;13:377–385. doi: 10.1016/0741-8329(96)00027-4. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Molina JC. Acute ethanol contamination of the amniotic fluid during gestational day 21: Postnatal changes in alcohol responsiveness in rats. Developmental Psychobiology. 1990;23:535–547. doi: 10.1002/dev.420230608. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Molina JC. Bradycardiac responses elicited by alcohol odor in rat neonates: Influence of in utero experience with ethanol. Psychopharmacology. 1992;106:491–496. doi: 10.1007/BF02244820. [DOI] [PubMed] [Google Scholar]
- Clareen SK, Smith DW. The fetal alcohol syndrome. New England Journal of Medicine. 1978;298:1063–1067. doi: 10.1056/NEJM197805112981906. [DOI] [PubMed] [Google Scholar]
- Coopersmith R, Leon M. Enhanced neural response to familiar olfactory cues. Science. 1984 Aug 24;225:849–851. doi: 10.1126/science.6474157. [DOI] [PubMed] [Google Scholar]
- Coopersmith R, Leon M. Enhanced neural response by adult rats to odors experienced early in life. Brain Research. 1986;371:400–403. doi: 10.1016/0006-8993(86)90384-7. [DOI] [PubMed] [Google Scholar]
- Cordoba NE, Molina JC, Basso AM, Orsingher OA. Perinatal undernutrition reduced ethanol preference in adult recovered rats. Physiology & Behavior. 1990;47:1111–1116. doi: 10.1016/0031-9384(90)90360-g. [DOI] [PubMed] [Google Scholar]
- Dominguez HD, Chotro MG, Molina JC. Alcohol in the amniotic fluid prior to cesarean delivery: Effects of subsequent exposure to the drug’s odor upon alcohol responsiveness. Behavioral Neural Biology. 1993;60:129–138. doi: 10.1016/0163-1047(93)90229-b. [DOI] [PubMed] [Google Scholar]
- Dominguez HD, Lopez MF, Chotro MG, Molina JC. Perinatal responsiveness to alcohol’s chemosensory cues as a function of prenatal alcohol administration during gestational days 17–20 in the rat. Neurobiology of Learning and Memory. 1996;65:103–112. doi: 10.1006/nlme.1996.0012. [DOI] [PubMed] [Google Scholar]
- Dominguez HD, Lopez MF, Molina JC. Neonatal responsiveness to alcohol odor and infant alcohol intake as a function of alcohol experience during late gestation. Alcohol. 1998;16:109–117. doi: 10.1016/s0741-8329(97)00169-9. [DOI] [PubMed] [Google Scholar]
- Driscoll CD, Streissguth AP, Riley EP. Prenatal alcohol exposure: Comparability of effects in human and animal models. Neurotoxicology and Teratology. 1990;12:231–237. doi: 10.1016/0892-0362(90)90094-s. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Ontogenesis of sensory function in birds and mammals. In: Tobach E, Aronson LR, Shaw E, editors. The biopsychology of development. New York: Academic Press; 1971. pp. 67–128. [Google Scholar]
- Hannigan JH, Spear LP, Spear NE, Goodlett CR. Alcohol and alcoholism: Effects on brain development. Mahwah, NJ: Erlbaum; 1999. [Google Scholar]
- Hudson R. From molecule to mind: The role of experience in shaping olfactory function. Journal of Comparative Physiology. 1999;185:297–304. doi: 10.1007/s003590050390. [DOI] [PubMed] [Google Scholar]
- Hudson R, Distel H. Induced peripheral sensitivity in the developing vertebrate olfactory system. Annals of the New York Academy of Science. 1998;855:109–115. doi: 10.1111/j.1749-6632.1998.tb10552.x. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Kraebel KS, Rabine H, Spear LP, Spear NE. Enhanced ethanol intake in preweanling rats following exposure to ethanol in a nursing context. Developmental Psychobiology. 1993;26:133–153. doi: 10.1002/dev.420260302. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Woo CC, Duong H, Nguyen V, Leon M. A learned odor evokes an enhanced Fos-like glomerular response in the olfactory bulb of young rats. Brain Research. 1995;699:192–200. doi: 10.1016/0006-8993(95)00896-x. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW, Ulleland CN, Streissguth AP. Patterns of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1:1267. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- Lemoine PH, Harousseau H, Borteyru JP, Meneut JC. Les enfants de parents alcoholiques: Anomalies observées apropos de 127 cas [Children of alcoholic parents: Anomalies observed concerning 127 cases] Ouest Medical. 1968;21:476–482. [Google Scholar]
- Martin DC, Martin JC, Streissguth AP, Lund CA. Suckling frequency and amplitude in newborns as a function of maternal drinking and smoking. In: Galanter M, editor. Currents in alcoholism. Vol. 5. New York: Grune & Stratton; 1977. pp. 359–366. [PubMed] [Google Scholar]
- McCollum JF, Woo CC, Leon M. Granule and mitral cell densities are unchanged following early olfactory preference training. Developmental Brain Research. 1997;99:118–120. doi: 10.1016/s0165-3806(96)00201-5. [DOI] [PubMed] [Google Scholar]
- McGivern RF, Clancy AN, Mousa S, Couri D, Noble EP. Prenatal alcohol exposure alters enkephalin levels, without affecting ethanol preference. Life Science. 1984;34:585–589. doi: 10.1016/0024-3205(84)90492-2. [DOI] [PubMed] [Google Scholar]
- McLean JH, Harley CW. Olfactory learning in the rat pup: A model that may permit visualization of a mammalian memory trace. Neuroreport. 2004;15:1691–1697. doi: 10.1097/01.wnr.0000134988.51310.c3. [DOI] [PubMed] [Google Scholar]
- Mennella JA. The transfer of alcohol to human milk: Sensory implications and effects on mother-infant interaction. In: Hannigan JH, Spear LP, Spear NE, Goodlett CR, editors. Alcohol: Effects on brain and development. Mahwah, NJ: Erlbaum; 1999. pp. 177–198. [Google Scholar]
- Mennella JA. Regulation of milk intake after exposure to alcohol in mothers’ milk. Alcoholism, Clinical and Experimental Research. 2001;25:590–593. [PMC free article] [PubMed] [Google Scholar]
- Mennella JA, Beauchamp GK. Infants’ exploration of scented toys: Effects of prior experiences. Chemical Senses. 1998;23:11–17. doi: 10.1093/chemse/23.1.11. [DOI] [PubMed] [Google Scholar]
- Miller MW. Circadian rhythm of cell proliferation in the telencephalic ventricular zone: Effect of in utero exposure. Brain Research. 1992a;595:17–24. doi: 10.1016/0006-8993(92)91447-m. [DOI] [PubMed] [Google Scholar]
- Miller MW. Development of the central nervous system: Effects of alcohol and opiates. New York: Wiley-Liss; 1992b. [Google Scholar]
- Molina JC, Chotro MG, Dominguez HD. Fetal alcohol learning resulting from alcohol contamination of the prenatal environment. In: Lecanuet JP, Fifer WP, Krasnegor NA, Smotherman WP, editors. Fetal development: A psychological perspective. Hillsdale, NJ: Erlbaum; 1995. pp. 419–438. [Google Scholar]
- Molina JC, Dominguez HD, Lopez MF, Pepino MY, Faas AE. The role of fetal and infantile experience with alcohol in later recognition and acceptance patterns of the drug. In: Hannigan JH, Spear LP, Spear NE, Goodlett CR, editors. Alcohol: Effects on brain and development. Mahwah, NJ: Erlbaum; 1999. pp. 199–228. [Google Scholar]
- Molina JC, Spear NE, Spear LP, Mennella JA, Lewis MJ. Alcohol and development: Beyond fetal alcohol syndrome. Developmental Psychobiology. 2007;49:227–242. doi: 10.1002/dev.20224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn JA, Harris SR, Weinberg J. Fetal alcohol syndrome: Review of the literature with implication for physical therapists. Physical Therapy. 1993;73:599–607. doi: 10.1093/ptj/73.9.599. [DOI] [PubMed] [Google Scholar]
- Oulette EM, Rosett HL, Rosman NP, Weiner L. Adverse effects on offspring of maternal alcohol abuse during pregnancy. New England Journal of Medicine. 1977;297:528–530. doi: 10.1056/NEJM197709082971003. [DOI] [PubMed] [Google Scholar]
- Pepino MY, Abate P, Spear NE, Molina JC. Heightened ethanol intake in infant and adolescent rats after nursing experiences with an ethanol-intoxicated dam. Alcoholism: Clinical and Experimental Research. 2004;28:895–905. doi: 10.1097/01.alc.0000128223.95184.c9. [DOI] [PubMed] [Google Scholar]
- Pepino MY, Kraebel KS, Lopez MF, Spear NE, Molina JC. Behavioral detection of low concentrations of ethanol in milk in the preweanling rat. Alcohol. 1998;15:337–353. doi: 10.1016/s0741-8329(97)00154-7. [DOI] [PubMed] [Google Scholar]
- Pepino MY, Lopez MF, Spear NE, Molina JC. Infant rats respond differently to alcohol after nursing from an alcohol-intoxicated dam. Alcohol. 1999;18:189–201. doi: 10.1016/s0741-8329(99)00003-8. [DOI] [PubMed] [Google Scholar]
- Pepino MY, Spear NE, Molina JC. Nursing experiences with an alcohol-intoxicated rat dam counteract appetitive conditioned responses toward alcohol. Alcoholism: Clinical and Experimental Research. 2001;25:18–24. [PubMed] [Google Scholar]
- Phillips DS, Stainbrook GL. Effects of early alcohol exposure upon adult learning abilities and taste preferences. Physiological Psychology. 1976;4:473–475. [Google Scholar]
- Ponce LF, Pautassi RM, Spear NE, Molina JC. Nursing from an ethanol-intoxicated dam induces short- and long-term disruptions in motor performance and enhances later self-administration of the drug. Alcoholism and Clinical Experimental Research. 2004;28:1039–1050. doi: 10.1097/01.alc.0000131298.32045.96. [DOI] [PubMed] [Google Scholar]
- Randall CL, Hughs SS, Williams CK, Anton RF. Effect of prenatal alcohol on consumption of alcohol and alcohol-induced sleep time in mice. Pharmacology Biochemistry and Behavior. 1983;18:325–329. doi: 10.1016/0091-3057(83)90194-6. [DOI] [PubMed] [Google Scholar]
- Reyes E, Garcia KD, Jones BC. Effects of the maternal consumption of alcohol on alcohol selection in rats. Alcohol. 1985;2:323–326. doi: 10.1016/0741-8329(85)90068-0. [DOI] [PubMed] [Google Scholar]
- Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. Journal of Neuroscience. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo E, Zhang C, Kronberg E, Restrepo D. Analysis of training-induced changes in ethyl acetate odor maps using a new computational tool to map the glomerular layer of the olfactory bulb. Chemical Senses. 2005;30:615–626. doi: 10.1093/chemse/bji055. [DOI] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, et al. Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratogen. 1997;56:317–326. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Schaal B, Marlier L, Soussignan R. Human fetuses learn odors from their pregnant mother’s diet. Chemical Senses. 2000;25:729–737. doi: 10.1093/chemse/25.6.729. [DOI] [PubMed] [Google Scholar]
- Schaal B, Orgeur P. Olfaction in utero: Can the rodent model be generalized? Quarterly Journal of Experimental Psychology: Journal of Comparative and Physiological Psychology. 1992;44(B):245–278. doi: 10.1080/02724999208250615. [DOI] [PubMed] [Google Scholar]
- Semke E, Distel H, Hudson R. Specific enhancement of olfactory receptor sensitivity associated with fetal learning of food odors in the rabbit. Naturwissenschaften. 1995;82:148–149. doi: 10.1007/BF01177279. [DOI] [PubMed] [Google Scholar]
- Smotherman WP. In utero chemosensory experience alters taste preferences and corticosterone responsiveness. Behavioral Neural Biology. 1982a;36:61–68. doi: 10.1016/s0163-1047(82)90245-x. [DOI] [PubMed] [Google Scholar]
- Smotherman WP. Odor aversion learning by the rat fetus. Physiology and Behavior. 1982b;29:769–771. doi: 10.1016/0031-9384(82)90322-5. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. The rat fetus in its environment: Behavioral adjustments to novel, familiar, aversive, and conditioned stimuli presented in utero. Behavioral Neuroscience. 1985;99:521–530. doi: 10.1037//0735-7044.99.3.521. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. Psychobiology of fetal experience in the rat. In: Krasnegor NA, Blass EM, Hofer MA, Smotherman WP, editors. Perinatal development: A psychobiological perspective. Orlando, FL: Academic Press; 1987. pp. 39–60. [Google Scholar]
- Smotherman WP, Robinson SR. Behavior of rat fetuses following chemical or tactile stimulation. Behavioral Neuroscience. 1988;102:24–34. doi: 10.1037//0735-7044.102.1.24. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. Rat fetuses respond to chemical stimuli in gas phase. Physiology and Behavior. 1990;47:863–868. doi: 10.1016/0031-9384(90)90010-2. [DOI] [PubMed] [Google Scholar]
- Spear NE, Molina JC. Consequences of early exposure to alcohol: How animal studies reveal later patterns of use and abuse in humans. In: Carroll M, Overmier B, editors. Animal research and human health. Washington, DC: American Psychological Association; 2001. pp. 85–99. [Google Scholar]
- Spear NE, Molina JC. Fetal or infantile exposure to ethanol promotes ethanol ingestion in adolescence and adulthood: A theoretical review. Alcoholism: Clinical and Experimental Research. 2005;29:909–929. doi: 10.1097/01.alc.0000171046.78556.66. [DOI] [PubMed] [Google Scholar]
- Stickrod G, Kimble DP, Smotherman WP. In utero taste/odor aversion conditioning in the rat. Physiology and Behavior. 1982;28:5–7. doi: 10.1016/0031-9384(82)90093-2. [DOI] [PubMed] [Google Scholar]
- Stratton K, Howe C, Battaglia F. Fetal alcohol syndrome: Diagnosis, epidemiology, prevention, and treatment. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Streissguth AP. Teratogenic and genetic influences on adolescent and adult alcohol use and abuse. Symposium organized and presented at the meeting of the Research Society on Alcoholism; Hilton Head, SC. 1998. Jun, [Google Scholar]
- Streissguth AP, Landesman-Dwyer S, Martin JC, Smith DW. Teratogenic effects of alcohol in humans and animals. Science. 1980 Jul 18;209:335–361. doi: 10.1126/science.6992275. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Leon M. Early olfactory learning induces an enhanced olfactory bulb response in young rats. Brain Research. 1986;392:278–282. doi: 10.1016/0165-3806(86)90256-7. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, McGaugh JL, Leon M. Norepinephrine-induced plasticity and one-trial olfactory learning in neonatal rats. Developmental Brain Research. 1991;60:219–228. doi: 10.1016/0165-3806(91)90050-s. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Leon M. Norepinephrine and learning-induced plasticity in infant rat olfactory system. Journal of Neuroscience. 1989;9:3998–4006. doi: 10.1523/JNEUROSCI.09-11-03998.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulleland C, Wennburg RP, Igo RP, Smith NJ. The offspring of alcoholic mothers. Pediatric Research. 1970;4:474. [Google Scholar]
- Van Dyke DC, MacKay L, Ziaylek EN. Management of severe feeding dysfunction in children with fetal alcohol syndrome. Clinical Pediactrics. 1982;21:336–339. doi: 10.1177/000992288202100603. [DOI] [PubMed] [Google Scholar]
- Vavrousek-Jakuba EM, Baker RA, Shoemaker WJ. Effect of ethanol on maternal and offspring characteristics: Comparison of the formulations fed during gestation. Alcoholism: Clinical and Experimental Research. 1991;15:129–135. doi: 10.1111/j.1530-0277.1991.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Olfactory associative conditioning in infant rats with brain stimulation as reward: I. Neurobehavioral consequences. Developmental Brain Research. 1990;53:215–221. doi: 10.1016/0165-3806(90)90009-n. [DOI] [PubMed] [Google Scholar]
- Woo CC, Coopersmith R, Leon M. Localized changes in olfactory bulb morphology associated with early olfactory learning. Journal of Comparative Neurology. 1987;263:113–125. doi: 10.1002/cne.902630110. [DOI] [PubMed] [Google Scholar]
- Woo CC, Oshita MH, Leon M. A learned odor decreases the number of Fos-immunopositive granule cells in the olfactory bulb of young rats. Brain Research. 1996;716:149–156. doi: 10.1016/0006-8993(96)00037-6. [DOI] [PubMed] [Google Scholar]
- Yates WR, Cadoret RJ, Troughton EP, Steward M, Giunta TA. Effect of fetal alcohol exposure on adult symptoms of nicotine, alcohol and drug dependence. Alcoholism: Clinical and Experimental Research. 1998;22:914–920. [PubMed] [Google Scholar]
- Youngentob SL, Kent PF. Enhancement of odorant-induced mucosal activity patterns in rats trained on an odorant identification task. Brain Research. 1995;670:82–88. doi: 10.1016/0006-8993(94)01275-m. [DOI] [PubMed] [Google Scholar]
- Youngentob S, Kent P, Sheehe P, Molina J, Spear NE, Young-entob L. The effect of gestational ethanol exposure on the behavioral and neurophysiologic olfactory response to ethanol odor in early postnatal and adult rats. Journal of Neuroscience. doi: 10.1037/0735-7044.121.6.1293. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]