Abstract
Background
Studies report a fundamental relationship between chemosensory function and the responsiveness to ethanol, its component orosensory qualities, and its odor as a consequence of fetal ethanol exposure. Regarding odor, fetal exposed rats display enhanced olfactory neural and behavioral responses to ethanol odor at postnatal (P) day 15. Although these consequences are absent in adults (P90), the behavioral effect has been shown to persist into adolescence (P37). Given the developmental timing of these observations, we explored the decay in the response to ethanol odor by examining ages between P37 and young adulthood. Moreover, we sought to determine whether the P15 neurophysiologic effect persists, at least, to P40.
Methods
Behavioral and olfactory epithelial (OE) responses of fetal ethanol exposed and control rats were tested at P40, P50, P60, or P70. Whole-body plethysmography was used to quantify each animal’s innate behavioral response to ethanol odor. We then mapped the odorant-induced activity across the OE in response to different odorants, including ethanol, using optical recording methods.
Results
Relative to controls, ethanol exposed animals showed an enhanced behavioral response to ethanol odor that, while significant at each age, decreased in magnitude. These results, in conjunction with previous findings, permitted the development of an ontologic odor response model of fetal exposure. The fitted model exemplifies that odor-mediated effects exist at birth, peak in adolescence and then decline, becoming absent by P90. There was no evidence of an effect on the odor response of the OE at any age tested.
Conclusions
Fetal exposure yields an enhanced behavioral response to ethanol odor that peaks in adolescence and wanes through young adulthood. This occurs absent an enhanced response of the OE. This latter finding suggests that by P40 the OE returns to an ethanol “neutral” status and that central mechanisms, such as ethanol-induced alterations in olfactory bulb circuitry, underlie the enhanced behavioral response. Our study provides a more comprehensive understanding of the ontogeny of fetal-ethanol-induced olfactory functional plasticity and the behavioral response to ethanol odor.
Keywords: Ontogeny, Fetal Ethanol Exposure, Olfactory Plasticity, Whole-Body Plethysmography, Optical Recording
BACKGROUND
Human studies point to a predictive relationship between fetal ethanol exposure and the risk for abuse in adolescence. Fetal ethanol exposure is, perhaps, the best predictor of ethanol abuse in this age group (Alati et al., 2006; Baer et al., 1998, 2003; Streissguth, 1998; Yates et al., 1998), with an inverse correlation between the age of first experience and the likelihood of continued abuse (Streissguth, 1998; Yates et al., 1998). Despite these findings, the antecedent factors contributing to this progressive pattern are still poorly understood. How does fetal exposure alter adolescent ethanol acceptability and what, if any, are the long-term risks of these effects?
Human and animal studies show that specific memories arise from fetal processing of ethanol’s chemosensory properties present in the amniotic fluid (e.g., Arias and Chotro, 2006; Bond and DiGiusto, 1976; Chotro and Molina, 1990, 1992; Chotro et al., 1996; Dominguez et al., 1996, 1998; Faas et al., 2000; Molina et al., 1995; Schaal and Orguer, 1992; Schaal et al., 2000). In this regard, recent studies have shown a fundamental relationship between chemosensory function and the responsiveness to ethanol, its component orosensory qualities, and its odor as a consequence of fetal exposure. Early postnatal day 15 (P15) rats exposed to ethanol throughout fetal development display an enhanced or tuned neural and behavioral response to ethanol odor that is specific to the exposure stimulus (Youngentob et al., 2007a). Moreover, the behavioral consequence is directly attributable to the neural effect of fetal exposure. A parallel study showed that ethanol intake is also enhanced in these animals (Youngentob et al., 2007b). Importantly, fetal ethanol exposure alters the olfactory system such that the observed elevated intake of ethanol is causally linked, in part, to the enhanced response to its odor (Youngentob and Glendinning, 2009). Although these consequences of fetal exposure are absent in adults (Youngentob et al., 2007a,b) they persist into adolescence (Eade et al., 2009; Middleton et al., 2009; Youngentob and Glendinning, 2009).
Taken together, the foregoing points to adolescence as a potentially key developmental transition point for the fetal-ethanol-induced effects on chemosensory function. Given the developmental timing of these prior studies, the present work focused on filling the gap in our understanding of the effect of in utero ethanol exposure on ethanol odor related responses (both behavioral and neurophysiologic), by examining ages between late adolescence and adulthood.
METHODS
Experimental Design
The present study utilized 144 experimental Long–Evans Hooded rats, the progeny of 18 dams. Each dam provided 8 experimental pups (4 males and 4 females). Four pairs of littermates (i.e., 1 male and 1 female) were individually randomized to 1 of 4 ages, i.e., P40, P50, P60, or P70. Following a single behavioral testing session, these same animals were killed for neurophysiological examination.
Animals
All animals were housed in a temperature and humidity controlled environment with a fixed 12-hour light/dark cycle at SUNY Upstate Medical University (Syracuse, NY). Experimental procedures were performed in accordance with the Universities Institutional Animal Care and Use Committee guidelines.
On gestational day 5 (G5), pregnant Long–Evans female rats (Harlan–Sprague–Dawley, Indianapolis, IN) were weighed and placed into weight-matched blocks of 3 dams each. The present study utilized 6 blocks of dams to generate all experimental animals. Within a single block, dams were randomly assigned to 1 of the 3 maternal treatments: ethanol exposed (ET), pair-fed (PF), or free choice (FC).
Ethanol was administered to the ET treatment group via ad libitum access to a liquid diet (L10251; Research Diets, New Brunswick, NJ) supplemented with increasing levels of ethanol (2.2% v/v G6 to G8, 4.5% v/v G9 to G10, 6.7% v/v G11 to G20) (e.g., Eade et al., 2009; Youngentob et al., 2007a,b). The highest concentration of ethanol in this diet provided the dams with 35% of their daily calories coming from ethanol. This concentration has been noted to model moderate ethanol intake (Driscoll et al., 1990; Vavrousek-Jakuba et al., 1991), with peak blood alcohol levels reaching approximately 150 mg/dl (Miller, 1992; Youngentob et al., 2007a). Importantly, this level of ethanol exposure is attained prior to the age at which the olfactory neurons of the fetus begin to transduce sensory information (G14) (Gesteland et al., 1982) and make synaptic connections with the olfactory bulb (OB; G14 to G15) (Farbman, 1991). Dams in both the PF and FC control groups were provided an iso-nutritive liquid diet supplemented with maltose/dextrin, providing equivalent caloric content as the ET diet (L10252; Research Diets). To control for potential nutritional deficits due to ET dams voluntarily consuming less diet, PF dams were restricted to the caloric intake of their respective weight-matched ET dam. Thus, PF dams were provided with access to the same volume of liquid diet that the respective ET dams had consumed on the previous day, as measured at 4:00 PM. Dams in the FC group were allowed ad libitum access to the liquid diet and water throughout gestation (e.g., Eade et al., 2009).
Litters from all 3 maternal treatment groups were fostered to non-experimental surrogate dams within 24 hours of birth. Each litter was sexed and culled to 10 pups, leaving no fewer than 4 pups of each sex, on the morning of P2. Only blocks of litters that provided the minimum 4 males and 4 females per treatment group required for our experiments were considered for this study. Animals were separated by sex at the time of weaning and remained housed with their same-sex littermates until the time of testing. One male and 1 female rat from each litter were randomly allocated to the P40, P50, P60, or P70 time points, providing a total of 12 ET, 12 PF, and 12 FC subjects per age group.
Behavioral Testing and Data
Ethanol odor responsivity was monitored using a high-throughput behavioral method originally developed for the trans-NIH mouse mutagenesis initiative (Youngentob, 2005). This fully automated procedure is based on the evaluation of an animal’s stimulus-induced reflexive sniffing response to odorant stimuli. Importantly, this technique requires no prior training of the animal to be tested and the outcome of the evaluation yields an operationally defined measure. Briefly, an unrestrained animal was individually placed in a 1.3 l Plexiglas testing chamber that permitted the rapid onset and clean out of ethanol odor. Stimulus generation and presentation occurred using a computer controlled standard flow-dilution olfactometer and electronic mass flow controllers (Teledyne Co., Hampton, VA). Each animal experienced a 40 trial air-only habituation period followed by a 5 block testing session. Within each block, air and 1 of 5 concentrations of ethanol odor (0.313%, 0.625%, 1.25%, 2.5%, and 5% of vapor saturation at 20°C) were randomly presented 10 times each on a fixed 6 seconds interval schedule (Eade and Youngentob, 2009; Eade et al., 2009; Middleton et al., 2009; Youngentob, 2005; Youngentob and Glendinning, 2009; Youngentob et al., 2007a).
In keeping with our previously established methods and procedures (ibid), whole-body plethysmography was used to determine the numerical values for a set of 14 respiratory measures (sniff frequency; the number of inspiratory and expiratory sniffs; the duration, volume, average flow rate, and peak flow rate of an inspiratory and expiratory sniff; the total inspiratory and expiratory volume; and the total apneic duration) in response to air or odorant presentation. Using principal components analysis (PCA) in conjunction with multivariate multiple regression, the 14 respiratory measures of stimulus-induced sniffing were incorporated into a model that defined a basic measure of ethanol odor-mediated response behavior for each tested animal within a specific data set. Specifically, PCA reduced the 14 derived respiratory measures to a fewer number of uncorrelated dimensions (i.e., usually 1 or 2 factors) that defined an animal’s behavioral response to each of the 5 concentrations of ethanol odor. For each factor, multivariate analysis estimated the coefficients for each odorant concentration tested plus a constant. The summation of the constant and the animal’s 5 respective factor values (1 for each concentration of odorant from the PCA) multiplied by their respective coefficients, provided a composite index value that represented the animals behavioral response to ethanol odor across all concentrations tested. In short, for each age group tested, the derived 14 × 5 data matrix for each animal (i.e., 14 response measures × 5 odorant concentrations) was reduced to either a single index value (for PCAs producing only 1 factor) or a pair of X and Y coordinates (for PCAs providing 2 resultant factors). This principal behavioral measure for each animal, i.e., the composite reflexive sniffing index (ibid), was used in subsequent analyses of specific hypotheses. Importantly, increases in the value of this measure in response to ethanol odorant stimulation are both predictive of and causally linked to enhanced ethanol intake as a consequence of prior fetal ethanol exposure (Youngentob and Glendinning, 2009).
Optical Recording of Odorant-Induced Epithelial Activity
At the completion of behavioral testing, the olfactory epithelium (OE) of each experimental animal was evaluated for its response to odorant stimulation using optical recording methods and a voltage-sensitive dye (e.g., Youngentob et al., 1995, 2003, 2007a). In keeping with our standard approach (ibid), the right nasal cavity was split in half, exposing the OE of the septum and turbinates. Each piece of tissue was soaked in a voltage-sensitive dye (di-4-ANEPPS; Invitrogen, Carlsbad, CA), rinsed with saline, and then placed in a Delrin chamber that contained a clear top, as well as stimulus input and output ports. The ports allowed for air or odorant stimuli to be introduced into the chamber and pulled across the OE from the region of the external naris to nasopharynx. OE responses to odorant stimuli were recorded onto a 120 × 120 pixel array of a Dalsa 12-bit digital CCD Camera (Dalsa, Waterloo, ON, Canada). A single presentation of 6 different odorants (i.e., propyl acetate, heptanal, ethanol, carvone, and ethylacetoacetate) was used as stimuli. A sixth odorant, amyl acetate, served as a standard stimulus for correction of tissue responsiveness over time and was presented twice, once at the beginning and again at the end of the testing session. All raw responses were adjusted for background fluorescence and corrected for baseline shifts due to photo bleaching. The magnitude of the average and peak response of each tissue to odorant stimulation was determined and used in later analysis.
RESULTS
As previously described in detail (e.g., Youngentob, 2005), whole-body plethysmography measures alterations in respiration (in response to odorant stimuli) by detecting and amplifying minute changes created by an unrestrained animal’s breathing inside a testing chamber. Unfortunately, in the current study, the amplification used for the P50 animals saturated a great deal of the plethysmograph signal, rendering the raw data unusable. As such, the P50 age group was removed from our behavioral and electrophysiological analyses. Nonetheless, as outlined below, loss of this data did not obviate our ability to fulfill the objectives of the study.
Analysis of Reflexive Sniffing Behavior by Age
Previously, we have shown that the chemosensory response to ethanol is enhanced in ET animals, relative to both PF and FC controls, when tested either during early postnatal development (i.e., P15: Youngentob et al., 2007a; Youngentob and Glendinning, 2009) or in adolescence (i.e., P37: Eade et al., 2009). Importantly, in these studies, the magnitude of the ET effect when compared with PF controls did not differ from the magnitude relative to FC controls. Therefore, based on these prior findings we focused our analysis on a specific a priori null-hypothesis. Namely, for each age group evaluated, we hypothesized no difference in the behavioral response to ethanol odor between ET versus FC animals. We chose to use FC animals since pair feeding, itself, can be considered a form of perturbation.
Regarding the above, to further justify focusing our evaluation on the ET versus FC comparison at each age, we first confirmed the previous observations that the potential untoward effect of nutritional restriction, due to pair feeding, is not a relevant treatment variable in the evaluation of the behavioral response to ethanol odor. To accomplish this, we calculated a standardized “effect size” for each comparison (i.e., ET vs. FC, ET vs. PF) at each age to eliminate any potential effect of scale across age groups and/or comparison (Eade and Youngentob, 2009). Recall that the composite reflexive sniffing index defines an animal’s odor-mediated behavioral response to ethanol. Further, this principal measure is derived, in part, by a PCA of 14 measures of reflexive sniffing behavior. For the P40, P60, and P70 age groups, the PCA yielded a 2-dimensional, 2-dimensional, and 1-dimensional solution, respectively. The “effect size” or magnitude of the differential effect between ET versus FC and ET versus PF treatment groups for the P40 and P60 comparisons was the standardized displacement vector between the mean locations in 2 dimensions. That is, for each dimension we standardized by dividing the magnitude of the difference by the standard deviation and then calculated the overall distance between the mean behavioral responses of the groups being compared (i.e., displacement vector) (ibid). This resulted in a single value that quantified the magnitude of treatment versus control effect. For the P70 group the effect size was the standardized displacement vector between the mean locations in 1 dimension. For each of the 3 ages, we found no evidence of a difference in the magnitude of the ET versus FC exposure effect as compared to the ET versus PF exposure effect [P40, t(15) = 0.976; P60, t(15) = 0.689; P70, t(15) = 0.203; all p’s > 0.20].
Consequently, given the above, we evaluated whether and to what extent there was an effect of ET versus FC at each experimental age. Recall from above that the P40 and P60 age groups produced a 2-dimensional composite sniffing index while the P70 group provided a single-dimensional behavioral measure. Therefore, to conduct analyses of the treatment effects within each age, multivariate ANOVAs were conducted on the 2-dimensional P40 and P60 index values while standard ANOVA was utilized for the P70 analysis. Interestingly, the results of these analyses revealed significant effects of prenatal treatment (ET vs. FC) on the behavioral response to ethanol odor at all 3 ages evaluated (P40, F2,14 = 13.36, p < 0.0006; P60, F2,14 = 5.66, p < 0.02; P70, F1,15 = 7.83, p < 0.02). Nonetheless, as shown in Fig. 1, the magnitude of the differential effect of ET exposure relative to FC controls declined with age. Relative to controls, the consequence of prenatal ethanol exposure on the behavioral response to ethanol odor decreased by approximately 44% between P40 and P70.
Fig. 1.
Ethanol effect size (mean ± SEM) at P40, P60, and P70. Relative to FC controls there was a 44% decline in the differential magnitude of the enhanced response to ethanol odor in ET animals between P40 and P70.
Sex effects were present at P40 (F2,14 = 3.85, p < 0.05) and P70 (F1,15 = 2.33, p < 0.02) with no evidence of a sex effect at P60 (F2,14 = 2.25, p > 0.14) or sex by treatment interaction at any age (P40, F2,14 = 0.95, p > 0.40; P60, F2,14 = 1.44, p > 0.26; P70, F1,15 = 0.27, p > 0.60). These findings indicate that although males and females (at P40 and P70) do not respond equally to ethanol odor, this effect is not specific to any given treatment.
An Ontologic Model of the Response to Ethanol Odor
The above results, coupled with previously published findings provided us with an opportunity to model the development and decay of the enhanced response to ethanol odor resulting from fetal ethanol exposure. To accomplish this, we used the following approach. (i) To approximate the growth of the enhanced odor response we first calculated an effect size between the ET versus FC P15 reflexive sniffing data illustrated in figure 3 of Youngentob and Glendinning (2009) (derived with an identical experimental design). As in the current study, this value represented the standardized magnitude of the differential effect of ET exposure relative to FC controls. This yielded a data point early in development that was lower in magnitude than the effect size observed at P40 in the present experiment (see Fig. 2). Since previous studies have shown that fetal exposure yields an enhanced odor response at P0 (e.g., Dominguez et al., 1998; Faas et al., 2000), we made the assumption that any curve describing the development of the enhanced odor response would not cross through the origin. Thus, as is typical in describing natural processes that begin small and approach a maximum over time, we modeled the growth portion of the effect with a sigmoid function. (ii) The decline in the magnitude of the enhanced odor response between P40 and P70 appeared to be linear. However, if the decay process were strictly linear then the size of the behavioral effect would continue to decline past zero to negative values. Thus, we made the assumption that the decay portion of the enhanced ethanol odor response would follow an archetypal biological decay process that, while approaching zero, would not cross it. Such a biological process, which begins at a maximum value and decreases at a constant relative rate, is best described by an exponential decay. (iii) Finally, to visualize the complete ontogeny of the prenatal ethanol effect we generated a mathematical model that superimposed these 2 processes upon one another. This model represents the interplay between the growth and decay of the behavioral effect, thus illustrating it as a single continuous biological process.
Fig. 2.
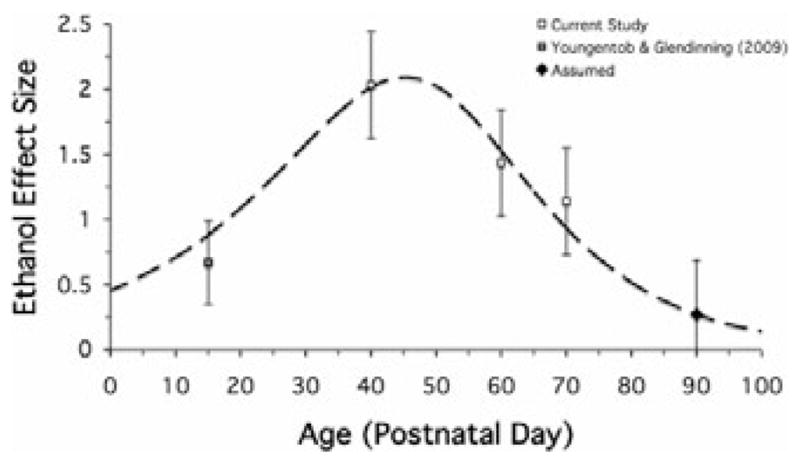
Ontologic odor response model of fetal exposure. A single model of fetal ethanol-induced behavioral effects accurately fits: (i) the observation that a response to ethanol odor should be present at birth (e.g., Dominguez et al., 1998; Faas et al., 2000); (ii) P15 data from previous work (Youngentob and Glendinning, 2009), (iii) P40, P60, and P70 data from the current study, and (iv) the assumption of a nonsignificant effect of prenatal exposure at P90. Further, this model estimates that the enhancement of the behavioral response to ethanol odor peaks within late adolescence (approximately P45).
The model generated by this approach is:
where t represents an animal’s age, C equals the maximal effect size of the growth component if it were unrestricted by the force of decay, r1 represents the intrinsic growth rate of the sigmoid curve, r2 is the exponential decay rate, and k corresponds to the time where the effect size of the growth component is 50% of its maximal value (C).
As can be seen in Fig. 2, the curve resulting from this mathematical model provides an excellent fit (i.e., falls within the standard errors) of the data resulting from the present study, the P15 data from previous work (Youngentob and Glendinning, 2009) and is consistent with the observation that the response to ethanol odor should be present at birth (e.g., Dominguez et al., 1998; Faas et al., 2000). Importantly, the curve models the previous finding that the enhanced odor-mediated effects of prenatal exposure are absent at P90 (Youngentob et al., 2007a). That is, the assumed P90 data point illustrated in Fig. 2 is not significantly different from zero (i.e., it falls within 1 SE of 0). Finally, the model estimates that the enhanced response to ethanol odor peaks at approximately P45 (i.e., late adolescence; Spear, 2000).
Response of the Olfactory Epithelium
Table 1 illustrates the resultant F-values obtained from the individual ANOVAs of the average (Ravg) and peak (Rpeak) responses of the OE at P40, P60, and P70. In keeping with previous studies of the response of the rat OE (e.g., Youngentob and Kent, 1995; Youngentob et al., 1995), both the septum and turbinates displayed a highly significant effect of odorant on the average and peak response at each age tested. By contrast, there was no evidence for an overall effect of maternal treatment at any age of testing. Further, no evidence of a significant interaction between maternal treatment and odorant appeared at any age.
Table 1.
Statistical Analysis of the Olfactory Epithelial Response
| Tissue | Odorant | Maternal treatment | O × MT interaction |
|---|---|---|---|
| P40 | |||
| Septum | |||
| Ravg | F4,144 = 23.48* | F2,144 = 0.51 | F8,144 = 0.37 |
| Rpeak | F4,144 = 21.99* | F2,144 = 0.57 | F8,144 = 0.37 |
| Turbinates | |||
| Ravg | F4,144 = 26.40* | F2,144 = 0.11 | F8,144 = 0.52 |
| Rpeak | F4,144 = 25.12* | F2,144 = 0.21 | F8,144 = 0.52 |
| P60 | |||
| Septum | |||
| Ravg | F4,144 = 11.33* | F2,144 = 0.88 | F8,144 = 0.30 |
| Rpeak | F4,144 = 9.45* | F2,144 = 0.92 | F8,144 = 0.28 |
| Turbinates | |||
| Ravg | F4,144 = 12.17* | F2,144 = 1.63 | F8,144 = 0.46 |
| Rpeak | F4,144 = 10.35* | F2,144 = 1.57 | F8,144 = 0.45 |
| P70 | |||
| Septum | |||
| Ravg | F4,139 = 8.71* | F2,139 = 2.23 | F8,139 = 0.30 |
| Rpeak | F4,139 = 7.72* | F2,139 = 2.06 | F8,139 = 0.27 |
| Turbinates | |||
| Ravg | F4,139 = 8.70* | F2,139 = 0.31 | F8,139 = 0.37 |
| Rpeak | F4,139 = 8.00* | F2,139 = 0.31 | F8,139 = 0.38 |
O, odorant; MT, maternal treatment; Ravg, response average; Rpeak, peak response.
p’s ≤ 0.0001.
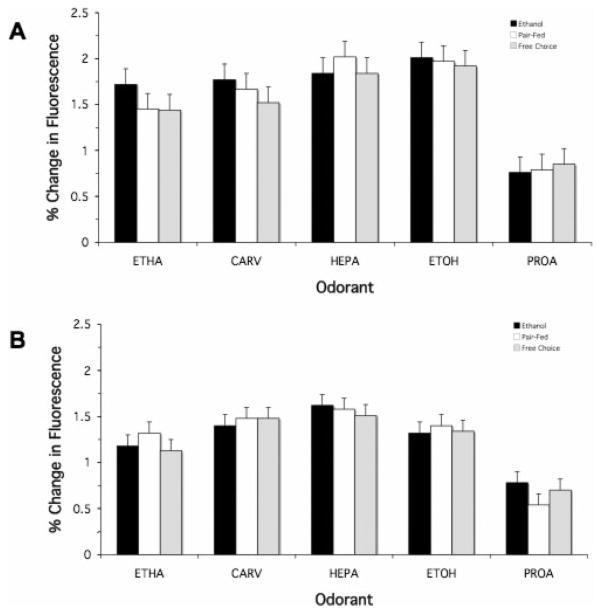
As an exemplar of the foregoing results, Fig. 3 illustrates the average response height (mean ± SEM) for both the septum and turbinates at P40 as a function of prenatal treatment and odorant. As can been in this figure, although, on average, the magnitude of the response varied with odorant there is remarkable similarity in the responses across maternal treatment groups.
Fig. 3.
Comparison by odorant and mucosal recording surface of the mean average response height as a function of maternal treatment. The magnitude of the mean average response (i.e., the height of the bars) is expressed in units of percent change in fluorescence (mean ± SEM). Septum (A) and turbinate (B) are illustrated for the P40 animals. For all test odorants, the responsivity of the OE was not altered as a function of prenatal treatment.
DISCUSSION
Much is known about the ability of the human (Faas et al., 2000; Schaal and Orguer, 1992; Schaal et al., 2000) and animal (e.g., Bilko et al., 1994; Hudson and Distel, 1998; Semke et al., 1995; Smotherman, 1982a,b; Smotherman and Robinson, 1985, 1987, 1988, 1990; Stickrod et al., 1982) fetus to learn about chemosensory stimuli present in the amniotic fluid, including ethanol (e.g., Chotro and Molina, 1992; Chotro et al., 1996). It has been suggested that this learning is a fundamental mechanism by which animals gain important information, in utero, about stimuli that are presumed to be important for their survival later in life, for example, identifying foods that are safe to eat (Hudson, 1999). With respect to ethanol, however, such an adaptive mechanism may work to the disadvantage of the animal by contributing to an increased risk for initial use and subsequent abuse of the drug.
Indeed, there is substantial evidence that the fetus can acquire information about ethanol-related sensory cues and display a memory of the prenatal experience with ethanol (e.g., Abate et al., 2000, 2001; Chotro and Molina, 1992; Chotro et al., 1996; Eade et al., 2009; Youngentob et al., 2007a). Importantly, prenatal exposure has been shown to modulate subsequent intake both in early postnatal (e.g., Chotro and Arias, 2003; Chotro and Molina, 1990; Spear and Molina, 2001, 2005; Youngentob et al., 2007b) and adolescent animals (Chotro et al., 2007; Molina et al., 2007; Spear and Molina, 2005). The question of whether the postnatal effects persist into adulthood, however, is less clear with studies reporting results ranging from enhanced acceptance (e.g., Bond and DiGiusto, 1976; Phillips and Stainbrook, 1976) to an absence of an effect (e.g., Abel and Yourk, 1979; McGivern et al., 1984; Youngentob et al., 2007b).
Recently, using a rodent model, we have demonstrated that fetal-ethanol-induced chemosensory plasticity is an important underlying determinant in the above observations. In summary of these findings, early postnatal rats exposed to gestational ethanol display enhanced ethanol intake (Youngentob et al., 2007b) as well as behavioral responses to ethanol odor that are mediated by an effect of maternal treatment on the response of the OE (Youngentob et al., 2007a). Although these parallel consequences are absent in adults (Youngentob et al., 2007a,b), the enhanced behavioral odor-mediated response persists into the period of adolescence (Eade et al., 2009; Middleton et al., 2009). Importantly, fetal ethanol exposure directly increases ethanol intake by altering the smell and taste systems such that the normally aversive odor and flavor attributes of ethanol become more acceptable (Youngentob and Glendinning, 2009).
In short, the available data suggest an epigenetic chemosensory mechanism by which maternal ethanol use is transferred to offspring. Moreover, the above studies highlight adolescence as a potential transition point for the chemosensory consequences of fetal ethanol exposure. Nevertheless, a large gap (in time) exists between late adolescence (P40) (an age up to which studies have uniformly demonstrated enhanced intake effects of fetal exposure) and adulthood (P90) (an age at which there are inconsistent or negative results). Why is it important to understand this gap? First, clinical and epidemiological studies demonstrate: (i) a predictive relationship between prenatal ethanol exposure and the increased risk for adolescent ethanol abuse (Alati et al., 2006; Baer et al., 1998, 2003; Streissguth, 1998; Yates et al., 1998); and (ii) that the earlier the age of postnatal experience the greater the probability of long-term abuse (Streissguth, 1998; Yates et al., 1998). Second, animal studies have shown that adolescent ethanol odor re-exposure (via a social interaction paradigm) augments the enhanced response to ethanol odor resulting from fetal exposure. This yields persistence of the enhanced odor response into adulthood (Eade et al., 2009). Moreover, the interaction of pre- and postnatal exposure can yield enhanced ethanol avidity relative to the effect of prenatal exposure alone (Chotro et al., 1996; Honey and Galef, 2003). Thus, given the relationship between chemosensory function and postnatal ethanol avidity, investigating the decay process of the olfactory mediated fetal exposure effect is clinically germane in terms of understanding the potential time frame for increased postnatal vulnerability.
Given the above perspective, the results of the present experiment expand on our prior work in several important ways. First, we demonstrated that the enhanced response to ethanol odor resulting from prior fetal exposure persists beyond adolescence and into young adulthood. Notably, however, the magnitude of the odor-mediated effect decreased with age. Second, the present data, in conjunction with previous results defining the early postnatal response to ethanol odor (Youngentob and Glendinning, 2009), permitted us to model the ontogeny of the fetal induced odor-mediated response. The resulting mathematical model superimposed a sigmoid curve defining the growth of the prenatal effect during postnatal development on an exponential curve that defined the decay. The resulting curve was consistent with the previous observations that the odor-mediated consequence of fetal exposure is present at birth (i.e., P0) (e.g., Dominguez et al., 1998; Faas et al., 2000) and absent in adulthood (P90) (Youngentob et al., 2007a). Moreover, the curve in Fig. 2 estimates that the developmental peak of the enhanced ethanol odor response falls within the age range of adolescence, a high risk period for establishing long-term patterns of abuse (see review Spear, 2000). Taken together, these data suggest that the postnatal vulnerability to the progressive consequences of pre- and postnatal ethanol exposure may persist over a significant period of the life span.
Regarding the above, one might suggest that the absence of the P50 data might have produced a substantially different curve. In this respect, we explored P50 ethanol effect sizes falling outside 1 SE of our present curve (both higher and lower). Although such manipulations do, indeed, shift the location (i.e., age) of the peak ethanol effect size, nonetheless, they do not appreciably change the curve and the peak ethanol effect size still falls within the age range of mid- to late adolescence (P40 to P50). That is, although the growth and decay rates of the model do slightly change, the resulting curve still falls within the error of all data points presented in Fig. 2. Further, these manipulations do not alter the accuracy of an assumed nonsignificant effect at P90.
In studies of the offspring of dams fed an ethanol containing diet throughout gestation, adequate nutritional control (i.e., pair feeding) is generally considered to be critical (e.g., see review Spear and Molina, 2005). However, with specific regard to chemosensory responses, previous studies of early postnatal and adolescent animals have shown that under-nutrition is not a pertinent variable in the evaluation of the behavioral (Eade et al., 2009; Youngentob and Glendinning, 2009; Youngentob et al., 2007a) and neural (Youngentob et al., 2007a) response to ethanol odor, as well as taste-mediated orosensory responses to ethanol (Youngentob and Glendinning, 2009). That is, PF and FC controls are not different from each other. The results of the present study extend upon these observations by demonstrating the generality of this finding across additional ages between late adolescence and young adulthood. Here again, we found that while the odor-mediated response to ethanol was enhanced in ET animals relative to both PF and FC controls, the magnitude of this effect did not differ as a function of the control used in the comparison.
Unlike the behavioral findings, we found no effect of prenatal treatment on the neural response of the OE to either odorant stimulation in general or ethanol in particular. This result for the P40 to P70 animals stands in stark contrast to the previous finding in P15 rats that a tuned neural response to ethanol odor mediated a significant proportion of the enhanced ethanol odor response (Youngentob et al., 2007a). Several considerations for the observed disconnect between the peripheral neural and behavioral response at these older ages is warranted. It is reasonable to expect that for the neural consequence to persist would require modification of the basal cell (progenitor) population such that as the OE expands during postnatal development (Weiler and Farbman, 1997) a clonal expansion of the relevant ethanol responsive olfactory sensory neurons (OSNs) would occur. These OSNs, in turn, would create new functional connections with the developing OB (Gheusi and Lledo, 2007; Mair et al., 1982; Weiler and Farbman, 1997). It is clear from our results, however, that the behavioral effect outlives the population of OSNs that were present during prenatal sensitization. That is, the OE has turned over many times in the P40 to P70 rats as a consequence of the normal continuing process of OSN cell death and cell renewal (e.g., Graziadei and Monti Graziadei, 1979, 1980). Thus, our data indicate that while the early effects on the OEs response to ethanol odor plays an important role in establishing the initial behavioral response (Youngentob et al., 2007a) it is not necessary for its maintenance. Nonetheless, it must be emphasized, that this does not obviate a role for the peripheral neural response under conditions in which postnatal sensitization (i.e., experience with ethanol) continues. Indeed, with continued odorant experience an enhanced response of the OE to the exposure stimulus would be expected (e.g., Wang et al., 1993; Youngentob and Kent, 1995). The exact mechanism by which this occurs is still unknown.
If the OEs response is only important to the initial establishment of the augmented behavioral effect then how might this occur? Data suggest that the OB should be viewed from the perspective of odor information processing and memory storage (e.g., Gheusi and Lledo, 2007; Wilson and Sullivan, 1994). Therefore, one potential hypothesis for the disconnect we observed is that OB changes occur as a result of the in utero pairing of the response to ethanol odor (augmented by the initial peripheral neural tuning) with its’ associative properties. Indeed, there are considerable descending monoaminergic and noradrenergic influences on OB function. These centrifugal inputs to the OB signal both arousal and reward and are necessary to modulate OB responses to learned odors (e.g., Gervais and Pager, 1983; Gervais et al., 1988; Sullivan et al., 1989; Wilson and Leon, 1988a,b). In short, during fetal ethanol exposure the corresponding activation of peripherally activated ethanol-responsive neurons and descending influences of ethanol on OB circuits may encode that ethanol odor has gained biological significance. This, in turn, would result in an enhanced odor-guided response to ethanol that outlives the initial peripheral effect. In this regard, recent evidence has shown that fetal ethanol exposure not only enhances the adolescent odor-mediated response to ethanol, but also alters the expression of OB genes involved in synaptic transmission and plasticity as well as neuronal development (both cell fate and axon/neurite outgrowth) (Middleton et al., 2009). Whether these OB effects are, indeed, the result of the forgoing scenario is a matter of conjecture at this time. Nonetheless, these data provide insight for potential underlying molecular mechanisms contributing to persistence of the odor-mediated effect.
CONCLUSIONS
The present study provides a comprehensive description of the ontogeny of fetal-ethanol-induced peripheral olfactory functional plasticity and the enhanced behavioral response to ethanolodor. In keeping with other work (see, for review, Spear, 2000), our ontologic growth and decay model of the enhanced ethanol odor-mediated response highlights adolescence as a key developmental transition point (Streissguth, 1998; Yates et al., 1998). Importantly, however, our model emphasizes that post-natal vulnerability to the potentially exacerbating interaction of pre- and postnatal exposure lasts over a considerable life span, encompassing young adulthood. Given the recent evidence (e.g., Youngentob and Glendinning, 2009) that a fundamental relationship exists between chemosensory function and the responsiveness to ethanol as a consequence of fetal exposure, the results of this study have important implications for under-standing the clinical progression of ethanol abuse patterns.
Acknowledgments
This study was supported by NIH-NIAAA Grant AA014871 (SLY).
Footnotes
AUTHORS‘ CONTRIBUTIONS
AME and SLY designed the study and prepared the manuscript; AME executed the study; and AME, PRS, and SLY were involved in data analysis and interpretation of the results. All authors reviewed the final manuscript.
References
- Abate P, Pepino MY, Dominguez HD, Spear NE, Molina JC. Fetal associative learning mediated through maternal alcohol intoxication. Alcohol Clin Exp Res. 2000;24:39–47. [PubMed] [Google Scholar]
- Abate P, Spear NE, Molina JC. Fetal and infantile alcohol-mediated associative learning in the rat. Alcohol Clin Exp Res. 2001;25:989–998. [PubMed] [Google Scholar]
- Abel EL, Yourk JL. Absence of effect of prenatal ethanol on adult emotionality and ethanol consumption in rats. J Stud Alcohol. 1979;40:547–553. doi: 10.15288/jsa.1979.40.547. [DOI] [PubMed] [Google Scholar]
- Alati R, Al Mamum A, Williams GM, O’Callagham M, Najman JM, Bor W. In utero alcohol exposure and prediction of alcohol disorders in early adulthood: a birth cohort study. Arch Gen Psychiatry. 2006;63:1009–1016. doi: 10.1001/archpsyc.63.9.1009. [DOI] [PubMed] [Google Scholar]
- Arias C, Chotro MG. Interactions between prenatal ethanol exposure and postnatal learning about ethanol in rat pups. Alcohol. 2006;40:51–59. doi: 10.1016/j.alcohol.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Baer JS, Barr HM, Bookstein FL, Sampson PD, Streissguth AP. Prenatal alcohol exposure and family history of alcoholism in the etiology of adolescent alcohol problems. J Stud Alcohol. 1998;59:533–543. doi: 10.15288/jsa.1998.59.533. [DOI] [PubMed] [Google Scholar]
- Baer JS, Sampson PD, Barr HM, Connor PD, Streissguth AP. A 21-year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. Arch Gen Psychiatry. 2003;60:377–385. doi: 10.1001/archpsyc.60.4.377. [DOI] [PubMed] [Google Scholar]
- Bilko A, Altbacker V, Hudson R. Transmission of food preference in the rabbit: the means of information transfer. Physiol Behav. 1994;56:907–912. doi: 10.1016/0031-9384(94)90322-0. [DOI] [PubMed] [Google Scholar]
- Bond NW, DiGiusto EL. Effects of prenatal alcohol consumption on open-field behaviour and alcohol preference in rats. Psychopharmacologia. 1976;46:163–165. doi: 10.1007/BF00421386. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Arias C. Prenatal exposure to ethanol increases ethanol consumption: a conditioned response? Alcohol. 2003;30:19–28. doi: 10.1016/s0741-8329(03)00037-5. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Arias C, Laviola G. Increased ethanol intake after prenatal ethanol exposure: studies with animals. Neurosci Biobehav Rev. 2007;31:181–191. doi: 10.1016/j.neubiorev.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Kraebel KS, McKinzie DL, Molina JC, Spear N. Prenatal and postnatal ethanol exposure influences preweanling rats’ behavioral and autonomic responding to ethanol odor. Alcohol. 1996;13:377–385. doi: 10.1016/0741-8329(96)00027-4. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Molina JC. Acute ethanol contamination of the amniotic fluid during gestational day 21: postnatal changes in alcohol responsiveness in rats. Dev Psychobiol. 1990;23:535–547. doi: 10.1002/dev.420230608. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Molina JC. Bradycardiac responses elicited by alcohol odor in rat neonates: influence of in utero experience with ethanol. Psycho-pharmacology. 1992;106:491–496. doi: 10.1007/BF02244820. [DOI] [PubMed] [Google Scholar]
- Dominguez HD, Lopez MF, Chotro MG, Molina JC. Perinatal responsiveness to alcohol’s chemosensory cues as a function of prenatal alcohol administration during gestational days 17–20 in the rat. Neurobiol Learn Mem. 1996;65:103–112. doi: 10.1006/nlme.1996.0012. [DOI] [PubMed] [Google Scholar]
- Dominguez HD, Lopez MF, Molina JC. Neonatal responsiveness to alcohol odor and infant alcohol intake as a function of alcohol experience during late gestation. Alcohol. 1998;16:109–117. doi: 10.1016/s0741-8329(97)00169-9. [DOI] [PubMed] [Google Scholar]
- Driscoll CD, Streissguth AP, Riley EP. Prenatal alcohol exposure: comparability of effects in human and animal models. Neurotoxicol Teratol. 1990;12:231–237. doi: 10.1016/0892-0362(90)90094-s. [DOI] [PubMed] [Google Scholar]
- Eade AM, Sheehe PR, Molina JC, Spear NE, Youngentob LM, Youngentob SL. The consequence of fetal ethanol exposure and adolescent odor re-exposure on the response to ethanol odor in adolescent and adult rats. Behav Brain Funct. 2009;5:3. doi: 10.1186/1744-9081-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eade AM, Youngentob SL. Adolescent ethanol experience alters immediate and long-term behavioral responses to ethanol odor in observer and demonstrator rats. Behav Brain Funct. 2009;5:23. doi: 10.1186/1744-9081-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faas AE, Sponton ED, Moya PR, Molina JC. Differential responsiveness to alcohol odor in human neonates: effects of maternal consumption during gestation. Alcohol. 2000;22:7–17. doi: 10.1016/s0741-8329(00)00103-8. [DOI] [PubMed] [Google Scholar]
- Farbman AI. Developmental Neurobiology of the Olfactory System. In: Getchell TV, Doty R, Bartoshuk L, Snow J, editors. Smell and Taste in Health and Disease. Raven Press; New York: 1991. pp. 19–33. [Google Scholar]
- Gervais R, Holley A, Keverne B. The importance of central noradrenergic influences on the olfactory bulb in the processing of learned olfactory cues. Chem Senses. 1988;13:1–12. [Google Scholar]
- Gervais R, Pager J. Olfactory bulb excitability selectively modified in behaving rats after local 6-hydroxydopamine treatment. Behav Brain Res. 1983;9:165–179. doi: 10.1016/0166-4328(83)90126-2. [DOI] [PubMed] [Google Scholar]
- Gesteland RC, Yancey RA, Farbman AI. Development of olfactory receptor neuron selectivity in the rat fetus. Neuroscience. 1982;7:3127–3136. doi: 10.1016/0306-4522(82)90235-4. [DOI] [PubMed] [Google Scholar]
- Gheusi G, Lledo PM. Control of early events in olfactory processing by adult neurogenesis. Chem Senses. 2007;32:397–409. doi: 10.1093/chemse/bjm012. [DOI] [PubMed] [Google Scholar]
- Graziadei PPC, Monti Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neuron. J Neurocytol. 1979;8:1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- Graziadei PPC, Monti Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. III. Deafferentation and reinnervation of the olfactory bulb following section of the fila olfactoria. J Neurocytol. 1980;9:145–162. doi: 10.1007/BF01205155. [DOI] [PubMed] [Google Scholar]
- Honey PL, Galef BG., Jr Ethanol consumption by rat dams during gestation, lactation and weaning increases ethanol consumption by their adolescent young. Dev Psychobiol. 2003;42:252–260. doi: 10.1002/dev.10098. [DOI] [PubMed] [Google Scholar]
- Hudson R. From molecule to mind. The role of experience in shaping olfactory function. J Comp Physiol A. 1999;185:297–304. doi: 10.1007/s003590050390. [DOI] [PubMed] [Google Scholar]
- Hudson R, Distel H. Induced peripheral sensitivity in the developing vertebrate olfactory system. Ann NY Acad Sci. 1998;855:109–115. doi: 10.1111/j.1749-6632.1998.tb10552.x. [DOI] [PubMed] [Google Scholar]
- Mair RG, Gellman RL, Gesteland RC. Postnatal proliferation and maturation of olfactory bulb neurons in the rat. Neuroscience. 1982;7:3105–3116. doi: 10.1016/0306-4522(82)90233-0. [DOI] [PubMed] [Google Scholar]
- McGivern RF, Clancy AN, Mousa S, Couri D, Noble EP. Prenatal alcohol exposure alters enkephalin levels without affecting ethanol preference. Life Sci. 1984;34:585–589. doi: 10.1016/0024-3205(84)90492-2. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Carrierfenster K, Mooney SM, Youngentob SL. Gestational ethanol exposure alters the behavioral response to ethanol odor and the expression of neurotransmission genes in the olfactory bulb of adolescent rats. Brain Res. 2009;1252:105–116. doi: 10.1016/j.brainres.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW. Effects of Alcohol and Opiates. Wiley-Liss; New York: 1992. Development of the Central Nervous System. [Google Scholar]
- Molina JC, Chotro MG, Dominguez HD. Fetal alcohol learning resulting from alcohol contamination of the prenatal environment. In: Lecanuet JP, Fifer WP, Krasnegor NA, Smotherman WP, editors. Fetal Development: A Psychobiological Perspective. Lawrence Erlbaum; Hillsdale, NJ: 1995. pp. 419–438. [Google Scholar]
- Molina JC, Spear NE, Spear LP, Mennella JA, Lewis MJ. The International Society for Developmental Psychobiology 39th Annual Meeting Symposium: Alcohol and development: Beyond fetal alcohol syndrome. Dev Psychobiol. 2007;49:227–242. doi: 10.1002/dev.20224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DS, Stainbrook GL. Effects of early alcohol exposure upon adult learning abilites and taste preferences. Physiol Psychol. 1976;4:473–475. [Google Scholar]
- Schaal B, Marlier L, Soussignan R. Human foetuses learn odours from their pregnant mother’s diet. Chem Senses. 2000;25:729–737. doi: 10.1093/chemse/25.6.729. [DOI] [PubMed] [Google Scholar]
- Schaal B, Orguer P. Olfaction in utero: can the rodent model be generalized? Q J Exp Psychol B. 1992;44:245–278. doi: 10.1080/02724999208250615. [DOI] [PubMed] [Google Scholar]
- Semke E, Distel H, Hudson R. Specific enhancement of olfactory receptor sensitivity associated with foetal learning of food odors in the rabbit. Naturwissenschaften. 1995;82:148–149. doi: 10.1007/BF01177279. [DOI] [PubMed] [Google Scholar]
- Smotherman WP. In utero chemosensory experience alters taste preferences and corticosterone responsiveness. Behav Neural Biol. 1982a;36:61–68. doi: 10.1016/s0163-1047(82)90245-x. [DOI] [PubMed] [Google Scholar]
- Smotherman WP. Odor aversion learning by the rat fetus. Physiol Behav. 1982b;29:769–771. doi: 10.1016/0031-9384(82)90322-5. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. The rat fetus in its environment: behavioral adjustments to novel, familiar, aversive, and conditioned stimuli presented in utero. Behav Neurosci. 1985;99:521–530. doi: 10.1037//0735-7044.99.3.521. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. Psychobiology of fetal experience in the rat. In: Krasnegor NA, Blass EM, Hofer MA, Smotherman WP, editors. Perianatal Development: A Psychobiological Perspective. Academic Press; Orlando, FL: 1987. pp. 39–60. [Google Scholar]
- Smotherman WP, Robinson SR. Behavior of rat fetuses following chemical or tactile stimulation. Behav Neurosci. 1988;102:24–34. doi: 10.1037//0735-7044.102.1.24. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. Rat fetuses respond to chemical stimuli in gas phase. Physiol Behav. 1990;47:863–868. doi: 10.1016/0031-9384(90)90010-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear NE, Molina JC. Consequences of early exposure to alcohol: how animal studies reveal later patterns of use and abuse in humans. In: Carroll M, Overrmier B, editors. Animal Research and Human Health. APA; Washington, DC: 2001. pp. 85–99. [Google Scholar]
- Spear NE, Molina JC. Fetal or infantile exposure to ethanol promotes ethanol ingestion in adolescence and adulthood: a theoretical review. Alcohol Clin Exp Res. 2005;29:909–929. doi: 10.1097/01.alc.0000171046.78556.66. [DOI] [PubMed] [Google Scholar]
- Stickrod G, Kimble DP, Smotherman WP. In utero taste/odor aversion conditioning in the rat. Physiol Behav. 1982;28:5–7. doi: 10.1016/0031-9384(82)90093-2. [DOI] [PubMed] [Google Scholar]
- Streissguth A. Teratogenic and genetic influences on adolescent and adult alcohol use and abuse. Symposium presented at the annual meeting of the Research Society on Alcoholism; Hilton Head, SC. Jun, 1998. [Google Scholar]
- Sullivan RM, Wilson DA, Leon M. Norepinephrine and learning-induced plasticity in infant rat olfactory system. J Neurosci. 1989;9:3998–4006. doi: 10.1523/JNEUROSCI.09-11-03998.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavrousek-Jakuba EM, Baker RA, Shoemaker WJ. Effect of ethanol on maternal and offspring characteristics: comparison of the formulations fed during gestation. Alcohol Clin Exp Res. 1991;15:129–135. doi: 10.1111/j.1530-0277.1991.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Wang HW, Wysocki CJ, Gold GH. Induction of olfactory receptor sensitivity in mice. Science. 1993;260:998–1000. doi: 10.1126/science.8493539. [DOI] [PubMed] [Google Scholar]
- Weiler E, Farbman AI. Proliferation in the rat olfactory epithelium: age-dependent changes. J Neurosci. 1997;17:3610–3622. doi: 10.1523/JNEUROSCI.17-10-03610.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA, Leon M. Spatial patterns of olfactory bulb single-unit responses to learned olfactory cues in young rats. J Neurophys. 1988a;59:1770–1782. doi: 10.1152/jn.1988.59.6.1770. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Leon M. Noradrenergic modulation of olfactory bulb excitability in the postnatal rat. Brain Res. 1988b;470:69–75. doi: 10.1016/0165-3806(88)90202-7. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Neurobiology of associative learning in the neonate: early olfactory learning. Behav Neural Biol. 1994;61:1–18. doi: 10.1016/s0163-1047(05)80039-1. [DOI] [PubMed] [Google Scholar]
- Yates WR, Cadoret RJ, Troughton EP, Stewart M, Giunta TS. Effect of fetal alcohol exposure on adult symptoms of nicotine, alcohol, and drug dependence. Alcohol Clin Exp Res. 1998;22:914–920. [PubMed] [Google Scholar]
- Youngentob SL. A method for the rapid automated assessment of olfactory function. Chem Senses. 2005;30:219–229. doi: 10.1093/chemse/bji017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Glendinning JI. Fetal ethanol exposure increases ethanol intake by making it smell and taste better. Proc Natl Acad Sci USA. 2009;106:5359–5364. doi: 10.1073/pnas.0809804106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Kent PF. Enhancement of odorant-induced mucosal activity patterns in rats trained on an odorant identification task. Brain Res. 1995;670:82–88. doi: 10.1016/0006-8993(94)01275-m. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Kent PF, Margolis FL. OMP deletion results in an alteration in odorant-induced mucosal activity patterns. J Neurophysiol. 2003;90:3864–3873. doi: 10.1152/jn.00806.2002. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Kent PF, Sheehe PR, Molina JC, Spear NE, Youngentob LM. Experience-induced fetal plasticity: the effect of gestational ethanol exposure on the behavioral and neurophysiologic olfactory response to ethanol odor in early postnatal and adult rats. Behav Neurosci. 2007a;121:1293–1305. doi: 10.1037/0735-7044.121.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Kent PF, Sheehe PR, Schwob JE, Tzoumaka E. Mucosal inherent activity patterns in the rat: evidence from voltage-sensitive dyes. J Neurophysiol. 1995;73:387–398. doi: 10.1152/jn.1995.73.1.387. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Molina JC, Spear NE, Youngentob LM. The effect of gestational ethanol exposure on voluntary intake in early postnatal and adult rats. Behav Neurosci. 2007b;121:1306–1315. doi: 10.1037/0735-7044.121.6.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]