Abstract
Rich evidence indicates that monoamine oxidase (MAO) A, the major enzyme catalysing the degradation of monoamine neurotransmitters, plays a key role in emotional regulation. Although MAOA deficiency is associated with reactive aggression in humans and mice, the involvement of this enzyme in defensive behaviour remains controversial and poorly understood. To address this issue, we tested MAOA knockout (KO) mice in a spectrum of paradigms and settings associated with variable degrees of threat. The presentation of novel inanimate objects induced a significant reduction in exploratory approaches and increase in defensive behaviours, such as tail-rattling, biting and digging. These neophobic responses were context-dependent and particularly marked in the home cage. In the elevated plus- and T-mazes, MAOA KO mice and wild-type (WT) littermates displayed equivalent locomotor activity and time in closed and open arms; however, MAOA KO mice featured significant reductions in risk assessment, as well as unconditioned avoidance and escape. No differences between genotypes were observed in the defensive withdrawal and emergence test. Conversely, MAOA KO mice exhibited a dramatic reduction of defensive and fear-related behaviours in the presence of predator-related cues, such as predator urine or an anaesthetized rat, in comparison with those observed in their WT littermates. The behavioural abnormalities in MAOA KO mice were not paralleled by overt alterations in sensory and microvibrissal functions. Collectively, these results suggest that MAOA deficiency leads to a general inability to appropriately assess contextual risk and attune defensive and emotional responses to environmental cues.
Keywords: Anxiety, defensive behaviour, exploration, monoamine oxidase A, predator urine
Introduction
Monoamine oxidase (MAO) A is the major enzyme catalysing the degradation of serotonin (5-hydroxytryptamine; 5-HT) and norepinephrine (NE) in the brain. Cogent evidence has shown that MAOA catalytic activity is a key determinant in emotional regulation, and is inversely related to the severity of aggressive and antisocial traits (Alia-Klein et al. 2008). A paradigmatic example of this link is Brunner syndrome, a genetic condition characterized by a nonsense point mutation in the MAOA gene, resulting in marked increases in urinary 5-HT levels, antisocial behaviour, reactive aggression and mild cognitive impairment (Brunner et al. 1993a).
The nosographic characterization of the endophenotypical abnormalities caused by MAOA deficiency is still highly elusive, in consideration of the low prevalence of Brunner syndrome (Hebebrand & Klug, 1995). A useful experimental tool to overcome this limitation, however, is afforded by MAOA knockout (KO) mice (Cases et al. 1995). Similar to MAOA-deficient individuals, these animals show elevated levels of 5-HT and NE in the brain, as well as heightened levels of reactive aggression in the resident-intruder task (Cases et al. 1995; Scott et al. 2008; Shih et al. 1999).
The reactive nature of the aggression associated with MAOA-deficiency strongly supports the existence of alterations in the appraisal of potential or actual danger (Blair, 2009). Accordingly, preliminary findings have shown that individuals with polymorphic variants associated with low MAOA activity display disturbances in the emotional processing of environmental and social cues (Brummett et al. 2008; Buckholtz & Meyer-Lindenberg, 2008; Caspi et al. 2002; Kumari et al. 2009; Lee & Ham, 2008; Williams et al. 2009).
Despite the well-established aggression towards conspecifics, the available evidence on the defensive and anxiety-like behaviours in MAOA KO mice remains incomplete and controversial. While few lines of research indicate that MAOA KO mice may have a heightened sensitivity to fear-inducing stimuli, such as a mild footshock (Kim et al. 1997), these mutants show reduced endocrine response to restraint stress (Popova et al. 2006) and lack of anxiety-related behaviours in several assays (Agatsuma et al. 2006; Popova et al. 2001; Scott et al. 2008; Vishnivetskaya et al. 2007).
This background prompted us to hypothesize that MAOA deficiency may result in a generalized alteration of threat assessment. We tested this possibility by analysing different domains of defensive and emotional reactivity of MAOA KO mice, including avoidance, neophobia, risk assessment and response to predator-associated cues and height. To this end, we used MAOAA863T KO mice, a mutant line featuring a spontaneous nonsense point mutation in the eighth exon of the Maoa gene (Scott et al. 2008). These mice were selected in view of the isomorphism between their mutation and that featured in Brunner syndrome (Scott et al. 2008), which makes them a valuable naturalistic model for this disorder. Furthermore, the background of these mice (129S6) is well-suited for studies of emotional reactivity in mutant mice (Marques et al. 2008; Paulus et al. 1999) and does not result in sensory deficits.
Methods
Animal husbandry
We used experimentally naive male 129S6/SvEvTac mice aged 3–4 months [n = 247; 118 wild type (WT) and 129 MAOAA863T KO], weighing 25–30 g. Animals were group-housed in cages with food and water available ad libitum. The room was maintained at 22 °C, on a 12-h light/dark cycle (lights on 06:00 hours). Light and sound were maintained at 10 lx and 70 dB for all behavioural tests unless otherwise indicated. Experimental procedures were in compliance with the National Institute of Health guidelines and approved by the Animal Use Committees of the University of Southern California and the University of Cagliari. For each experiment, the number of each group was defined based on statistical power calculations based on preliminary studies conducted within our experimental settings.
Novel object exploration
The experiment was performed as previously described (Bortolato et al. 2009). Mice (WT = 9, MAOA KO = 11) were tested within a grey Plexiglas cubic box (20 × 20 × 20 cm). Mice were acclimated to the chamber for 15 min. Twenty-four hours later, two novel, identical black plastic cylinders (8 cm heigh × 3.5 cm in diameter) were symmetrically placed at equal distance (4 cm) from the centre and affixed to the floor of the box. Mice were placed in a corner, facing the centre, and left undisturbed for 15 min. Their start position was rotated and counterbalanced for each genotype throughout the test.
The same experimental protocol was used in two alternative contextual situations with separate groups of animals:
in moderately familiar cages (after 2 consecutive days of acclimation, for 15 min/d), to study the impact of contextual adjustment on object exploration (WT = 8, MAOA KO = 8);
in their home cages (commercial Makrolon mouse caging units; size: 28 × 17 × 12 cm; 0.5 cm of bedding layer) (WT = 8, MAOA KO = 8). Twenty-four hours following acclimation to the new home cage, two novel objects were affixed to the cage floor, at equal distances (7 cm) from the centre.
For each trial, we analysed: locomotor activity (defined as the number of crossings on a grid super-imposed onto the image of each cage in a video monitor); tail-rattling; latency to first exploratory approach; total duration and number of exploratory approaches. Exploration was defined as sniffing or touching objects with the snout; climbing or sitting on the object was not considered exploration.
Elevated plus-maze and T-maze
The test was performed as previously described (Bortolato et al. 2009). Briefly, we used a black Plexiglas apparatus consisting of two open (25 × 5 cm) and two closed arms (25 × 5 × 5 cm), which extended from a central platform (5 × 5 cm) positioned 60 cm from the ground. Mice (WT = 8, MAOA KO = 8) were individually placed on the central platform facing an open arm. Behaviour was recorded for 5 min. Measures included: entries and duration in the open and closed arms and the central platform; frequency of stretch-attend postures and head dips (defined as previously described; Rodgers et al. 1992); number of faecal boli. In a second experiment, one of the closed arms was blocked with a black Plexiglas panel, in order to convert the apparatus into a T-maze. Using a different set of mice (WT = 15, MAOA KO = 15), we employed a simplified variation of the protocol previously described (Carvalho-Netto & Nunes-de-Souza, 2004). Mice were initially placed at the end of the accessible closed arm, facing the central platform, and their latency to exit this compartment was recorded (with a 3-min time limit). Animals were then positioned at the end of an open arm, facing the central platform, and their latency to escape into the closed arm was measured (with a 5-min time limit).
Defensive withdrawal
We used the protocol described by Bortolato et al. (2009). Mice (WT = 8, MAOA KO = 8) were individually placed inside a cylindrical aluminium chamber (7 cm diameter × 11 cm length) located along one of the four walls of a dimly lit (10 lx) black Plexiglas open field (40 × 40 × 40 cm), with the open end facing the centre. Mice were allowed to freely explore the environment for 15 min. Behaviours were recorded and monitored by an observer unaware of the genotype. Behavioural measures included: latency to exit the chamber; transitions between the chamber and open field; time spent in the chamber.
Emergence test
The emergence test was performed as previously described (Holmes et al. 2003; Liu et al. 2007) with minor variations. A black Plexiglas rectangular arena (40 × 10 × 20 cm) was divided by a guillotine door into two compartments. The first compartment (start chamber, 10 × 10 × 20 cm) was covered with a black ceiling, while the second compartment (open chamber, 30 × 10 × 20 cm) was left uncovered. Light and sound were maintained at 300 lx (central compartment and goal chamber) and 70 dB, respectively. Mice (WT = 8, MAOA KO = 9) were individually placed in the start chamber for 10 min acclimation. The door was raised at 6 cm from the floor and the animal was allowed to freely explore the open chamber for 5 min.
Using different groups of animals, the same test was performed to test whether the emergence behaviour of MAOA KO mice may be conditioned by the following elements: an object (tennis ball, 7 cm in diameter) at 5 cm from the door; the same object impregnated with 10 ml of diluted bobcat urine (v/v, 1: 1) (Lexington Outdoors Inc., USA); a male Long–Evans adult rat, previously anaesthetized with pentobarbital (50 mg/kg i.p.), with the snout at 7 cm from the guillotine door. For each test, we measured the latency to emerge from the start chamber, the number of transitions across the guillotine door, the percent time spent in each chamber and sniffing the object (or rat) and the number of faecal boli.
Defensive burying
The defensive burying test was based on objects impregnated with predator urine, as previously described (Campbell et al. 2003). Mice (WT = 27, MAOA KO = 31) were individually placed into new home cages filled with 2 cm of sawdust. Following a 24 h familiarization period, mice were exposed to a pair of wooden blocks (3 × 3 × 3 cm), previously impregnated with 1 ml of diluted bobcat urine (v/v, 1: 1) (Lexington Outdoors Inc.). Water-impregnated objects were used as controls. Blocks were placed in the centre of the cage at equal distances apart. The number and duration of digging bouts were measured for the 5-min periods before (baseline) and after placing the blocks.
Visual cliff test
The visual cliff test was used to ascertain the presence of overt visual deficits (Fox, 1965) in MAOA KO mice. The apparatus consisted of two pedestals (30 × 30 × 36 cm), featuring a black/white checkerboard pattern on their surface. The pedestals were placed 25 cm apart and connected by a 1-cm-thick, transparent Plexiglas (85 × 36 cm) platform. Mice (WT = 9, MAOA KO = 11) were placed in the middle of the ledge area at the edge of the apparent cliff, and their activity was recorded for 5 min by a video camera positioned above the apparatus. The latency of each mouse to cross the cliff ledge was measured.
Buried food test
Olfactory activity of animals was evaluated as described by (Yang & Crawley, 2009). Mini chocolate-cereal chips (weight ~1.0 g) were used as the food stimulus. For three consecutive nights, one chip was placed into each cage to establish odour familiarization. Chip consumption was verified every morning. Mice (WT = 12, MAOA KO = 11) were deprived of food for 24 h prior to testing. Mice were individually exposed to a standard clean cage with a 3-cm-thick layer of clean bedding for a 5-min acclimation period. The animal was briefly removed and a familiar food pellet was buried 1 cm beneath the surface. Food and animal placement were randomized. The latency to retrieve the chip was measured with a 15 min cut-off time.
Object recognition under total darkness
The object recognition test was performed under total darkness to verify the haptic function of microvibrissae in exploration (Brecht et al. 1997). Novel object exploration was studied in WT and MAOA KO mice as described above (with 1 d of cage acclimation), under either regular environmental light or total darkness (with an infrared camera to videotape behaviour). Ninety min later, animals were returned to the same cage for 15 min, under the same light conditions as in the first exploration trials. The cage contained one object identical to those used in the previous trial (familiar object) and a different novel object with equivalent odour but different size (rectangular block, 6 cm × 3 cm × 3 cm) and texture. Positions of familiar and novel objects were counter-balanced throughout the experiment. Each object was used only once throughout the experiment. A novelty exploration index (NEI) was calculated as the ratio of the duration of the exploratory approaches targeting the novel object over the time of exploration of both objects.
Statistical analyses
Normality and homoscedasticity of data distribution were verified using the Kolmogorov–Smirnov and Bartlett’s tests. Parametric analyses were performed with one-way or two-way ANOVA, as appropriate, followed by Tukey’s test with Spjøtvoll–Stoline correction for post-hoc comparisons. Non-parametric comparisons were performed by Mann–Whitney and Kruskal–Wallis tests as appropriate. Correlation analyses were performed by multiple regression. Comparisons of categorical data were performed by Fisher’s exact test. Significance threshold was set at p = 0.05.
Results
Novel object exploration
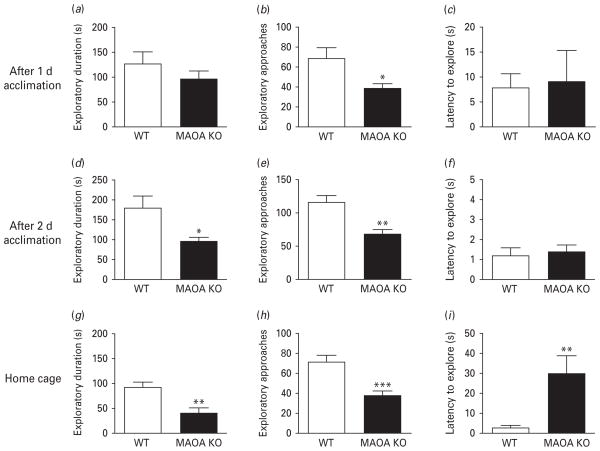
We first compared the exploratory and defensive responses of MAOA KO mice to novel objects in a standard cage, following 1 d acclimation. Under these conditions, no significant difference was found in either the exploratory duration (Fig. 1a) [F(1, 18) = 1.13, n.s.; ANOVA] or in the latency to the first approach (Fig. 1c) [H(1) = 0.24, n.s.; Kruskal–Wallis]. Conversely, the number of approaches was found to be significantly lower in MAOA KO mice than WT counterparts (Fig. 1b) [F(1, 18) = 7.23, p < 0.05]. Object presentation also elicited tail-rattling responses in some (33%) MAOA-deficient mice, but not in WT conspecifics; however, the occurrence of this phenomenon was not found significantly different between the two genotypes (Fisher’s exact test). Crossings were comparable between genotypes (data not shown) [F(1, 15) = 1.89, n.s.]. Notably, no significant correlation was found between locomotor activity and exploratory duration (data not shown).
Fig. 1.
MAOA KO mice display contextual-specific alterations in novel object exploration. (a–c) MAOA-deficient mice exhibit a decrease in exploratory approaches, but not duration or latency to explore a foreign object under standard conditions. (d–f) Environmental acclimation resulted in a reduction of exploratory duration and approaches, but not latency in MAOA KO mice. (g–i) Contextual familiarity produced a decrease in exploratory duration and approaches, and a significant increase in latency to explore the novel objects. All values are represented as means ± S.E.M. * p < 0.05, ** p < 0.01, *** p < 0.001 compared to WT mice.
The same test, conducted in a different group of mice after 2 d acclimation to the cage, elicited significant reductions in both duration (Fig. 1d) [H(1) = 4.41, p < 0.05] and number of exploratory approaches (Fig. 1e) [F(1, 14) = 11.09, p < 0.01] from MAOA KO mice compared to their WT counterparts. Latency to explore, however, was comparable between genotypes (Fig. 1f) [F(1, 12) = 0.15, n.s.]. Notably, tail-rattling was observed in a high percentage (62.5%) of MAOA KO mice, but not in WT animals (p < 0.05, Fisher’s exact test). Although the number of crossings was reduced in MAOA KO mice (data not shown) [F(1, 14) = 5.74, p < 0.05], linear regression analysis on the exploratory duration showed no significant difference between genotypes (data not shown).
In their home cages, MAOA-deficient mice exhibited significant reductions in both the duration (Fig. 2g) [F(1, 15) = 11.39, p < 0.01] and number (Fig. 2h) [F(1, 15) = 22.6, p < 0.001] of exploratory approaches in their home cages. MAOA KO mice also displayed significant increases in the latency to explore (Fig. 2i) [H(1) = 8.72, p < 0.01] and tail-rattling responses (62.5% of occurrence among MAOA KO mice; 0% in WT) (p < 0.05, Fisher’s exact test) compared to WT littermates. The number of crossings (data not shown) [F(1, 15) = 0.00, n.s.] was equivalent between genotypes. Moreover, the locomotion and exploratory activity were not significantly correlated (data not shown).
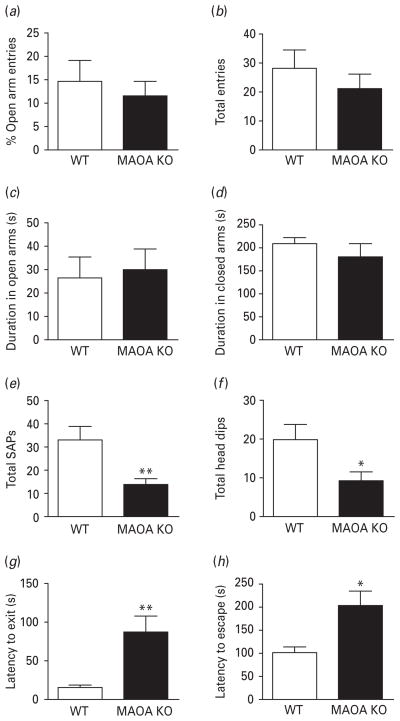
Fig. 2.
MAOA KO mice exhibit a reduction in risk-assessment and escape behaviours in the elevated plus-maze and elevated T-maze paradigms. (a–d) Both genotypes show equivalent measures for anxiety-related parameters in the elevated plus-maze task. (e, f) MAOA-deficient mice display a significant reduction in stretch-attend postures and head dips compared to WT littermates. (g, h) MAOA KO mice exhibit a significant increase in the latencies to exit a closed arm and to escape from an open arm in the elevated T-maze assay. All values are represented as means ± S.E.M. * p < 0.05, ** p < 0.01 compared to WT mice. SAPs, Stretch-attend postures.
Elevated plus-maze
In agreement with previous studies on other lines of MAOA-deficient mice (Popova et al. 2001), MAOA KO mice showed comparable percent open-arm entries (Fig. 2a) [F(1, 14) = 0.32, n.s.], percent closed-arm entries (data not shown) [F(1, 14) = 0.16, n.s.], and total arm entries (Fig. 2b) [F(1, 14) = 0.80, n.s.] to their WT counterparts. Moreover, both genotypes displayed an equivalent duration in the open arms (Fig. 2c) [F(1, 14) = 0.07, n.s.], closed arms (Fig. 2d) [F(1, 14) = 0.78, n.s.], and on the central platform (data not shown) [F(1, 14) = 0.80, n.s.], respectively. We also examined the risk assessment and exploration by measuring stretch-attend postures and head dips, respectively (Rodgers & Johnson, 1995). MAOA KO mice displayed fewer stretch-attend postures (Fig. 2e) [F(1, 14) = 8.88, p < 0.01] and a lower number of head dips (Fig. 2f) [F(1, 14) = 5.21, p < 0.05] than WT mice. Conversely, the number of faecal boli was comparable between genotypes (data not shown) [F(1, 14) = 0.05, n.s.].
Elevated T-maze
MAOA KO mice displayed a significantly longer latency to exit the closed arm (Fig. 2g) [U(15, 15) = 49, p < 0.01]. Similarly, MAOA-deficient mice exhibited a significant increase in latency to escape to the closed arm (Fig. 2h) [U(14, 13) = 46.5, p < 0.05]. These results indicate that MAOA KO mice display reductions in both exploratory activity and escape behaviours.
Defensive withdrawal
No significant differences were found between MAOA KO and WT mice in any behavioural parameter (data not shown).
Emergence test
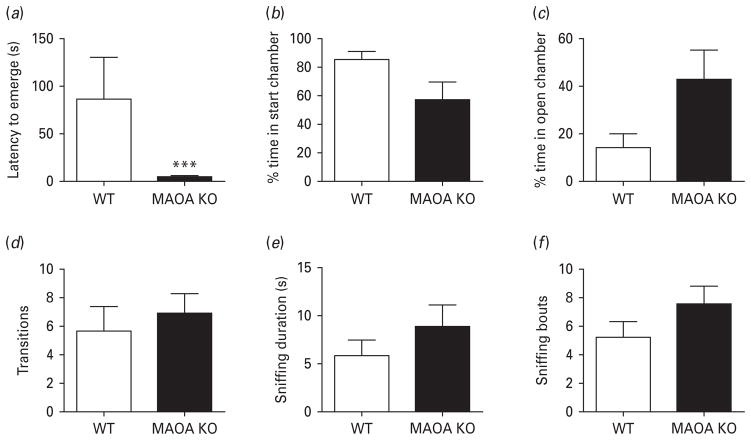
Both genotypes showed similar behavioural responses in the emergence test under standard conditions and in the presence of a foreign object in the open chamber (data not shown). In contrast, the introduction of an object impregnated with predator urine produced an increase in fear-related parameters in WT, but not in MAOA KO mice. Specifically, MAOA KO mice exhibited a significant reduction in the latency to exit the start chamber (Fig. 3a) [U(8, 8) = 0.00, p < 0.001]. No differences were detected between genotypes in the percent time in the start chamber (Fig. 3b) [U(7, 9) = 16, n.s.], percent time in open chamber (Fig. 3c) [U(7, 9) = 16; n.s.], transitions (Fig. 3d) [F(1, 15) = 0.32, n.s.], sniffing duration (Fig. 3e) [F(1, 15) = 0.56, n.s.], and sniffing bouts (Fig. 3f) [F(1, 15) = 1.94, n.s.].
Fig. 3.
MAOA KO mice display a reduction in fear-related behaviours in the emergence test with an object impregnated with predator urine. (a) MAOA KO mice exhibit a significant decrease in the latency to emerge from the start chamber compared to WT mice. (b–f) No significant differences were detected between genotypes in the percent time in the start chamber, percent time in the open chamber, number of transitions, object sniffing bouts and duration. All values are represented as means ± S.E.M. *** p < 0.001 compared to WT mice.
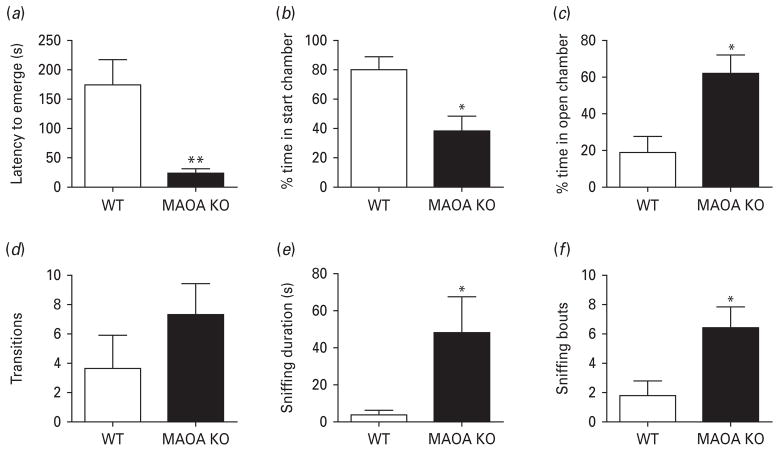
The presence of an anaesthetized rat in the open chamber also elicited a marked increase in fear-related responses in WT mice, but not in MAOA KO littermates. In particular, the latter displayed a significant decrease in latency to emerge from the start chamber (Fig. 4a) [U(5, 9) = 2.00, p < 0.01] compared to WT counterparts. This reduction in latency was accompanied by a significant decrease in percent time spent in the start chamber (Fig. 4b) [F(1, 14) = 7.53, p < 0.05] and a significant increase in the percent time spent in the open chamber (Fig. 4c) [F(1, 14) = 7.53, p < 0.05]. Moreover, MAOA KO mice exhibited a significant increase in sniffing duration (Fig. 4e) [F(1, 14) = 5.79, p < 0.05], and sniffing bouts (Fig. 4f) [F(5, 10) = 1.94, p < 0.05], but not the number of transitions (Fig. 4d) [F(1, 13) = 1.38, n.s.].
Fig. 4.
MAOA KO mice display a marked reduction in fear-related behaviours in the presence of an anaesthetized rat. (a, b) MAOA KO mice exhibit a significant decrease in the latency to emerge and the percent time in the start chamber compared to WT mice. (c, d) Similarly, MAOA KO mice show an increase in the percent time in the open chamber, but no significant alterations in locomotor activity. (e, f) Moreover, MAOA-deficient mice engaged in significantly more predator sniffing behaviour than their WT counterparts. All values are represented as means ± S.E.M. * p < 0.05, ** p < 0.01 compared to WT mice.
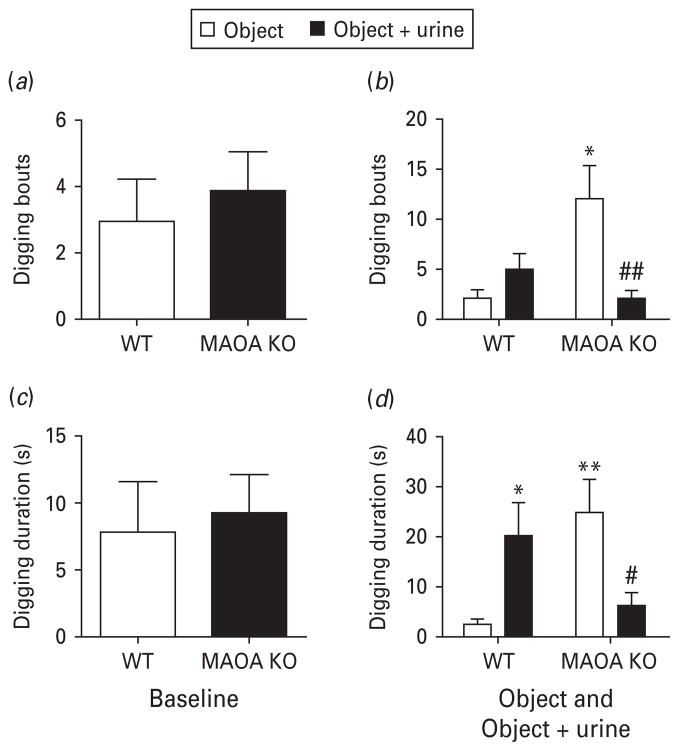
Defensive burying
We exposed mice to foreign objects, either odourless or impregnated with predator urine, in their home cage. Both genotypes showed comparable baseline digging frequency (Fig. 5a) [F(1, 49) = 0.28, n.s.] and duration (Fig. 5c) [F(1, 48) = 0.09, n.s.]. Although the presentation of odourless objects induced a significant increase in digging responses (in both digging bouts and duration) in MAOA KO mice compared to WT littermates, predator urine-impregnated objects elicited higher digging activity in WT than MAOA KO mice, in both frequency (Fig. 5b) [H(3) = 10.09; p < 0.05] and overall duration (Fig. 5d) [H(3) = 11.63, p < 0.01]. Post-hoc analysis revealed significant differences between genotypes in digging frequency in the absence (p < 0.05), but not in the presence of urine (p < 0.10). Moreover, urine-impregnated object presentation elicited a significant difference in digging bouts (p < 0.01) in MAOA-deficient compared to odourless objects. In line with these findings, significant differences in digging duration were detected between genotypes exposed to the odourless object (p < 0.01). Differences in digging duration between genotypes in the presence of predator urine were not significant (p < 0.06). The presence of urine-impregnated objects also induced a significant difference in WT (p < 0.05) and MAOA KO mice (p < 0.05) compared to odourless objects.
Fig. 5.
MAOA KO mice display maladaptive digging reactions to unfamiliar objects in the absence and presence of predator urine. (a, c) Both genotypes show equivalent baseline digging activity. (b, d) In contrast, the presentation of an odourless object induced a robust digging response in MAOA-deficient mice. Impregnation of the object with predator urine elicited a marked decrease in digging behaviour in MAOA KO mice compared to their WT counterparts. All values are represented as means ± S.E.M. * p < 0.05, ** p < 0.01, *** p < 0.001 compared to WT mice exposed to the odourless foreign object. # p < 0.05, ## p < 0.01 compared to MAOA KO mice exposed to the odourless novel object.
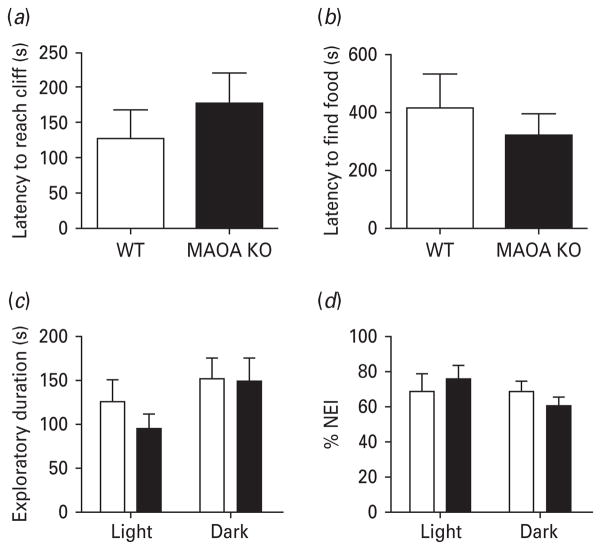
Assessment of visual, olfactory and microvibrissal functions
We then investigated whether some of the exploratory alterations in MAOA KO mice may be attributed to alterations in visual and olfactory sensitivity, or in the haptic function of the microvibrissae. In the visual cliff paradigm, both MAOA KO mice and WT littermates exhibited comparable visual acuity, as measured by their latency to reach the cliff [F(1, 18) = 0.69, n.s.] (Fig. 6a). Similarly, the equivalent latency to locate the hidden chocolate chips in the buried food test (Fig. 6b) [F(1, 21) = 0.46, n.s.], signified the lack of differences in olfactory sensitivity between MAOA KO and WT mice.
Fig. 6.
MAOA KO mice do not display alterations in sensory function. (a, b) MAOA KO mice exhibit comparable latencies to reach a cliff and locate buried food in the visual cliff and buried food tests, respectively. (c, d) In the novel object interaction and recognition task, MAOA KO mice (■) show comparable exploratory activity and percent novelty exploration indices compared to WT mice (□) in the absence of light, indicating intact microvibrissae function. All values are represented as means ± S.E.M. %NEI, Percent novelty exploration index.
In the object recognition test, we did not detect any difference between WT and MAOA KO mice in exploratory duration (Fig. 6c) [F(1, 32) = 0.56, n.s.] and NEI (Fig. 6d) [F(1, 28) = 0.00, n.s.], irrespective of the light conditions. These results suggest that the behavioural impairments in MAOA KO mice are not associated with an overt impairment of the microvibrissal function (Brecht et al. 1997) despite the alterations in barrel fields displayed by these animals (Cases et al. 1995).
Discussion
The results of the present study show that MAOA KO mice exhibit a broad spectrum of maladaptive defensive responses to contextual cues. Interestingly, abnormalities in defensive responsiveness featured by MAOA KO mice were multidirectional, in relation to the degree of potential danger associated with the environmental elements. On one hand, novel inanimate objects elicited high levels of neophobia in MAOA KO mice, manifested as defensive behaviours (including tail-rattling, biting and digging) and reduced exploratory activity, particularly if introduced in familiar environments. On the other hand, MAOA KO mice showed a paradoxical reduction of their unconditioned fear-related and escape responses across several settings associated with a higher level of potential danger, such as the open arms of an elevated T-maze or the presence of predator urine or a rat. In substantial agreement with previous findings (Agatsuma et al. 2006; Popova et al. 2001; Scott et al. 2008; Vishnivetskaya et al. 2007), the ethological conflict between protected and unprotected environments (in the elevated plus-maze, defensive withdrawal and emergence paradigms) did not induce marked behavioural variations in MAOA KO mice. These alterations were probably supported by a parallel decrement in both avoidance/fear and approach/exploration responses, as clearly documented by the elevated T-maze test (Torrejais et al. 2008; Viana et al. 1994). Interestingly, the behavioural deficits in MAOA KO mice were not accompanied by overt alterations in locomotor, visual, olfactory and microvibrissal functions, suggesting that their impairments may result from a general impairment in their emotional regulation.
Taken together, our findings suggest that MAOA deficiency leads to maladaptive emotional and defensive reactivity to environmental cues. In particular, MAOA KO mice exhibited a distinct inability to attune their responses to the situational content of their milieu, as indicated by the inappropriateness of their defensive behaviours (For a more comprehensive review on this concept, please see Blanchard & Blanchard, 2008). Specifically, the reduction in stretch-attend postures in the elevated plus-maze (Rodgers et al. 1992) and the manifestation of maladaptive responses, such as climbing a predator or rattling the tail in response to novel, innocuous objects, suggest a general impairment in risk assessment and goal-directed performance in MAOA KO mice. This conceptual framework may account for the dysregulated aggressive and stress-induced responses in MAOA KO mice (Cases et al. 1995; Kim et al. 1997; Popova et al. 2006).
Of note, the paradoxical responses of MAOA KO mice to neutral and fear-inducing stimuli are markedly reminiscent of the deficits in facial affect processing in schizophrenia and autism (Bolte & Poustka, 2003; Dawson et al. 2004; Gur et al. 2007; Hall et al. 2008; Phillips et al. 1999; Surguladze et al. 2006). In fact, schizophrenia patients have been shown to exhibit overactivation of fear systems in response to neutral stimuli (Hall et al. 2008; Seiferth et al. 2008) and decreased emotional response to fearful faces (Phillips et al. 1999; but see Morris et al. 2009 for contrasting evidence), as well as disturbances in contextual appraisal (Green et al. 2007). Interestingly, these deficits have been linked to negative symptoms (Van’t Wout et al. 2007), including flat affect, stereotypical and rigid behaviour. MAOA activity has actually been linked to a number of psychiatric disturbances characterized by perseverative behaviours and low flexibility, such as autism spectrum disorders and obsessive compulsive disorder (Camarena et al. 2001; Chugani, 2002; Cohen et al. in press, 2003; Davis et al. 2008; Yoo et al. 2009). In line with this possibility, the behavioural changes in MAOA KO mice may reflect their limited range of adaptive responses and behavioural flexibility compared to WT mice. Based on the level of danger associated with a given context and/or situation, the behaviour of MAOA KO mice may therefore appear more or less defensive and fearful, insofar as it is compared with the broader, multi-faceted behavioural repertoire of WT mice.
Alternatively, the neurochemical changes induced by MAOA deficiency may lead to specific alterations for each domain of defensive and emotional reactivity. In keeping with this possibility, both 5-HT and NE have been extensively shown to play different roles in the regulation of defensive behaviours (Bondi et al. 2007; Dringenberg et al. 2003; Graeff, 1993; Grahn et al. 2002).
Our experiments documented a general reduction of exploratory activity in MAOA KO mice across a broad spectrum of paradigms, which was not associated with a reduction in locomotor activity. This finding suggests that MAOA KO mice may display a lower level of inquisitiveness. This view is supported by previous findings, attesting poor novelty-seeking and reward-dependence traits in male carriers of the low-activity variant of MAOA polymorphism (Shiraishi et al. 2006). Interestingly, the gradual familiarization of MAOA KO mice to the external environment induced a progressive enhancement of the defensive responses targeting novel objects. This finding may indicate that the sharp contrast between the relative acquaintance with the context and the novelty of the objects may enhance the salience (and the anxiogenic valence) of the latter, thereby leading MAOA KO mice to channel their aversive responses on them.
While novel object exploration in an unfamiliar environment is widely regarded as a dependable model of state-anxiety (Belzung & Le Pape, 1994), the same task in a familiar context has been proposed to measure trait-anxiety (Avgustinovich et al. 2000). This premise may suggest that the anxiety-like behaviour of MAOA KO mice may be an innate, enduring characteristic, rather than a transient alteration of emotional responsiveness. Moreover, high trait-anxiety may also partially contribute to the poor environmental adaptation and habituation (Hare et al. 2008) previously observed in MAOA-deficient mice (Agatsuma et al. 2006).
Previous studies have shown that long-term treatment with MAOA inhibitors in adult rodents induces a decrease in defensive behaviour against predators (Griebel et al. 1998), but an enhancement in exploratory activity (Steckler et al. 2001). These findings indicate that the emotional alterations featured by MAOA KO mice are at least partially due to neurodevelopmental alterations. Indeed, several studies have shown that the sensorimotor cortex deficits in these animals are due to neurodevelopmental alterations based on the excessive 5-HT levels and 5-HT1B receptor hyperactivation in the first days of postnatal life (Cases et al. 1995; Salichon et al. 2001; Vitalis et al. 1998). Additionally, while most behavioural alterations of MAOA KO mice – such as the elevated aggressiveness and fear conditioning – cannot be reproduced by pharmacological inhibition of this enzyme in adulthood, early treatment with MAOA inhibitors has been shown to induce antisocial behaviour and emotional impairments in rodents (Mejia et al. 2002; Whitaker-Azmitia et al. 1994).
MAOA KO mice have been shown to display alterations of the barrel fields (Cases et al. 1995), the cortical representations of the mystacial vibrissae in the rodent snout (Erzurumlu & Jhaveri, 1990). These formations play a key role in the functional coordination of the mystacial vibrissae in the rodent snout (Luhmann et al. 2005), and their impairment has been shown to result in profound alterations of perceptual processing, exploratory activity, threat response and sensory integration of environmental stimuli (Cases et al. 1996; Dowman & Ben-Avraham, 2008; Hurwitz et al. 1990; Sanders et al. 2001; Straube et al. 2009). Nevertheless, we did not observe any significant change in the exploration and recognition of novel objects in the absence of light. This finding shows that, irrespective of the presence of visual cues or odour differences, MAOA KO and WT mice did not display differences in object recognition, thereby ruling out the role of microvibrissae in the exploratory deficits displayed by the mutant genotype. In line with this concept, we found that MAOAA863T KO mice did not display any overt impairment of olfactory and visual perception, the other two sensory modalities used in object exploration by rodents. In particular, our results on the visual cliff paradigm suggest that the abnormal development of retinal projections previously documented in C3H MAOA KO mice (Tg8) (Upton et al. 1999) do not exert profound effects on visual acuity and discrimination in our line.
Our data expand and complement previous clinical evidence showing a link between low MAOA activity and impairments in threat processing (Kumari et al. 2009; Lee & Ham, 2008; Williams et al. 2009), stress response (Jabbi et al. 2007) and decision-making (Ibanez et al. 2000; Meyer-Lindenberg et al. 2006; Perez de Castro et al. 2002). These alterations are likely to account for the aberrant aggressiveness observed in individuals with low MAOA activity (Alia-Klein et al. 2008; Brunner et al. 1993a, b; Caspi et al. 2002; Foley et al. 2004; Jacob et al. 2005; Nilsson et al. 2006). In spite of these similarities, several limitations advocate extreme caution in the interpretation of our findings with respect to their translational validity: first, psychiatric disorders cannot be fully recapitulated in murine models, and data from these findings may not fully translate to clinical settings; second, the lack of molecular bases to the observed alterations does not allow understanding of the mechanisms of MAOA in the regulation of defensive responses.
In conclusion, these findings show that MAOA KO mice exhibit a biphasic change in defensive reactivity towards environmental cues: while neutral objects induce high levels of neophobia and fear-like behaviours, risk-laden contexts evoked a reduction in risk assessment and escape behaviour. These paradigms bear marked similarity with the deficits in emotional processing observed in several psychiatric disorders, including schizophrenia and autism (Bölte & Poustka, 2003; Dawson et al. 2004; Gur et al. 2007; Hall et al. 2008; Phillips et al. 1999; Surguladze et al. 2006). Additionally, our study highlights the role of MAOA in the processing of adaptive, goal-directed responses to contextual cues. Further studies are warranted to validate the endophenotypic traits identified in MAOA KO mice throughout our experiments, and elucidate their neurobiological substrates.
Acknowledgments
This study was supported by R01MH39085 and R37MH39085 (MERIT Award) grants and the Boyd and Elsie Welin Professorship (to J. S.) as well as by the Zumberge Research Individual Grant (to M. B.). We are grateful to Lauren Burgeno, Kyle Bickel, Paradai Adisayathepkul and Simone Tambaro for their valuable support in the execution of the experiments.
Footnotes
Statement of Interest
None.
References
- Agatsuma S, Lee M, Zhu H, Chen K, et al. Monoamine oxidase A knockout mice exhibit impaired nicotine preference but normal responses to novel stimuli. Human Molecular Genetics. 2006;15:2721–2731. doi: 10.1093/hmg/ddl206. [DOI] [PubMed] [Google Scholar]
- Alia-Klein N, Goldstein RZ, Kriplani A, Logan J, et al. Brain monoamine oxidase A activity predicts trait aggression. Journal of Neuroscience. 2008;28:5099–5104. doi: 10.1523/JNEUROSCI.0925-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avgustinovich DF, Lipina TV, Bondar NP, Alekseyenko OV, et al. Features of the genetically defined anxiety in mice. Behavior Genetics. 2000;30:101–109. doi: 10.1023/a:1001999020138. [DOI] [PubMed] [Google Scholar]
- Belzung C, Le Pape G. Comparison of different behavioral test situations used in psychopharmacology for measurement of anxiety. Physiology & Behavior. 1994;56:623–628. doi: 10.1016/0031-9384(94)90311-5. [DOI] [PubMed] [Google Scholar]
- Blair RJR. The neurobiology of aggression. In: Charney DS, Nestler EJ, editors. Neurobiology of Mental Illness. Oxford: Oxford University Press; 2009. pp. 1307–1320. [Google Scholar]
- Blanchard D, Blanchard RJ. Defensive behaviors, fear, and anxiety. In: Blanchard R, Blanchard DC, Griebel G, Nutt DJ, editors. Handbook of Anxiety and Fear. Amsterdam: Academic Press; 2008. pp. 63–79. [Google Scholar]
- Bolte S, Poustka F. The recognition of facial affect in autistic and schizophrenic subjects and their first-degree relatives. Psychological Medicine. 2003;33:907–915. doi: 10.1017/s0033291703007438. [DOI] [PubMed] [Google Scholar]
- Bondi CO, Barrera G, Lapiz MD, Bedard T, et al. Noradrenergic facilitation of shock-probe defensive burying in lateral septum of rats, and modulation by chronic treatment with desipramine. Progress in Neuropsychopharmacology & Biological Psychiatry. 2007;31:482–495. doi: 10.1016/j.pnpbp.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Godar SC, Davarian S, Chen K, et al. Behavioral disinhibition and reduced anxiety-like behaviors in monoamine oxidase B-deficient mice. Neuropsychopharmacology. 2009;34:2746–2757. doi: 10.1038/npp.2009.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht M, Preilowski B, Merzenich MM. Functional architecture of the mystacial vibrissae. Behavioural Brain Research. 1997;84:81–97. doi: 10.1016/s0166-4328(97)83328-1. [DOI] [PubMed] [Google Scholar]
- Brummett BH, Boyle SH, Siegler IC, Kuhn CM, et al. HPA axis function in male caregivers: effect of the monoamine oxidase-A gene promoter (MAOA-uVNTR) Biological Psychology. 2008;79:250–255. doi: 10.1016/j.biopsycho.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner HG, Nelen M, Breakefield XO, Ropers HH, et al. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993a;262:578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- Brunner HG, Nelen MR, van Zandvoort P, Abeling NG, et al. X-linked borderline mental retardation with prominent behavioral disturbance: phenotype, genetic localization, and evidence for disturbed monoamine metabolism. American Journal of Human Genetics. 1993b;52:1032–1039. [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer-Lindenberg A. MAOA and the neurogenetic architecture of human aggression. Trends in Neuroscience. 2008;31:120–129. doi: 10.1016/j.tins.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Camarena B, Rinetti G, Cruz C, Gomez A, et al. Additional evidence that genetic variation of MAO A gene supports a gender subtype in obsessive-compulsive disorder. American Journal of Medical Genetics. 2001;105:279–282. doi: 10.1002/ajmg.1323. [DOI] [PubMed] [Google Scholar]
- Campbell T, Lin S, DeVries C, Lambert K. Coping strategies in male and female rats exposed to multiple stressors. Physiology & Behavior. 2003;78:495–504. doi: 10.1016/s0031-9384(03)00033-7. [DOI] [PubMed] [Google Scholar]
- Carvalho-Netto EF, Nunes-de-Souza RL. Use of the elevated T-maze to study anxiety in mice. Behavioural Brain Research. 2004;148:119–132. doi: 10.1016/s0166-4328(03)00184-0. [DOI] [PubMed] [Google Scholar]
- Cases O, Seif I, Grimsby J, Gaspar P, et al. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases O, Vitalis T, Seif I, De Maeyer E, et al. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: role of a serotonin excess during the critical period. Neuron. 1996;16:297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Chugani DC. Role of altered brain serotonin mechanisms in autism. Molecular Psychiatry. 2002;7 (Suppl 2):S16–17. doi: 10.1038/sj.mp.4001167. [DOI] [PubMed] [Google Scholar]
- Cohen IL, Liu X, Lewis ME, Chudley A, et al. Autism severity is associated with child and maternal MAOA genotypes. Clinical Genetics. doi: 10.1111/j.1399-0004.2010.01471.x. (in press) [DOI] [PubMed] [Google Scholar]
- Cohen IL, Liu X, Schutz C, White BN, et al. Association of autism severity with a monoamine oxidase A functional polymorphism. Clinical Genetics. 2003;64:190–197. doi: 10.1034/j.1399-0004.2003.00115.x. [DOI] [PubMed] [Google Scholar]
- Davis LK, Hazlett HC, Librant AL, Nopoulos P, et al. Cortical enlargement in autism is associated with a functional VNTR in the monoamine oxidase A gene. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2008;147B:1145–1151. doi: 10.1002/ajmg.b.30738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, Carver L, Panagiotides H, et al. Young children with autism show atypical brain responses to fearful vs. neutral facial expressions of emotion. Developmental Science. 2004;7:340–359. doi: 10.1111/j.1467-7687.2004.00352.x. [DOI] [PubMed] [Google Scholar]
- Dowman R, Ben-Avraham D. An artificial neural network model of orienting attention toward threatening somatosensory stimuli. Psychophysiology. 2008;45:229–239. doi: 10.1111/j.1469-8986.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Dennis KE, Tomaszek S, Martin J. Orienting and defensive behaviors elicited by superior colliculus stimulation in rats: effects of 5-HT depletion, uptake inhibition, and direct midbrain or frontal cortex application. Behavioural Brain Research. 2003;144:95–103. doi: 10.1016/s0166-4328(03)00065-2. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Jhaveri S. Thalamic axons confer a blueprint of the sensory periphery onto the developing rat somatosensory cortex. Brain Research. Developmental Brain Research. 1990;56:229–234. doi: 10.1016/0165-3806(90)90087-f. [DOI] [PubMed] [Google Scholar]
- Foley DL, Eaves LJ, Wormley B, Silberg JL, et al. Childhood adversity, monoamine oxidase a genotype, and risk for conduct disorder. Archives of General Psychiatry. 2004;61:738–744. doi: 10.1001/archpsyc.61.7.738. [DOI] [PubMed] [Google Scholar]
- Fox MW. The visual cliff test for the study of visual depth perception in the mouse. Animal Behaviour. 1965;13:232–233. doi: 10.1016/0003-3472(65)90040-0. [DOI] [PubMed] [Google Scholar]
- Graeff FG. Role of 5-HT in defensive behavior and anxiety. Reviews in the Neurosciences. 1993;4:181–211. doi: 10.1515/revneuro.1993.4.2.181. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Hammack SE, Will MJ, O’Connor KA, et al. Blockade of alpha1 adrenoreceptors in the dorsal raphe nucleus prevents enhanced conditioned fear and impaired escape performance following uncontrollable stressor exposure in rats. Behavioural Brain Research. 2002;134:387–392. doi: 10.1016/s0166-4328(02)00061-x. [DOI] [PubMed] [Google Scholar]
- Green MJ, Waldron JH, Coltheart M. Emotional context processing is impaired in schizophrenia. Cognitive Neuropsychiatry. 2007;12:259–280. doi: 10.1080/13546800601051847. [DOI] [PubMed] [Google Scholar]
- Griebel G, Curet O, Perrault G, Sanger DJ. Behavioral effects of phenelzine in an experimental model for screening anxiolytic and anti-panic drugs: correlation with changes in monoamine-oxidase activity and monoamine levels. Neuropharmacology. 1998;37:927–935. doi: 10.1016/s0028-3908(98)00077-x. [DOI] [PubMed] [Google Scholar]
- Gur RE, Loughead J, Kohler CG, Elliott MA, et al. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Archives of General Psychiatry. 2007;64:1356–1366. doi: 10.1001/archpsyc.64.12.1356. [DOI] [PubMed] [Google Scholar]
- Hall J, Whalley HC, McKirdy JW, Romaniuk L, et al. Overactivation of fear systems to neutral faces in schizophrenia. Biological Psychiatry. 2008;64:70–73. doi: 10.1016/j.biopsych.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, et al. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebebrand J, Klug B. Specification of the phenotype required for men with monoamine oxidase type A deficiency. Human Genetics. 1995;96:372–376. doi: 10.1007/BF00210430. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Lesch KP, Crawley JN, et al. Mice lacking the serotonin transporter exhibit 5-HT(1A) receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology. 2003;28:2077–2088. doi: 10.1038/sj.npp.1300266. [DOI] [PubMed] [Google Scholar]
- Hurwitz BE, Dietrich WD, McCabe PM, Watson BD, et al. Sensory-motor deficit and recovery from thrombotic infarction of the vibrissal barrel-field cortex. Brain Research. 1990;512:210–220. doi: 10.1016/0006-8993(90)90628-o. [DOI] [PubMed] [Google Scholar]
- Ibanez A, Perez de Castro I, Fernandez-Piqueras J, Blanco C, et al. Pathological gambling and DNA polymorphic markers at MAO-A and MAO-B genes. Molecular Psychiatry. 2000;5:105–109. doi: 10.1038/sj.mp.4000654. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Korf J, Kema IP, Hartman C, et al. Convergent genetic modulation of the endocrine stress response involves polymorphic variations of 5-HTT, COMT and MAOA. Molecular Psychiatry. 2007;12:483–490. doi: 10.1038/sj.mp.4001975. [DOI] [PubMed] [Google Scholar]
- Jacob CP, Muller J, Schmidt M, Hohenberger K, et al. Cluster B personality disorders are associated with allelic variation of monoamine oxidase A activity. Neuropsychopharmacology. 2005;30:1711–1718. doi: 10.1038/sj.npp.1300737. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Shih JC, Chen K, Chen L, et al. Selective enhancement of emotional, but not motor, learning in monoamine oxidase A-deficient mice. Proceedings of the National Academy of Sciences USA. 1997;94:5929–5933. doi: 10.1073/pnas.94.11.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Barkataki I, Goswami S, Flora S, et al. Dysfunctional, but not functional, impulsivity is associated with a history of seriously violent behaviour and reduced orbitofrontal and hippocampal volumes in schizophrenia. Psychiatry Research. 2009;173:39–44. doi: 10.1016/j.pscychresns.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Lee BT, Ham BJ. Monoamine oxidase A-uVNTR genotype affects limbic brain activity in response to affective facial stimuli. Neuroreport. 2008;19:515–519. doi: 10.1097/WNR.0b013e3282f94294. [DOI] [PubMed] [Google Scholar]
- Liu GX, Cai GQ, Cai YQ, Sheng ZJ, et al. Reduced anxiety and depression-like behaviors in mice lacking GABA transporter subtype 1. Neuropsychopharmacology. 2007;32:1531–1539. doi: 10.1038/sj.npp.1301281. [DOI] [PubMed] [Google Scholar]
- Luhmann HJ, Huston JP, Hasenohrl RU. Contralateral increase in thigmotactic scanning following unilateral barrel-cortex lesion in mice. Behavioural Brain Research. 2005;157:39–43. doi: 10.1016/j.bbr.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Marques JM, Olsson IA, Ogren SO, Dahlborn K. Evaluation of exploration and risk assessment in pre-weaning mice using the novel cage test. Physiology & Behavior. 2008;93:139–147. doi: 10.1016/j.physbeh.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Mejia JM, Ervin FR, Baker GB, Palmour RM. Monoamine oxidase inhibition during brain development induces pathological aggressive behavior in mice. Biological Psychiatry. 2002;52:811–821. doi: 10.1016/s0006-3223(02)01418-x. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, Hariri A, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proceedings of the National Academy of Sciences USA. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RW, Weickert CS, Loughland CM. Emotional face processing in schizophrenia. Current Opinion in Psychiatry. 2009;22:140–146. doi: 10.1097/YCO.0b013e328324f895. [DOI] [PubMed] [Google Scholar]
- Nilsson KW, Sjoberg RL, Damberg M, Leppert J, et al. Role of monoamine oxidase A genotype and psychosocial factors in male adolescent criminal activity. Biological Psychiatry. 2006;59:121–127. doi: 10.1016/j.biopsych.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Dulawa SC, Ralph RJ, Mark AG. Behavioral organization is independent of locomotor activity in 129 and C57 mouse strains. Brain Research. 1999;835:27–36. doi: 10.1016/s0006-8993(99)01137-3. [DOI] [PubMed] [Google Scholar]
- Perez de Castro I, Ibanez A, Saiz-Ruiz J, Fernandez-Piqueras J. Concurrent positive association between pathological gambling and functional DNA polymorphisms at the MAO A and the 5-HT transporter genes. Molecular Psychiatry. 2002;7:927–928. doi: 10.1038/sj.mp.4001148. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Williams L, Senior C, Bullmore ET, et al. A differential neural response to threatening and non-threatening negative facial expressions in paranoid and non-paranoid schizophrenics. Psychiatry Research. 1999;92:11–31. doi: 10.1016/s0925-4927(99)00031-1. [DOI] [PubMed] [Google Scholar]
- Popova NK, Maslova LN, Morosova EA, Bulygina VV, et al. MAO A knockout attenuates adrenocortical response to various kinds of stress. Psychoneuroendocrinology. 2006;31:179–186. doi: 10.1016/j.psyneuen.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Popova NK, Skrinskaya YA, Amstislavskaya TG, Vishnivetskaya GB, et al. Behavioral characteristics of mice with genetic knockout of monoamine oxidase type A. Neuroscience and Behavioral Physiology. 2001;31:597–602. doi: 10.1023/a:1012364910091. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Cole JC, Cobain MR, Daly P, et al. Anxiogenic-like effects of fluprazine and eltoprazine in the mouse elevated plus-maze: profile comparisons with 8-OH-DPAT, CGS 12066B, TFMPP and mCPP. Behavioural Pharmacology. 1992;3:621–634. [PubMed] [Google Scholar]
- Rodgers RJ, Johnson NJ. Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacology, Biochemistry, and Behavior. 1995;52:297–303. doi: 10.1016/0091-3057(95)00138-m. [DOI] [PubMed] [Google Scholar]
- Salichon N, Gaspar P, Upton AL, Picaud S, et al. Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase a and 5-ht transporter knock-out mice. Journal of Neuroscience. 2001;21:884–896. doi: 10.1523/JNEUROSCI.21-03-00884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MJ, Dietrich WD, Green EJ. Behavioral, electrophysiological, and histopathological consequences of mild fluid-percussion injury in the rat. Brain Research. 2001;904:141–144. doi: 10.1016/s0006-8993(01)02424-6. [DOI] [PubMed] [Google Scholar]
- Scott AL, Bortolato M, Chen K, Shih JC. Novel monoamine oxidase A knock out mice with human-like spontaneous mutation. Neuroreport. 2008;19:739–743. doi: 10.1097/WNR.0b013e3282fd6e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiferth NY, Pauly K, Habel U, Kellermann T, et al. Increased neural response related to neutral faces in individuals at risk for psychosis. Neuroimage. 2008;40:289–297. doi: 10.1016/j.neuroimage.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Shih JC, Ridd MJ, Chen K, Meehan WP, et al. Ketanserin and tetrabenazine abolish aggression in mice lacking monoamine oxidase A. Brain Research. 1999;835:104–112. doi: 10.1016/s0006-8993(99)01478-x. [DOI] [PubMed] [Google Scholar]
- Shiraishi H, Suzuki A, Fukasawa T, Aoshima T, et al. Monoamine oxidase A gene promoter polymorphism affects novelty seeking and reward dependence in healthy study participants. Psychiatric Genetics. 2006;16:55–58. doi: 10.1097/01.ypg.0000199447.62044.ef. [DOI] [PubMed] [Google Scholar]
- Steckler T, Rammes G, Sauvage M, van Gaalen MM, et al. Effects of the monoamine oxidase A inhibitor moclobemide on hippocampal plasticity in GR-impaired transgenic mice. Journal of Psychiatric Research. 2001;35:29–42. doi: 10.1016/s0022-3956(00)00040-6. [DOI] [PubMed] [Google Scholar]
- Straube T, Schmidt S, Weiss T, Mentzel HJ, et al. Dynamic activation of the anterior cingulate cortex during anticipatory anxiety. Neuroimage. 2009;44:975–981. doi: 10.1016/j.neuroimage.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Surguladze S, Russell T, Kucharska-Pietura K, Travis MJ, et al. A reversal of the normal pattern of parahippocampal response to neutral and fearful faces is associated with reality distortion in schizophrenia. Biological Psychiatry. 2006;60:423–431. doi: 10.1016/j.biopsych.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Torrejais JC, Rosa CC, Boerngen-Lacerda R, Andreatini R. The elevated T-maze as a measure of two types of defensive reactions: a factor analysis. Brain Research Bulletin. 2008;76:376–379. doi: 10.1016/j.brainresbull.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Upton AL, Salichon N, Lebrand C, Ravary A, et al. Excess of serotonin (5-HT) alters the segregation of ispilateral and contralateral retinal projections in monoamine oxidase A knock-out mice: possible role of 5-HT uptake in retinal ganglion cells during development. Journal of Neuroscience. 1999;19:7007–7024. doi: 10.1523/JNEUROSCI.19-16-07007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Wout M, van Dijke A, Aleman A, Kessels RP, et al. Fearful faces in schizophrenia: the relationship between patient characteristics and facial affect recognition. Journal of Nervous and Mental Disease. 2007;195:758–764. doi: 10.1097/NMD.0b013e318142cc31. [DOI] [PubMed] [Google Scholar]
- Viana MB, Tomaz C, Graeff FG. The elevated T-maze: a new animal model of anxiety and memory. Pharmacology, Biochemistry, and Behavior. 1994;49:549–554. doi: 10.1016/0091-3057(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Vishnivetskaya GB, Skrinskaya JA, Seif I, Popova NK. Effect of MAO A deficiency on different kinds of aggression and social investigation in mice. Aggressive Behavior. 2007;33:1–6. doi: 10.1002/ab.20161. [DOI] [PubMed] [Google Scholar]
- Vitalis T, Cases O, Callebert J, Launay JM, et al. Effects of monoamine oxidase A inhibition on barrel formation in the mouse somatosensory cortex: determination of a sensitive developmental period. Journal of Comparative Neurology. 1998;393:169–184. doi: 10.1002/(sici)1096-9861(19980406)393:2<169::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Zhang X, Clarke C. Effects of gestational exposure to monoamine oxidase inhibitors in rats: preliminary behavioral and neurochemical studies. Neuropsychopharmacology. 1994;11:125–132. doi: 10.1038/npp.1994.42. [DOI] [PubMed] [Google Scholar]
- Williams LM, Gatt JM, Kuan SA, Dobson-Stone C, et al. A polymorphism of the MAOA gene is associated with emotional brain markers and personality traits on an antisocial index. Neuropsychopharmacology. 2009;34:1797–1809. doi: 10.1038/npp.2009.1. [DOI] [PubMed] [Google Scholar]
- Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. Current Protocols in Neuroscience. 2009;Chapter 8(Unit 8):24. doi: 10.1002/0471142301.ns0824s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo HJ, Lee SK, Park M, Cho IH, et al. Family- and population-based association studies of monoamine oxidase A and autism spectrum disorders in Korean. Neuroscience Research. 2009;63:172–176. doi: 10.1016/j.neures.2008.11.007. [DOI] [PubMed] [Google Scholar]