Abstract
Immunogenetic analysis of the B cell receptors (BCRs) has been a richly rewarding field for unraveling the pathogenesis of human lymphomas, including CLL. A biased immunoglobulin gene repertoire is seen as evidence for selection of CLL progenitor cells by antigen. Additional corroborative evidence is provided by the differential prognosis of cases with distinct mutational status of the clonotypic BCRs. However, perhaps the strongest immunogenetic evidence for the importance of interactions with microenvironment in driving CLL development and evolution is the existence of subsets of patients with quasi-identical, stereotyped BCRs, collectively accounting for a remarkable one-third of the entire cohort. These observations have been instrumental in shaping the notion that CLL ontogeny is functionally driven and dynamic, rather than a simple stochastic process. From a clinical perspective, ample evidence indicates that immunogenetic information can be used for the biologically and clinically rational categorization of CLL, with important potential implications for basic, translational and clinical research.
Introduction
Chronic lymphocytic leukemia (CLL) is a disease of aged populations and the most common adult leukemia in the Western world. It is a chronic, incurable disease and very heterogeneous in terms of response to treatment: in fact, some patients reach complete and prolonged remissions, while others relapse early and need several lines of treatment.1 This clinical heterogeneity is linked to and likely reflects the underlying molecular and cellular heterogeneity of the disease.2
Several lines of research have demonstrated beyond doubt that CLL can be subdivided into subgroups with distinct biological features, extending from genomic aberrations3 to immune signaling4 via receptor molecules of both innate (e.g. Toll-like receptors) and adaptive nature (i.e. the B cell receptor, BCR), with the latter so far thought to play perhaps a more pivotal role.2
The importance of antigenic stimulation through the BCR in CLL development and evolution is evidenced by: (i) restrictions in the immunoglobulin heavy variable (IGHV) gene repertoire expressed by the clonotypic BCRs; (ii) different prognosis of patients with different IGHV gene mutational status; and, (iii) the existence of subsets of patients sharing BCRs with restricted, quasi-identical immunoglobulin sequences (stereotyped BCRs).
With the widespread application of assays for the determination of IGHV gene mutational status in a clinical context, a large amount of information became available for both biological investigations and prognostication. Thanks to these and other developments, CLL is now considered as the prototype for cancers where micro environmental interactions are critical in the onset, expansion and progression of the disease.2
Origins of Immunoglobulin Diversity
B cells are key players in adaptive immune responses and crucial in the recognition and elimination of both exogenous as well as autologous threats to homeostasis.5 This fundamental function of B cells is dependent on a two-step process, where the creation of diverse antigen receptors represents the first step and selection by antigen the second. Indeed, the immune system relies on the formation of an extremely diverse population of B cells, each expressing on its surface numerous identical immunoglobulin (IG) molecules, which serve as the antigen receptors for adaptive immune responses (B-cell receptor, BCR).
Each IG molecule is composed of four polypeptide chains; two identical heavy chains (HC) and two identical light chains (LC).6 Each IG chain can be subdivided into a variable (V) and a constant (C) region. The V region is the part of the molecule that recognizes antigen, while the C region has effector functions. Each V region comprises of four areas of relatively limited diversity, known as the framework regions (FRs), interspersed with three hypervariable regions, known as complementarity determining regions (CDRs), which confer the IG molecule its unique specificity.
BCR diversity rests on the combined effect of molecular events taking place during B cell maturation. First, the random assembly of one each of multiple distinct variable (V), diversity [(D) – for IG heavy chains only] and joining (J) genes, referred to as combinatorial diversity, leads to a huge variety of combinations and corresponding molecular structures.7,8 A significant boost in diversity emerges during V(D)J recombination, since (i) nucleotides may be trimmed from the ends of the recombining genes; and, (ii) random nucleotides may be added at the V-D, D-J or V-J junctions located within CDR3, the most diverse part of the V region (“junctional diversity”). Just considering the combinatorial events of the heavy and light chain gene loci, there are greater than 1.6 x 106 possible combinations for IG BCR.
In later phases of B cell ontogeny, diversity is exponentially increased as a result of further modifications by the somatic hypermutation (SHM) and class-switch recombination (CSR) processes, both driven by antigen encounter and orchestrated by an enzymatic activity called activation-induced cytidine deaminase (AID).9 SHM is characterized by the introduction of mutations within rearranged genes, which increases antibody diversity and produces antibodies with higher specificity.10 CSR replaces the constant (IGHC) gene to be expressed from IGHM to IGHG or IGHE or IGHA, switching antibody production from IgM to a different class, such as IgG, IgE or IgA, without changing antigen specificity.11 SHM and CSR have been estimated to increase the potential for variation 103–106 fold. Hence, altogether, the B-cell repertoire comprises of, in principle, 1012 different specificities. In other words, the probability that two independent B-cell clones carry exactly the same IG BCR by chance alone is virtually negligible (10–12).
The unique IG BCR expressed by each B cell clone can be viewed as its molecular ‘identity’. Therefore, it is no surprise that the study of immunoglobulin (IG) genes has been instrumental in understanding immune physiology and pathology. In fact, immunogenetics has provided an upgraded perception into both the ontogenetic derivation of B cell malignancies and the possible involvement of interactions with antigens in their onset and evolution.12,13 In this respect, CLL is a model disease for showing how IG gene analysis can assist in understanding the processes underlying lymphomagenesis.2
Immunoglobulin Genes in CLL: Early Days
The immunogenetic history of CLL can be traced back to the 1990s when pioneer studies reported restrictions in immunoglobulin heavy variable (IGHV) gene usage14,15 and distinctive antigen-binding sites among unrelated cases;16,17 however, the series were small, thus hindering definitive conclusions. A major step forward occurred in 1998, when Chiorazzi’s group published the first comprehensive study confirming that the IGHV gene repertoire of CLL is restricted and also different from that of normal IgM+ B cells, with certain genes, such as IGHV1-69, IGHV4-34, and IGHV3-7, clearly over-represented in CLL.18 Pronounced restrictions were also recognized regarding the usage of IGHD and IGHJ genes. Indeed, only five IGHD genes were used by almost half of CLL cases, with the IGHD3-3 gene being the most frequent. Also, the repertoire of IGHJ genes was characterized by predominance of the IGHJ4 and IGHJ6 genes. Focusing on rearrangements of the predominant IGHV genes, specific associations were identified with certain IGHD and IGHJ genes, best depicted by rearrangements utilizing the IGHV1-69 gene, which were strongly biased toward the usage of the IGHD3-3 and the IGHJ6 genes. On the contrary, weaker or no biases were noted for cases expressing other IGHV genes, particularly IGHV4-34, IGHV3-7 and IGHV3-23. Furthermore, the imprint of SHM was not uniform among IGHV genes: for example, the IGHV1-69 gene carried very few or no mutations as opposed to the IGHV3-7, IGHV3-23 and IGHV4-34 genes, which were significantly mutated.18 These biases in the IG gene repertoire were justifiably taken as evidence for the implication of antigens in CLL development, thus seriously challenging the then prevailing concept of CLL as a neoplasm of antigen-naïve B cells.
With hindsight, the turning point in the immunogenetic research of CLL came in 1999 when the Hamblin and Stevenson and Chiorazzi groups independently demonstrated that the mutational status of the rearranged IGHV genes directly correlated with patient survival.19,20 As a general principle, patients carrying mutated IGHV genes generally follow a more indolent course than those with unmutated IGHV genes, who tend to show evidence of advanced, progressive disease, adverse cytogenetic profiles, clonal evolution, and resistance to therapy.
Immunoglobulin Genes in CLL: Coming of Age, Alias Prognostication and Beyond
The load of somatic mutations across the sequence of the rearranged IGHV genes was the first highly accurate molecular marker for disease prognostication and remains one of the strongest independent prognostic markers in CLL. Importantly, it is independent of the actual tumor burden and also does not change during the clinical course. With the realization of its potential clinical utility came a flurry of sequencing activity focused on the IG genes: it was reassuring that all studies corroborated the general rule: “unmutated-bad prognosis, mutated-good prognosis”.
Yet, this general rule could not be applied in all cases, as first shown by Rosenquist’s group who reported that usage of the IGHV3-21 gene in CLL IG BCRs may represent an adverse prognostic factor, regardless of the actual SHM load.21 Another open issue concerns the usage of a cut-off value for assigning a case to the mutated or unmutated category. In particular, both pioneering studies on the prognostic impact of IGHV gene mutational status have suggested a cut-off value of 98% identity with the closest germline IGHV gene as the most reliable discriminator between unmutated (≥98%) and mutated cases (<98%).19,20 This choice was necessitated by practical considerations since in 1999 the complete sequence of the human genome was not available, therefore, in principle, the observed differences from the germline could represent polymorphisms rather than somatic mutations. The cut-off value was a short cut to exclude potential polymorphic variant sequences, thus bypassing the need to sequence the corresponding germline gene in each patient.22
The 98% identity cut-off value has been confirmed by all subsequent studies and is still considered the best, though approximate, discriminator for clinical prognostication. That notwithstanding, one has to keep in mind that this cut-off is statistically rather than biologically relevant:23 in other words, it has very questionable utility for purposes other than clinical prognostication, especially in view of the fact that even a low level of mutations can be functionally relevant.24 Furthermore, even when used for prognostication, caution is clearly recommended for cases of “borderline” SHM status, which have been reported to comprise of a mixture of benign and malignant cases rather than a homogeneous group with moderate malignancy.25
A final parameter to be taken into account is the differential impact of SHM in rearrangements utilizing different IGHV genes, first noted by Chiorazzi’s group18 and subsequently confirmed in a large study by our group where we showed that the IGHV gene repertoires of subgroups of cases with <98% (“mutated”), 98-98.9% (“borderline mutated”), 99-99.9% (“minimally mutated”) or 100% identity to the germline (“truly unmutated”) differed significantly.26 On these grounds, we suggested that the presence of little or no mutations in CLL IG BCRs should not be viewed as a marker of naivety rather it could reflect selective pressures for maintaining the germline configuration.
The accumulation of IG BCR sequence data brought unprecedented surprises. For instance, following traditional immunological thinking, no-one could have anticipated that approximately half of CLL cases utilizing the IGHV3-21 gene carry distinctive IGs with highly restricted VH CDR3s and biased pairing with lambda light chains utilizing the IGLV3-21 gene.27 Considering how remote the chances are that this might happen by chance alone, this finding was rightly considered as evidence for common antigenic drive, perhaps of pathogenic significance.
Stereotyped BCRs: CLL Development is not Stochastic
A peculiar finding in early immunogenetic studies of the IG repertoire in CLL was that unrelated CLL cases could carry highly similar VH CDR3s characterized by shared amino acid motifs.16,17 This was confirmed by the landmark study by Chiorazzi’s group, who reported the existence of subsets of BCR IGs with highly restricted VH CDR3s and proposed that a common antigen could be selecting out clones eventually leading to the observed restrictions.28
With hindsight, this aspect of CLL immunobiology remained of somewhat marginal interest until the IGHV3-21 story broke the news. However, rather than a noteworthy exception, it turned out that sequence restriction was rather common as it was reported independently by different groups in both Europe and the US.29,30 Interestingly, even in these initial, rather small-scale studies (compared to what would follow –see below), certain themes emerged: homologous BCRs were present among both mutated and unmutated cases; different types of homologous BCRs existed, enabling the grouping of different cases into subsets based on common sequence features of the BCR IG, in particular the VH CDR3. These highly similar BCRs were referred to as “stereotyped”,28 clearly fulfilling the definition of stereotype as “something conforming to a fixed or general pattern”.
Right from the start, stereotyped BCR IGs were seen as the strongest immunogenetic evidence that selection rather than serendipity drives CLL development. Several aspects and implications of stereotypy, however, remained to be elucidated.
BCR Stereotypy: How to Identify it and How Frequent is it?
In all studies of BCR stereotypy, the focus is on the IG heavy chains, in particular the VH CDR3, on the grounds that the more similar the primary VH CDR3 sequences of two IGs, the more similar their folding and, perhaps, their specificities.31
The first set of criteria for the definition of subsets with stereotyped IGHV/IGHD/IGHJ rearrangements was proposed in 2004 by Chiorazzi’s group:28
usage of the same IGHV/IGHD/IGHJ germline genes,
usage of the same IGHD gene reading frame, and
VH CDR3 amino acid identity ≥60%, in line with established bioinformatics concepts for evaluating sequence conservation in protein sequences (e.g. amino acid substitution matrices such as BLOSUM62).32
In 2007, we modified the clustering algorithm allowing sequences to be clustered together even when their IGHV genes differed, provided the criterion of VH CDR3 sequence conservation was met.33 The validity of this approach is exemplified by a cluster now defined as subset #1, defined by usage of the IGHD6-19 gene in reading frame 3 and the IGHJ4 gene in association with different IGHV genes (namely IGHV1-2, IGHV1-3, IGHV1-18, IGHV1-8, IGHV5-a, IGHV5-51, IGHV7-4-1). Interestingly, all these IGHV genes are members of the same IGHV phylogenetic clan,34,35 i.e. they are phylogenetically linked and carry related germline sequences, thus they can produce overall homologous VH domains when recombining with identical IGHD and IGHJ genes. Subset #1 is not a conglomeration of sequences simply happening to look similar: independent studies have demonstrated beyond doubt that the cases assigned to subset #1 have similarities extending from primary IG gene sequences to genomic, functional and clinical features.26,33,36-38
The main findings of studies based on this clustering approach, which are still valid today, can be summarized as follows:
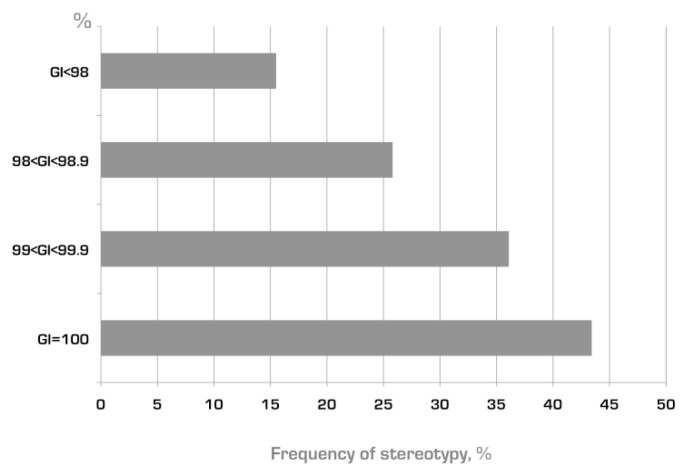
BCR IG stereotypes exist among both mutated and unmutated CLL, though significantly more frequently in the latter (Figure 1).
different versions of BCR IG stereotypes can be defined based on shared VH CDR3 amino acid sequence patterns which are distinct for each subset; accordingly, there are many different subsets with distinct stereotyped BCRs.
the relative size of each subset can differ markedly, from just a pair to large numbers of cases with homologous BCRs; indeed, certain major subsets are remarkably populated - e.g. subset #1 mentioned above and subset #2 (utilizing the IGHV3-21 gene) each account for ~2.5% of the entire cohort!26,36
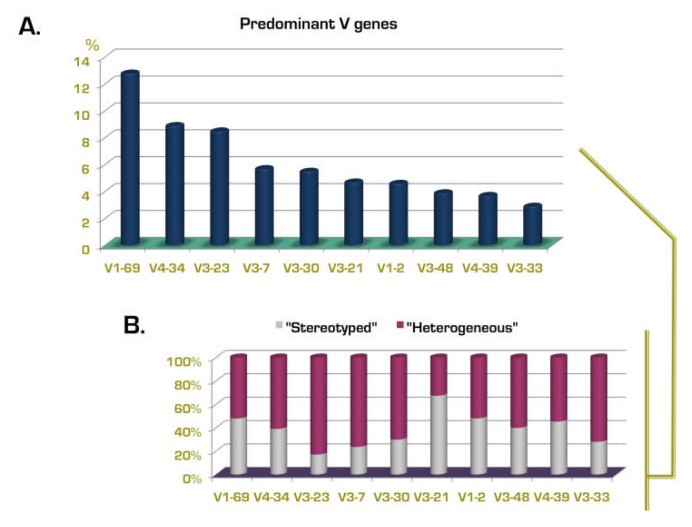
different genes show a markedly different “propensity” to be used in stereotyped rearrangements in CLL (Figure 2) – in other words, the frequency of stereotyped rearrangements exceeded 30% in cases using certain IGHV genes (e.g. IGHV3-21, IGHV1-69, IGHV1-2, IGHV1-3, IGHV4-39, IGHV3-48), whereas it was very low (<5%) for other IGHV genes (e.g. IGHV3-7, IGHV3-74).33
subsets with stereotyped VH CDR3 are often characterized by restricted IG light chain gene usage and CDR3 features.33,39,40
selected subsets may be associated with distinctive clinical/phenotypic features or outcome (see following section), raising the possibility that a particular antigen binding site can be critical in determining clinical presentation and possibly also prognosis.33,36
the frequency of BCR IG stereotypy in CLL can exceed 25% or one-fourth of the entire cohort.
Figure 1. Frequency of BCR IG stereotypy among subgroups of rearrangements of different mutational status.
Almost half of truly unmutated rearrangements (100% identity to the germline, GI) are assigned to subsets with stereotyped VH CDR3 regions, while the frequency of stereotypy showed a trend toward decrease when the mutational load of the rearrangements was increasing. Based on data from Agathangelidis et al. 2012.47
Figure 2. IGHV gene repertoire in CLL and relation to BCR IG stereotypy.
Relative frequency (%) of the 10 predominant IGHV genes in CLL (A), and their distribution among the “stereotyped” and “heterogeneous” subsets (B). The majority of IGHV3-21 cases carry stereotyped B cell receptors, whereas most rearrangements of the IGHV3-23 gene exhibit heterogeneity within their VH CDR3s. Based on data from Agathangelidis et al. 2012.47
All these observations spurred great enthusiasm and, at the same time, also raised many important questions. It was reasonably felt that the observed sequence restrictions in CLL could reflect a corresponding restriction also in terms of the selecting antigens. Not paradoxically, the quest for antigens41-45 went along with the quest for subsets. However, the progressively increasing amount of IG sequence information rendered the previously used methods for identifying BCR IG stereotypy problematic as they lacked efficiency, robustness, and, importantly, sensitivity.
In order to overcome these limitations, we developed purpose-built bioinformatics methods based on sequence pattern discovery, enabling the reliable identification of VH CDR3 sequence similarities regardless of the IGHV/IGHD/IGHJ genes used. We first applied this approach in a study of 2662 patients with CLL where we reported that CLL actually consists of two different categories, based on the BCR repertoire, with important biological and ontogenetic differences.46 The first includes cases with heterogeneous BCRs (non-clustered cases), while the second (almost 30% of cases) is characterized by a remarkably high frequency of BCR stereotypy (clustered cases).
A pivotal aspect of this new approach was the ability to recognize more distant relationships between sequences, which can form the basis for the discovery of subsets at successive hierarchically higher levels, characterized by more broadly shared sequence patterns and, hence, greater size. Intriguingly, high-level clusters were found to be still characterized by striking IGHV repertoire restrictions, with only six IGHV genes (IGHV1-69, IGHV1-3, IGHV1-2, IGHV3-21, IGHV4-34, IGHV4-39) accounting for >80% of cases.46 A corollary of this finding is that the IG gene repertoire restrictions reported as typical of CLL are in essence a property of the category of clustered cases with stereotyped IG BCRs, whereas non-clustered cases exhibit a much less skewed repertoire.
The analysis of the IG repertoire in CLL, including BCR stereotypy, recently culminated in a study of 7596 IG VH (IGHV-IGHD-IGHJ) sequences from 7424 CLL patients, three times the size of the largest published series.47 In that study, we used an updated version of our purpose-built clustering algorithm, now requiring that only sequences carrying IGHV genes of the same clan can be assigned to the same subset, and also adopted more stringent criteria, including the requirement for identical VH CDR3 lengths and identical sequence pattern offsets (i.e. exact locations within the VH CDR3 region) between connected sequences.
This analysis provided strong evidence to our previous claim46 that CLL indeed comprises two distinct categories, one with stereotyped and the other with heterogeneous IG, in an approximate ratio of 1:2. Furthermore, it offered answers to several questions regarding BCR stereotypy:
the existence of subsets of cases with stereotyped BCRs expressing different yet phylogenetically related IGHV genes is a consistent feature of the CLL IG repertoire: in addition to the well-established subset #1 (see above), several other examples came to the fore, including subsets #12 (IGHV1-2 and IGHV1-46), #59 (IGHV1-58 and IGHV1-69) and #77 (IGHV4-4 and IGHV4-59). Therefore, it is possible that related genes might be favored for selection by particular antigens perhaps because specificity is endowed by only a few critical IGHV-encoded residues in association with a distinctive VH CDR3
not all CLL will end up belonging to stereotyped subsets, even if the cohort size increases significantly, as also supported by random simulations
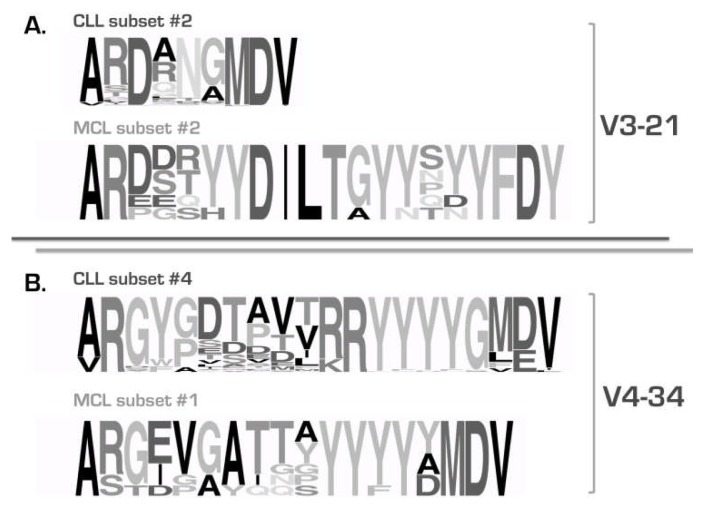
the BCR IG stereotypes in CLL are fundamentally different from those recently reported in other B cell malignancies (Figure 3), e.g. splenic marginal-zone lymphoma48,49 and mantle cell lymphoma,50 alluding to distinct, disease-biased selective and ontogenetic processes
the sequence patterns defining subsets can be broadly divided into two types: (i) “mainly combinatorial”, i.e. largely encoded by the germline sequences of the D-REGION and 5’J-REGION of specific combinations of IGHD-IGHJ genes; and, (ii) “combinatorial+junctional”, i.e. encoded in part by the N-diversity regions (N1 and N2) leading to restricted motifs at the IGHV-IGHD and/or IGHD-IGHJ gene junctions
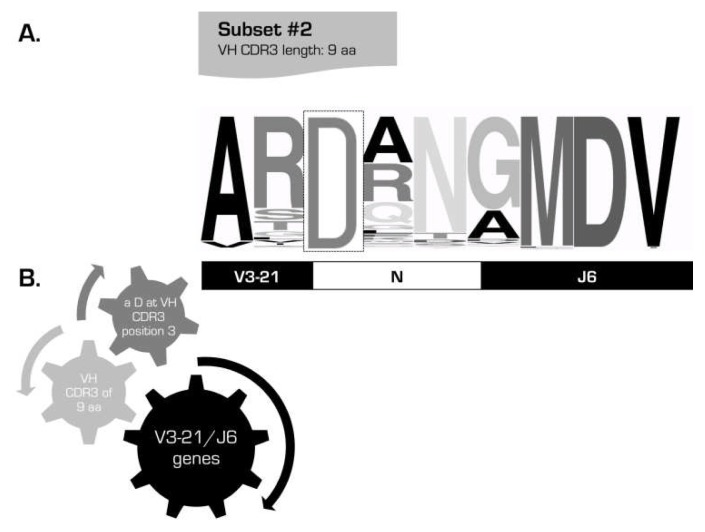
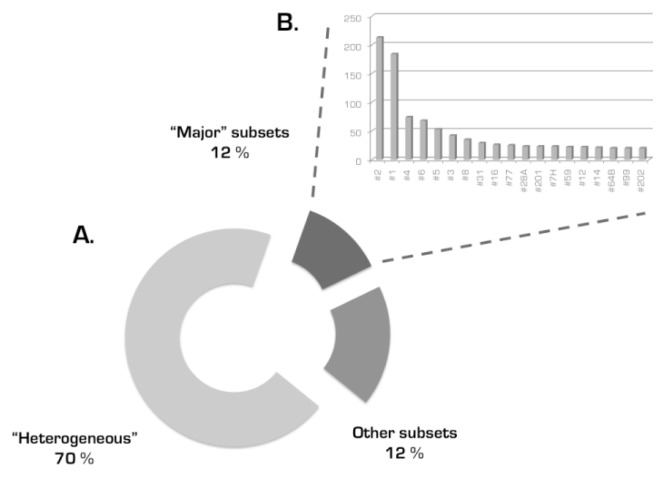
the widely shared sequence patterns characteristic of major subsets could cover the entire VH CDR3 or be comprised of a few, even a single, strategically positioned residue; a fascinating example of the latter is subset #2 with 213 IGHV3-21 expressing CLL cases (2.8% of the cohort) characterized by a very short VH CDR3 sequence (9 amino acids) with a “landmark” amino acid (aspartic acid, D) at position 107, between the amino acids qualified as IGHV- or IGHJ-encoded (Figure 4)
the major stereotyped subsets collectively account for a substantial proportion of the IG CLL repertoire (Figure 5). In more detail, 19 different subsets with 20 or more (up to 213) sequences (defined as major) collectively included 943 cases and, thus, accounted for 41% of the stereotyped cases and 12% of the cohort, respectively. In other words, one-in-eight CLL patients were found to belong to a major subset!
Figure 3. CLL stereotypes are “disease-specific”.
The comparative analysis of VH CDR3 sequences in CLL showed that they were clearly distinct, in terms of amino acid composition and VH CDR3 length, from those identified in MCL carrying the same IGHV genes: IGHV3-21 (A) and IGHV4-34 (B). The height of symbols within the stack indicates the relative frequency of each amino or nucleic acid at that position. Modified from Agathangelidis et al. 2012.47
Figure 4. Subset #2: a unique length and a single VG CDR3 residue define a subset.
Sequence logo of the VH CDR3 region of cases belonging to subset #2, one of the largest subsets in CLL. The height of symbols within the stack indicates the relative frequency of each amino or nucleic acid at that position (A). Set of criteria required for the assignment of rearrangements to this particular subset (B). Rearrangements assigned to this subset can be simply identified by the usage of the IGHV3-21 gene, a VH CDR3 of 9 amino acids (aa), and an acidic residue (D) at the third position of the VH CDR3.
Figure 5. A significant fraction of the entire CLL cohort is represented by a limited number of VH CDR3 stereotypes.
Altogether, these “major” subsets accounted for 12% of cases in the recent study by Agathangelidis et al (A). The magnitude of these subsets ranged from 20 to 213 sequences (B).47
IG Genes as a Prognostic Marker in CLL: Beyond IGHV Gene Mutational Status
The first hints that not only the mutational status of IGHV genes but also other features of the IG BCRs can be prognostically relevant in CLL came in 2002 with the realization that usage of the IGHV3-21 gene was associated with adverse prognosis independently of the load of SHM –in fact, most IGHV3-21 rearrangements were mutated.21 It did not take long before additional reports were published about genes associated with a distinctive prognosis regardless the actual SHM status -e.g. IGHV3-23 (adverse),51 IGHV3-30 and IGHV3-72 (both favorable).52,53 However, except for the IGHV3-21 gene, where the supportive evidence is strong,54,55 these results were obtained in retrospective and mostly small series, hence caution is warranted.
Given how much attraction stereotyped BCRs have held for scientists working on CLL, it was reasonable to explore whether BCR stereotypy might be reflected in stereotyped clinical presentation and outcome. Chiorazzi’s group were the first to report associations between BCR stereotypy and clinical features for a subset of cases expressing stereotyped IGHV4-39/IGHD6-13/IGHJ5 BCRs (now known as subset #8) who experienced aggressive clinical courses complicated by severe recurrent infections, Richter’s transformation, or the occurrence of second solid tumors.56 Of note, a recent collaborative study from Italy independently reported that this particular BCR stereotype is associated with the highest risk for Richter’s transformation among all CLL subgroups analyzed.57
A turning point in searching for clinical implications of BCR stereotypy was the realization that among IGHV3-21 CLL those cases carrying stereotyped BCRs typical of subset #2 uniformly expressed CD38 and had progressive disease, whereas cases with heterogeneous IGHV3-21 BCRs exhibited variable CD38 expression and experienced variable clinical courses.58 On these grounds, it was suggested for the first time that prognostic information might be gleaned by defining not only the usage of specific genes (e.g. IGHV3-21) and their mutational load, but also the molecular features of the BCR IG among cases utilizing similar IGHV genes. Since the original publication, several studies have independently shown that expression of stereotyped subset #2 BCRs is associated with shorter time to progression and presence of other poor-prognostic markers.33,36,55
Another clinically relevant example is offered by CLL subset #4 which is defined by the expression of stereotyped IGHV4-34/IGKV2-30 BCRs of the G isotype 59 showing distinctive patterns of SHM.26,60,61 Cases assigned to subset #4 are significantly younger at diagnosis and have been reported to follow a very indolent disease course compared to either the entire cohort or the subgroup of cases expressing non-subset #4 IGHV4-34 BCRs.33
Altogether the available evidence suggests that the biological behavior of CLL malignant B cells, which underlies clonal history and evolution, may be guided by the functional antigen reactivity profile of the BCR. Therefore, the grouping of CLL cases based on shared features of the primary IG gene sequences can be functionally and prognostically relevant.
Inferring the Role of Antigen in CLL: Hints from Immunogenetic Analysis and Links to BCR Igs With Distinctive Molecular Features
Over the past decade, defining which antigens are recognized by CLL BCRs has been a matter of intense investigation going along with the increasing interest in BCR molecular features as a prognostic indicator for CLL. Earlier studies, from more than two decades ago, had pointed out the autoantigenic and polyreactive nature of CLL-derived monoclonal antibodies (mAbs) (Table 1).62-64 Major developments came with the advent of new technologies for obtaining the CLL mAbs. Thanks to the new technological possibilities, it was demonstrated that self- and poly- reactivity is inversely correlated with the mutational load of the BCR IG 41 and that CLL mAbs could exhibit common patterns of antigen binding (Table 1).43,45,65
Table 1.
Summary of reactivities reported for CLL.
| Study | Antigens involved in CLL | BCR IG | Reference |
|---|---|---|---|
| Bröker BM et al. 1988 | IgG-Fc, ssDNA, dsDNA, histones, cardiolipin, cytoskeletal components | unknown | [62] |
| Sthoeger ZM et al. 1989 | ssDNA, dsDNA, rabbit gamma globulin (RGG) | unknown | [63] |
| Borche L et al. 1990 | actin, tubulin and myosin, ssDNA, rabbit gamma globulin (RGG) | IGHV1 and IGHV4 genes Mutation status unknown | [64] |
| Hervé M et al. 2005 | LPS, cytoplasmic structures, DNA, insulin | various | [41] |
| Lanemo MA et al. 2008 | Human tissue samples (e.g. tonsil, stomach chief cells, vascular endothelial cells) FM55M2 melanoma cells, rat aortic smooth muscle cells (SMCs), Jurkat T cells, HepG2 cells, apoptotic Jurkat cells, oxidized low density lipoprotein (oxLDL), cardiolipin, vimentin, filamin B cofilin-1, PRAP-1, S. pneumoniae capsular polysaccharides and phosphorylcholine | various | [42] |
| Catera R et al. 2008 | Healthy HEp-2 cells, apoptotic RAMOS B cells, apoptotic Jurkat T cells Metabolites of lipid peroxidation conjugated to BSA (MDA-BSA, POVPC-BSA, HNE-BSA), Phosphorylcholine (PC)-BSA, oxLDL, tubulin, Sm, Ku, snRNP A, BB’, and C, CENP-B | various | [43] |
| Chu CC et al. 2008 | Non muscle myosin heavy chain IIA (MYHIIA) | IGHV1-69 unmutated (subset #6) | [45] |
| Chu CC et al. 2010 | MYHIIA-exposed apoptotic cells (MEACs) | IGHV1-2, IGHV1-3 UM and IGHV1-18 M (subset #1)/IGHV1-69 UM (subset #6)/IGHV4-39 UM (subset #8)/IGHV1-69 and IGHV3-21 UM (subset#9)/IGHV1-2 UM (subset#28)/IGHV4-b UM/IGHV1-3 UM | [44] |
| Binder M et al. 2010 | Vimentin, calreticulin (on viable stroma cells) | IGHV1-2 and IGHV1-3 UM (subset #1) | [70] |
| Hatzi K et al. 2006 | Streptococcus pyogenes, Enterococcus faecium, Enterococcus faecalis, Enterobacter cloacae | various | [65] |
| Landgren O et al. 2007 | Streptococcus pneumonia, Haemophilus influenza (population based study) | unknown | [73] |
| Kostareli E et al. 2009 | CMV, EBV (Real Time PCR approach) | IGHV4-34 M (subset #4) | [74] |
| Steininger C et al. 2009 | CMV seropositivity (population based study ) | unknown | [75] |
| Kostareli et al. 2012 | IgG-Fc (with possible link to HCV) | IGHV4-59 M (subset #13) | [77] |
| Steininger C et al. 2012 | pUL32 protein of CMV | IGHV1-69 UM, IGHV3-21 M and UM | [76] |
An emerging theme from these antigen reactivity studies is that CLL mAbs with distinctive BCR IG molecular features (i.e. IGHV gene usage and mutational status, BCR IG stereotypy) react with molecules present on apoptotic cells, including cytoskeletal proteins, as well as bacterial antigens.42,43,45,66 This is similar to the reactivity profile of natural antibodies, which are produced in the absence of external antigenic stimulation and play a crucial role in immediate host defenses against a wide range of pathogens.67-69
An intriguing finding of these studies was that stereotyped BCR IGs from different subsets showed distinct patterns of antigen reactivity and, more importantly, that CLL mAbs from cases assigned to the same subset exhibited similar (stereotyped) binding profiles.42-45,66 For instance, reactivity against lipid oxidation products created during apoptosis were mainly linked to the unmutated CLL and strong binders of such antigens concerned CLL mABs from stereotyped subsets #1, #6, #8, #9 and #32; on the other hand, vimentin was shown to be recognized by different stereotyped CLL mAbs [IGHV3-30, subset #32;42 IGHV4-39, subset #8 );45 IGHV1-2 or IGHV1-3, subset #1]70 (Table 1).
More recently, Chu et al. showed that non-muscle myosin heavy chain IIA (MYHIIA) expressed on a subpopulation of apoptotic cells (called myosin-exposed apoptotic cells or MEACs) was the antigenic target of CLL mAbs from subset #6 (IGHV1-69/IGHD3-16/IGHJ3 stereotyped BCRs).44 In a subsequent study from the same group, it was shown that MEAC reactivity was not confined to subset #6 mAbs rather it was exhibited by several other CLL mAbs, though variably. MEAC reactivity was far more frequent among unmutated rather than mutated CLL. Of note, mAbs from the same stereotyped subsets showed concordant patterns of MEAC reactivity.44,71
Self is not the only target of CLL BCRs. Several antigens recognized by CLL mAbs have been functionally associated with certain microbial infections. For instance, molecular mimicry driven by Streptococcus pneumoniae capsular polysaccharides and oxidized LDL (oxLDL) has been reported as a critical link between autoreactivity and alloreactivity of CLL mAbs.72 Furthermore, certain CLL Abs have been shown to react against various Gram-positive and Gram-negative bacterial strains (Streptococcus pyogenes, Enterococcus faecium, Enterococcus faecalis, Enterobacter cloacae); notably, UM-CLL mAbs exhibited the highest level of reactivity, with the unmutated IGHV1-69 mAbs being the dominant binders.65 Perhaps relevant to these observations, a recent epidemiologic study suggested that recurrent respiratory tract infections caused by Streptococcus pneumoniae and Haemophilus influenzae are associated with an increased risk of CLL (Table 1).73
Viral infections have also been suggested to drive subgroups of CLL cases. For instance, persistent infections by EBV and CMV have been correlated with the stereotyped IGHV4-34 subset #4,74 while higher herpesvirus-specific CMV seropositivity has been reported in selected CLL cohorts compared with the general population.75 Along these lines, IGHV1-69 and IGHV3-21 CLL mAbs have been found to react with the CMV pUL32 protein (Table 1).76 Furthermore, the hepatitis C Virus (HCV) has been recently associated with CLL, albeit indirectly. In particular, we showed that the stereotyped IGHV4-59/IGKV3-20 CLL mAbs of subset #13 can exhibit rheumatoid factor (RF) activity and are linked to HCV infection. Interestingly, we found similar sequences to those of CLL in subset #13 in other entities (including splenic marginal-zone lymphoma, myoepithelial sialadenitis in primary Sjogren’s syndrome, mixed cryoglobulinemia type II and a rheumatoid factor), some with positive HCV serology, others not. The existence of RF with restricted IG gene sequences in various conditions including CLL may allude to cross-reactivity or molecular mimicry of the antigenic elements selecting the clonogenic progenitors yet resulting in distinct pathological conditions.77
BCR- and non-BCR-Mediated Modes of Interaction of CLL Cells with the Microenvironment: a Dynamic and Extensive Cross-Talk
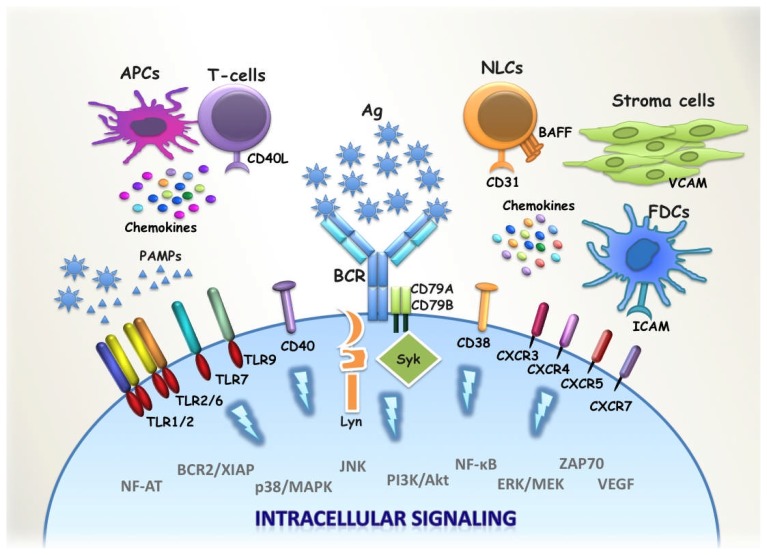
BCR is of undisputable significance in CLL development and evolution. BCR is not only engaged in antigen recognition but, importantly, it is the start point of intracellular cascades involving downstream kinases, such as Syk, Btk and PI3Kδ (Figure 6).78 Interestingly, the kinases involved in BCR signalling have been identified as attractive targets for molecular therapeutic approaches.79,80
Figure 6. BCR and non-BCR modalities of interactions of CLL cells with their microenvironment.
The malignant clone is dependent on prosurvival signals conveyed by cell-cell contacts and interactions with soluble factors secreted by T-cells, stromal cells, Nurse-like cells (NLCs), Follicular dendritic cells (FDCs) or other Antigen-Presenting Cells (APCs). The cross-talk between the malignant clone and its millieu is initiated by specific ligand biding to receptor molecules such as the BCR, TLRs, CD38, CD40 and CXCRs. Among the most prominent ligand-receptor interactions are the specific antigenic stimulus of the BCR as well as the triggering of TLRs after recognition of MAMPs. Ligand-Receptor engagement activates a number of different intracellular cascades, which control cell cycle, apoptosis, proliferation and migration.
CLL cells preferentially reside in spleen, lymph nodes and bone marrow, where the microenvironment is supportive for expansion since the antigenic stimulus is present together with other promoting signals. Normally, most antigens initiate antibody production by up-regulating CD40L on T cells. CD40/CD40L interaction stimulates B cells to migrate to the germinal center where they undergo SHM and CSR.81,82 CLL cells have been shown to be generally responsive to CD40 ligation, whereas CD40L stimulation has been shown to up-regulate inhibitor of apoptosis proteins such as survinin.83 However, Scielzo et al recently showed that CLL cells exhibit differential patterns of responsiveness upon in vitro CD40L stimulation, linked to distinct activation of intracellular signaling pathways (i.e. phoshorylation of IKKα/β, upregulation of BCL2, MCL1) and, intriguingly, to clinical outcome. CD40L-independent cases seem to be less dependent on CD40/CD40L microenvironmental signals, likely because of a higher autonomous proliferative and survival potential and interestingly these cases exhibited a more aggressive disease phenotype.84
The crosstalk between CLL cells and components of the adaptive and innate immune system in their microenvironment as well as the exact contribution of the various receptor molecules of CLL cell progenitors or the malignant cells themselves in promoting malignant transformation and progression are still unclear. Recently, we investigated the role of innate immune receptors (Toll-like receptors, TLRs) in CLL (Figure 6). We found that distinct TLR signatures define mutated versus unmutated CLL cases. Furthermore, CLL cases assigned to different subsets with stereotyped BCRs exhibit subset-biased TLR signaling expression profiles.37 We also showed that TLRs are functional in CLL cells yet in a heterogeneous fashion and that CLL cases assigned to certain stereotyped subsets exhibit distinct patterns of TLR functionality and/or TLR tolerance. For instance, CLL subsets #1 and #4 do not only differ in disease outcome (subset #1 aggressive, subset #4 indolent) but are also characterized by distinct gene expression profiles of the TLR signaling pathway and distinct responsiveness to TLR ligands.38 These findings indicate that CLL cells receive prosurvival signals via both BCR-dependent and -independent pathways, with stereotyped subsets perhaps representing functionally distinct entities with distinct natural histories and patterns of cross-talk with the microenvironment.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
References
- 1.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 2.Chiorazzi N, Ferrarini M. Cellular origin(s) of chronic lymphocytic leukemia: cautionary notes and additional considerations and possibilities. Blood. 2011;117:1781–1791. doi: 10.1182/blood-2010-07-155663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dohner H, Stilgenbauer S, Benner A, Leupolt E, Krober A, Bullinger L, Dohner K, Bentz M, Lichter P. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 4.Stevenson FK, Krysov S, Davies AJ, Steele AJ, Packham G. B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2011;118:4313–4320. doi: 10.1182/blood-2011-06-338855. [DOI] [PubMed] [Google Scholar]
- 5.Cooper MD, Alder MN. The evolution of adaptive immune systems. Cell. 2006;124:815–822. doi: 10.1016/j.cell.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Janeway CA, Jr, Travers P, Walport M, Shlomchik MJ. The immune system in health and disease. 6th ed Garland Science Publishing; New York, NY: 2005. Immunobiology. 2005. [Google Scholar]
- 7.Schlissel MS. Regulating antigen-receptor gene assembly. Nat Rev Immunol. 2003;3:890–899. doi: 10.1038/nri1225. [DOI] [PubMed] [Google Scholar]
- 8.Maizels N. Immunoglobulin gene diversification. Annu Rev Genet. 2005;39:23–46. doi: 10.1146/annurev.genet.39.073003.110544. [DOI] [PubMed] [Google Scholar]
- 9.Fritz EL, Papavasiliou FN. Cytidine deaminases: AIDing DNA demethylation? Genes Dev. 2010;24:2107–2114. doi: 10.1101/gad.1963010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu M, Schatz DG. Balancing AID and DNA repair during somatic hypermutation. Trends Immunol. 2009;30:173–181. doi: 10.1016/j.it.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Durandy A, Taubenheim N, Peron S, Fischer A. Pathophysiology of B-cell intrinsic immunoglobulin class switch recombination deficiencies. Adv Immunol. 2007;94:275–306. doi: 10.1016/S0065-2776(06)94009-7. [DOI] [PubMed] [Google Scholar]
- 12.Stevenson FK, Sahota SS, Ottensmeier CH, Zhu D, Forconi F, Hamblin TJ. The occurrence and significance of V gene mutations in B cell-derived human malignancy. Adv Cancer Res. 2001;83:81–116. doi: 10.1016/S0065-230X(01)83004-9. [DOI] [PubMed] [Google Scholar]
- 13.Dunn-Walters D, Thiede C, Alpen B, Spencer J. Somatic hypermutation and B-cell lymphoma. Philos Trans R Soc Lond B Biol Sci. 2001;356:73–82. doi: 10.1098/rstb.2000.0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroeder HW, Jr, Dighiero G. The pathogenesis of chronic lymphocytic leukemia: analysis of the antibody repertoire. Immunol Today. 1994;15:288–294. doi: 10.1016/0167-5699(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 15.Efremov DG, Ivanovski M, Siljanovski N, Pozzato G, Cevreska L, Fais F, Chiorazzi N, Batista FD, Burrone OR. Restricted immunoglobulin VH region repertoire in chronic lymphocytic leukemia patients with autoimmune hemolytic anemia. Blood. 1996;87:3869–3876. [PubMed] [Google Scholar]
- 16.Hashimoto S, Dono M, Wakai M, Allen SL, Lichtman SM, Schulman P, Vinciguerra VP, Ferrarini M, Silver J, Chiorazzi N. Somatic diversification and selection of immunoglobulin heavy and light chain variable region genes in IgG+ CD5+ chronic lymphocytic leukemia B cells. J Exp Med. 1995;181:1507–1517. doi: 10.1084/jem.181.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson TA, Rassenti LZ, Kipps TJ. Ig VH1 genes expressed in B cell chronic lymphocytic leukemia exhibit distinctive molecular features. J Immunol. 1997;158:235–246. [PubMed] [Google Scholar]
- 18.Fais F, Ghiotto F, Hashimoto S, Sellars B, Valetto A, Allen SL, Schulman P, Vinciguerra VP, Rai K, Rassenti LZ, Kipps TJ, Dighiero G, Schroeder HW, Jr, Ferrarini M, Chiorazzi N. Chronic lymphocytic leukemia B cells express restricted sets of mutated and unmutated antigen receptors. J Clin Invest. 1998;102:1515–1525. doi: 10.1172/JCI3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL, Buchbinder A, Budman D, Dittmar K, Kolitz J, Lichtman SM, Schulman P, Vinciguerra VP, Rai KR, Ferrarini M, Chiorazzi N. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–1847. [PubMed] [Google Scholar]
- 20.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–1854. [PubMed] [Google Scholar]
- 21.Tobin G, Thunberg U, Johnson A, Thorn I, Soderberg O, Hultdin M, Botling J, Enblad G, Sallstrom J, Sundstrom C, Roos G, Rosenquist R. Somatically mutated Ig V(H)3-21 genes characterize a new subset of chronic lymphocytic leukemia. Blood. 2002;99:2262–2264. doi: 10.1182/blood.V99.6.2262. [DOI] [PubMed] [Google Scholar]
- 22.Forconi F, Sahota SS, Lauria F, Stevenson FK. Revisiting the definition of somatic mutational status in B-cell tumors: does 98% homology mean that a V(H)-gene is unmutated? Leukemia. 2004;18:882–883. doi: 10.1038/sj.leu.2403312. [DOI] [PubMed] [Google Scholar]
- 23.Ghia P, Stamatopoulos K, Belessi C, Moreno C, Stilgenbauer S, Stevenson F, Davi F, Rosenquist R. ERIC recommendations on IGHV gene mutational status analysis in chronic lymphocytic leukemia. Leukemia. 2007;21:1–3. doi: 10.1038/sj.leu.2404457. [DOI] [PubMed] [Google Scholar]
- 24.Barbas SM, Ditzel HJ, Salonen EM, Yang WP, Silverman GJ, Burton DR. Human autoantibody recognition of DNA. Proc Natl Acad Sci U S A. 1995;92:2529–2533. doi: 10.1073/pnas.92.7.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamblin TJ, Davis ZA, Oscier DG. Determination of how many immunoglobulin variable region heavy chain mutations are allowable in unmutated chronic lymphocytic leukaemia - long-term follow up of patients with different percentages of mutations. Br J Haematol. 2008;140:320–323. doi: 10.1111/j.1365-2141.2007.06928.x. [DOI] [PubMed] [Google Scholar]
- 26.Murray F, Darzentas N, Hadzidimitriou A, Tobin G, Boudjogra M, Scielzo C, Laoutaris N, Karlsson K, Baran-Marzsak F, Tsaftaris A, Moreno C, Anagnostopoulos A, Caligaris-Cappio F, Vaur D, Ouzounis C, Belessi C, Ghia P, Davi F, Rosenquist R, Stamatopoulos K. Stereotyped patterns of somatic hypermutation in subsets of patients with chronic lymphocytic leukemia: implications for the role of antigen selection in leukemogenesis. Blood. 2008;111:1524–1533. doi: 10.1182/blood-2007-07-099564. [DOI] [PubMed] [Google Scholar]
- 27.Tobin G, Thunberg U, Johnson A, Eriksson I, Soderberg O, Karlsson K, Merup M, Juliusson G, Vilpo J, Enblad G, Sundstrom C, Roos G, Rosenquist R. Chronic lymphocytic leukemias utilizing the VH3-21 gene display highly restricted Vlambda2-14 gene use and homologous CDR3s: implicating recognition of a common antigen epitope. Blood. 2003;101:4952–4957. doi: 10.1182/blood-2002-11-3485. [DOI] [PubMed] [Google Scholar]
- 28.Messmer BT, Albesiano E, Efremov DG, Ghiotto F, Allen SL, Kolitz J, Foa R, Damle RN, Fais F, Messmer D, Rai KR, Ferrarini M, Chiorazzi N. Multiple distinct sets of stereotyped antigen receptors indicate a role for antigen in promoting chronic lymphocytic leukemia. J Exp Med. 2004;200:519–525. doi: 10.1084/jem.20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Widhopf GF, 2nd, Rassenti LZ, Toy TL, Gribben JG, Wierda WG, Kipps TJ. Chronic lymphocytic leukemia B cells of more than 1% of patients express virtually identical immunoglobulins. Blood. 2004;104:2499–2504. doi: 10.1182/blood-2004-03-0818. [DOI] [PubMed] [Google Scholar]
- 30.Tobin G, Thunberg U, Karlsson K, Murray F, Laurell A, Willander K, Enblad G, Merup M, Vilpo J, Juliusson G, Sundstrom C, Soderberg O, Roos G, Rosenquist R. Subsets with restricted immunoglobulin gene rearrangement features indicate a role for antigen selection in the development of chronic lymphocytic leukemia. Blood. 2004;104:2879–2885. doi: 10.1182/blood-2004-01-0132. [DOI] [PubMed] [Google Scholar]
- 31.Zemlin M, Klinger M, Link J, Zemlin C, Bauer K, Engler JA, Schroeder HW, Jr, Kirkham PM. Expressed murine and human CDR-H3 intervals of equal length exhibit distinct repertoires that differ in their amino acid composition and predicted range of structures. J Mol Biol. 2003;334:733–749. doi: 10.1016/j.jmb.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Henikoff S, Henikoff JG. Performance evaluation of amino acid substitution matrices. Proteins. 1993;17:49–61. doi: 10.1002/prot.340170108. [DOI] [PubMed] [Google Scholar]
- 33.Stamatopoulos K, Belessi C, Moreno C, Boudjograh M, Guida G, Smilevska T, Belhoul L, Stella S, Stavroyianni N, Crespo M, Hadzidimitriou A, Sutton L, Bosch F, Laoutaris N, Anagnostopoulos A, Montserrat E, Fassas A, Dighiero G, Caligaris-Cappio F, Merle-Beral H, Ghia P, Davi F. Over 20% of patients with chronic lymphocytic leukemia carry stereotyped receptors: Pathogenetic implications and clinical correlations. Blood. 2007;109:259–270. doi: 10.1182/blood-2006-03-012948. [DOI] [PubMed] [Google Scholar]
- 34.Kirkham PM, Mortari F, Newton JA, Schroeder HW., Jr Immunoglobulin VH clan and family identity predicts variable domain structure and may influence antigen binding. EMBO J. 1992;11:603–609. doi: 10.1002/j.1460-2075.1992.tb05092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vargas-Madrazo E, Lara-Ochoa F, Ramirez-Benites MC, Almagro JC. Evolution of the structural repertoire of the human V(H) and Vkappa germline genes. Int Immunol. 1997;9:1801–1815. doi: 10.1093/intimm/9.12.1801. [DOI] [PubMed] [Google Scholar]
- 36.Bomben R, Dal Bo M, Capello D, Forconi F, Maffei R, Laurenti L, Rossi D, Del Principe MI, Zucchetto A, Bertoni F, Rossi FM, Bulian P, Cattarossi I, Ilariucci F, Sozzi E, Spina V, Zucca E, Degan M, Lauria F, Del Poeta G, Efremov DG, Marasca R, Gaidano G, Gattei V. Molecular and clinical features of chronic lymphocytic leukaemia with stereotyped B cell receptors: results from an Italian multicentre study. Br J Haematol. 2009;144:492–506. doi: 10.1111/j.1365-2141.2008.07469.x. [DOI] [PubMed] [Google Scholar]
- 37.Arvaniti E, Ntoufa S, Papakonstantinou N, Touloumenidou T, Laoutaris N, Anagnostopoulos A, Lamnissou K, Caligaris-Cappio F, Stamatopoulos K, Ghia P, Muzio M, Belessi C. Toll-like receptor signaling pathway in chronic lymphocytic leukemia: distinct gene expression profiles of potential pathogenic significance in specific subsets of patients. Haematologica. 2011;96:1644–1652. doi: 10.3324/haematol.2011.044792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ntoufa S, Vardi A, Papakonstantinou N, Anagnostopoulos A, Aleporou-Marinou V, Belessi C, Ghia P, Caligaris-Cappio F, Muzio M, Stamatopoulos K. Distinct Innate Immunity Pathways to Activation and Tolerance in Subgroups of Chronic Lymphocytic Leukemia with Distinct Immunoglobulin Receptors. Mol Med. 2012 doi: 10.2119/molmed.2011.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Widhopf GF, 2nd, Goldberg CJ, Toy TL, Rassenti LZ, Wierda WG, Byrd JC, Keating MJ, Gribben JG, Rai KR, Kipps TJ. Nonstochastic pairing of immunoglobulin heavy and light chains expressed by chronic lymphocytic leukemia B cells is predicated on the heavy chain CDR3. Blood. 2008;111:3137–3144. doi: 10.1182/blood-2007-02-073130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hadzidimitriou A, Darzentas N, Murray F, Smilevska T, Arvaniti E, Tresoldi C, Tsaftaris A, Laoutaris N, Anagnostopoulos A, Davi F, Ghia P, Rosenquist R, Stamatopoulos K, Belessi C. Evidence for the significant role of immunoglobulin light chains in antigen recognition and selection in chronic lymphocytic leukemia. Blood. 2009;113:403–411. doi: 10.1182/blood-2008-07-166868. [DOI] [PubMed] [Google Scholar]
- 41.Herve M, Xu K, Ng YS, Wardemann H, Albesiano E, Messmer BT, Chiorazzi N, Meffre E. Unmutated and mutated chronic lymphocytic leukemias derive from self-reactive B cell precursors despite expressing different antibody reactivity. J Clin Invest. 2005;115:1636–1643. doi: 10.1172/JCI24387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lanemo Myhrinder A, Hellqvist E, Sidorova E, Soderberg A, Baxendale H, Dahle C, Willander K, Tobin G, Backman E, Soderberg O, Rosenquist R, Horkko S, Rosen A. A new perspective: molecular motifs on oxidized LDL, apoptotic cells, and bacteria are targets for chronic lymphocytic leukemia antibodies. Blood. 2008;111:3838–3848. doi: 10.1182/blood-2007-11-125450. [DOI] [PubMed] [Google Scholar]
- 43.Catera R, Silverman GJ, Hatzi K, Seiler T, Didier S, Zhang L, Herve M, Meffre E, Oscier DG, Vlassara H, Scofield RH, Chen Y, Allen SL, Kolitz J, Rai KR, Chu CC, Chiorazzi N. Chronic lymphocytic leukemia cells recognize conserved epitopes associated with apoptosis and oxidation. Mol Med. 2008;14:665–674. doi: 10.2119/2008-0102.Catera. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chu CC, Catera R, Zhang L, Didier S, Agagnina BM, Damle RN, Kaufman MS, Kolitz JE, Allen SL, Rai KR, Chiorazzi N. Many chronic lymphocytic leukemia antibodies recognize apoptotic cells with exposed nonmuscle myosin heavy chain IIA: implications for patient outcome and cell of origin. Blood. 2010;115:3907–3915. doi: 10.1182/blood-2009-09-244251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chu CC, Catera R, Hatzi K, Yan XJ, Zhang L, Wang XB, Fales HM, Allen SL, Kolitz JE, Rai KR, Chiorazzi N. Chronic lymphocytic leukemia antibodies with a common stereotypic rearrangement recognize nonmuscle myosin heavy chain IIA. Blood. 2008;112:5122–5129. doi: 10.1182/blood-2008-06-162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Darzentas N, Hadzidimitriou A, Murray F, Hatzi K, Josefsson P, Laoutaris N, Moreno C, Anagnostopoulos A, Jurlander J, Tsaftaris A, Chiorazzi N, Belessi C, Ghia P, Rosenquist R, Davi F, Stamatopoulos K. A different ontogenesis for chronic lymphocytic leukemia cases carrying stereotyped antigen receptors: molecular and computational evidence. Leukemia. 2010;24:125–132. doi: 10.1038/leu.2009.186. [DOI] [PubMed] [Google Scholar]
- 47.Agathangelidis A, Darzentas N, Hadzidimitriou A, Brochet X, Murray F, Yan XJ, Davis Z, van Gastel-Mol EJ, Tresoldi C, Chu CC, Cahill N, Giudicelli V, Tichy B, Pedersen LB, Foroni L, Bonello L, Janus A, Smedby K, Anagnostopoulos A, Merle-Beral H, Laoutaris N, Juliusson G, di Celle PF, Pospisilova S, Jurlander J, Geisler C, Tsaftaris A, Lefranc MP, Langerak AW, Oscier DG, Chiorazzi N, Belessi C, Davi F, Rosenquist R, Ghia P, Stamatopoulos K. Stereotyped B-cell receptors in one third of chronic lymphocytic leukemia: towards a molecular classification with implications for targeted therapeutic interventions. Blood. 2012. [DOI] [PMC free article] [PubMed]
- 48.Zibellini S, Capello D, Forconi F, Marcatili P, Rossi D, Rattotti S, Franceschetti S, Sozzi E, Cencini E, Marasca R, Baldini L, Tucci A, Bertoni F, Passamonti F, Orlandi E, Varettoni M, Merli M, Rizzi S, Gattei V, Tramontano A, Paulli M, Gaidano G, Arcaini L. Stereotyped patterns of B-cell receptor in splenic marginal zone lymphoma. Haematologica. 2010;95:1792–1796. doi: 10.3324/haematol.2010.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bikos V, Darzentas N, Hadzidimitriou A, Davis Z, Hockley S, Traverse-Glehen A, Algara P, Santoro A, Gonzalez D, Mollejo M, Dagklis A, Gangemi F, Bosler DS, Bourikas G, Anagnostopoulos A, Tsaftaris A, Iannitto E, Ponzoni M, Felman P, Berger F, Belessi C, Ghia P, Papadaki T, Dogan A, Degano M, Matutes E, Piris MA, Oscier D, Stamatopoulos K. Over 30% of patients with splenic marginal zone lymphoma express the same immunoglobulin heavy variable gene: ontogenetic implications. Leukemia. 2012. [DOI] [PubMed]
- 50.Hadzidimitriou A, Agathangelidis A, Darzentas N, Murray F, Delfau-Larue MH, Pedersen LB, Lopez AN, Dagklis A, Rombout P, Beldjord K, Kolstad A, Dreyling MH, Anagnostopoulos A, Tsaftaris A, Mavragani-Tsipidou P, Rosenwald A, Ponzoni M, Groenen P, Ghia P, Sander B, Papadaki T, Campo E, Geisler C, Rosenquist R, Davi F, Pott C, Stamatopoulos K. Is there a role for antigen selection in mantle cell lymphoma? Immunogenetic support from a series of 807 cases. Blood. 2011;118:3088–3095. doi: 10.1182/blood-2011-03-343434. [DOI] [PubMed] [Google Scholar]
- 51.Bomben R, Dal-Bo M, Benedetti D, Capello D, Forconi F, Marconi D, Bertoni F, Maffei R, Laurenti L, Rossi D, Del Principe MI, Luciano F, Sozzi E, Cattarossi I, Zucchetto A, Rossi FM, Bulian P, Zucca E, Nicoloso MS, Degan M, Marasca R, Efremov DG, Del Poeta G, Gaidano G, Gattei V. Expression of mutated IGHV3-23 genes in chronic lymphocytic leukemia identifies a disease subset with peculiar clinical and biological features. Clin Cancer Res. 2010;16:620–628. doi: 10.1158/1078-0432.CCR-09-1638. [DOI] [PubMed] [Google Scholar]
- 52.Del Giudice I, Chiaretti S, Tavolaro S, De Propris MS, Maggio R, Mancini F, Peragine N, Santangelo S, Marinelli M, Mauro FR, Guarini A, Foa R. Spontaneous regression of chronic lymphocytic leukemia: clinical and biologic features of 9 cases. Blood. 2009;114:638–646. doi: 10.1182/blood-2008-12-196568. [DOI] [PubMed] [Google Scholar]
- 53.Dal-Bo M, Del Giudice I, Bomben R, Capello D, Bertoni F, Forconi F, Laurenti L, Rossi D, Zucchetto A, Pozzato G, Marasca R, Efremov DG, Guarini A, Del Poeta G, Foa R, Gaidano G, Gattei V. B-cell receptor, clinical course and prognosis in chronic lymphocytic leukaemia: the growing saga of the IGHV3 subgroup gene usage. Br J Haematol. 2011;153:3–14. doi: 10.1111/j.1365-2141.2010.08440.x. [DOI] [PubMed] [Google Scholar]
- 54.Thorselius M, Krober A, Murray F, Thunberg U, Tobin G, Buhler A, Kienle D, Albesiano E, Maffei R, Dao-Ung LP, Wiley J, Vilpo J, Laurell A, Merup M, Roos G, Karlsson K, Chiorazzi N, Marasca R, Dohner H, Stilgenbauer S, Rosenquist R. Strikingly homologous immunoglobulin gene rearrangements and poor outcome in VH3-21-using chronic lymphocytic leukemia patients independent of geographic origin and mutational status. Blood. 2006;107:2889–2894. doi: 10.1182/blood-2005-06-2227. [DOI] [PubMed] [Google Scholar]
- 55.Oscier D, Wade R, Davis Z, Morilla A, Best G, Richards S, Else M, Matutes E, Catovsky D. Prognostic factors identified three risk groups in the LRF CLL4 trial, independent of treatment allocation. Haematologica. 2010;95:1705–1712. doi: 10.3324/haematol.2010.025338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghiotto F, Fais F, Valetto A, Albesiano E, Hashimoto S, Dono M, Ikematsu H, Allen SL, Kolitz J, Rai KR, Nardini M, Tramontano A, Ferrarini M, Chiorazzi N. Remarkably similar antigen receptors among a subset of patients with chronic lymphocytic leukemia. J Clin Invest. 2004;113:1008–1016. doi: 10.1172/JCI19399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rossi D, Spina V, Cerri M, Rasi S, Deambrogi C, De Paoli L, Laurenti L, Maffei R, Forconi F, Bertoni F, Zucca E, Agostinelli C, Cabras A, Lucioni M, Martini M, Magni M, Deaglio S, Ladetto M, Nomdedeu JF, Besson C, Ramponi A, Canzonieri V, Paulli M, Marasca R, Larocca LM, Carbone A, Pileri SA, Gattei V, Gaidano G. Stereotyped B-cell receptor is an independent risk factor of chronic lymphocytic leukemia transformation to Richter syndrome. Clin Cancer Res. 2009;15:4415–4422. doi: 10.1158/1078-0432.CCR-08-3266. [DOI] [PubMed] [Google Scholar]
- 58.Ghia P, Stamatopoulos K, Belessi C, Moreno C, Stella S, Guida G, Michel A, Crespo M, Laoutaris N, Montserrat E, Anagnostopoulos A, Dighiero G, Fassas A, Caligaris-Cappio F, Davi F. Geographic patterns and pathogenetic implications of IGHV gene usage in chronic lymphocytic leukemia: the lesson of the IGHV3-21 gene. Blood. 2005;105:1678–1685. doi: 10.1182/blood-2004-07-2606. [DOI] [PubMed] [Google Scholar]
- 59.Potter KN, Mockridge CI, Neville L, Wheatley I, Schenk M, Orchard J, Duncombe AS, Packham G, Stevenson FK. Structural and functional features of the B-cell receptor in IgG-positive chronic lymphocytic leukemia. Clin Cancer Res. 2006;12:1672–1679. doi: 10.1158/1078-0432.CCR-05-2164. [DOI] [PubMed] [Google Scholar]
- 60.Sutton LA, Kostareli E, Hadzidimitriou A, Darzentas N, Tsaftaris A, Anagnostopoulos A, Rosenquist R, Stamatopoulos K. Extensive intraclonal diversification in a subgroup of chronic lymphocytic leukemia patients with stereotyped IGHV4-34 receptors: implications for ongoing interactions with antigen. Blood. 2009;114:4460–4468. doi: 10.1182/blood-2009-05-221309. [DOI] [PubMed] [Google Scholar]
- 61.Kostareli E, Sutton LA, Hadzidimitriou A, Darzentas N, Kouvatsi A, Tsaftaris A, Anagnostopoulos A, Rosenquist R, Stamatopoulos K. Intraclonal diversification of immunoglobulin light chains in a subset of chronic lymphocytic leukemia alludes to antigen-driven clonal evolution. Leukemia. 2010;24:1317–1324. doi: 10.1038/leu.2010.90. [DOI] [PubMed] [Google Scholar]
- 62.Broker BM, Klajman A, Youinou P, Jouquan J, Worman CP, Murphy J, Mackenzie L, Quartey-Papafio R, Blaschek M, Collins P, et al. Chronic lymphocytic leukemic (CLL) cells secrete multispecific autoantibodies. J Autoimmun. 1988;1:469–481. doi: 10.1016/0896-8411(88)90068-6. [DOI] [PubMed] [Google Scholar]
- 63.Sthoeger ZM, Wakai M, Tse DB, Vinciguerra VP, Allen SL, Budman DR, Lichtman SM, Schulman P, Weiselberg LR, Chiorazzi N. Production of autoantibodies by CD5-expressing B lymphocytes from patients with chronic lymphocytic leukemia. J Exp Med. 1989;169:255–268. doi: 10.1084/jem.169.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Borche L, Lim A, Binet JL, Dighiero G. Evidence that chronic lymphocytic leukemia B lymphocytes are frequently committed to production of natural autoantibodies. Blood. 1990;76:562–569. [PubMed] [Google Scholar]
- 65.Hatzi K, Catera R, Ferrarini M, Fischetti V, Herve M, Meffre E, Chu CC, Chiorazzi N. B-Cell Chronic Lymphocytic Leukemia (B-CLL) Cells Express Antibodies Reactive with Antigenic Epitopes Expressed on the Surface of Common Bacteria. Blood (ASH Annual Meeting Abstracts) 2006;108 Abstract 25. [Google Scholar]
- 66.Seiler T, Woelfle M, Yancopoulos S, Catera R, Li W, Hatzi K, Moreno C, Torres M, Paul S, Dohner H, Stilgenbauer S, Kaufman MS, Kolitz JE, Allen SL, Rai KR, Chu CC, Chiorazzi N. Characterization of structurally defined epitopes recognized by monoclonal antibodies produced by chronic lymphocytic leukemia B cells. Blood. 2009;114:3615–3624. doi: 10.1182/blood-2009-01-197822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goodyear CS, Silverman GJ. B cell superantigens: a microbe’s answer to innate-like B cells and natural antibodies. Springer Semin Immunopathol. 2005;26:463–484. doi: 10.1007/s00281-004-0190-2. [DOI] [PubMed] [Google Scholar]
- 68.Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol. 2005;26:347–362. doi: 10.1007/s00281-004-0182-2. [DOI] [PubMed] [Google Scholar]
- 69.Schwartz-Albiez R, Monteiro RC, Rodriguez M, Binder CJ, Shoenfeld Y. Natural antibodies, intravenous immunoglobulin and their role in autoimmunity, cancer and inflammation. Clin Exp Immunol. 2009;158(Suppl 1):43–50. doi: 10.1111/j.1365-2249.2009.04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Binder M, Lechenne B, Ummanni R, Scharf C, Balabanov S, Trusch M, Schluter H, Braren I, Spillner E, Trepel M. Stereotypical chronic lymphocytic leukemia B-cell receptors recognize survival promoting antigens on stromal cells. PLoS One. 2010;5:e15992. doi: 10.1371/journal.pone.0015992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stamatopoulos K. Antigens in CLL: themes and variations. Blood. 2010;115:3855–3856. doi: 10.1182/blood-2010-02-270249. [DOI] [PubMed] [Google Scholar]
- 72.Binder CJ, Horkko S, Dewan A, Chang MK, Kieu EP, Goodyear CS, Shaw PX, Palinski W, Witztum JL, Silverman GJ. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003;9:736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 73.Landgren O, Rapkin JS, Caporaso NE, Mellemkjaer L, Gridley G, Goldin LR, Engels EA. Respiratory tract infections and subsequent risk of chronic lymphocytic leukemia. Blood. 2007;109:2198–2201. doi: 10.1182/blood-2006-08-044008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kostareli E, Hadzidimitriou A, Stavroyianni N, Darzentas N, Athanasiadou A, Gounari M, Bikos V, Agathagelidis A, Touloumenidou T, Zorbas I, Kouvatsi A, Laoutaris N, Fassas A, Anagnostopoulos A, Belessi C, Stamatopoulos K. Molecular evidence for EBV and CMV persistence in a subset of patients with chronic lymphocytic leukemia expressing stereotyped IGHV4-34 B-cell receptors. Leukemia. 2009;23:919–924. doi: 10.1038/leu.2008.379. [DOI] [PubMed] [Google Scholar]
- 75.Steininger C, Rassenti LZ, Vanura K, Eigenberger K, Jager U, Kipps TJ, Mannhalter C, Stilgenbauer S, Popow-Kraupp T. Relative seroprevalence of human herpes viruses in patients with chronic lymphocytic leukaemia. Eur J Clin Invest. 2009;39:497–506. doi: 10.1111/j.1365-2362.2009.02131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Steininger C, Widhopf GF, 2nd, Ghia EM, Morello CS, Vanura K, Sanders R, Spector D, Guiney D, Jager U, Kipps TJ. Recombinant antibodies encoded by IGHV1-69 react with pUL32, a phosphoprotein of cytomegalovirus and B-cell superantigen. Blood. 2012;119:2293–2301. doi: 10.1182/blood-2011-08-374058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kostareli E, Gounari M, Janus A, Murray F, Brochet X, Giudicelli V, Pospisilova S, Oscier D, Foroni L, di Celle PF, Tichy B, Pedersen LB, Jurlander J, Ponzoni M, Kouvatsi A, Anagnostopoulos A, Thompson K, Darzentas N, Lefranc MP, Belessi C, Rosenquist R, Davi F, Ghia P, Stamatopoulos K. Antigen receptor stereotypy across B-cell lymphoproliferations: the case of IGHV4-59/IGKV3-20 receptors with rheumatoid factor activity. Leukemia. 2012 May;26(5):1127–31. doi: 10.1038/leu.2011.311. doi: 10.1038/leu.2011.311. [DOI] [PubMed] [Google Scholar]
- 78.Scharenberg AM, Humphries LA, Rawlings DJ. Calcium signalling and cell-fate choice in B cells. Nat Rev Immunol. 2007;7:778–789. doi: 10.1038/nri2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suljagic M, Longo PG, Bennardo S, Perlas E, Leone G, Laurenti L, Efremov DG. The Syk inhibitor fostamatinib disodium (R788) inhibits tumor growth in the Emu- TCL1 transgenic mouse model of CLL by blocking antigen-dependent B-cell receptor signaling. Blood. 2010;116:4894–4905. doi: 10.1182/blood-2010-03-275180. [DOI] [PubMed] [Google Scholar]
- 80.Song Z, Lu P, Furman RR, Leonard JP, Martin P, Tyrell L, Lee FY, Knowles DM, Coleman M, Wang YL. Activities of SYK and PLCgamma2 predict apoptotic response of CLL cells to SRC tyrosine kinase inhibitor dasatinib. Clin Cancer Res. 2010;16:587–599. doi: 10.1158/1078-0432.CCR-09-1519. [DOI] [PubMed] [Google Scholar]
- 81.Schattner EJ. CD40 ligand in CLL pathogenesis and therapy. Leuk Lymphoma. 2000;37:461–472. doi: 10.3109/10428190009058499. [DOI] [PubMed] [Google Scholar]
- 82.Grdisa M. Influence of CD40 ligation on survival and apoptosis of B-CLL cells in vitro. Leuk Res. 2003;27:951–956. doi: 10.1016/S0145-2126(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 83.Granziero L, Ghia P, Circosta P, Gottardi D, Strola G, Geuna M, Montagna L, Piccoli P, Chilosi M, Caligaris-Cappio F. Survivin is expressed on CD40 stimulation and interfaces proliferation and apoptosis in B-cell chronic lymphocytic leukemia. Blood. 2001;97:2777–2783. doi: 10.1182/blood.V97.9.2777. [DOI] [PubMed] [Google Scholar]
- 84.Scielzo C, Apollonio B, Scarfo L, Janus A, Muzio M, Ten Hacken E, Ghia P, Caligaris-Cappio F. The functional in vitro response to CD40 ligation reflects a different clinical outcome in patients with chronic lymphocytic leukemia. Leukemia. 2011;25:1760–1767. doi: 10.1038/leu.2011.149. [DOI] [PubMed] [Google Scholar]