Abstract
Background:
MicroRNAs (miRNAs) are small noncoding RNAs that silence target gene expression posttranscriptionally, and their impact on gene expression has been reported in various diseases. It has been reported that the expression of the hypoxia-inducible factor-1α (HIF-1α) is reduced and that of p53 is increased in lungs from patients with COPD. However, the role of miRNAs associated with these genes in lungs from patients with COPD is unknown.
Methods:
Lung tissue samples from 55 patients were included in this study. Total RNA, miRNA, and protein were extracted from lung tissues and used for reverse transcriptase polymerase chain reaction and Western blot analysis. Cell culture experiments were performed using cultured human pulmonary microvascular endothelial cells (HPMVECs).
Results:
miR-34a and miR-199a-5p expressions were increased, and the phosphorylation of AKT was decreased in the lung tissue samples of patients with COPD. The miR-199a-5p expression was correlated with HIF-1α protein expression in the lungs of patients with COPD. Transfection of HPMVECs with the miR-199a-5p precursor gene decreased HIF-1α protein expression, and transfection with the miR-34a precursor gene increased miR-199a-5p expression.
Conclusions:
These data suggest that miR-34a and miR-199a-5p contribute to the pathogenesis of COPD, and these miRNAs may also affect the HIF-1α-dependent lung structure maintenance program.
MicroRNAs (miRNAs) are noncoding RNA molecules that modulate gene expression by binding to complementary sequences in the coding- or the 3′-untranslated region of target mRNAs. miRNAs can regulate cell proliferation, differentiation, and apoptosis,1‐3 and their roles in responses to injury or adaptation to chronic stress are under intense investigation. Emerging data indicate that miRNAs affect the responsiveness of cells to signaling molecules, such as transforming growth factor-β, notch, and epidermal growth factor.4 The role of miRNAs in the pathogenesis of respiratory diseases has been examined. Schembri et al5 described miRNAs as modulators of smoking-induced gene expression changes in human airway epithelium and reported that miR-218 and miR-128b were decreased in expression. Xi et al6 showed that cigarette smoke condensate increased the expression of miR-31 in airway cells, and Izzotti et al7 investigated the impact of cigarette smoking on mouse lungs. miRNAs are likely to play a role in the pathogenesis of lung cancer,8 and involvement of miRNA let-7d has been suggested in idiopathic pulmonary fibrosis.9 In addition, Pottelberge et al10 reported reduced expression of let-7c and miR-125b in sputum samples from patients with COPD.
Apoptosis of lung endothelial cells has been described in emphysematous lungs,11 and vascular endothelial growth factor (VEGF) receptor inhibition12 or conditional knockout of the VEGF ligand in mouse lungs13 results in emphysema. We have recently reported the decreased expression of hypoxia inducible factor-1α (HIF-1α) protein and decreased phosphorylation of AKT in the lung tissues from patients with COPD.14 However, to our knowledge, the role of miRNAs in the pathogenesis of COPD regarding HIF-1α and AKT interaction has not yet been investigated. A few studies have implicated the AKT pathway in the regulation of HIF-1α.15‐17 Rane et al18 recently reported that constitutive active adenovirus AKT gene transfection caused the decrease of miR-199a-5p expression in neonatal cardiac myocytes, which are known to control HIF-1α protein expression.19 Further, it has been reported that replenishing of miR-199a-5p also regulates the hypoxic stabilization of p53.18 The p53 protein expression is increased in tissues from patients with COPD/emphysema,14,20 and p53 regulates miR-34a expression in response to DNA damage.21,22 Combining these observations, we hypothesized that in lung tissue samples from patients with COPD, the low HIF-1α protein expression would be related to an increased expression of miR-199a-5p and miR-34a and to AKT inactivation. Here, we measured miR-199a-5p and miR-34a expression in the lung tissues from patients with COPD/emphysema and found large expression changes of both of these miRNAs. To address the mechanisms of interactions between these miRNAs and the expression of HIF-1α, we performed experiments in cultured human pulmonary microvascular endothelial cells (HPMVECs) using microRNA precursor gene transfection and AKT and p53 gene silencing. These cell culture results support the concept that HIF-1α and the important angiogenesis factor VEGF are under miRNA control.
Materials and Methods
Lung Tissue Samples
Lung tissue samples from 55 patients were included in this study. We divided the patients into four groups according to the GOLD (Global Initiative for Chronic Obstructive Lung Disease) classification. Ten samples were from patients with normal lung function (no COPD); the remainder of the samples were from patients with mild (n = 13; stage I, GOLD classification), moderate (n = 17; stage II, GOLD classification), and severe (n = 15; stage III and IV, GOLD classification) COPD. Table 1 summarizes the patient characteristics. Samples were obtained from the National Institutes of Health Lung Tissue Repository Consortium (LTRC). The diagnosis of emphysema was made by the LTRC pathologist based on histologic examination, and all of the lung tissue samples from patients with COPD had centrilobular emphysema.
Table 1.
—Characteristics of Patients
| Characteristics | No COPD | Mild COPD | Moderate COPD | Severe COPD |
| GOLD | … | Stage I | Stage II | Stage III, IV |
| No. of patients | 10 | 13 | 17 | 15 |
| Age, y | 56.8 ± 14.0 | 71.7 ± 5.8 | 66.7 ± 10.4 | 56.7 ± 6.4 |
| Sex, male (female) | 0 (10) | 11 (2) | 12 (5) | 8 (7) |
| FEV1/FVC, % | 81.2 ± 5.5 | 62.6 ± 5.4 | 58.2 ± 7.3 | 28.7 ± 9.2 |
| FEV1 % predicted | 92.7 ± 8.6 | 91.8 ± 13.5 | 61.3 ± 8.3 | 21.3 ± 7.4 |
| BDR, % | 2.2 ± 3.3 | 5.8 ± 5.8 | 5.5 ± 4.7 | 4.5 ± 5.4 |
| Smoking, pack-years | 0 | 30.4 ± 32.2 | 32.4 ± 35.2 | 43.2 ± 38.6 |
| Current smoker, No. | 0 | 2 | 0 | 0 |
| Medication, No. | ||||
| Oral corticosteroid | 0 | 0 | 1 | 1 |
| Inhaled corticosteroid | 0 | 0 | 0 | 7 |
| Diagnosis of lung cancer, No. | 3 | 7 | 13 | 2 |
Values are expressed as means ± SD. BDR = bronchodilator response; GOLD = Global Initiative for Obstructive Lung Disease.
All lung tissue samples were maintained at −80°C until processing. All of the patients gave informed consent to have their tissue banked in the National Institutes of Health LTRC. The study was approved by the institutional review board of both of our institutions (Protocol: COMIRB 07-0269).
Cell Culture
HPMVECs were purchased from Lonza. They were cultured in endothelial cell growth medium supplemented with 5% fetal bovine serum (Lonza). The cells were cultured in 175-cm2 tissue culture flasks in a cell-culture incubator (37°C, 5% CO2 and 95% air) and used in all the experiments at the sixth passage after trypsinization. After reaching confluence, media were changed, and after addition of different concentrations (10 nM to 1 μM) of AKT inhibitors (MK2206 and perifosine) the cells were cultured for another 24 h. Some cells were transfected with AKT1 small interfering RNAs (siRNAs) or miR34a or miR199a-5p precursor miRNA and cultured for 48 h after transfection. In additional experiments, p53 siRNA-transfected cells were cultured for another 24 h with or without 10 μM of nutlin, an agent reported to induce p53.23 After incubation, the cells were harvested and used for reverse transcriptase polymerase chain reaction (RT-PCR) and Western blot analysis. See e-Appendix 1 (378.2KB, pdf) for chemicals, real-time RT-PCR analysis of mRNA and miRNA, Western blot analysis, and transfection of siRNA or miRNA precursors into HPMVECs.
Statistical Analysis
Results are expressed as mean ± SE. Statistical analysis was performed using analysis of variance with Bonferroni corrections for multiple comparisons. Correlations were analyzed by the Pearson correlation coefficient. Comparisons were considered statistically significant at P < .05.
Results
Patient Characteristics
The data and characteristics describing the patients from which the lung tissue samples (LTRC) have been derived are displayed in Table 1. The lung samples from never smokers with normal lung function were all from female patients. Although a histopathologic diagnosis of lung cancer had been made in 24 of 55 patients, the tissue samples investigated were all histologically cancer-free and represented tissue highly remote from any tumor. The LTRC data set did not provide blood gas information for all of the patients of this study; thus, unfortunately, we cannot distinguish between hypoxemic and nonhypoxemic patients. All of the COPD lung samples were from smokers, and the pack-year smoking history was comparable for the patients in GOLD stages I to IV.
mRNA and miRNA Expression in the Lung Tissue Samples From Patients With COPD
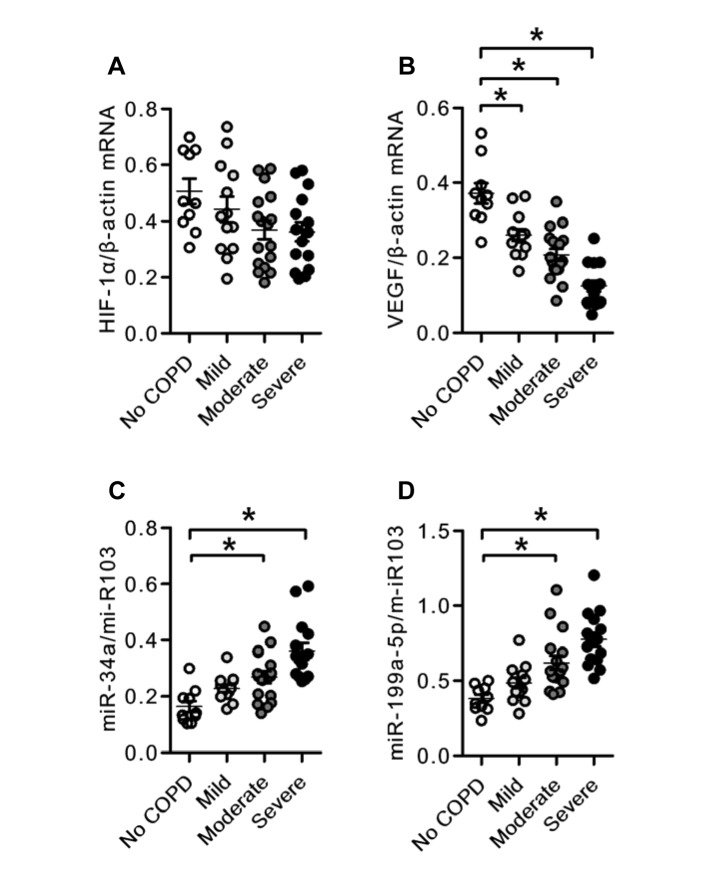
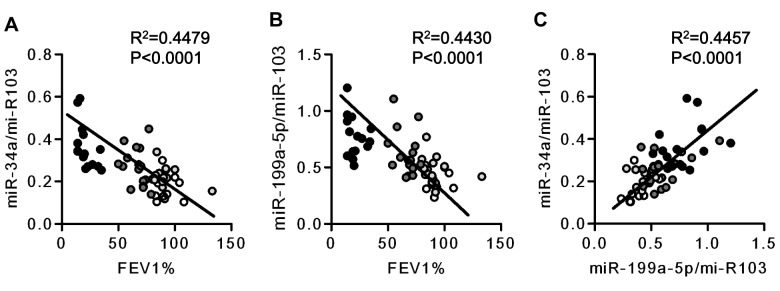
We categorized the tissue samples according to the severity of the patients’ lung disease as mild, moderate, and severe. When the tissue extracts were analyzed for gene and miRNA expression, we found that there was a severity-related decrease in the expression of the gene encoding VEGF (Fig 1B), thus confirming earlier published data.11 The measurement of miRNA species referenced to the stable miR-103 revealed increased expression of miR-34a and miR-199a-5p in COPD lung tissues as compared with normal control tissues (Figs 1C, 1D). A correlation analysis using FEV1 % predicted revealed a strong association between FEV1 % predicted and the expression of miR-34a and miR-199a-5p (Figs 2A, 2B). Although seven of 15 patients had a history of receiving inhaled corticosteroids and two patients with COPD had a history of oral corticosteroid treatment, all of the patients receiving inhaled corticosteroid treatment had severe COPD, and there were no significant differences in the expression of miR-34a and miR-199a-5p between the patients with or without steroid treatment (data not shown).
Figure 1.
mRNA and micro RNA (miRNA) expression in the lungs from patients with COPD. A, Individual data derived from reverse transcriptase polymerase chain reaction (RT-PCR) analysis of HIF-1α mRNA expression in the lungs from patients with COPD. B, Individual data derived from RT-PCR analysis of VEGF mRNA expression in the lungs from patients with COPD. C, Individual data derived from RT-PCR analysis of miR-34a miRNA expression in the lungs from patients with COPD. D, Individual data derived from RT-PCR analysis of miR-199a-5p miRNA expression in the lungs from patients with COPD. VEGF mRNA expression was significantly decreased in the lungs of patients with COPD compared with the expression of control (functionally and histologically normal) lungs. The expression of miR-34a and miR-199a-5p was significantly increased in the lungs from patients with COPD compared with the expression in control lungs. Data are expressed as mean ± SE *P < .05 vs no COPD. HIF-1α = hypoxia inducible factor-1α; VEGF = vascular endothelial growth factor.
Figure 2.
Relationship between miRNA expression in the lungs from patients with COPD and FEV1%. A, Correlation analysis between miR-34a expression and FEV1%. B, Correlation analysis between miR-199a-5p expression and FEV1%. A strong inverse correlation with FEV1% was noted of both miR-34a and miR-199a-5p miRNA expression. C, A strong correlation was found between miR-34a miRNA expression and miR-199a-5p miRNA expression. ○ = no COPD,  = mild COPD,
= mild COPD,  = moderate COPD, and ● = severe COPD. FEV1% = FEV1 % predicted. See Figure 1 legend for expansion of other abbreviation.
= moderate COPD, and ● = severe COPD. FEV1% = FEV1 % predicted. See Figure 1 legend for expansion of other abbreviation.
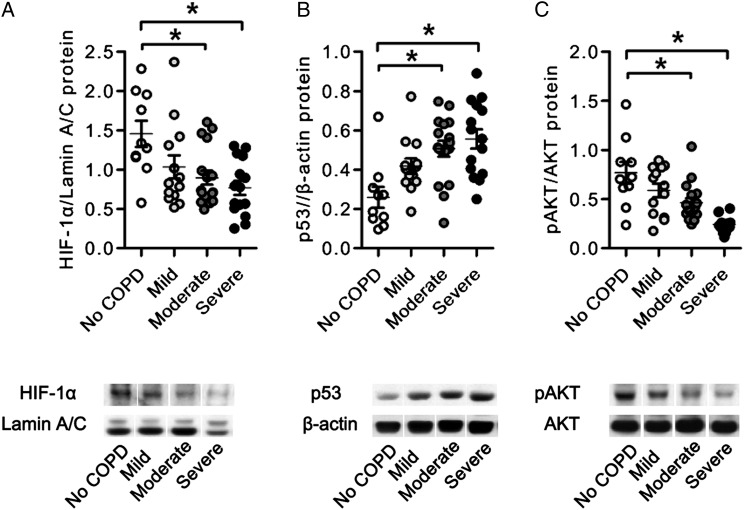
Alterations in Lung Tissue Protein Expression
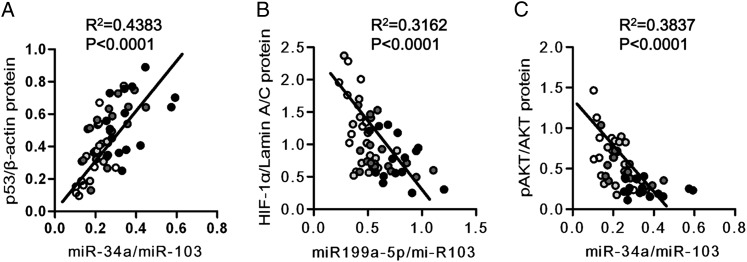
As can been seen in Figure 3, the lung tissue expression of HIF-1α protein was reduced in the samples of patients with COPD, and the amount of phosphorylated AKT (pAKT) referenced to total AKT was also reduced. The p53 protein was increased in the samples of patients with COPD. We next assessed and found that there were strong inverse correlations between miR-199a-5p and HIF-1α protein, between miR-199a-5p and pAKT, and between miR-34a and pAKT. In contrast, there was a positive correlation between miR-34a and p53 protein expression (Fig 4), as previously reported in cancer cells.
Figure 3.
Protein expression in the lungs from patients with COPD. Representative photographs of Western blot analyses of HIF-1α, lamin A/C, pAKT, AKT, p53, and β-actin in lung nuclear and cytosolic protein extracts from patients with COPD. A, Individual data derived from Western blot analyses of HIF-1α nuclear protein expression relative to lamin A/C nuclear protein expression. B, Individual data derived from Western blot analyses of p53 protein expression relative to β-actin or AKT protein expression. C, Individual data derived from Western blot analyses of pAKT protein expression relative to β-actin or AKT protein expression. The phosphorylation of AKT and expression of HIF-1α protein was decreased in the lungs from patients with COPD compared with control lungs. The expression of p53 protein was increased in the lungs from patients with COPD compared with control lungs. Data are expressed as mean ± SE *P < .05 vs no COPD. pAKT = phosphorylated AKT. See Figure 1 legend for expansion of other abbreviation.
Figure 4.
Correlations of AKT phosphorylation and correlation between miR-34a and p53 and between miR-199a-5p and HIF-1α in the lungs from patients with COPD. Correlation analysis between pAKT, HIF-1α, or p53 protein expression, and miR-34a or miR-199a-5p expression. A, A strong correlation was seen between p53 protein expression and miR-34a expression. B, A moderate correlation was seen between HIF-1α protein expression and miR-199a-5p expression. C, A moderate inverse correlation was seen between pAKT and miR34a. ○ = no COPD,  = mild COPD,
= mild COPD,  = moderate COPD, and ● = severe COPD. See Figure 1 and 3 legends for expansion of abbreviations.
= moderate COPD, and ● = severe COPD. See Figure 1 and 3 legends for expansion of abbreviations.
Gene Transfection Experiments in HPMVECs
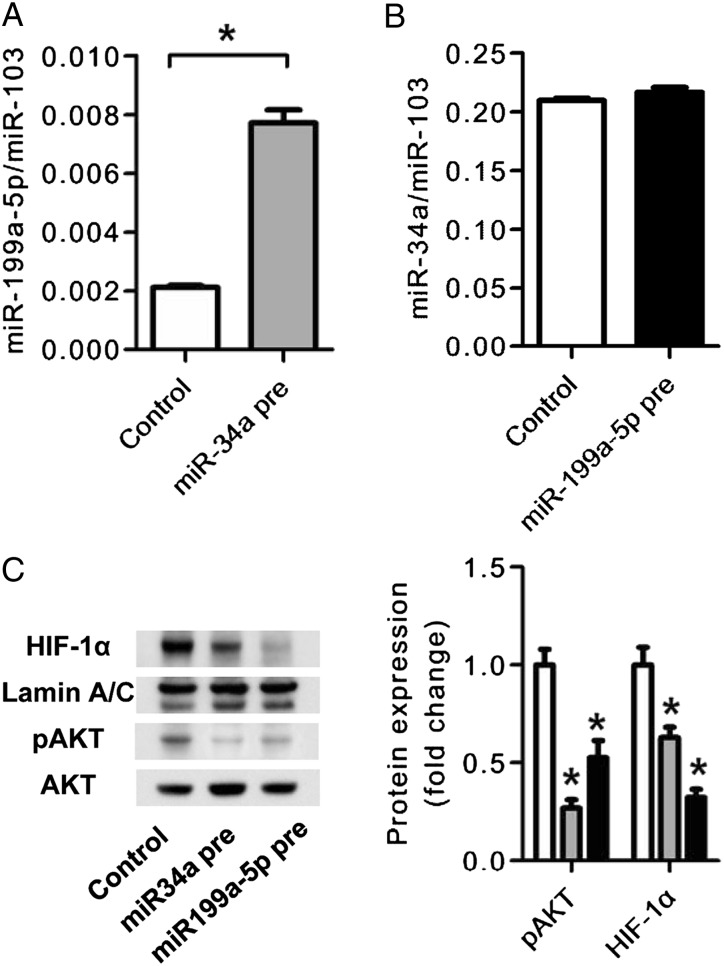
The next set of experiments was designed to examine whether the observed correlations in the patient tissues simply reflected chance relationships or whether they were indicative of a functional control by miRNA species of the expression of genes and proteins. To begin examining potential mechanisms that could explain the correlations between expressed miRNA and proteins (Figs 2, 4), we conducted experiments using cultured HPMVECs. We unexpectedly found a strong correlation between FEV1 % predicted and the expression of miR-199a-5p and miR-34a (Figs 2A, 2B). Furthermore, the tissue expression of miR-34a and miR-199a-5p was correlated (Fig 2C), and subsequently we wished to examine possible transcriptional interactions between these two miRNAs. We, thus, transfected HPMVECs either with miR-34a or miR-199a-5p precursor RNA and found that transfection of the endothelial cells with the miR-34a precursor increased the expression of miR-199a-5p but did not change the expression of miR-34a (Figs 5A, 5B). However, transfection of HPMVEC with either miRNA precursor decreased the expression of HIF-1α protein and of pAKT, thus providing evidence that in these endothelial cells HIF-1α gene expression is controlled by both miR-34a and miR-199a-5p (Fig 5C).
Figure 5.
Effects of miR-34a and miR-199a-5p transfection in cultured human pulmonary microvascular endothelial cells (HPMVECs). A, RT-PCR analysis of miR-199a-5p expression in miR-34a precursor miRNA-transfected HPMVECs. The miR34a pre increased the expression of miR-199a-5p compared with the control precursor miRNA transfection. B, RT-PCR analysis of miR-34a expression in miR-199a-5p precursor miRNA-transfected HPMVECs. The miR199a-5p pre had no effect on the expression of miR34a. C, Western blot analysis of HIF-1α, lamin A/C, pAKT, and AKT in miR-34a and miR-199a-5p precursor-transfected HPMVECs. The miR-199a-5p precursor transfection (gray bars) decreased HIF-1α, and the miR-34a precursor transfection (black bars) decreased pAKT expression compared with control precursor miRNA transfection (open bars). Data are expressed as mean ± SE (n = 6 wells or dishes for each group). *P < .05 vs control. miR34a pre = miR-34a precursor transfection; miR-199a-5p pre = miR-199-a-59 precursor transfection. See Figure 1 and 3 legends for expansion of other abbreviations.
p53 and pAKT Control miR199a-5p Expression
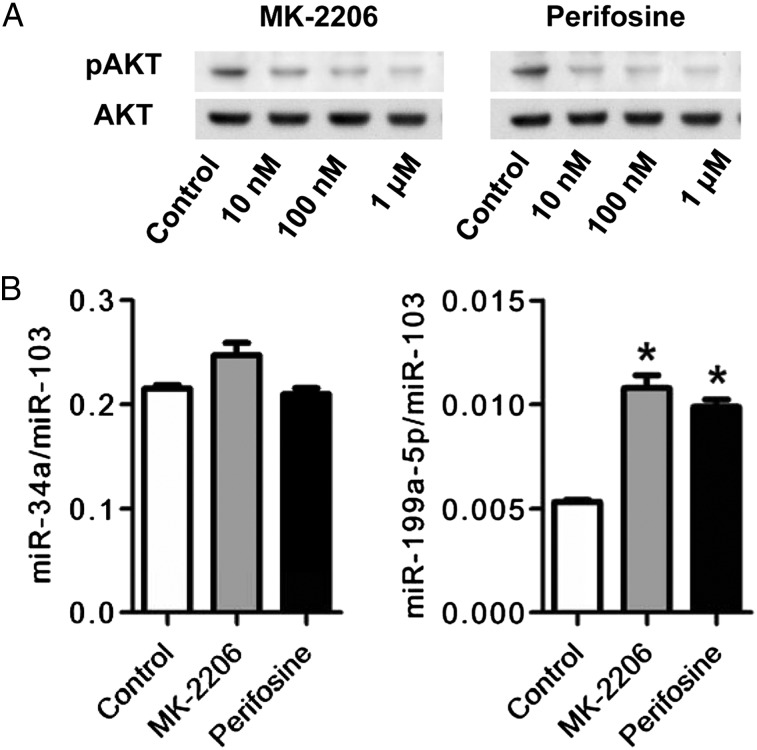
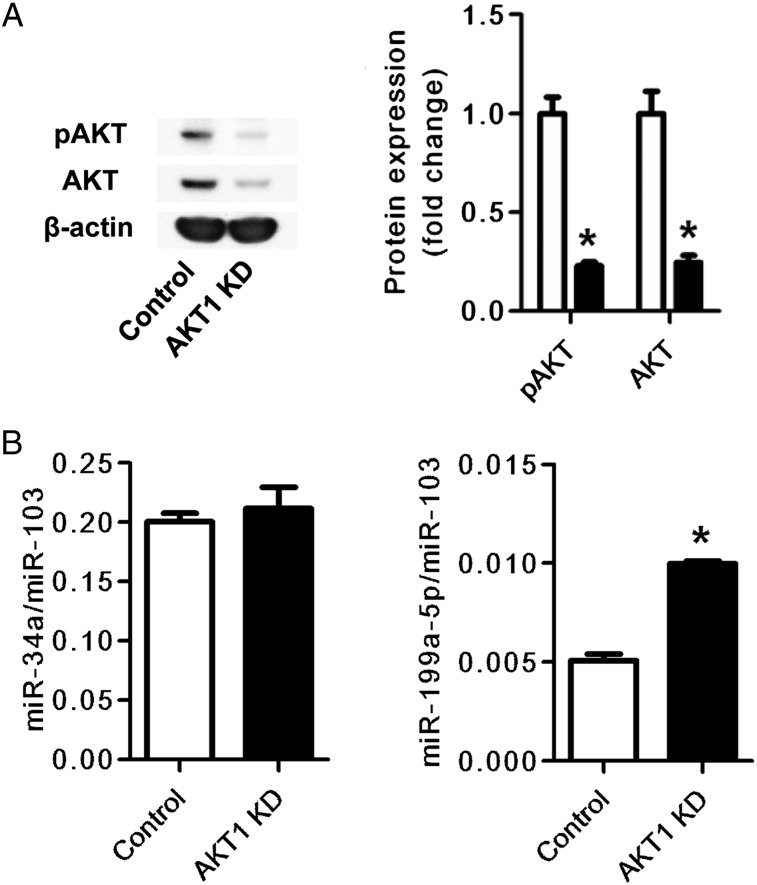
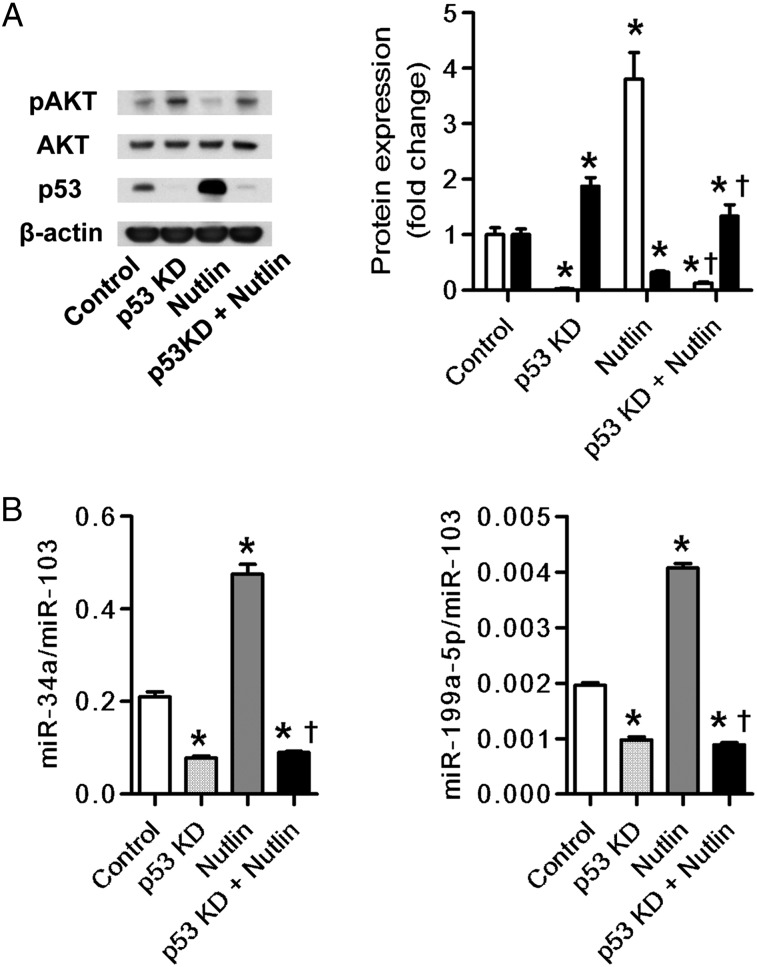
To examine whether the AKT phosphorylation affected the expression of either miR-34a or miR-199a-5p, we used two different pharmacologic inhibitors of AKT phosphorylation (MK-2206 and perifosine). The inhibitors decreased the expression of pAKT and increased the expression of miR-199a-5p, but they did not affect the expression of miR-34a (Figs 6A, 6B).These data were supported by additional data that demonstrate that gene knockdown of AKT1 increased miR-199a-5p expression in HPMVECs but did not affect miR-34a expression (Figs 7A, 7B). Nutlin, known as an inducer of the p53 protein,23 increased miR-34a and miR-199a-5p expression and decreased AKT phosphorylation. The knockdown of the p53 gene suppressed the nutlin-induced expression of miR-34a and miR-199a-5p (Fig 8).
Figure 6.
Effects of AKT inhibition on miR-34a and miR-199a-5p expression in cultured HPMVECs. A, Western blot analysis of pAKT and AKT shows that AKT phosphorylation was dose-dependently suppressed by both MK-2206 and perifosine starting at a concentration of 10 nM. B, RT-PCR analysis of miR-34a and miR-199a-5p expression and Western blot analysis of AKT phosphorylation in cultured HPMVECs treated with MK-2206 and perifosine. Both AKT inhibitors, MK-2206 (gray bars) and perifosine (black bars) increased the expression of miR-199a-5p expression. Data are expressed as mean ± SE (n = 6 wells or dishes for each group). *P < .05 vs control. See Figure 1, 3, and 5 legends for expansion of abbreviations.
Figure 7.
Effects of AKT1 gene silencing on miR-34a and miR-199a-5p expression in cultured HPMVECs. A, Western blot analysis of pAKT and AKT shows that AKT and pAKT expression was suppressed in AKT1 gene-silenced cells (AKT1 KD) compared with control siRNA-transfected cells (Control). The bar graph shows protein expression of pAKT and AKT in AKT1 siRNA-transfected cells (closed bars) relative to control siRNA-transfected cells (open bars). B, RT-PCR analysis of miR-34a and miR-199a-5p expression in AKT1 gene-silenced HPMVECs. The miR-199a-5p expression was increased in AKT1 gene-silenced HPMVECs (AKT1 KD) compared with control siRNA-treated cells (Control). Data are expressed as mean ± SE (n = 6 wells or dishes for each group). *P < .05 vs control. KD = knock down. See Figure 1, 3, and 5 legends for expansion of abbreviations.
Figure 8.
Effects of p53 expression on miR-34a and miR-199a-5p expression in cultured HPMVECs. A, Western blot analysis shows increased expression of p53 protein (open bars) in nutlin-treated cells and the suppressed p53 expression by p53 gene silencing (p53 KD). Phosphorylation of AKT (closed bars) was decreased in nutlin-treated cells and the decrease of AKT phosphorylation was in turn suppressed by p53 gene silencing. B, RT-PCR and Western blot analysis in p53 siRNA-transfected HPMVECs treated with or without 10 μM of nutlin. The expression of miR34a and miR199a-5p was significantly increased by nutlin, and this increase was completely suppressed by p53 gene silencing (p53KD). Data are expressed as mean ± SE (n = 6 wells or dishes for each group). *P < .05 vs control. †P < .05 vs nutlin-treated cells. See Figure 1, 3, 5, and 7 legends for expansion of abbreviations.
Discussion
The destruction of lung tissue in patients with COPD and emphysema has been well characterized by histologic studies and inflammatory and per se noninflammatory pathomechanisms have been proposed and discussed,24 also in the context of a homeostatic adult lung structure maintenance program.25 We have reported a decreased expression of HIF-1α protein in lungs from patients with COPD and varying degrees of lung function impairment.14 Here, to our knowledge, we report for the first time the increased lung tissue expression of miR-34a and miR-199a-5p in a new cohort of patients with COPD/emphysema. We also show that the increased expression of these miRNA species was associated with impaired lung function, as assessed by FEV1 % predicted. Because of reports demonstrating that the expression of the important transcription factor HIF-1α was under the control of miRNA,19,26,27 we conducted experiments in HPMVECs to investigate interactions between miRNA and HIF-1α expression. In view of other data demonstrating increased p53 expression in COPD/emphysema lung tissues14,20 and in view of the established interaction between p53 and HIF-1α protein expression,28,29 we also examined the interaction between p53 and HIF-1α expression in HPMVECs.
As a starting point for our lung tissue analysis, we measured HIF-1α and VEGF expression in all of the 55 lung samples and confirmed in this large cohort the previously published results of decreased VEGF expression.30 In this present data set, there was only a statistical trend for a decreased lung tissue HIF-1α mRNA expression (Fig 1A). Although a global miRNA expression analysis of the tissues is feasible, we focused on the analysis of miR-34a and miR-199a-5p; these miRNA have previously been shown to be involved in the control of HIF-1α and VEGF protein expression19,26 or were shown to be controlled by p53.21,22 We have previously proposed, based on the expression analysis of lung tissues from patients with COPD, that the reduction of lung tissue histone deacetylase 2 expression may be related to the regulation of both p53 and HIF-1α14 and to lung cell apoptosis and maintenance of pulmonary microvessels.29 Here, we analyzed the expression of miRNAs, known to control the expression of these proteins, and found that the expression of miR-34a and miR-199a-5p was related to lung disease severity (Figs 1C, 1D) and further that there was a correlation between the expression of miR-34a and miR-199a-5p (Fig 2C). Although all of the patients with COPD categorized by GOLD criteria were smokers or ex-smokers, neither miRNA species related to the pack-years of smoking or to the diagnosis of lung cancer. Figure 3 shows that there is also a disease severity-dependent decrease in lung tissue HIF-1α and pAKT protein. The p53 protein expression was positively correlated with the expression of miR-34a, and the HIF-1α protein expression was inversely correlated with the expression of miR-199a-5p (Figs 4A, 4B).
Because endothelial cells in the lungs of patients with COPD/emphysema undergo apoptosis,11 and there is evidence of lung endothelial cell dysfunction,12,31 we examined in human lung microvascular cells the transcriptional interactions between HIF-1α and miR-34a and miR-199a-5p. To answer the question whether the expression of either one of these two miRNAs affected the expression of the other miRNA, cells were transfected with their precursor miRNAs, and transfection with precursor miR-34a upregulated the expression of miR-199a-5p (Fig 5), supporting the idea that the positive correlation between the expression of these two miRNAs shown in the lung tissue extracts of patients with COPD (Fig 2C) had not occurred by chance. Western blot analysis showed that the transfection of HPMVECs with either precursor miR-34a or precursor miR-199a-5p decreased AKT phosphorylation and HIF-1α protein expression, suggesting that miRNA-34a controlled the expression of pAKT and possibly also of miR-199a-5p. To further evaluate whether miR-34a indeed was upstream of AKT, two different blockers of AKT phosphorylation and knockdown of the AKT1 gene were used, and the data shown in Figure 6 are consistent with the idea that AKT controls the expression of miR-199a-5p but not of miR-34a. In addition, the knockdown of p53 robustly decreased the expression of these miRNAs, suggesting a p53 control upstream of the miR-34a and miR-199a-5p. These results were confirmed when the nutlin-induced p53 expression was accompanied by miR-34a and miR-199a-5p overexpression, which in turn was abrogated in HPMVECs in which p53 had been knocked down (Fig 8). When the results of these HPMVEC gene and protein expression data are taken together, a signaling pathway emerges that connects p53, AKT, and miRNA in the control of HIF-1α protein expression and in turn HIF-1α protein expression in the control of AKT. The importance of HIF-1α in the regulation of pulmonary vascular endothelial cell gene expression in response to hypoxia has been demonstrated by Manalo et al,32 in particular the several-fold increased expression of the genes encoding prostacyclin synthase, lysyl oxidase, VEGF, and peroxisome proliferator activator receptor-γ.
Here we have connected for the first time, to our knowledge, in nonmalignant cells (HPMVECs) the role of miRNA in the transcriptional control of genes and the consequences for protein expression of two transcription factors that shape the tissue response to hypoxia and genotoxic stress. The regulation of HIF-1α by miR-199a-5p in neonatal cardiomyocytes18 and in HPMVEC has been illustrated by Gonsalves and Kalra.19 In cultured melanoma cells, miR-34a inhibits cell proliferation,33 and there is a consensus that miR-34a, a direct p53 target, mediates induction of apoptosis22 via a number of miR-34a-responsive cell cycle progression and angiogenesis genes, and because ectopic expression of miR-34a induces apoptosis or senescence21 and decreases AKT phosphorylation.33 Finally, and pointing toward complex interactions, in ovarian cancer cells the upregulation of HIF-1α expression was shown to be abrogated by an AKT inhibitor and AKT siRNA interference.17 The schematic in Figure 9 illustrates our attempt to connect signaling events both upstream and downstream from VEGF. Kasahara et al11 reported decreased expression of the VEGFR2 (KDR) in human lungs with emphysema, and Yasuo et al14 also reported decreased AKT phosphorylation and decreased HIF-1α but increased p53 expression. Here, we report increased expression of miR-199a-5p, which may be regulated by the inactivation of AKT and connected with the decreased expression of HIF-1α. Further, we report increased expression of miR-34a, induced by p53 upregulation; both may be associated with AKT inactivation in human lungs with emphysema (Fig 9). By highlighting the actions of p53, miR-34a, and miR-199a-5p in lung endothelial cells, and postulating that these actions affected the expression of the HIF-1α protein in the lungs from patients with COPD/emphysema, we do not intend to limit the role of HIF-1α in lung structure maintenance to the homeostasis of lung vessels. The proposed signaling interactions are likely to apply equally to the control of HIF-1α expression in the response of stressed lung epithelial cells.34
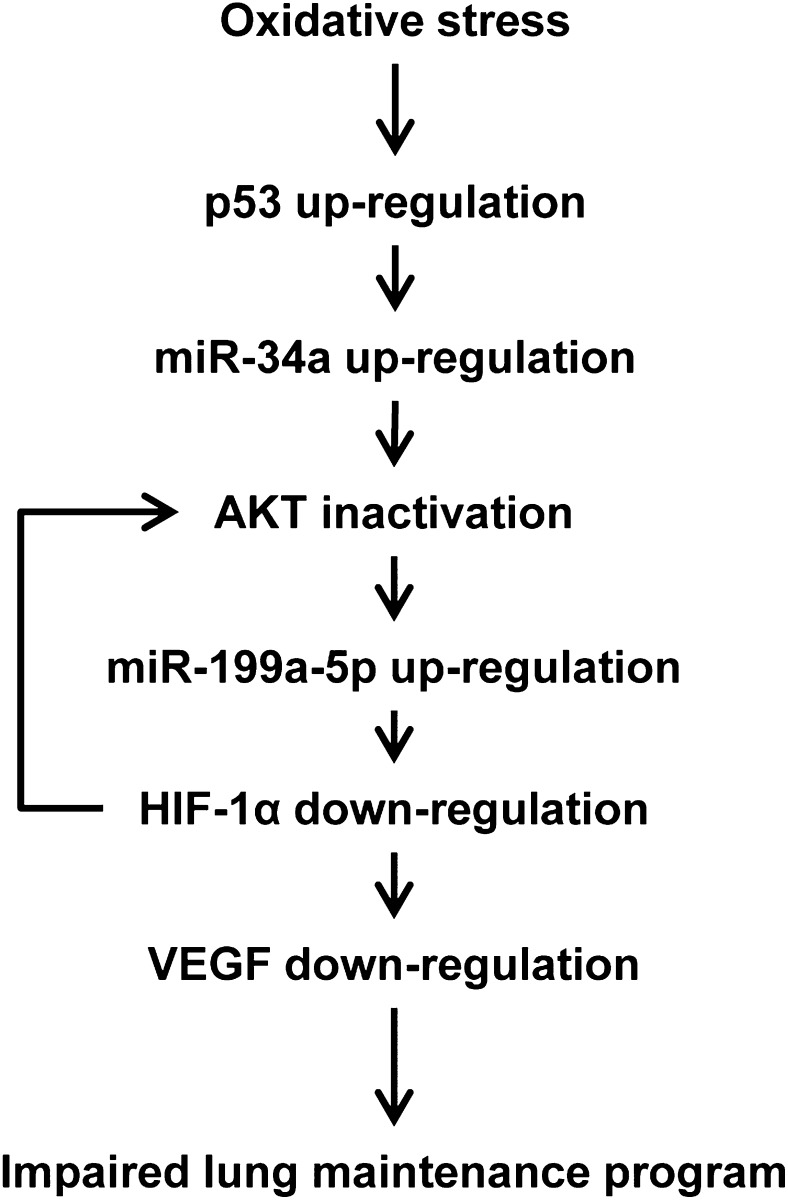
Figure 9.
Schematic depicting cellular and molecular interactions that play a role in the homeostatic maintenance of the adult lung structure. Oxidative stress increases p53 protein expression. Upregulated p53 induces miR-34a expression and reduces AKT phosphorylation. In contrast, AKT inactivation causes upregulation of miR-199a-5p, which reduces the expression of HIF-1α. Reduced expression of HIF-1α and reduced HIF-1α transactivation impair VEGF expression and phosphorylation of AKT, which could cause lung cell apoptosis and emphysema. See Figure 1 and 3 legends for expansion of abbreviations.
In the present study of COPD tissues, > 40% of the patients had lung cancer. Because it is well known that p53 is closely related to the pathogenesis of lung cancer, and HIF-1α levels are higher in lung cancer tissue samples,35,36 there is a possibility that the decreased expression of HIF-1α and overexpression of p53 and miR-34a are related to lung cancer. Although we could not find significant differences of p53, HIF-1α, and miR-34a expression between the samples from with and without lung cancer (data not shown), future studies will continue to examine a potential impact of lung cancer on gene expression in COPD lung tissue samples.
The interpretation of our human lung tissue gene and protein expression results is limited by the unavailability of blood gas data, and, thus, a potential influence of hypoxemia could not be evaluated. It is apparent that each lung tissue sample provides only one snapshot of a chronic disease process and that the mechanistic manipulations of gene knockdown and increased expression could not be performed using the patients’ diseased tissues. In addition, COPD is a heterogeneous disease. In the present study, the patients were categorized according to the FEV1 % predicted, not according to the severity of the lung tissue destruction. However, all of the tissue samples from patients with COPD showed centrilobular emphysema, and none of the lung tissues from patients without COPD had high levels of miR-34a or miR-199a-5p expression. We believe that HIF-1α and these miRNAs play a part in the pathogenesis and development of emphysema.
Because we measured the miRNAs and protein expressions from whole lung tissue samples, we could not determine which cells contribute these signals. However, we reported the decreased expression of HIF-1α in lung tissues from patients with COPD in both alveolar epithelial cells and pulmonary vascular endothelial cells.14 Based on this previous report, we speculate that the decreased expression of HIF-1α and increased expression of miR-199a-5p in both pulmonary endothelial cells and alveolar septal cells affect the pulmonary endothelial cell survival in COPD lung tissues, and the survival of the endothelial cells may also play a part in the lung maintenance program and pathogenesis of pulmonary emphysema.
In conclusion, we show, using a large number of tissue samples, that miR-34a and miR-199a-5p are overexpressed in lungs from patients with COPD/emphysema, when compared with the expression levels in histologically normal lungs. Manipulations of gene and protein expression in human lung microvascular endothelial cells shed light on a complicated miRNA-dependent mechanism of HIF-1α protein expression control.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Dr Mizuno: contributed to designing and organizing the experiments, carrying out the data analysis, and writing of the manuscript.
Dr Bogaard: contributed to the design of the study and revising the manuscript.
Dr Gomez-Arroyo: contributed to laboratory measurements, data analysis, and writing of the manuscript.
Dr Alhussaini: contributed to laboratory measurements, data analysis, and writing of the manuscript.
Dr Kraskauskas: contributed to laboratory measurements, data analysis, and writing of the manuscript.
Dr Cool: contributed to the design of the study, examination of the lung tissues, and writing of the manuscript.
Dr Voelkel: contributed to conceiving the ideas, supervising the study, and writing the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The National Institutes of Health/National Heart, Lung and Blood Institute Lung Tissue Repository provided the human tissue samples and supported the studies.
Other contributions: We thank Vita Kraskauskiene, BS, for expert technical assistance. This work was performed at the Victoria Johnson Center for Obstructive Lung Diseases, Virginia Commonwealth University, Richmond, VA.
Additional information: The e-Appendix can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- HIF-1α
hypoxia-inducible factor-1α
- HPMVEC
human pulmonary microvascular endothelial cell
- LTRC
Lung Tissue Repository Consortium
- miRNA
microRNA
- pAKT
phosphorylated AKT
- PVDF
polyvinylidene difluoride
- RT-PCR
reverse transcriptase polymerase chain reaction
- siRNA
small interfering RNA
- VEGF
vascular endothelial growth factor
Funding/Support: This study was supported by the National Institutes of Health [Grant N01-HR-46160-3], and funds from the Victoria Johnson Center for Lung Research of the Virginia Commonwealth University.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11(4):441-450 [DOI] [PubMed] [Google Scholar]
- 2.Gangaraju VK, Lin H. MicroRNAs: key regulators of stem cells. Nat Rev Mol Cell Biol. 2009;10(2):116-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23175-205 [DOI] [PubMed] [Google Scholar]
- 4.Inui M, Martello G, Piccolo S. MicroRNA control of signal transduction. Nat Rev Mol Cell Biol. 2010;11(4):252-263 [DOI] [PubMed] [Google Scholar]
- 5.Schembri F, Sridhar S, Perdomo C, et al. MicroRNAs as modulators of smoking-induced gene expression changes in human airway epithelium. Proc Natl Acad Sci U S A. 2009;106(7):2319-2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xi S, Yang M, Tao Y, et al. Cigarette smoke induces C/EBP-β-mediated activation of miR-31 in normal human respiratory epithelia and lung cancer cells. PLoS ONE. 2010;5(10):e13764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Izzotti A, Larghero P, Balansky R, Pfeffer U, Steele VE, De Flora S. Interplay between histopathological alterations, cigarette smoke and chemopreventive agents in defining microRNA profiles in mouse lung. Mutat Res. 2011;717(1-2):17-24 [DOI] [PubMed] [Google Scholar]
- 8.Wu X, Piper-Hunter MG, Crawford M, et al. MicroRNAs in the pathogenesis of lung cancer. J Thorac Oncol. 2009;4(8):1028-1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandit KV, Corcoran D, Yousef H, et al. Inhibition and role of let-7d in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;182(2):220-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pottelberge GR, Mestdagh P, Bracke KR, et al. MicroRNA expression in induced sputum of smokers and patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183(7):898-906 [DOI] [PubMed] [Google Scholar]
- 11.Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med. 2001;163(3 pt 1):737-744 [DOI] [PubMed] [Google Scholar]
- 12.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106(11):1311-1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang K, Rossiter HB, Wagner PD, Breen EC. Lung-targeted VEGF inactivation leads to an emphysema phenotype in mice. J Appl Physiol. 2004;97(4):1559-1566 [DOI] [PubMed] [Google Scholar]
- 14.Yasuo M, Mizuno S, Kraskauskas D, et al. Hypoxia inducible factor-1α in human emphysema lung tissue. Eur Respir J. 2011;37(4):775-783 [DOI] [PubMed] [Google Scholar]
- 15.Jiang BH, Jiang G, Zheng JZ, Lu Z, Hunter T, Vogt PK. Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth Differ. 2001;12(7):363-369 [PubMed] [Google Scholar]
- 16.Kim CH, Cho YS, Chun YS, Park JW, Kim MS. Early expression of myocardial HIF-1alpha in response to mechanical stresses: regulation by stretch-activated channels and the phosphatidylinositol 3-kinase signaling pathway. Circ Res. 2002;90(2):E25-E33 [DOI] [PubMed] [Google Scholar]
- 17.Hua K, Din J, Cao Q, et al. Estrogen and progestin regulate HIF-1alpha expression in ovarian cancer cell lines via the activation of Akt signaling transduction pathway. Oncol Rep. 2009;21(4):893-898 [DOI] [PubMed] [Google Scholar]
- 18.Rane S, He M, Sayed D, Yan L, Vatner D, Abdellatif M. An antagonism between the AKT and beta-adrenergic signaling pathways mediated through their reciprocal effects on miR-199a-5p. Cell Signal. 2010;22(7):1054-1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonsalves CS, Kalra VK. Hypoxia-mediated expression of 5-lipoxygenase-activating protein involves HIF-1alpha and NF-kappaB and microRNAs 135a and 199a-5p. J Immunol. 2010;184(7):3878-3888 [DOI] [PubMed] [Google Scholar]
- 20.Morissette MC, Vachon-Beaudoin G, Parent J, Chakir J, Milot J. Increased p53 level, Bax/Bcl-x(L) ratio, and TRAIL receptor expression in human emphysema. Am J Respir Crit Care Med. 2008;178(3):240-247 [DOI] [PubMed] [Google Scholar]
- 21.Hermeking H. p53 enters the microRNA world. Cancer Cell. 2007;12(5):414-418 [DOI] [PubMed] [Google Scholar]
- 22.Chang TC, Wentzel EA, Kent OA, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26(5):745-752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Secchiero P, Corallini F, Rimondi E, et al. Activation of the p53 pathway down-regulates the osteoprotegerin expression and release by vascular endothelial cells. Blood. 2008;111(3):1287-1294 [DOI] [PubMed] [Google Scholar]
- 24.Taraseviciene-Stewart L, Voelkel NF. Molecular pathogenesis of emphysema. J Clin Invest. 2008;118(2):394-402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuder RM, Yoshida T, Fijalkowka I, Biswal S, Petrache I. Role of lung maintenance program in the heterogeneity of lung destruction in emphysema. Proc Am Thorac Soc. 2006;3(8):673-679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rane S, He M, Sayed D, et al. Downregulation of miR-199a derepresses hypoxia-inducible factor-1alpha and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res. 2009;104(7):879-886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taguchi A, Yanagisawa K, Tanaka M, et al. Identification of hypoxia-inducible factor-1 alpha as a novel target for miR-17-92 microRNA cluster. Cancer Res. 2008;68(14):5540-5545 [DOI] [PubMed] [Google Scholar]
- 28.Sano M, Minamino T, Toko H, et al. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446(7134):444-448 [DOI] [PubMed] [Google Scholar]
- 29.Mizuno S, Yasuo M, Bogaard HJ, Kraskauskas D, Natarajan R, Voelkel NF. Inhibition of histone deacetylase causes emphysema. Am J Physiol Lung Cell Mol Physiol. 2011;300(3):L402-L413 [DOI] [PubMed] [Google Scholar]
- 30.Suzuki M, Betsuyaku T, Nagai K, et al. Decreased airway expression of vascular endothelial growth factor in cigarette smoke-induced emphysema in mice and COPD patients. Inhal Toxicol. 2008;20(3):349-359 [DOI] [PubMed] [Google Scholar]
- 31.Giordano RJ, Lahdenranta J, Zhen L, et al. Targeted induction of lung endothelial cell apoptosis causes emphysema-like changes in the mouse. J Biol Chem. 2008;283(43):29447-29460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manalo DJ, Rowan A, Lavoie T, et al. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105(2):659-669 [DOI] [PubMed] [Google Scholar]
- 33.Yan D, Zhou X, Chen X, et al. MicroRNA-34a inhibits uveal melanoma cell proliferation and migration through downregulation of c-Met. Invest Ophthalmol Vis Sci. 2009;50(4):1559-1565 [DOI] [PubMed] [Google Scholar]
- 34.Shimoda LA, Semenza GL. HIF and the lung: role of hypoxia-inducible factors in pulmonary development and disease. Am J Respir Crit Care Med. 2011;183(2):152-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giatromanolaki A, Koukourakis MI, Sivridis E, et al. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer. 2001;85(6):881-890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koukourakis MI, Papazoglou D, Giatromanolaki A, et al. C2028T polymorphism in exon 12 and dinucleotide repeat polymorphism in intron 13 of the HIF-1alpha gene define HIF-1alpha protein expression in non-small cell lung cancer. Lung Cancer. 2006;53(3):257-262 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement