Abstract
Embryonic development is controlled by a small set of signal transduction pathways, with vastly different phenotypic outcomes depending on the time and place of their recruitment. How the same molecular machinery can elicit such specific and distinct responses, remains one of the outstanding questions in developmental biology. Part of the answer may lie in the high inherent genetic complexity of these signaling cascades, as observed for the Wnt-pathway. The mammalian genome encodes multiple Wnt proteins and receptors, each of which show dynamic and tightly controlled expression patterns in the embryo. Yet how these components interact in the context of the whole organism remains unknown.
Here we report the generation of a novel, inducible transgenic mouse model that allows spatiotemporal control over the expression of Wnt5a, a protein implicated in many developmental processes and multiple Wnt-signaling responses. We show that ectopic Wnt5a expression from E10.5 onwards results in a variety of developmental defects, including loss of hair follicles and reduced bone formation in the skull. Moreover, we find that Wnt5a can have dual signaling activities during mouse embryonic development. Specifically, Wnt5a is capable of both inducing and repressing β-catenin/TCF signaling in vivo, depending on the time and site of expression and the receptors expressed by receiving cells. These experiments show for the first time that a single mammalian Wnt protein can have multiple signaling activities in vivo, thereby furthering our understanding of how signaling specificity is achieved in a complex developmental context.
Keywords: Wnt5a, Ror2, development, hair follicles, calvarial mesenchyme, mouse model
Introduction
In all multicellular animals, tissue morphogenesis is regulated by the concerted activities of a limited number of developmental signal transduction pathways, including Wnt, Hedgehog and Notch (Gerhart, 1999). Over the past thirty years our knowledge regarding the molecular nature of the intracellular signaling events triggered by pathway activation has steadily increased. However, we have only begun to scratch the surface when it comes to understanding how these same molecular pathways can be used over and over again, at multiple developmental sites and time points, with vastly different phenotypic outcomes. One reason for our apparent lack of understanding signal transduction in the context of an intact, multicellular animal, is the obvious intricacy of the organism under study: At any one time a given cell will find itself capable of receiving simultaneous inputs from multiple sources, including the extracellular environment and neighboring cells, and in a developing organism these surroundings are rapidly and continually changing. Equally important however, is the high complexity of the developmental signaling pathways themselves. An illustrative example is that of the Wnt-pathway, a major player in development, physiology and disease (Croce and McClay, 2008; Klaus and Birchmeier, 2008; MacDonald et al., 2009), which uses a large number of extracellular ligands (Wnts) and transmembrane receptors (Fzd) to elicit a diverse array of intracellular signaling responses.
Both ligands and receptors belong to large, multi-gene families: The mammalian genome encodes 19 Wnt and 10 Fzd homologues, totaling 190 different potential ligand/receptor pairings. Of note, this complexity is conserved in lower organisms, with the sea anemone Nematostella showing conservation of 11 of the 12 Wnt subfamilies (Kusserow et al., 2005). Further complication arises from the presence of additional (co-)receptors, and secreted inhibitors or co-activators, which help shape the signaling response (see for instance (Mii and Taira, 2009)). Furthermore, Wnt proteins can elicit multiple intracellular responses (James et al., 2008; Komiya and Habas, 2008). The best-characterized response downstream of Wnt/Fzd binding results in the activation of β-catenin/TCF transcriptional complexes (hereafter ‘Wnt/β-catenin signaling’) and requires the recruitment of LRP5/6 co-receptors (Pinson et al., 2000; Tamai et al., 2000; Wehrli et al., 2000). For many years, Wnt proteins themselves were classified as either ‘canonical’ or ‘non-canonical’ based on their (in)ability to activate Wnt/β-catenin signaling (Du et al., 1995; Shimizu et al., 1997; Wong et al., 1994). This strict subdivision was challenged however, by the finding that a given Wnt can be turned into an activator of Wnt/β-catenin signaling when provided with the appropriate receptor (He et al., 1997).
In light of this complexity, a key question then is how signaling specificity is achieved in a developmental context. One step towards answering this question will come from determining whether different, and if so which, combinations of ligands and receptors trigger specific signaling outcomes. To date however, it has proven a challenge to determine binding affinities for specific Wnt/Fzd complexes. Most efforts have stalled due to the hydrophobic nature of the Wnt proteins and the concomitant difficulty of purifying them. Although this hurdle has been overcome for some Wnt proteins (Mikels and Nusse, 2006; Willert et al., 2003), it has prevented comprehensive quantitative biochemical analyses in vitro. Both Wnts and Fzds display tightly regulated and highly dynamic spatiotemporal expression patterns within the developing organism (Fischer et al., 2007; Summerhurst et al., 2008; Witte et al., 2009), suggesting equally dynamic ligand/receptor interactions. Although some Wnt/receptor pairings appear to be more likely than others (Carmon and Loose, 2010; Hsieh et al., 1999; Mikels and Nusse, 2006; Takada et al., 2005; Wu and Nusse, 2002), at present it remains largely unknown which ligands engage which receptors under which circumstances.
One particularly intriguing Wnt protein is Wnt5a, which has been implicated in many developmental processes and which can activate multiple intracellular signaling responses. Our lab previously showed that mouse Wnt5a, which is typically not found to activate a β-catenin/TCF responsive reporter gene, was able to induce Wnt/β-catenin signaling in 293 cells overexpressing Fzd4 and LRP5 (Mikels and Nusse, 2006). In their absence however, the cells responded to Wnt5a by inhibiting Wnt3A-mediated signaling through β-catenin/TCF. The capacity of Wnt5a to inhibit Wnt/β-catenin signaling has been reported in multiple systems and organisms (Li et al., 2010; Olson and Gibo, 1998; Topol et al., 2003; Torres et al., 1996) and is mediated through the receptor tyrosine kinase Ror2, which has been reported to function as either a co-receptor or a bona-fide Wnt-receptor (Green et al., 2007; Grumolato et al., 2010; Mikels et al., 2009; Nishita et al., 2010). In addition to the above, Wnt5a can also induce alternative signaling responses that appear to proceed independently from β-catenin. Although many of these remain poorly characterized at the biochemical level, they too have been reported to involve Fzd as well as Ror2 receptors (Nishita et al., 2010; Nomachi et al., 2008; Oishi et al., 2003; Sato et al., 2010).
Wnt5a-knockout mice have a variety of phenotypes, including skeletal defects (Yamaguchi et al., 1999) and multiple defects associated with internal organs (Allgeier et al., 2008; Andersson et al., 2008; Cervantes et al., 2009; Cha et al., 2004; He et al., 2008; Kim et al., 2005; Li et al., 2002; Qian et al., 2007; Roarty et al., 2009; Tai et al., 2009). Interestingly, the phenotype of Ror-knockout mice resembles that of Wnt5a-knockout mice. Both display dwarfism, craniofacial defects, limb abnormalities and intestinal elongation defects (Ho et al., 2012; Oishi et al., 2003; Takeuchi et al., 2000; Yamada et al., 2010). In addition, both Ror2 and Wnt5a loss-of-function alleles have been associated with increased Wnt/β-catenin signaling (Gao et al., 2011; Mikels et al., 2009; Topol et al., 2003). Taken together, these studies suggest the existence of a Wnt5a/Ror2 pathway that inhibits Wnt/β-catenin signaling in vivo. However, it is unknown whether this is the sole or dominant activity of Wnt5a. In particular, the question of whether mammalian Wnt5a also has the capacity to activate Wnt/β-catenin signaling in vivo remains unexplored.
The severe developmental phenotype observed in conventional Wnt5a-knockout mice precludes a straightforward analysis of Wnt5a-signaling. To uncover the potential signaling activities of Wnt5a during mammalian development, we therefore generated a novel, inducible transgenic mouse model that allows tight spatiotemporal control over Wnt5a expression. Our experiments reveal multiple phenotypes caused by ectopic Wnt5a expression, some of which can be linked to the inhibition of Wnt/β-catenin signaling and others to its activation. First, overexpression of Wnt5a in the developing skin results in a loss of hair follicle formation, coinciding with a decrease in β-catenin/TCF reporter activity in the dermis and revealing a previously unrecognized role for Wnt5a/Ror2 in patterning of the skin. Second, Wnt5a overexpression causes delayed bone formation in the developing skull. Unexpectedly, this is preceded by an increase in β-catenin/TCF reporter activity in the calvarial mesenchyme, suggesting that Wnt5a can induce Wnt/β-catenin signaling in vivo. Taken together, our studies uncover novel roles for Wnt5a and its receptors during development and reveal a dual signaling capacity for Wnt5a in an intact, complex organism.
Materials and Methods
Animals
The tetO-Flag-Wnt5A transgenic construct was generated by cloning a Flag-tagged version of mouse Wnt5a downstream of an artificial signal sequence into the pTRE-tight vector (Clontech). Fourteen independent mouse lines (F5A-1 through F5A-14) were generated initially by injecting the transgenic construct into FVB oocytes. Four additional founder lines (F5A-15 through F5A-18) were generated in a second round of injection. The vast majority of experiments in this paper were performed with line F5A-5, which we have since replaced with line F5A-17. TetO-Flag-Wnt5A mice were kept on an FVB background, although all complex crosses were performed on a mixed background. Rosa26-rtTA-M2 mice (Hochedlinger et al., 2005) were obtained from Jackson Laboratories (stock #006965). Axin2-lacZ (Lustig et al., 2002) and TOPGAL (DasGupta and Fuchs, 1999) reporter mice were obtained from Dr. W. Birchmeier (Max Delbruck Center, Berlin-Buch, Germany) and Dr. E. Fuchs (Rockefeller University, New York, USA), respectively. The tetO-Dkk mice (Chu et al., 2004) were obtained from Dr. S. Millar (University of Pennsylvania, Philadelphia, USA), Ror2-knockout mice (Takeuchi et al., 2000) from Dr. Y. Minami (Kobe University, Kobe, Japan) and Wnt5a-knockout mice (Yamaguchi et al., 1999) were a gift from Dr. E. Vladar and Dr. J. Axelrod (Stanford University, USA). Mice were housed at the Stanford University Medical Center. All experiments were approved by the Stanford University Animal Care and Use Committee and performed according to NIH guidelines.
Genotyping
Tail clippings were lysed overnight at 55°C in Direct PCR lysis reagent (Viagen Biotech, Inc., Los Angeles, CA, USA) supplemented with proteinase K (100 ug/ml, Roche). Following heat inactivation at 85°C, the lysate was used for PCR with the following primers:
tetO-Wnt5A (transgene specific product, annealing at 55°C):
Fwd: ACAAAGACGATGACGACAAGC
Rev: CGCACCTTCTCCAATGTACTG
Rosa26-rtTA-M2 (transgene specific product, annealing at 60°C):
Fwd: CTGGGAGTTGAGCAGCCTAC
Rev: AGAGCACAGCGGAATGACTT
Axin2-lacZ and TOPGAL (transgene specific product, annealing at 55°C):
Fwd: ATCCTCTGCATGGTCAGGTC
Rev: CGTGGCCTGATTCATTCC
Ror2-knockout (WT band 500 bp; KO band 200 bp, annealing at 61°C):
WT Fwd: CTTAACTGTTCTAGGTCAAGTATG
WT Rev: CCTACTATAGACTCTGATCCTTCTGCC
Mutant Rev: ATCGCCTTCTATCGCCTTCTTGACGAG
Wnt5a-knockout (WT band 484 bp; KO band 400 bp, annealing at 55°C):
WT Fwd: GAGGAGAAGCGCAGTCAATC WT
Rev: CATCTCAACAAGGGCCTCAT
Mutant Fwd: GCCAGAGGCCACTTGTGTAG
Induction of tetO-Wnt5a transgene expression in vivo
Timed matings were set up and the morning on which a vaginal plug was discovered was designated E0.5. Induction of transgene expression during embryogenesis from the indicated timepoints onwards was achieved by dissolving doxycycline (Sigma) in the drinking water of pregnant dams. Unless otherwise indicated, mice received a final concentration of 1–2 mg/ml doxycycline. The doxycycline containing drinking water was refreshed three times per week.
Induction of tetO-Wnt5a transgene expression in vitro
Primary mouse embryo fibroblasts (MEFs) were isolated and cultured in a 3T3 protocol according to Jacobs et al. (Jacobs et al., 1999). Early passage MEFs from tetO-Wnt5A transgenic embryos or wildtype littermates were infected with a mix of pBabe-TBX2 (blasticidin resistance) and pBabe-rtTA3 (puromycin resistance) retroviruses to allow for immortalization and inducible expression of the tetO-Wnt5a transgene, respectively. Alternatively, transgene expression was induced in double-heterozygous tetO-Wnt5a;R26-rtTA MEFs. Cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, glutamine, penicillin/streptomycin and 50 μM β-mercaptoethanol under 5% CO2 at 37°C in humidifying conditions. To induce Wnt5a transgene expression, cells were cultured in the presence of 1 ug/ml doxycycline overnight (TBX2-infected MEFs), or in the presence of a concentration series of doxycycline for 3–5 days (tetO-Wnt5a;R26-rtTA MEFs), after which cells were lysed to extract RNA or protein.
Quantitative RT-PCR
RNA was isolated using an RNeasy Mini Kit (Qiagen) with on column DNAse digestion. cDNA was synthesized using random hexamer primers and the Thermoscript II RT-PCR system for First-Strand Synthesis (Invitrogen). Quantitative PCRs were performed on a Roche LightCycler (Roche) using the LightCycler Fast Start DNA Master Plus SYBR green I mix (Roche) with the following primers:
tetO-Wnt5a:
Fwd: GCGTGGCTATGACCAGTTTA
Rev: CTACAAATGTGGTATGGCTGATTA
mGAPDH:
Fwd: CTGGTGCTGCCAAGGCT
Rev: CTGCTTCACCACCTTCTTGATGTCATCATA
Protein isolation and Western blotting
Cells were lysed in RIPA buffer supplemented with Complete Protease Inhibitor Cocktail (Roche). Equal amounts of lysate (or purified Wnt5a protein; see below) were run on a 10% SDS/PAGE gel. Following Western blot transfer, membranes were blocked in TBST with 3% milk and 2% BSA. Wnt5a protein was detected using a primary antibody directed against the Flag tag (M1, Sigma) or Wnt5a (RnD), a secondary HRP-conjugated antibody (Santa Cruz Biotechnology) and ECL (Perkin Elmer). Ror2 protein expression was detected using hybridoma supernatant (1:100) containing the anti-Ror2 mouse monoclonal previously generated by our lab ((Mikels et al., 2009), available through the Developmental Studies Hybridoma Bank). An antibody detecting tubulin (Sigma) was used as a loading control.
Wnt5a protein purification and luciferase assays
Flag-tagged Wnt5a protein was purified from stably transfected S2 cells by consecutive affinity chromatography and gel filtration steps as described previously (Mikels and Nusse, 2006; Willert et al., 2003). Peak fractions were identified by Western blot analysis and used in luciferase assays. To test the efficacy of Flag-Wnt5a in inhibiting the activity of Wnt3a, 293T cells transfected with the SuperTOPFLASH luciferase reporter and CMV-β-galactosidase for normalization were treated with different combinations of purified Wnt3a and Wnt5a. To test the ability of Flag-Wnt5a to induce β-catenin/TCF signaling, 293T cells were transfected with SuperTOPFLASH, CMV-β-galactosidase, Fzd4 and LRP5, and treated with purified Wnt3a or Wnt5a. Following overnight stimulation with purified Wnt proteins, cells were lysed and luciferase and β-galactosidase activities were measured using the Tropix Dual Light Reporter Gene Assay System (Applied Biosystems) on a Berthold luminometer (Berthold Technologies).
Histology and immunohistochemistry
Tissue samples were fixed in 4% paraformaldehyde, washed in PBS, dehydrated through a graded ethanol series, cleared in orange terpene and embedded in paraffin. For wholemount analysis of dorsal skins, samples were flat mounted and imaged under a dissecting scope (Leica) when they were in 70% ethanol. Contrast and color in these images were enhanced in Photoshop in order to better visualize pigmentation. Tissue blocks were sectioned on a microtome at 3–6 μm thickness, mounted on Superfrost glass microscope slides (Fisher Scientific) and left to dry at 37°C overnight. For further processing, sections were deparaffinized in orange terpene and rehydrated in a graded ethanol series. For H&E staining, slides were stained in hematoxilin (Sigma) and eosin Y (Sigma). For immunohistochemistry, antigen retrieval was performed in Tris/EDTA (pH 9.0). Endogenous peroxidase activity was blocked by incubating the sections with 0.3% H2O2. Slides were blocked using the Vector M.O.M. kit (Vectorlabs). Primary antibody incubation was performed with the anti-Ror2 mouse monoclonal (1:1000) described previously (Mikels et al., 2009) or a rabbit polyclonal antibody raised against Wnt5a (previously generated in our lab) at room temperature for 4 hours or overnight. After this, tissue sections were further processed using the Vectastain ABC system (Vectorlabs). Antibody binding was detected with VIP or DAB substrate (Vectorlabs). Following dehydration in a graded ethanol series and orange terpene, coverslips were sealed with Cytoseal-60 (Thermo Scientific). Images were acquired on a Zeiss upright microscope equipped with an Axiocam CCD camera.
Detection of endogenous AP and lacZ reporter gene activity
To visualize endogenous alkaline phosphatase (AP) activity in primary hair follicles, E14.5 embryos were processed according to Nagy et al. with minor modifications (Nagy et al., 2007). Embryos were fixed in 4% PFA for 30 minutes, rinsed in PBS and AP buffer, after which AP activity was detected with BM Purple substrate (Roche) in the dark at room temperature. Wholemount X-gal staining was performed according to Hogan (Hogan, 1994) with minor modifications. Organs from E14.5 or E16.5 embryos were microdissected following fixation (in PBS with 0.2% glutaraldehyde, 5 mM EGTA (pH 8.0) and 2 mM MgCl2). Tissues were washed in detergent rinse (PBS with 2 mM MgCl2, 0.01% sodium deoxycholate and 0.02% NP-40) and stained in staining solution (PBS with 2 mM MgCl2, 0.01% sodium deoxycholate, 0.02% NP-40, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide and 1 mg/ml X-gal) in the dark at room temperature overnight. Following staining, tissues were washed in detergent rinse, postfixed in 4% paraformaldehyde and processed for paraffin embedding. Wholemount tissues were photographed under a dissecting scope (Leica) when samples were in 70% ethanol. Paraffin sections of X-gal stained tissues were counterstained with nuclear fast red. In all cases, tetO-Wnt5a;R26rtTA double-transgenics were compared to control mice from the same litter, the samples of which processed at the same time and for the same duration and analyzed simultaneously.
Skeletal stainings
Skulls and skeletons from newborn mice were processed for Alizarin Red and Alcian Blue staining to detect bone and cartilage (McLeod, 1980). In short, tissues were fixed in 100% ethanol, washed in 100% acetone, stained in Alizarin Red and Alcian Blue for three days at 37°C, cleared in 1% KOH and processed through a graded glycerol series. Cleared specimens were stored in 100% glycerol and imaged under a dissecting scope (Leica).
Quantification of hair follicle number, animal size and bone length
To quantify the number of hair follicles in newborn skin, images of H&E stained sections were imported in Image J. A freehand line was drawn to trace and measure the length (in pixels) of a continuous stretch of longitudinally oriented skin (as determined by the orientation of the sub-dermal muscle fibers). The number of hair follicles in this stretch of skin was counted by hand. Hair follicle number was divided by the length of the skin in pixels. The resulting ratio, designated the ‘number of hair follicles per unit length’ was plotted.
To determine the relative size of newborn mice, images of double-heterozygotes photographed together with littermate controls were imported in Image J. A freehand line was drawn to trace and measure (in pixels) the distance from the nose, along the back of the animal to the tip of the tail. For each control littermate, this measurement was set at 100% and the size reduction in double-heterozygotes was calculated accordingly.
To quantify the reduction in bone length, images of Alazarin Red and Alcian Blue stained limbs were imported in Image J. A straight line was drawn from the distal to the proximal end of the bone. The measured distance (in pixels) was then used as a relative measure for bone length.
Results
Generation and characterization of tetO-Wnt5a transgenic mice
To uncover the potential signaling activities of Wnt5a in vivo, we generated an inducible transgenic mouse model allowing tight spatiotemporal control over Wnt5a expression. For this purpose, we cloned a Flag-tagged version of the mouse Wnt5a gene downstream of a tetracycline inducible promoter (Fig. 1A and Fig. S1A in the supplementary data). Activity of Flag-tagged Wnt5a was comparable to that of the untagged protein (Fig. S1B–D in the supplementary data), allowing us to routinely purify large quantities of bioactive Flag-Wnt5a according to previously published protocols (Mikels and Nusse, 2006). Transgenic mice were generated by oocyte injection of the tetO-Flag-Wnt5a (hereafter tetO-Wnt5a) construct. Mouse embryo fibroblasts from individual transgenic founder lines were tested in vitro for doxycycline-inducible expression of the transgene by quantitative RT-PCR (Fig. S2 in the supplementary data) and Western blot analysis (Fig. 1B) to identify lines that showed robust induction of Flag-Wnt5a expression. Furthermore, transgene induction was shown to be dose-dependent by testing a concentration series of doxycycline (Fig. S2 in the supplementary data). All phenotypes discussed below were confirmed to be present in at least two independent tetO-Wnt5a lines.
Figure 1. A novel transgenic mouse model allowing inducible Wnt5a overexpression.
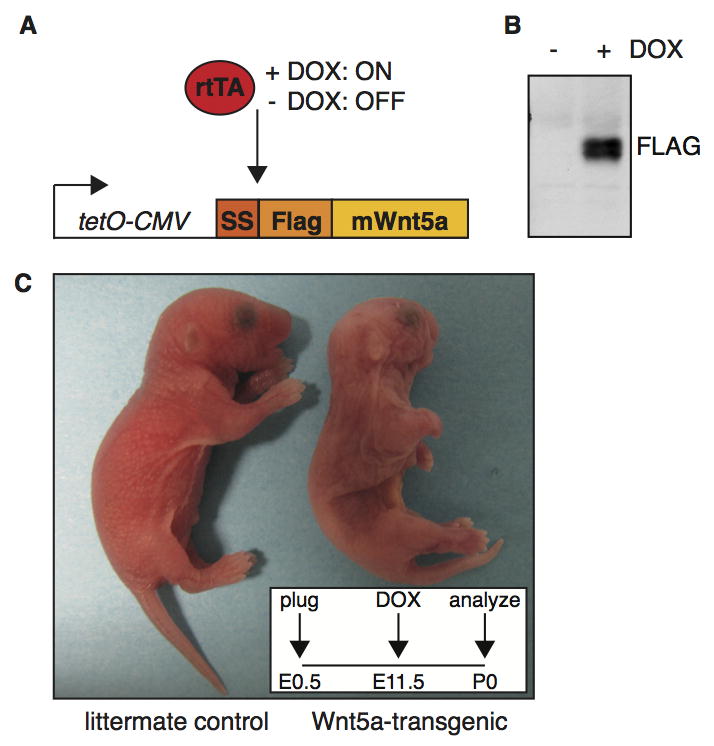
(A) Schematic representation of the tetO-Wnt5A transgenic construct and the experimental strategy. Mouse Wnt5a was cloned downstream of an artificial signal sequence (SS) in frame with an N-terminal FLAG tag under the control of a doxycycline inducible promoter. Transgene expression is only switched on in the presence of both an rtTA driver and doxycycline (DOX). (B) Western blot analysis illustrating that Wnt5a protein can be detected with an anti-Flag antibody in tetO-Wnt5A transgenic mouse embryo fibroblasts infected with pBabe-rtTA3 in the presence (right) but not in the absence (left) of doxycycline. (C) External appearance of a newborn tetO-Wnt5a;R26rtTA double-transgenic animal (right) and a littermate control (left) following treatment with doxycycline from E11.5-P0. Wnt5a overexpressing mice are smaller, have shortened limbs and an easily identifiable craniofacial phenotype. Insert depicts treatment schedule.
Generalized overexpression of Wnt5A throughout embryonic development is lethal
Transgene expression can be induced in vivo by crossing tetO-Wnt5a mice to a strain expressing the appropriate rtTA driver, after which the expression of Wnt5A can be switched on during embryonic development by the administration of doxycycline in the drinking water of pregnant dams (Fig. 1A). To determine the effect of broad Wnt5a overexpression during embryonic development, we set up timed matings between tetO-Wnt5a mice and mice expressing the rtTA-M2 driver from the Rosa26 locus ((Hochedlinger et al., 2005), hereafter R26rtTA). Expectant mothers were treated with doxycycline for various amounts of time during pregnancy, after which newborn mice were analyzed at birth. In the absence of doxycycline, the different genotypes were observed at the expected Mendelian ratios (data not shown). However, when doxycycline treatment was started on or prior to E7.5, no tetO-Wnt5A;R26rtTA double-heterozygotes were recovered among the offspring (Table 1, P = 0.0015). In contrast, treatment from E13.5 onwards again yielded the expected ratios of tetO-Wnt5A;R26rtTA double-heterozygotes at birth (Table 1). Since we were able to recover tetO-Wnt5A;R26rtTA double-transgenic mice at birth when doxycycline treatment was started on or after E10.5 (see below), this suggests a critical time window prior to E10.5 when generalized overexpression of Wnt5a is lethal.
Table 1. Mendelian ratios of tetO-Wnt5a;R26rtTA mice.
Offspring from a cross between parents heterozygous for either tetO-Wnt5a or R26rtTA was born at the expected Mendelian ratios when doxycycline treatment of pregnant mothers was started from E13.5 onwards. In contrast, no double-transgenic offspring was recovered at birth when Wnt5a overexpression was induced from, or prior to, E7.5.
| tetO-Wnt5a;R26rtTA | Wnt5a induced ≤ E7.5*
|
Wnt5a induced ≥ E13.5**
|
||
|---|---|---|---|---|
| Expected | Observed | Expected | Observed | |
|
|
|
|
||
| wt;wt | 8.5 | 8 | 9 | 10 |
| wt;het | 8.5 | 10 | 9 | 6 |
| het;wt | 8.5 | 16 | 9 | 7 |
| het;het | 8.5 | 0 | 9 | 13 |
n=34, P=0.0015 (Chi Square Test)
n=36, P=0.34 (Chi Square Test)
Wnt5a overexpression during the second half of pregnancy results in a variety of defects
Double heterozygous tetO-Wnt5A;R26rtTA mice that were born following transgene induction from E13.5 onwards were slightly smaller than their control littermates, had trouble breathing and did not survive (data not shown). We next determined that the earliest timepoint at which doxycycline treatment could be initiated while still allowing double-heterozygotes to be recovered at birth, was E10.5. These mice were stillborn and were easily identified by their external appearance. When doxycycline treatment was started at E11.5 or E12.5, double heterozygous animals showed a similar aberrant gross morphology and an approximate 10% reduction in size compared to their control littermates (Fig. 1C and Fig. S3 in the supplementary data). Closer inspection of these newborn tetO-Wnt5A;R26rtTA mice revealed phenotypes in multiple tissues. Most strikingly, virtually all double-transgenic mice lacked most of their intestinal tract. Whereas these mice had a stomach and hindgut, the small intestine was largely absent (Fig. S4 in the supplementary data). In addition, double heterozygotes had lung defects (van Amerongen and Nusse, unpublished data) as well as abnormalities in the skin and multiple skeletal malformations (see below).
Global Wnt5a overexpression causes a Wnt/β-catenin loss-of-function phenotype in the skin
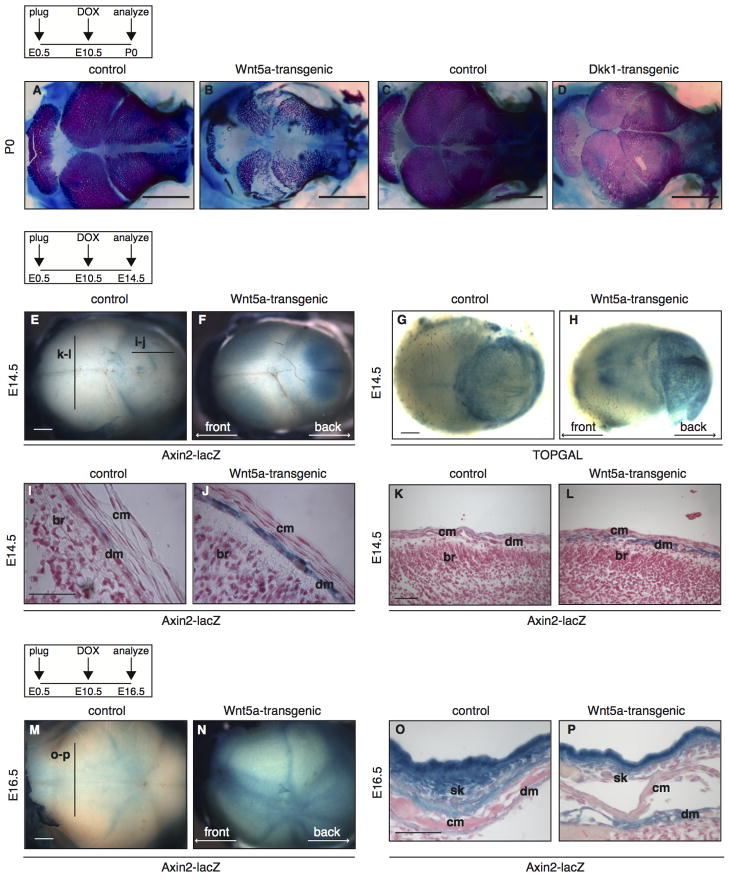
Given the reported role for Wnt5a in repressing Wnt/β-catenin signaling in vivo, both in normal development and disease (Nemeth et al., 2007; Roarty et al., 2009; Topol et al., 2003), we hypothesized that at least some of the phenotypes observed in tetO-Wnt5a;R26rtTA mice would reflect this particular signaling activity of Wnt5a. To distinguish between this and potential other activities of Wnt5a, we decided to compare the phenotype of mice overexpressing Wnt5a to that of mice overexpressing the known secreted Wnt/β-catenin inhibitor Dickkopf1 (Dkk1) (Glinka et al., 1998). Since the effects of global Dkk1 overexpression have not been reported, we crossed both the tetO-Wnt5a mice and previously generated tetO-Dkk mice (Chu et al., 2004), to R26rtTA driver mice and compared newborn tetO-Wnt5a;R26rtTA and tetO-Dkk;R26rtTA pups following doxycycline treatment from E10.5 onwards. To our surprise, the gross phenotype of generalized Dkk overexpression was much less severe than that of Wnt5a overexpression. TetO-Dkk;R26rtTA mice were born alive at the expected Mendelian ratios and could not be distinguished from control littermates based on their overall appearance (data not shown). Moreover, tetO-Dkk;R26rtTA double-transgenic mice were readily recovered when doxycycline treatment was initiated at E7.5, in contrast to what we observed for tetO-Wnt5a;R26rtTA mice (data not shown).
Overexpression of Dkk1 in the basal layers of the developing epidermis was previously reported to cause a complete loss of hair follicles in the dorsal skin and to result in aberrant and reduced vibrissiae formation (Andl et al., 2002). This phenotype was recapitulated in tetO-Dkk;R26rtTA double-transgenic mice that were treated with doxycycline from E10.5 onwards (Fig. 2A–B and data not shown). Interestingly, we observed a similar phenotype in the skin of double-transgenic tetO-Wnt5a;R26rtTA mice (Fig. 2C–D and Fig. S5 in the supplementary data). Loss of hair follicle formation is not only observed in mice overexpressing Dkk1, but also in mice conditionally deficient for β-catenin (Huelsken et al., 2001) and in mice displaying the combined loss of TCF3/TCF4 (Nguyen et al., 2009). Therefore, this phenotype bears the hallmarks of a typical Wnt/β-catenin loss-of-function phenotype.
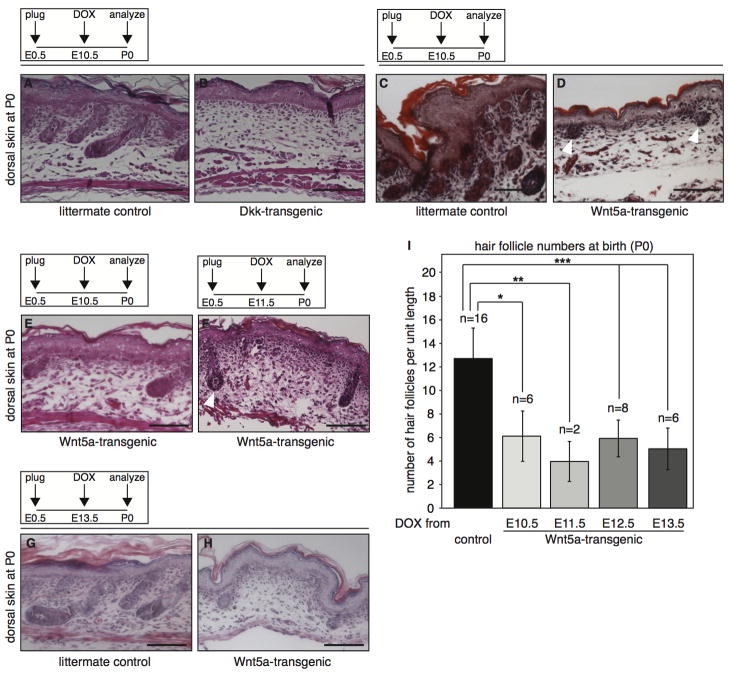
Figure 2. Global overexpression of Wnt5a or Dkk1 results in loss of hair follicle formation in the dorsal skin.
(A–D) H&E stained histological tissue sections from the dorsal skin of tetO-Dkk;R26rtTA (B) and tetO-Wnt5a;R26rtTA (D) double-transgenic mice and their respective littermate controls (A and C), showing that both Wnt5a and Dkk1 overexpression results in loss of hair follicle formation. Transgene induction was achieved by administering doxycycline from E10.5 to P0. (E–H) H&E stained histological tissue sections showing representative images of the dorsal skin from newborn tetO-Wnt5a;R26rtTA mice, illustrating that a reduction in hair follicle numbers occurs regardless of whether doxycycline treatment was initiated at E10.5 (E), E11.5 (F) or E13.5 (G–H). In addition to their overall number being lower, remaining hair follicles were less mature, although some did show signs of keratinization (white arrowhead in F).
(I) Graph depicting the quantification of hair follicle numbers in the skin of newborn mice overexpressing Wnt5a from E10.5-P0, E11.5-P0, E12.5-P0 or E13.5-P0 relative to littermate controls. Sagittal sections from a total of 38 skins were counted. Control samples (n = 16) derived from the different doxycycline treated litters were pooled to calculate the average number of hair follicles per unit length in control skin. A comparable and statistically significant reduction in hair follicle numbers was observed in all Wnt5a overexpressing newborn skins (* p < 1×10−4 for onset at E10.5, ** p < 5×10−4 for onset at E11.5 and *** p < 1×10−5 for onset at E12.5 and E13.5, T-test). Error bars indicate standard deviation. Scale bar is 100 μm in A–H.
Wnt5a overexpression inhibits the second wave of hair follicle formation
Whereas Dkk overexpression resulted in the complete loss of hair follicles in the dorsal skin (Fig. 2B), some hair follicles were found to be remaining in the skin of Wnt5a-overexpressing mice (Fig. 2D). In most cases however, these follicles failed to progress beyond the embryonic hair germ or hair peg stage (white arrowheads in Fig. 2D). Quantification of the number of remaining follicles in the dorsal skin of tetO-Wnt5A;R26rtTA mice showed a clear decrease at the time of birth (Fig. 2I). Of note, the absolute reduction in hair follicle number did not change with earlier onset of doxycyline treatment. Regardless of when Wnt5a overexpression was initiated between E10.5 and E13.5, there was a 58% (+/− 8%) decrease in the number of hair follicles that could be counted at birth. In addition, the extent to which remaining hair follicles developed was also comparable (Fig. E–H). While some hairs appeared to mature normally (arrowhead in Fig. 2F), on average the growth of remaining hair follicles was delayed compared to control skin (compare for instance Fig. 2H to 2G).
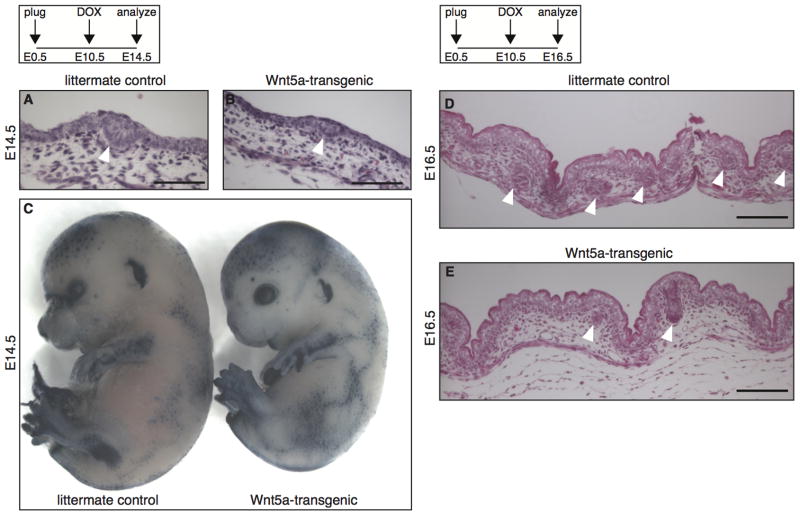
Hair follicle formation in the mouse skin occurs in three waves (reviewed in (Duverger and Morasso, 2009), the first of which takes place around E14.5. When we compared sections from the developing skin of E14.5 embryos treated with doxycycline from E10.5 onwards, hair placodes were readily detectable in both control and Wnt5a-overexpressing skin (Fig. 3A–B), suggesting that this primary wave of hair follicle formation was initiated normally. This was confirmed by wholemount analysis of E14.5 embryos, in which primary hair follicle formation can be visualized by alkaline phosphatase staining (Fig. 3C). At E16.5 however, when the second wave of hair follicle formation commences, the total number of hair follicles in the skin from Wnt5a overexpressing mice appeared to be reduced (Fig. 3D–E). Taken together these results demonstrate that Wnt5a overexpression does not affect the initial formation of hair placodes at E14.5, but results in a reduction in the number of hair follicles at birth. Thus, we conclude that Wnt5a overexpression affects the second and third, but not the first, wave of hair follicle formation in the skin.
Figure 3. Wnt5a overexpression inhibits the second, but not the first wave of hair follicle formation.
(A–B) H&E stained histological tissue sections of E14.5 dorsal skin, showing that hair placodes (white arrowheads) are formed in both tetO-Wnt5a;R26rtTA double-heterozygous embryos and littermate controls when doxycycline is administered from E10.5–E14.5. (C) Wholemount alkaline phosphatase staining of E14.5 embryos, revealing a normal pattern of primary hair follicle induction in Wnt5a-transgenic mice (right) following doxycycline administration from E10.5–E14.5. (D–E) H&E stained histological tissue sections of E16.5 dorsal skin, showing a reduction in the number of hair follicles (white arrowheads) in Wnt5a-transgenic mice following doxycycline administration from E10.5–E16.5. Scale bars are 100 μm in A–B and D–E.
Wnt5a overexpression affects hair follicle spacing through inhibition of Wnt/β-catenin signaling in the dermis
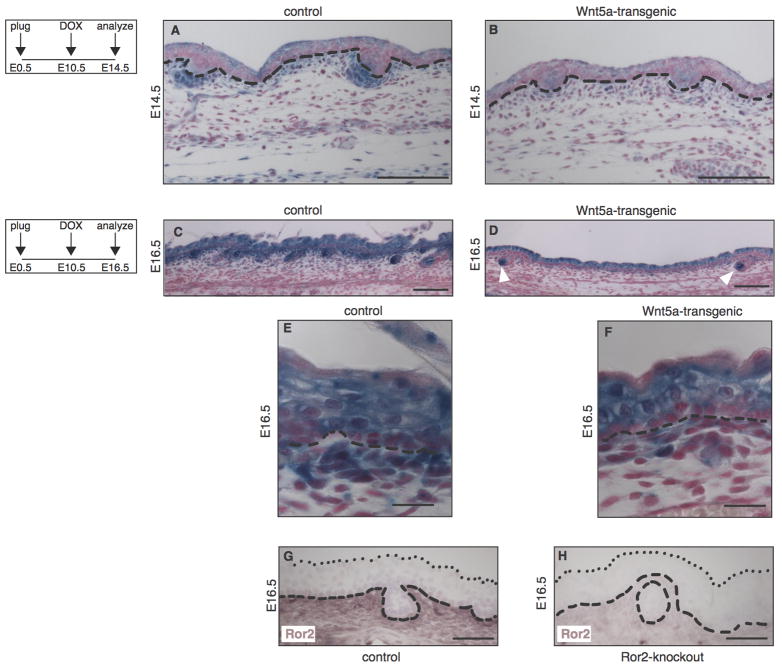
To test if Wnt5a overexpression indeed resulted in inhibition of Wnt/β-catenin signaling in the skin, we crossed a known Wnt/β-catenin reporter strain, Axin2-lacZ (Lustig et al., 2002), into our Wnt5a-transgenic mouse model and analyzed double-heterozygous tetO-Wnt5a;R26rtTA embryos carrying an Axin2-lacZ allele for changes in reporter gene expression. At E14.5, when hair follicle formation has been initiated, Axin2-lacZ is broadly expressed in wildtype skin (Fig. 4A), with the strongest expression seen in dermal condensates adjacent to hair placodes and in the outer layers of the epidermis. In addition, prominent Axin2-lacZ expression can be observed in a thin layer of dermal cells immediately underlying the basal epidermis. The overall expression of Axin2-lacZ is markedly reduced in the skin of tetO-Wnt5a;R26rtTA double-transgenic littermates (Fig. 4B). In particular, most of the expression observed in the dermis of control embryos is absent in Wnt5a-transgenic mice. At E16.5, Axin2-lacZ expression in control mice is widespread and observed in all layers of the epidermis, as well as in the dermal papilla of growing hair follicles and in the first five cell layers of the dermis (Fig. 4C,E). In mice overexpressing Wnt5a, growing hair follicles showed strong, and apparently unchanged, Axin2-lacZ staining in the dermal papilla (Fig. 4D, white arrowheads). Similarly, expression in most of the epidermis appears to be unaffected. In contrast, Axin2-lacZ expression is specifically reduced in the upper dermis of tetO-Wnt5a;R26rtTA double-transgenic (Fig. 4D,F).
Figure 4. Loss of Wnt/β-catenin signaling at sites of Ror2 expression in the dermis of Wnt5a-overexpressing mice.
(A–F) Histological tissue sections of wholemount X-gal stained dorsal skin, demonstrating that expression of the Wnt/β-catenin reporter Axin2-lacZ is markedly reduced in Wnt5a-transgenic embryos at both E14.5 (A–B) and E16.5 (C–F). Mice depicted in A and B are littermates, as are the mice depicted in C and D. The activity of an independent Wnt/β-catenin reporter strain, TOPGAL, was also reduced at these timepoints (data not shown). (A–B) At E14.5 the overall activity of Axin2-lacZ is lower throughout the epidermis and dermis of Wnt5a overexpressing mice, but expression of the reporter is particularly reduced in the dermal condensates. (C–D) At E16.5 the Axin2-lacZ signal in the dermal papilla of existing hair follicles remains largely unaffected (white arrowheads in D). (E–F) Close-ups of the control and Wnt5a overexpressing skins shown in C and D, showing that the most dramatic reduction in Wnt/β-catenin reporter gene expression is seen in a thin layer of cells just underlying the basal layer of the epidermis in tetO-Wnt5a;R26rtTA double-heterozygous mice (F) but not in littermate controls (E).
(G–H) Immunohistochemical detection of endogenous Ror2 protein expression in the dermis of control (G) but not Ror2-knockout (H) skin at E16.5. Dashed lines indicate the boundary between epidermis and dermis. Dotted lines in G and H indicate the outermost cell layer of the epidermis. Scale bars are 100 μm in A–B, 20 μm in C and D, and 50 μm in E–H.
The tyrosine kinase receptor Ror2 can transduce a Wnt5a signal resulting in inhibition of Wnt/β-catenin signaling in vitro. We therefore hypothesized that Ror2 might play a similar role in the developing skin. Ror2 has been reported to be expressed in the skin, but the pattern has not been studied in detail (Al-Shawi et al., 2001; DeChiara et al., 2000; Hu et al., 2010). Using an antibody directed against mouse Ror2, we were able to detect endogenous Ror2 protein expression in the developing skin at both E14.5 (data not shown) and E16.5 (Fig. 4G–H) in control, but not Ror2-deficient animals. The highest levels of Ror2 were expressed in the upper dermis, precisely where Wnt5a-overexpression results in inhibition of the Wnt/β-catenin dependent luciferase reporter Axin2-lacZ. Of note, we found that Axin2-lacZ reporter gene expression in this stripe of cells was unchanged in tetO-Dkk;R26rtTA mice, which is in agreement with the fact that Dkk inhibits β-catenin/TCF signaling in a Ror2-independent fashion (Fig. S6 in the supplementary data). Taken together, our data support a model in Wnt5a/Ror2 inhibits dermal Wnt/β-catenin signaling to control the progression of hair follicle formation during embryonic development.
To test the requirement for Ror2 in the Wnt5a-mediated loss of hair follicle formation, we sought to rescue the effects of Wnt5a overexpression in the skin by introducing a Ror2 loss-of-function allele (Takeuchi et al., 2000). Unfortunately, the interpretation of these rescue experiments was not straightforward. In agreement with published data (Hu et al., 2010), we initially did not detect any obvious defects in the initiation or progression of hair follicle formation in sections from the dorsal skin of Ror2-deficient mice at either E16.5 or P0 (Fig. S7A–D in the supplementary data). However, upon wholemount analyses of the dorsal skin from newborn animals, we noticed a large variation in hair follicle density in Ror2-null mice (Fig. S7E–G in the supplementary data). Since hair follicles in (early) anagen are easily identifiable as dark spots in wholemount preparations of the skin from newborn, pigmented animals, it thus appears that Ror2-deficient mice show aberrant hair patterning or pigmentation, albeit with incomplete penetrance. Consequently, we did not observe restoration to wildtype numbers of hair follicles in Ror2-deficient tetO-Wnt5a;R26rtTA animals compared to tetO-Wnt5a;R26rtTA littermates that were wildtype or heterozygous for Ror2 at either high (1 mg/ml doxycycline), intermediate (0.4 mg/ml doxycycline) or low (0.2 mg/ml doxycycline) levels of Wnt5a-transgene expression (Fig. S8 in the supplementary data). In contrast, some of these animals showed quite aberrant patterning, in which areas with hair follicles at low density were bordered by regions in which hair follicles appeared to be totally absent (Fig. S8O in the supplementary data). This observation suggests a precarious balance in Wnt5a/Ror2 signaling in the developing skin, the disruption of which affects hair follicle patterning and spacing.
Wnt5a overexpression inhibits bone formation
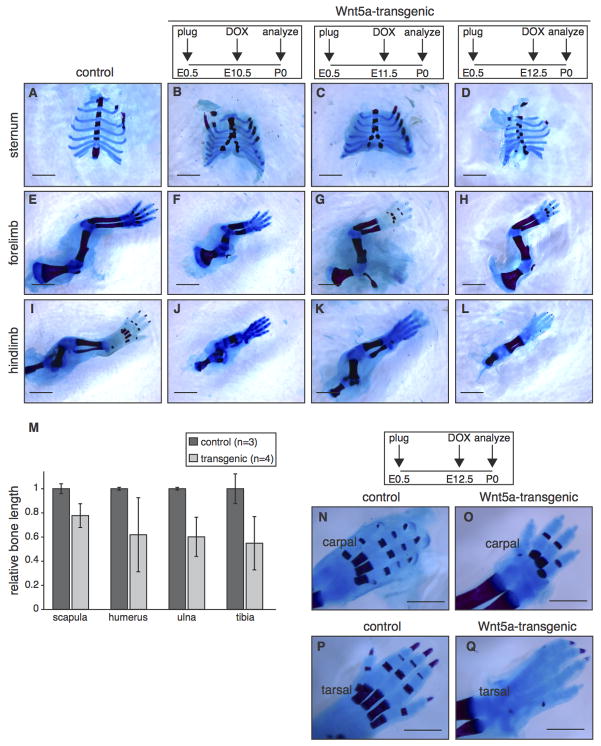
The external appearance of tetO-Wnt5A;R26rtTA transgenic mice also suggested prominent skeletal defects. Compared to control littermates, mice overexpressing Wnt5a had shortened limbs and prominent craniofacial abnormalities (Fig. 1C and Fig. S5 in the supplementary data), reminiscent of the phenotype previously reported for Col2a1-Wnt5a transgenic mice (Yang et al., 2003). Closer inspection of skeletons from newborn mice revealed that Wnt5a overexpression in the embryo (from either E10.5, E11.5 or E12.5 until birth) caused a split sternum (Fig. 5A–D) and resulted in reduced outgrowth of the long bones in both fore- and hindlimbs (Fig. 5E–M). Although shortened, long bones were fully ossified at the time of birth. In contrast, ossification in the digits was visibly delayed in Wnt5a-transgenic neonates, with reduced bone formation in the metacarpal and phalangeal bones. This delay was more prominent in the hindlimb than in the forelimb: When mice were treated with doxycycline from E12.5 onwards, bone formation was detectable in carpal (Fig. 5N–O), but absent in tarsal bones (Fig. 5P–Q).
Figure 5. Global overexpression of Wnt5a causes multiple skeletal defects.
(A–L) Wholemount preparations of newborn Wnt5a-overexpressing and control skeletons, stained with Alizarin Red and Alcian Blue to visualize bone (red) and cartilage (blue) following transgene expression from E10.5-P0 (B,F,J), E11.5-P0 (C,G,K) or E12.5-P0 (D,H,L). TetO-Wnt5a;R26rtTA double-heterozygotes develop a split sternum (A–D) and have shortened bones in the fore– (E–H) and hindlimbs (I–L) at the time of birth. (M) Graph depicting the relative length of flat (scapula) and long bones (humerus, ulna and tibia) in control (n=3) and Wnt5a-overexpressing mice (n=4), revealing that limb outgrowth is more severely impaired than overall body size (Figure S3C in the supplementary data). Data were pooled for animals treated with doxycycline from E10.5-P0, E11.5-P0 and E12.5-P0. Error bars indicate standard deviation. (N–Q) Close-up images, demonstrating that bone formation in the extremities is delayed in Wnt5a-overexpressing mice compared to littermate controls. This effect is more pronounced for tarsal (compare Q to P) than for carpal bones (compare O to N). Scale bar is 2 mm in (A–L) and 1 mm in (N–Q).
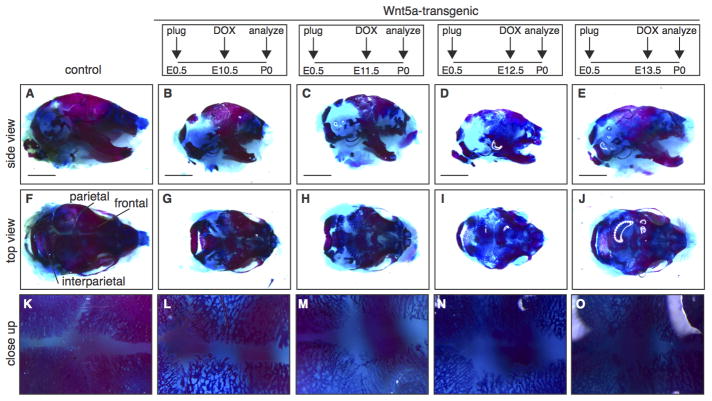
We observed the most prominent reduction in bone formation in the calvaria. Here, both the neural crest-derived frontal bones and the mesoderm-derived parietal and interparietal bones were affected (Fig. 6A–J). Compared to control littermates, the meshwork of bone in the calvaria of newborn tetO-Wnt5a;R26rtTA mice was less condensed (Fig. 6K–O), suggesting either a delay or a block in ossification. This phenotype was most apparent when doxycline treatment was commenced between E10.5 and E12.5, but even Wnt5a transgene expression from E13.5 onwards resulted in reduced density of the calvarial bones (Fig. 6O). Since bone formation in the limbs occurs through endochondral ossification of cartilage intermediates, whereas bone formation in the skull occurs directly through intramembranous ossification of a mesenchymal progenitor, these results suggest that Wnt5a overexpression affects both processes.
Figure 6. Delayed calvarial ossification in Wnt5a-transgenic mice.
Wholemount preparations of newborn Wnt5a-overexpressing and control skulls, stained with Alizarin Red and Alcian Blue to visualize bone (red) and cartilage (blue) following transgene expression from E10.5-P0 (B,G,L) to E11.5 (C,H,M) or E12.5 (D,I,N) or E13.5 (E,J,O), demonstrating that calvarial bone formation is reduced in the skull of Wnt5a-overexpressing mice compared to control littermates (A,F,K). (F–J) are top views of the skulls depicted in (A–E) and (K–O) are close-ups of the same samples. Scale bar is 2 mm.
Reduced calvarial ossification is preceded by gain of Wnt/β-catenin signaling in the meninges
Wnt/β-catenin signaling is known to control multiple steps of bone development (for reviews see (Leucht et al., 2008; Williams and Insogna, 2009)). It is generally considered to promote bone formation, as illustrated by the association of LRP5 gain and loss of function mutations with familial high and low bone mass phenotypes, respectively (Cui et al., 2011; Gong et al., 2001; Kwee et al., 2005; Little et al., 2002). In fact, administration of Wnt ligands is seen as a powerful tool to potentially increase bone mass upon tissue damage or aging (Minear et al., 2010). Although Dkk1 has previously been shown to negatively affect bone formation (Morvan et al., 2006), we did not observe any defects in calvarial ossification in tetO-Dkk;R26rtTA-transgenic mice following doxycycline administration from E10.5-P0 (Fig. 7A–D). We therefore hypothesized that the reduced bone formation in the skull of newborn tetO-Wnt5a;R26rtTA double-heterozygous mice might be due to a separate signaling activity of Wnt5a, distinct from its ability to inhibit β-catenin/TCF signaling.
Figure 7. Global Wnt5a overexpression causes an increase in Wnt/β-catenin signaling in the developing meninges.
(A–D) Wholemount preparations of the skulls from newborn mice, stained with Alizarin Red and Alcian Blue to visualize bone (red) and cartilage (blue), showing that overexpression of Wnt5a (compare B to A), but not Dkk1 (compare D to C) from E10.5-P0 results in reduced calvarial ossification at birth. (E–H) Wholemount preparations of X-gal stained E14.5 embryos, demonstrating that expression of the Wnt/β-catenin reporters Axin2-lacZ (compare F to E) and TOPGAL (compare H to G) is increased in the developing skull of Wnt5a-overexpressing mice compared to control littermates. The skin was removed to better visualize the calvarial mesenchyme. (I–L) Histological tissue sections of the samples depicted in (E–F) following paraffin embedding, showing that the increase in Axin2-lacZ activity is restricted to a thin layer of cells immediately underlying the calvarial mesenchyme and overlying the brain, consistent with the location of the developing meninges. The sagittal (I–J) and coronal (K–L) planes of sectioning are indicated with the black lines in panel (E). (M–N) Wholemount preparations of X-gal stained E16.5 embryos, demonstrating that expression of the Wnt/β-catenin reporter Axin2-lacZ (compare N to M) is increased in the developing skull of Wnt5a-overexpressing mice compared to control littermates. The skin was removed to better visualize the developing skull. (O–P) Independent coronal tissue sections of wholemount X-gal stained E16.5 embryos, showing an increase in Axin2-lacZ activity in the meninges. Scale bars are 2 mm in A–D, 1 mm in E–H and M–N, 100 μm in I–J and 50 μm in K–L and O–P. (br) brain, (cm) calvarial mesenchyme, (dm) dura mater, (sk) skin.
To test this, we again crossed the Axin2-lacZ reporter mice into the tetO-Wnt5a;R26rtTA background to score changes in Wnt/β-catenin activity. Independently, we also crossed in TOPGAL, a second Wnt/β-catenin reporter strain (DasGupta and Fuchs, 1999). To our surprise, we observed a consistent increase in β-catenin/TCF signaling in the heads of mice overexpressing Wnt5a at E14.5 and E16.5 (Fig. 7E–P and Fig. S9 in the supplementary data). Both Axin2-lacZ (Fig. 7E–F and 7M–N) and TOPGAL (Fig. 7G–H) reporter activity showed an increase in tetO-Wnt5a;R26rtTA double-transgenics compared to littermate controls. Thus, in contrast to the skin, where we found Wnt5a overexpression to inhibit β-catenin/TCF signaling, Wnt5a causes activation of Wnt/β-catenin signaling in the developing skull (see also Fig. S10 in the supplementary data).
Upon isolating the tissue with increased β-catenin/TCF signaling by microdissection, we found it to be located between the surface ectoderm and the brain, consistent with the location of the developing calvarial mesenchyme. However, when we analyzed Axin2-lacZ reporter gene expression in paraffin embedded tissue sections of E14.5 and E16.5 heads, we found that the increase in Wnt/β-catenin signaling was restricted to the dura mater, which is the outermost layer of the developing meninges (Fig. 7I–L and 7O–P). The meninges is a neural crest derived tissue that develops in close association with the underlying brain and the overlying frontal and parietal bones (Jiang et al., 2002). Of note, meningeal defects have previously been shown to adversely affect bone formation during embryonic development (Ito et al., 2003; Vivatbutsiri et al., 2008). Conversely, an intact dura mater has been shown to aid in osteogenic repair following calvarial defects (Levi et al., 2011). These studies, as well as our own, suggest important crosstalk between the meninges and the developing or healing calvaria. Our data suggest a previously unrecognized role for Wnt/β-catenin signaling in this process.
Discussion
It has long been debated whether individual Wnt ligands have multiple signaling activities in vivo and, if so, how signaling specificity is achieved in a complex, developing multicellular organism. In recent years it has become evident that a single Wnt protein can indeed initiate multiple downstream signaling events. For instance, both Wnt5a and Wnt11 are required for axis specification in the early Xenopus embryo (Cha et al., 2008; Tao et al., 2005), a process that is driven by Wnt/β-catenin signaling. Yet each protein is also well known to have a β-catenin-independent role in controlling convergent extension movements at later developmental stages (Kim et al., 2008; Wallingford et al., 2001). The finding that purified mouse Wnt5a was able to both activate and inhibit Wnt/β-catenin signaling in vitro (Mikels and Nusse, 2006), suggested that a similar paradigm might apply to mammalian Wnt proteins. Existing mouse models however, did not allow perturbation of Wnt5a expression in a controlled manner, precluding the testing of this hypothesis in vivo.
Here we describe the generation of a novel, inducible transgenic mouse model, which offers many advantages over existing Wnt5a-knockout and —transgenic mouse lines (Adamska et al., 2005; Baxley et al., 2011; Yamaguchi et al., 1999; Yang et al., 2003), including tight spatial and temporal control over the onset and duration of Wnt5a overexpression. For our first analysis, we chose to study the effects of broad Wnt5a overexpression during embryonic development, using a Rosa26-rtTA-M2 driver (Hochedlinger et al., 2005). We found that elevated levels of Wnt5a expression caused a variety of developmental defects that diminished in severity with lower levels (achieved by lowering the concentration of doxycycline) as well as later onset of transgene induction. By focusing on two of these phenotypes in more detail, we were able to uncover previously unrecognized roles for the dermis in determining hair follicle formation and patterning of the skin, as well as for Wnt/β-catenin signaling in the meninges in controlling calvarial bone formation. Interestingly, we were able to link these phenotypes to different signaling activities of Wnt5a: On the one hand, we found that Wnt5a/Ror2 signaling inhibits Wnt/β-catenin signaling in the dermis (Fig. 4), thereby affecting the second and third wave of hair follicle formation. On the other hand, Wnt5a caused an increase in Wnt/β-catenin signaling in the meninges (Fig. 7), preceding diminished ossification of the calvarial mesenchyme. To our knowledge, this is the first report of potential dual signaling activities for a mammalian Wnt ligand during embryonic development.
Wnt-signaling controls multiple aspects of hair follicle morphogenesis, both during embryonic development and in postnatal hair turnover. In addition to directing hair follicle formation, Wnt/β-catenin signaling has also been shown to control hair follicle spacing. Establishment of the hair follicle pattern in the skin occurs according to a reaction-diffusion model, with Wnt proteins serving as activators and Dkk proteins as diffusible inhibitors (Plikus et al., 2011; Schlake and Sick, 2007; Sick et al., 2006). We find that the Wnt5a-mediated inhibition of hair follicle formation (Figs. 2–3) coincides with a reduction in dermal Wnt/β-catenin signaling (Fig. 4). Ror2 is normally expressed throughout the dermis during this time (Fig. 4) and endogenous Wnt5a is similarly expressed from E14.5 onwards, although its expression later becomes concentrated in dermal condensates (Reddy et al., 2001). We therefore propose that endogenous Wnt5a/Ror2 signaling might help control hair follicle spacing by globally dampening Wnt/β-catenin signaling, thereby preventing the precocious or extraneous hair follicle formation that can result from ectopic β-catenin/TCF signaling (Narhi et al., 2008).
The fact that we were unable to rescue the loss of hair follicle formation in tetO-Wnt5a;R26rtTA animals by introducing a Ror2-null allele is puzzling in light of our earlier findings: Ror2 directly binds Wnt5a through its CRD domain and expression of Ror2 is sufficient to confer Wnt5a-mediated inhibition of β-catenin/TCF signaling, suggesting that it can act as a genuine Wnt receptor (Mikels and Nusse, 2006). However, other studies have proposed that Ror2 functions as a co-receptor for Fzd (Grumolato et al., 2010; Ho et al., 2012; Nishita et al., 2010; Yamamoto et al., 2008). As such, Ror2 might be involved in, but not be critically required for, the Wnt5a-mediated inhibition of β-catenin/TCF signaling, especially when the levels of Wnt5a are not limiting. One complicating factor in interpreting these experiments is our finding that loss of Ror2 alone causes a reduction in the number of hair follicles in the skin of some, but not all, animals (Fig. S7 in the supplementary data). Incomplete penetrance is something we have also observed for other phenotypes in the Ror2-knockout mice (RvA and RN, unpublished data) and might reflect functional redundancy with Ror1, which shows overlapping expression with Ror2 in some tissues during embryonic development (Al-Shawi et al., 2001; Matsuda et al., 2001). At present, we have no explanation for the defects in hair follicle spacing in Ror2-deficient mice, although it leaves open the possibility of a non-cell autonomous role for Ror2, in which it could function to sequester multiple Wnt ligands (both activators and inhibitors of Wnt/β-catenin signaling) similar to its C. elegans homologue cam1 (Green et al., 2007).
We uncovered a second signaling activity for Wnt5a in the developing skull. Here, Wnt5a overexpression caused the induction of Wnt/β-catenin signaling in the meninges (Fig. 7). This was associated with reduced calvarial ossification at birth (Figs. 6–7). Compared to endochondral bone formation, which occurs through a cartilage intermediate, the process of intramembranous ossification remains relatively poorly understood. As first glance our findings appear counterintuitive to a paradigm in which Wnt/β-catenin signaling is generally considered to promote bone formation. However, it was recently shown that high levels of Wnt/β-catenin signaling inhibit the osteogenic differentiation of embryonic calvarial mesenchymal cells, while inducing osteogenesis in mature calvarial osteoblasts (Quarto et al., 2010). Whereas Wnt/β-catenin signaling therefore appears to be required for bone formation, earlier activation of the pathway might result in the prolonged maintenance of a mesenchymal progenitor state. Although at present we have no proof that in tetO-Wnt5a;R26rtTA double-transgenic animals the increase in β-catenin/TCF signaling in the dura mater at E14.5 and E16.5 is directly responsible for the reduced bone formation observed at birth, it is generally well accepted that the dura mater affects osteogenesis in the overlying calvarial mesenchyme through the secretion of paracrine growth factors (Li et al., 2007; Spector et al., 2002; Vivatbutsiri et al., 2008). We therefore hypothesize that the enhanced Wnt/β-catenin signaling in the dura mater alters the production of one or more of these factors. Although the endogenous role of Wnt-signaling in dura mater biology has not been studied, both ligands and receptors are expressed in the developing meninges as suggested by publicly available expression databases (e.g. Gene Expression Database at http://www.informatics.jax.org). These include Fzd4, which can induce Wnt/β-catenin signaling in response to Wnt5a (Mikels and Nusse, 2006) and which might therefore be responsible for the observed increase in Axin2-lacZ and TOPGAL reporter activity in our Wnt5a overexpressing mice. At present however, we cannot exclude that other signaling activities of Wnt5a (such as a Ror2-mediated induction of osteoclastogenesis) underlie the bone loss observed in the skull and limbs of tetO-Wnt5a;R26rtTA double-transgenic mice (Maeda et al., 2012).
Of note, the block in calvarial ossification was more sensitive to dose than the loss of hair follicles in the skin or the loss of intestinal tissue. The latter two were still observed when mice were treated with lower levels of doxycycline (Figs. S4 and S8 in the supplementary data). In contrast, loss of bone formation in the skull was only detected when mice received 1–2 mg/ml doxycycline. This suggests that the Wnt/β-catenin loss of function phenotype is preserved at lower levels, whereas the induction of Wnt/β-catenin signaling requires higher levels of Wnt5a transgene expression. It is tempting to speculate that this might reveal something about the affinity of Wnt5a for its receptors: Much higher levels of Wnt5a might be required to induce activation of Wnt/β-catenin signaling through a particular Fzd receptor than to inhibit it via Ror2. By incorporating loss-of-function alleles for different receptors, as we have done here for Ror2, the tetO-Wnt5a mouse model might help elucidate the factors that control the specificity of ligand-receptor interactions. Furthermore, as the arsenal of rtTA drivers expands, it will be possible to induce tissue-specific overexpression at multiple sites, both in the embryo and in the adult. We have already observed vast differences in phenotypic outcome depending on the promoter used to drive Wnt5a overexpression in the developing lung (RvA and RN, unpublished data).
Although our system relies on the ectopic expression of Wnt5a, which may exert its activities at different sites than the endogenous protein, tight control over the onset and levels of ligand expression offers an important advantage in dissecting the signaling responses in a complex, developing organism. Follow-up studies using conditional knockout mice will be required to test the requirement for endogenous Wnt5a, or another Wnt protein, in the biological processes discussed here. In conclusion, although Wnt5a is generally not associated with signaling through β-catenin/TCF, our current study suggests that it is able to do so in vivo. While it remains to be determined whether Wnt5a indeed activates Wnt/β-catenin signaling under physiological conditions in mammals, this finding is of special interest given the dual, and confusing, roles reported for Wnt5a in oncogenesis. Wnt5a has been ascribed both oncogenic and tumor suppressor properties in human malignancies (Kurayoshi et al., 2006; McDonald and Silver, 2009; O’Connell et al., 2010; Peng et al., 2011; Roman-Gomez et al., 2007; Yamamoto et al., 2010; Ying et al., 2007). The current data suggest that especially when overexpressed at ectopic sites, its signaling activities should be carefully analyzed and the possibility of Wnt5a driving β-catenin/TCF signaling should be considered.
Supplementary Material
Highlights.
A novel, inducible transgenic mouse model allows spatiotemporal control over Wnt5a expression.
Wnt5a is capable of both inducing and repressing Wnt/β-catenin signaling during mammalian development.
Ectopic expression of Wnt5a results in hair follicle loss, intestinal malformation and reduced bone formation.
Acknowledgments
We thank Orly Liel Wapinski for help in the initial characterization of the Wnt5a-transgenic founder lines, Tim Blauwkamp for valuable discussions, and Catriona Logan and Xinhong Lim for comments on the manuscript. RvA was supported by an EMBO Long Term Fellowship (ALTF 122-2007) and a KWF fellowship from the Dutch Cancer Society. CF was supported by the Swiss National Science Foundation. RN is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamska M, Billi AC, Cheek S, Meisler MH. Genetic interaction between Wnt7a and Lrp6 during patterning of dorsal and posterior structures of the mouse limb. Dev Dyn. 2005;233:368–372. doi: 10.1002/dvdy.20437. [DOI] [PubMed] [Google Scholar]
- Al-Shawi R, Ashton SV, Underwood C, Simons JP. Expression of the Ror1 and Ror2 receptor tyrosine kinase genes during mouse development. Dev Genes Evol. 2001;211:161–171. doi: 10.1007/s004270100140. [DOI] [PubMed] [Google Scholar]
- Allgeier SH, Lin TM, Vezina CM, Moore RW, Fritz WA, Chiu SY, Zhang C, Peterson RE. WNT5A selectively inhibits mouse ventral prostate development. Dev Biol. 2008;324:10–17. doi: 10.1016/j.ydbio.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson ER, Prakash N, Cajanek L, Minina E, Bryja V, Bryjova L, Yamaguchi TP, Hall AC, Wurst W, Arenas E. Wnt5a regulates ventral midbrain morphogenesis and the development of A9–A10 dopaminergic cells in vivo. PLoS One. 2008;3:e3517. doi: 10.1371/journal.pone.0003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Baxley SE, Jiang W, Serra R. Misexpression of Wingless-Related MMTV Integration Site 5A in Mouse Mammary Gland Inhibits the Milk Ejection Response and Regulates Connexin43 Phosphorylation. Biol Reprod. 2011 doi: 10.1095/biolreprod.111.091645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmon KS, Loose DS. Development of a bioassay for detection of Wnt-binding affinities for individual frizzled receptors. Anal Biochem. 2010;401:288–294. doi: 10.1016/j.ab.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes S, Yamaguchi TP, Hebrok M. Wnt5a is essential for intestinal elongation in mice. Dev Biol. 2009;326:285–294. doi: 10.1016/j.ydbio.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha KB, Douglas KR, Potok MA, Liang H, Jones SN, Camper SA. WNT5A signaling affects pituitary gland shape. Mech Dev. 2004;121:183–194. doi: 10.1016/j.mod.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Cha SW, Tadjuidje E, Tao Q, Wylie C, Heasman J. Wnt5a and Wnt11 interact in a maternal Dkk1-regulated fashion to activate both canonical and non-canonical signaling in Xenopus axis formation. Development. 2008;135:3719–3729. doi: 10.1242/dev.029025. [DOI] [PubMed] [Google Scholar]
- Chu EY, Hens J, Andl T, Kairo A, Yamaguchi TP, Brisken C, Glick A, Wysolmerski JJ, Millar SE. Canonical WNT signaling promotes mammary placode development and is essential for initiation of mammary gland morphogenesis. Development. 2004;131:4819–4829. doi: 10.1242/dev.01347. [DOI] [PubMed] [Google Scholar]
- Croce JC, McClay DR. Evolution of the Wnt pathways. Methods Mol Biol. 2008;469:3–18. doi: 10.1007/978-1-60327-469-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Niziolek PJ, Macdonald BT, Zylstra CR, Alenina N, Robinson DR, Zhong Z, Matthes S, Jacobsen CM, Conlon RA, Brommage R, Liu Q, Mseeh F, Powell DR, Yang QM, Zambrowicz B, Gerrits H, Gossen JA, He X, Bader M, Williams BO, Warman ML, Robling AG. Lrp5 functions in bone to regulate bone mass. Nat Med. 2011;17:684–691. doi: 10.1038/nm.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- DeChiara TM, Kimble RB, Poueymirou WT, Rojas J, Masiakowski P, Valenzuela DM, Yancopoulos GD. Ror2, encoding a receptor-like tyrosine kinase, is required for cartilage and growth plate development. Nat Genet. 2000;24:271–274. doi: 10.1038/73488. [DOI] [PubMed] [Google Scholar]
- Du SJ, Purcell SM, Christian JL, McGrew LL, Moon RT. Identification of distinct classes and functional domains of Wnts through expression of wild-type and chimeric proteins in Xenopus embryos. Mol Cell Biol. 1995;15:2625–2634. doi: 10.1128/mcb.15.5.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duverger O, Morasso MI. Epidermal patterning and induction of different hair types during mouse embryonic development. Birth Defects Res C Embryo Today. 2009;87:263–272. doi: 10.1002/bdrc.20158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer T, Guimera J, Wurst W, Prakash N. Distinct but redundant expression of the Frizzled Wnt receptor genes at signaling centers of the developing mouse brain. Neuroscience. 2007;147:693–711. doi: 10.1016/j.neuroscience.2007.04.060. [DOI] [PubMed] [Google Scholar]
- Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, Andre P, Robinson J, Sood R, Minami Y, Economides AN, Yang Y. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell. 2011;20:163–176. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J. 1998 Warkany lecture: signaling pathways in development. Teratology. 1999;60:226–239. doi: 10.1002/(SICI)1096-9926(199910)60:4<226::AID-TERA7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- Green JL, Inoue T, Sternberg PW. The C. elegans ROR receptor tyrosine kinase, CAM-1, non-autonomously inhibits the Wnt pathway. Development. 2007;134:4053–4062. doi: 10.1242/dev.005363. [DOI] [PubMed] [Google Scholar]
- Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, Arroyave R, Vijayakumar S, Economides AN, Aaronson SA. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 2010;24:2517–2530. doi: 10.1101/gad.1957710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Xiong W, Yu X, Espinoza-Lewis R, Liu C, Gu S, Nishita M, Suzuki K, Yamada G, Minami Y, Chen Y. Wnt5a regulates directional cell migration and cell proliferation via Ror2-mediated noncanonical pathway in mammalian palate development. Development. 2008;135:3871–3879. doi: 10.1242/dev.025767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Saint-Jeannet JP, Wang Y, Nathans J, Dawid I, Varmus H. A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science. 1997;275:1652–1654. doi: 10.1126/science.275.5306.1652. [DOI] [PubMed] [Google Scholar]
- Ho HY, Susman MW, Bikoff JB, Ryu YK, Jonas AM, Hu L, Kuruvilla R, Greenberg ME. Wnt5a-Ror-Dishevelled signaling constitutes a core developmental pathway that controls tissue morphogenesis. Proc Natl Acad Sci U S A. 2012;109:4044–4051. doi: 10.1073/pnas.1200421109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121:465–477. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Hogan B. Manipulating the mouse embryo a laboratory manual. 2. Cold Spring Harbor Press; Plainview, N.Y: 1994. [Google Scholar]
- Hsieh JC, Rattner A, Smallwood PM, Nathans J. Biochemical characterization of Wnt-frizzled interactions using a soluble, biologically active vertebrate Wnt protein. Proc Natl Acad Sci U S A. 1999;96:3546–3551. doi: 10.1073/pnas.96.7.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Lefort K, Qiu W, Nguyen BC, Rajaram RD, Castillo E, He F, Chen Y, Angel P, Brisken C, Dotto GP. Control of hair follicle cell fate by underlying mesenchyme through a CSL-Wnt5a-FoxN1 regulatory axis. Genes Dev. 2010;24:1519–1532. doi: 10.1101/gad.1886910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Ito Y, Yeo JY, Chytil A, Han J, Bringas P, Jr, Nakajima A, Shuler CF, Moses HL, Chai Y. Conditional inactivation of Tgfbr2 in cranial neural crest causes cleft palate and calvaria defects. Development. 2003;130:5269–5280. doi: 10.1242/dev.00708. [DOI] [PubMed] [Google Scholar]
- Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–168. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- James RG, Conrad WH, Moon RT. Beta-catenin-independent Wnt pathways: signals, core proteins, and effectors. Methods Mol Biol. 2008;468:131–144. doi: 10.1007/978-1-59745-249-6_10. [DOI] [PubMed] [Google Scholar]
- Jiang X, Iseki S, Maxson RE, Sucov HM, Morriss-Kay GM. Tissue origins and interactions in the mammalian skull vault. Dev Biol. 2002;241:106–116. doi: 10.1006/dbio.2001.0487. [DOI] [PubMed] [Google Scholar]
- Kim GH, Her JH, Han JK. Ryk cooperates with Frizzled 7 to promote Wnt11-mediated endocytosis and is essential for Xenopus laevis convergent extension movements. J Cell Biol. 2008;182:1073–1082. doi: 10.1083/jcb.200710188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Schleiffarth JR, Jessurun J, Sumanas S, Petryk A, Lin S, Ekker SC. Wnt5 signaling in vertebrate pancreas development. BMC Biol. 2005;3:23. doi: 10.1186/1741-7007-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, Yasui W, Kikuchi A. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res. 2006;66:10439–10448. doi: 10.1158/0008-5472.CAN-06-2359. [DOI] [PubMed] [Google Scholar]
- Kusserow A, Pang K, Sturm C, Hrouda M, Lentfer J, Schmidt HA, Technau U, von Haeseler A, Hobmayer B, Martindale MQ, Holstein TW. Unexpected complexity of the Wnt gene family in a sea anemone. Nature. 2005;433:156–160. doi: 10.1038/nature03158. [DOI] [PubMed] [Google Scholar]
- Kwee ML, Balemans W, Cleiren E, Gille JJ, Van Der Blij F, Sepers JM, Van Hul W. An autosomal dominant high bone mass phenotype in association with craniosynostosis in an extended family is caused by an LRP5 missense mutation. J Bone Miner Res. 2005;20:1254–1260. doi: 10.1359/JBMR.050303. [DOI] [PubMed] [Google Scholar]
- Leucht P, Minear S, Ten Berge D, Nusse R, Helms JA. Translating insights from development into regenerative medicine: the function of Wnts in bone biology. Semin Cell Dev Biol. 2008;19:434–443. doi: 10.1016/j.semcdb.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Levi B, Nelson ER, Li S, James AW, Hyun JS, Montoro DT, Lee M, Glotzbach JP, Commons GW, Longaker MT. Dura Mater Stimulates Human Adipose-Derived Stromal to Undergo Bone Formation in Mouse Calvarial Defects. Stem Cells. 2011 doi: 10.1002/stem.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Xiao J, Hormi K, Borok Z, Minoo P. Wnt5a participates in distal lung morphogenesis. Dev Biol. 2002;248:68–81. doi: 10.1006/dbio.2002.0729. [DOI] [PubMed] [Google Scholar]
- Li J, Ying J, Fan Y, Wu L, Ying Y, Chan AT, Srivastava G, Tao Q. WNT5A antagonizes WNT/beta-catenin signaling and is frequently silenced by promoter CpG methylation in esophageal squamous cell carcinoma. Cancer Biol Ther. 2010;10:617–624. doi: 10.4161/cbt.10.6.12609. [DOI] [PubMed] [Google Scholar]
- Li S, Quarto N, Longaker MT. Dura mater-derived FGF-2 mediates mitogenic signaling in calvarial osteoblasts. Am J Physiol Cell Physiol. 2007;293:C1834–1842. doi: 10.1152/ajpcell.00135.2007. [DOI] [PubMed] [Google Scholar]
- Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, Lappe MM, Spitzer L, Zweier S, Braunschweiger K, Benchekroun Y, Hu X, Adair R, Chee L, FitzGerald MG, Tulig C, Caruso A, Tzellas N, Bawa A, Franklin B, McGuire S, Nogues X, Gong G, Allen KM, Anisowicz A, Morales AJ, Lomedico PT, Recker SM, Van Eerdewegh P, Recker RR, Johnson ML. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Kobayashi Y, Udagawa N, Uehara S, Ishihara A, Mizoguchi T, Kikuchi Y, Takada I, Kato S, Kani S, Nishita M, Marumo K, Martin TJ, Minami Y, Takahashi N. Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat Med. 2012;18:405–412. doi: 10.1038/nm.2653. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Nomi M, Ikeya M, Kani S, Oishi I, Terashima T, Takada S, Minami Y. Expression of the receptor tyrosine kinase genes, Ror1 and Ror2, during mouse development. Mech Dev. 2001;105:153–156. doi: 10.1016/s0925-4773(01)00383-5. [DOI] [PubMed] [Google Scholar]
- McDonald SL, Silver A. The opposing roles of Wnt-5a in cancer. Br J Cancer. 2009;101:209–214. doi: 10.1038/sj.bjc.6605174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod MJ. Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology. 1980;22:299–301. doi: 10.1002/tera.1420220306. [DOI] [PubMed] [Google Scholar]
- Mii Y, Taira M. Secreted Frizzled-related proteins enhance the diffusion of Wnt ligands and expand their signalling range. Development. 2009;136:4083–4088. doi: 10.1242/dev.032524. [DOI] [PubMed] [Google Scholar]
- Mikels A, Minami Y, Nusse R. Ror2 receptor requires tyrosine kinase activity to mediate Wnt5A signaling. J Biol Chem. 2009;284:30167–30176. doi: 10.1074/jbc.M109.041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minear S, Leucht P, Jiang J, Liu B, Zeng A, Fuerer C, Nusse R, Helms JA. Wnt proteins promote bone regeneration. Sci Transl Med. 2010;2:29ra30. doi: 10.1126/scitranslmed.3000231. [DOI] [PubMed] [Google Scholar]
- Morvan F, Boulukos K, Clement-Lacroix P, Roman Roman S, Suc-Royer I, Vayssiere B, Ammann P, Martin P, Pinho S, Pognonec P, Mollat P, Niehrs C, Baron R, Rawadi G. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res. 2006;21:934–945. doi: 10.1359/jbmr.060311. [DOI] [PubMed] [Google Scholar]
- Nagy A, Gertsenstein M, Vintersten K, Behringer R. Staining mouse embryos for alkaline phosphatase activity. CSH Protoc 2007. 2007 doi: 10.1101/pdb.prot4776. pdb prot4776. [DOI] [PubMed] [Google Scholar]
- Narhi K, Jarvinen E, Birchmeier W, Taketo MM, Mikkola ML, Thesleff I. Sustained epithelial beta-catenin activity induces precocious hair development but disrupts hair follicle down-growth and hair shaft formation. Development. 2008;135:1019–1028. doi: 10.1242/dev.016550. [DOI] [PubMed] [Google Scholar]
- Nemeth MJ, Topol L, Anderson SM, Yang Y, Bodine DM. Wnt5a inhibits canonical Wnt signaling in hematopoietic stem cells and enhances repopulation. Proc Natl Acad Sci U S A. 2007;104:15436–15441. doi: 10.1073/pnas.0704747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H, Merrill BJ, Polak L, Nikolova M, Rendl M, Shaver TM, Pasolli HA, Fuchs E. Tcf3 and Tcf4 are essential for long-term homeostasis of skin epithelia. Nat Genet. 2009;41:1068–1075. doi: 10.1038/ng.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishita M, Itsukushima S, Nomachi A, Endo M, Wang Z, Inaba D, Qiao S, Takada S, Kikuchi A, Minami Y. Ror2/Frizzled complex mediates Wnt5a-induced AP-1 activation by regulating Dishevelled polymerization. Mol Cell Biol. 2010;30:3610–3619. doi: 10.1128/MCB.00177-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomachi A, Nishita M, Inaba D, Enomoto M, Hamasaki M, Minami Y. Receptor tyrosine kinase Ror2 mediates Wnt5a-induced polarized cell migration by activating c-Jun N-terminal kinase via actin-binding protein filamin A. J Biol Chem. 2008;283:27973–27981. doi: 10.1074/jbc.M802325200. [DOI] [PubMed] [Google Scholar]
- O’Connell MP, Fiori JL, Xu M, Carter AD, Frank BP, Camilli TC, French AD, Dissanayake SK, Indig FE, Bernier M, Taub DD, Hewitt SM, Weeraratna AT. The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A signaling in metastatic melanoma. Oncogene. 2010;29:34–44. doi: 10.1038/onc.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- Olson DJ, Gibo DM. Antisense wnt-5a mimics wnt-1-mediated C57MG mammary epithelial cell transformation. Exp Cell Res. 1998;241:134–141. doi: 10.1006/excr.1998.4030. [DOI] [PubMed] [Google Scholar]
- Peng C, Zhang X, Yu H, Wu D, Zheng J. Wnt5a as a predictor in poor clinical outcome of patients and a mediator in chemoresistance of ovarian cancer. Int J Gynecol Cancer. 2011;21:280–288. doi: 10.1097/IGC.0b013e31820aaadb. [DOI] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Plikus MV, Baker RE, Chen CC, Fare C, de la Cruz D, Andl T, Maini PK, Millar SE, Widelitz R, Chuong CM. Self-organizing and stochastic behaviors during the regeneration of hair stem cells. Science. 2011;332:586–589. doi: 10.1126/science.1201647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, Steel KP, Dai X, Chen P. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306:121–133. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarto N, Behr B, Longaker MT. Opposite spectrum of activity of canonical Wnt signaling in the osteogenic context of undifferentiated and differentiated mesenchymal cells: implications for tissue engineering. Tissue Eng Part A. 2010;16:3185–3197. doi: 10.1089/ten.tea.2010.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, Morrisey EE, Millar SE. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev. 2001;107:69–82. doi: 10.1016/s0925-4773(01)00452-x. [DOI] [PubMed] [Google Scholar]
- Roarty K, Baxley SE, Crowley MR, Frost AR, Serra R. Loss of TGF-beta or Wnt5a results in an increase in Wnt/beta-catenin activity and redirects mammary tumour phenotype. Breast Cancer Res. 2009;11:R19. doi: 10.1186/bcr2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman-Gomez J, Jimenez-Velasco A, Cordeu L, Vilas-Zornoza A, San Jose-Eneriz E, Garate L, Castillejo JA, Martin V, Prosper F, Heiniger A, Torres A, Agirre X. WNT5A, a putative tumour suppressor of lymphoid malignancies, is inactivated by aberrant methylation in acute lymphoblastic leukaemia. Eur J Cancer. 2007;43:2736–2746. doi: 10.1016/j.ejca.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Sato A, Yamamoto H, Sakane H, Koyama H, Kikuchi A. Wnt5a regulates distinct signalling pathways by binding to Frizzled2. Embo J. 2010;29:41–54. doi: 10.1038/emboj.2009.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlake T, Sick S. Canonical WNT signalling controls hair follicle spacing. Cell Adh Migr. 2007;1:149–151. doi: 10.4161/cam.1.3.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Julius MA, Giarre M, Zheng Z, Brown AM, Kitajewski J. Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth Differ. 1997;8:1349–1358. [PubMed] [Google Scholar]
- Sick S, Reinker S, Timmer J, Schlake T. WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science. 2006;314:1447–1450. doi: 10.1126/science.1130088. [DOI] [PubMed] [Google Scholar]
- Spector JA, Greenwald JA, Warren SM, Bouletreau PJ, Crisera FE, Mehrara BJ, Longaker MT. Co-culture of osteoblasts with immature dural cells causes an increased rate and degree of osteoblast differentiation. Plast Reconstr Surg. 2002;109:631–642. doi: 10.1097/00006534-200202000-00033. discussion 643–634. [DOI] [PubMed] [Google Scholar]
- Summerhurst K, Stark M, Sharpe J, Davidson D, Murphy P. 3D representation of Wnt and Frizzled gene expression patterns in the mouse embryo at embryonic day 11.5 (Ts19) Gene Expr Patterns. 2008;8:331–348. doi: 10.1016/j.gep.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai CC, Sala FG, Ford HR, Wang KS, Li C, Minoo P, Grikscheit TC, Bellusci S. Wnt5a knock-out mouse as a new model of anorectal malformation. J Surg Res. 2009;156:278–282. doi: 10.1016/j.jss.2009.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada R, Hijikata H, Kondoh H, Takada S. Analysis of combinatorial effects of Wnts and Frizzleds on beta-catenin/armadillo stabilization and Dishevelled phosphorylation. Genes Cells. 2005;10:919–928. doi: 10.1111/j.1365-2443.2005.00889.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Takeda K, Oishi I, Nomi M, Ikeya M, Itoh K, Tamura S, Ueda T, Hatta T, Otani H, Terashima T, Takada S, Yamamura H, Akira S, Minami Y. Mouse Ror2 receptor tyrosine kinase is required for the heart development and limb formation. Genes Cells. 2000;5:71–78. doi: 10.1046/j.1365-2443.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie CC, Lin X, Heasman J. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005;120:857–871. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Yang-Snyder JA, Purcell SM, DeMarais AA, McGrew LL, Moon RT. Activities of the Wnt-1 class of secreted signaling factors are antagonized by the Wnt-5A class and by a dominant negative cadherin in early Xenopus development. J Cell Biol. 1996;133:1123–1137. doi: 10.1083/jcb.133.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivatbutsiri P, Ichinose S, Hytonen M, Sainio K, Eto K, Iseki S. Impaired meningeal development in association with apical expansion of calvarial bone osteogenesis in the Foxc1 mutant. J Anat. 2008;212:603–611. doi: 10.1111/j.1469-7580.2008.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford JB, Vogeli KM, Harland RM. Regulation of convergent extension in Xenopus by Wnt5a and Frizzled-8 is independent of the canonical Wnt pathway. Int J Dev Biol. 2001;45:225–227. [PubMed] [Google Scholar]
- Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407:527–530. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Williams BO, Insogna KL. Where Wnts went: the exploding field of Lrp5 and Lrp6 signaling in bone. J Bone Miner Res. 2009;24:171–178. doi: 10.1359/jbmr.081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte F, Dokas J, Neuendorf F, Mundlos S, Stricker S. Comprehensive expression analysis of all Wnt genes and their major secreted antagonists during mouse limb development and cartilage differentiation. Gene Expr Patterns. 2009;9:215–223. doi: 10.1016/j.gep.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Wong GT, Gavin BJ, McMahon AP. Differential transformation of mammary epithelial cells by Wnt genes. Mol Cell Biol. 1994;14:6278–6286. doi: 10.1128/mcb.14.9.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Nusse R. Ligand receptor interactions in the Wnt signaling pathway in Drosophila. J Biol Chem. 2002;277:41762–41769. doi: 10.1074/jbc.M207850200. [DOI] [PubMed] [Google Scholar]
- Yamada M, Udagawa J, Matsumoto A, Hashimoto R, Hatta T, Nishita M, Minami Y, Otani H. Ror2 is required for midgut elongation during mouse development. Dev Dyn. 2010;239:941–953. doi: 10.1002/dvdy.22212. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Oue N, Sato A, Hasegawa Y, Matsubara A, Yasui W, Kikuchi A. Wnt5a signaling is involved in the aggressiveness of prostate cancer and expression of metalloproteinase. Oncogene. 2010;29:2036–2046. doi: 10.1038/onc.2009.496. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Nishimura O, Misaki K, Nishita M, Minami Y, Yonemura S, Tarui H, Sasaki H. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev Cell. 2008;15:23–36. doi: 10.1016/j.devcel.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Yang Y, Topol L, Lee H, Wu J. Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development. 2003;130:1003–1015. doi: 10.1242/dev.00324. [DOI] [PubMed] [Google Scholar]
- Ying J, Li H, Chen YW, Srivastava G, Gao Z, Tao Q. WNT5A is epigenetically silenced in hematologic malignancies and inhibits leukemia cell growth as a tumor suppressor. Blood. 2007;110:4130–4132. doi: 10.1182/blood-2007-06-094870. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.